Increased Nitrogen Retention and Reduced Methane Emissions of Beef Cattle Grazing Legume vs. Grass Irrigated Pastures in the Mountain West USA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pasture Establishment
2.2. Grazing and Feeding
2.3. Plant Production, Intake, Nutritive Value and Chemical Composition
2.4. Enteric Methane Emissions
2.5. Methane Analysis
2.6. Fecal Output (2017–2018)
2.7. Blood and Urine Collection and Nitrogen Balance (2017–2018)
2.8. Statistical Analysis
3. Results
4. Discussion
4.1. Nutritional Quality and Intake
4.2. Methane Emissions
4.3. Nitrogen Retention and Emissions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADF | Acid detergent fiber |
| BFT | Birdsfoot trefoil |
| BUN | Blood urea nitrogen |
| BW | Body weight |
| CO2 | Carbon dioxide |
| Cr2O3 | Chromic oxide |
| CMV | Cicer milkvetch |
| CT | Condensed tannins |
| CF | Crude fat |
| CP | Crude protein |
| DM | Dry matter |
| DMI | Dry matter intake |
| GHG | Greenhouse gas |
| IVTDMD | In vitro true dry matter digestibility |
| LSMEANS | Least squares means |
| MBG | Meadow bromegrass |
| CH4 | Methane |
| NDFIP | NDF insoluble protein |
| NIRS | Near infrared spectroscopy |
| aNDF | Neutral detergent fiber |
| N | Nitrogen |
| N2O | Nitrous oxide |
| NFC | Non-fiber carbohydrates |
| P2O5 | Phosphorus pentoxide |
| K2O | Potassium oxide |
| RPM | Rising plate meter |
| SF6 | Sulfur hexafluoride |
| TMR | Total mixed ration |
| USU | Utah State University |
References
- Watson, R. Living Beyond Our Means: Natural Assets and Human Well-Being; Millennium Ecosystem Assessment; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Villalba, J.J.; A Beauchemin, K.; Gregorini, P.; Macadam, J.W. Pasture chemoscapes and their ecological services. Transl. Anim. Sci. 2019, 3, 829–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffin, A.W.; Sclater, V.; Swain, H.; Ponce-Campos, G.E.; Seymour, L. Ecosystem Services in Working Lands of the Southeastern USA. Front. Sustain. Food Syst. 2021, 5, 541590. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Lee, M.R.F. Review: Use of human-edible animal feeds by ruminant livestock. Anim. Int. J. Anim. Biosci. 2018, 12, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- USDA-ERS. Feedgrains Sector at a Glance; USDA Economic Research Service: Washington, DC, USA, 2021.
- Shen, J.; Melaku, N.D.; Treu, R.; Wang, J. Inventories of methane and nitrous oxide emissions from animal and crop farms of 69 municipalities in Alberta, Canada. J. Clean. Prod. 2019, 234, 895–911. [Google Scholar] [CrossRef]
- Hendy, C.R.C.; Kleih, U.; Crawshaw, R.; Phillips, M. Livestock and the Environment: Finding a Balance: Interactions between Livestock Production Systems and the Environment: Impact Domain: Concentrate Feed Demand; United Nations Food and Agriculture Organization: Rome, Italy, 1995. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Capper, J.L. Is the Grass Always Greener? Comparing the Environmental Impact of Conventional, Natural and Grass-Fed Beef Production Systems. Animals 2012, 2, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Christensen, R.G.; Yang, S.Y.; Eun, J.-S.; Young, A.J.; Hall, J.O.; MacAdam, J.W. Effects of feeding birdsfoot trefoil hay on neutral detergent fiber digestion, nitrogen utilization efficiency, and lactational performance by dairy cows. J. Dairy Sci. 2015, 98, 7982–7992. [Google Scholar] [CrossRef]
- Hunt, S.R.; Griggs, T.C.; MacAdam, J.W. Change in birdsfoot trefoil (Lotus corniculatus L.) nutritive value with stem elongation, flowering and pod formation. In Proceedings of the European Grassland Federation Change in Birdsfoot Trefoil (Lotus corniculatus) Nutritive Value with Stem Elongation, Flowering and Pod Formation, Aberystwyth, Wales, 7–11 September 2014; Hopkins, A., Ed.; Grassland Science in Europe: Aberystwyth, Wales, 2014; pp. 884–886. [Google Scholar]
- Hunt, S.R.; MacAdam, J.W.; Griggs, T.C. Lignification and tannin localization during the development of birdsfoot trefoil stems. Crop. Sci. 2014, 54, 1876–1886. [Google Scholar] [CrossRef]
- Dewhurst, R.J. Milk production from silage: Comparison of grass, legume and maize silages and their mixtures. Agric. Food Sci. 2013, 22, 57–69. [Google Scholar] [CrossRef] [Green Version]
- Archimède, H.; Eugène, M.; Marie Magdeleine, C.; Boval, M.; Martin, C.; Morgavi, v.; Lecomte, P.; Doreau, M. Comparison of methane production between C3 and C4 grasses and legumes. Anim. Feed Sci. Technol. 2011, 166–167, 59–64. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Henry Janzen, H.; Little, S.M.; McAllister, T.A.; McGinn, S.M. Life cycle assessment of greenhouse gas emissions from beef production in western Canada: A case study. Agric. Syst. 2010, 103, 371–379. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Villalba, J.J. Beneficial Effects of Temperate Forage Legumes that Contain Condensed Tannins. Agriculture 2015, 5, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Chail, A.; Legako, J.F.; Pitcher, L.R.; Griggs, T.C.; Ward, R.E.; Martini, S.; MacAdam, J.W. Legume finishing provides beef with positive human dietary fatty acid ratios and consumer preference comparable with grain-finished beef. J. Anim. Sci. 2016, 94, 2184–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lees, G.L.; Howarth, R.E.; Goplen, B.P. Morphological characteristics of leaves from some legume forages: Relation to digestion and mechanical strength. Can. J. Bot. 1982, 60, 2126–2132. [Google Scholar] [CrossRef]
- Waghorn, G.C.; Ulyatt, M.J.; John, A.; Fisher, M.T. The effect of condensed tannins on the site of digestion of amino acids and other nutrients in sheep fed on Lotus corniculatus L. Br. J. Nutr. 1987, 57, 115–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.B.; Waldron, B.L.; Larson, S.R.; Peel, M.D. Registration of ‘Cache’ Meadow Bromegrass. Crop Sci. 2004, 44, 2263–2264. [Google Scholar] [CrossRef]
- Stewart, E.K.; Beauchemin, K.A.; Dai, X.; MacAdam, J.W.; Christensen, R.G.; Villalba, J.J. Effect of tannin-containing hays on enteric methane emissions and nitrogen partitioning in beef cattle. J. Anim. Sci. 2019, 97, 3286–3299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; MacAdam, J.W.; Villalba, J.J.; Dai, X. In vitro digestibility of mountain-grown irrigated perennial legume, grass and forb forages is influenced by elevated non-fibrous carbohydrates and plant secondary compounds. J. Sci. Food Agric. 2021, 101, 334–340. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Delaby, L.; Moloney, A.; Boland, T.; Lewis, E. Nutritive value of forage legumes used for grazing and silage. Ir. J. Agric. Food Res. 2009, 48, 167–187. [Google Scholar]
- Villalba, J.J.; Ates, S.; MacAdam, J.W. Non-fiber Carbohydrates in Forages and Their Influence on Beef Production Systems. Front. Sustain. Food Syst. 2021, 5, 566338. [Google Scholar] [CrossRef]
- MacAdam, J.W.; Hunt, S.R. Using a Rising Plate Meter to Determine Paddock Size for Rotational Grazing; Utah State University: Logan, UT, USA, 2015. [Google Scholar]
- Pinares-Patiño, C.S.; Franco, F.E.; Molano, G.; Kjestrup, H.; Sandoval, E.; MacLean, S.; Battistotti, M.; Koolaard, J.; Laubach, J. Feed intake and methane emissions from cattle grazing pasture sprayed with canola oil. Livest. Sci. 2016, 184, 7–12. [Google Scholar] [CrossRef]
- Box, L.A.; Edwards, G.R.; Bryant, R.H. Milk production and urinary nitrogen excretion of dairy cows grazing plantain in early and late lactation. N. Z. J. Agric. Res. 2017, 60, 470–482. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, 15th ed.; Association of Analytical Chemists: McLean, VA, USA, 1990. [Google Scholar]
- AOAC. Official Method of Analysis, 19th ed.; Association of Analytical Chemists: Washington DC, USA, 2012. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses; USDA Agricultural Research Service: Washington, DC, USA, 1970.
- Peters, J. Wisconsin Procedures for Soil Testing, Plant Analysis and Feed & Forage Analysis; University of Wisconsin: Madison, WI, USA, 2013. [Google Scholar]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Research Council, National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Undersander, D.; Moore, J.E. Relative Forage Quality. Focus Forage Wis. Coop. Ext. 2002, 4, 1–2. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Weiss, W.P.; Conrad, H.R.; St. Pierre, N.R. A theoretically-based model for predicting total digestible nutrient values off orages and concentrates. Anim. Feed Sci. Technol. 1992, 39, 95–110. [Google Scholar] [CrossRef]
- Grabber, J.H.; Zeller, W.E.; Mueller-Harvey, I. Acetone enhances the direct analysis of procyanidin and prodelphinidin-based condensed tannins in Lotus species by the butanol−HCl−iron assay. J. Agric. Food Chem. 2013, 61, 2669–2678. [Google Scholar] [CrossRef]
- Hagerman, A.E. Extraction of phenolics from plants, Sephadex LH 20 and separation of tannin from non-tannin phenolics. In The Tannin Handbook; Miami University: Oxford, OH, USA, 2011. [Google Scholar]
- Johnson, K.; Huyler, M.; Westberg, H.; Lamb, B.; Zlmmerman, P. Measurement of Methane Emissions from Ruminant Livestock Using a SF6 Tracer Technique. Environ. Sci. Technol. 1994, 28, 359–362. [Google Scholar] [CrossRef]
- Johnson, K.A.; Westberg, H.H.; Michal, J.J.; Cossalman, M.W. The SF6 Tracer Technique: Methane Measurement from Ruminants. In Measuring Methane Production from Ruminants; Springer: Dordrecht, The Netherlands, 2007; pp. 33–67. [Google Scholar]
- Williams, S.R.O.; Moate, P.J.; Hannah, M.C.; Ribaux, B.E.; Wales, W.J.; Eckard, R.J. Background matters with the SF6 tracer method for estimating enteric methane emissions from dairy cows: A critical evaluation of the SF6 procedure. Anim. Feed Sci. Technol. 2011, 170, 265–276. [Google Scholar] [CrossRef]
- Fenton, T.W.; Fenton, M. An improved procedure for the determination of chromic oxide in feed and feces. Can. J. Anim. Sci. 1979, 59, 631–663. [Google Scholar] [CrossRef]
- Parker, W.J.; McCutcheon, S.N.; Carr, D.H. Effect of herbage type and level of intake on the release of chromic oxide from intraruminal controlled release capsules in sheep. N. Z. J. Agric. Res. 1989, 32, 537–546. [Google Scholar] [CrossRef]
- Kolver, E.S.; Muller, L.D. Performance and Nutrient Intake of High Producing Holstein Cows Consuming Pasture or a Total Mixed Ration. J. Dairy Sci. 1998, 81, 1403–1411. [Google Scholar] [CrossRef]
- Bargo, F.; Muller, L.D.; Delahoy, J.E.; Cassidy, T.W. lk Response to Concentrate Supplementation of High Producing Dairy Cows Grazing at Two Pasture Allowances. J. Dairy Sci. 2002, 85, 1777–1792. [Google Scholar] [CrossRef] [Green Version]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Getachew, G.; Robinson, P.H.; DePeters, E.J.; Taylor, S. Relationships between chemical composition, dry matter degradation and in vitro gas production of several ruminant feeds. Anim. Feed Sci. Technol. 2004, 111, 57–71. [Google Scholar] [CrossRef]
- Allen, M.S. Physical constraints on voluntary intake of forages by ruminants. J. Anim. Sci. 1996, 74, 3063–3075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.; Hummel, J.; Clauss, M. The relationship between forage cell wall content and voluntary food intake in mammalian herbivores. Mammal Rev. 2010, 40, 221–245. [Google Scholar] [CrossRef] [Green Version]
- Angela, R.; Moss, A.R.; Jouany, J.-P.; Newbold, J. Methane production by ruminants: Its contribution to global warming. Ann. Zootech. 2000, 49, 231–253. [Google Scholar]
- Johnson, K.A.; Johnson, D.E. Methane emissions from cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef]
- Undersander, D.; Albert, B.; Cosgrove, D.; Johnson, D.; Peterson, P. Pastures for Profit: A Guide to Rotational Grazing; University of Wisconsin Cooperative Extension and University of Minnesota Extension Service; Cooperative Extension Publishing, University of Wisconsin-Extension: Madison, WI, USA, 2002. [Google Scholar]
- Sun, X.; Henderson, G.; Cox, F.; Molano, G.; Harrison, S.J.; Luo, D.; Janssen, P.H.; Pacheco, D. Lambs Fed Fresh Winter Forage Rape (Brassica napus L.) Emit Less Methane than Those Fed Perennial Ryegrass (Lolium perenne L.), and Possible Mechanisms behind the Difference. PLoS ONE 2015, 10, e0119697. [Google Scholar] [CrossRef]
- Woodward, S.L.; Waghorn, G.C.; Laboyrie, P.G. Condensed tannins in birdsfoot trefoil (Lotus corniculatus) reduce methane emissions from dairy cows. Proc. N. Z. Soc. Anim. Prod. 2004, 64, 160–164. [Google Scholar]
- Moreira, G.D.; Lima, P.D.M.T.; Borges, B.O.; Primavesi, O.; Longo, C.; McManus, C.; Abdalla, A.; Louvandini, H. Tropical tanniniferous legumes used as an option to mitigate sheep enteric methane emission. Trop. Anim. Health Prod. 2013, 45, 879–882. [Google Scholar] [CrossRef]
- Wang, S.; Terranova, M.; Kreuzer, M.; Marquardt, S.; Eggerschwiler, L.; Schwarm, A. Supplementation of pelleted hazel (Corylus avellana) leaves decreases methane and urinary nitrogen emissions by sheep at unchanged forage intake. Sci. Rep. 2018, 8, 5427. [Google Scholar] [CrossRef] [PubMed]
- Hristov, A.N.; Bannink, A.; Crompton, L.A.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.K.; Bayat, A.R.; Yáñez-Ruiz, D.R.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Anim. Sci. 2018, 102, 5811–5852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, W.B.; Rickert, K.G.; McKeon, G.M.; Carter, J.O. Simulation studies of nitrogen concentration in the diet of sheep grazing Mitchell and mulga grasslands in western Queensland. Aust. J. Agric. Res. 2000, 51, 163–172. [Google Scholar] [CrossRef]
- Farghaly, M.M.; Abdullah, M.A.M.; Youssef, M.I.M.; Abdel-Rahim, I.R.; Abouelezz, K. Effect of feeding hydroponic barley sprouts to sheep on feed intake, nutrient digestibility, nitrogen retention, rumen fermentation and ruminal enzymes activity. Livest. Sci. 2019, 228, 31–37. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Chaturvedi, O.H.; Karim, S.A.; Singh, V.K.; Sisodiya, S.L. Effect of different levels of concentrate allowances on rumen fluid pH, nutrient digestion, nitrogen retention and growth performance of weaner lambs. Small Rumin. Res. 2007, 72, 178–186. [Google Scholar] [CrossRef]
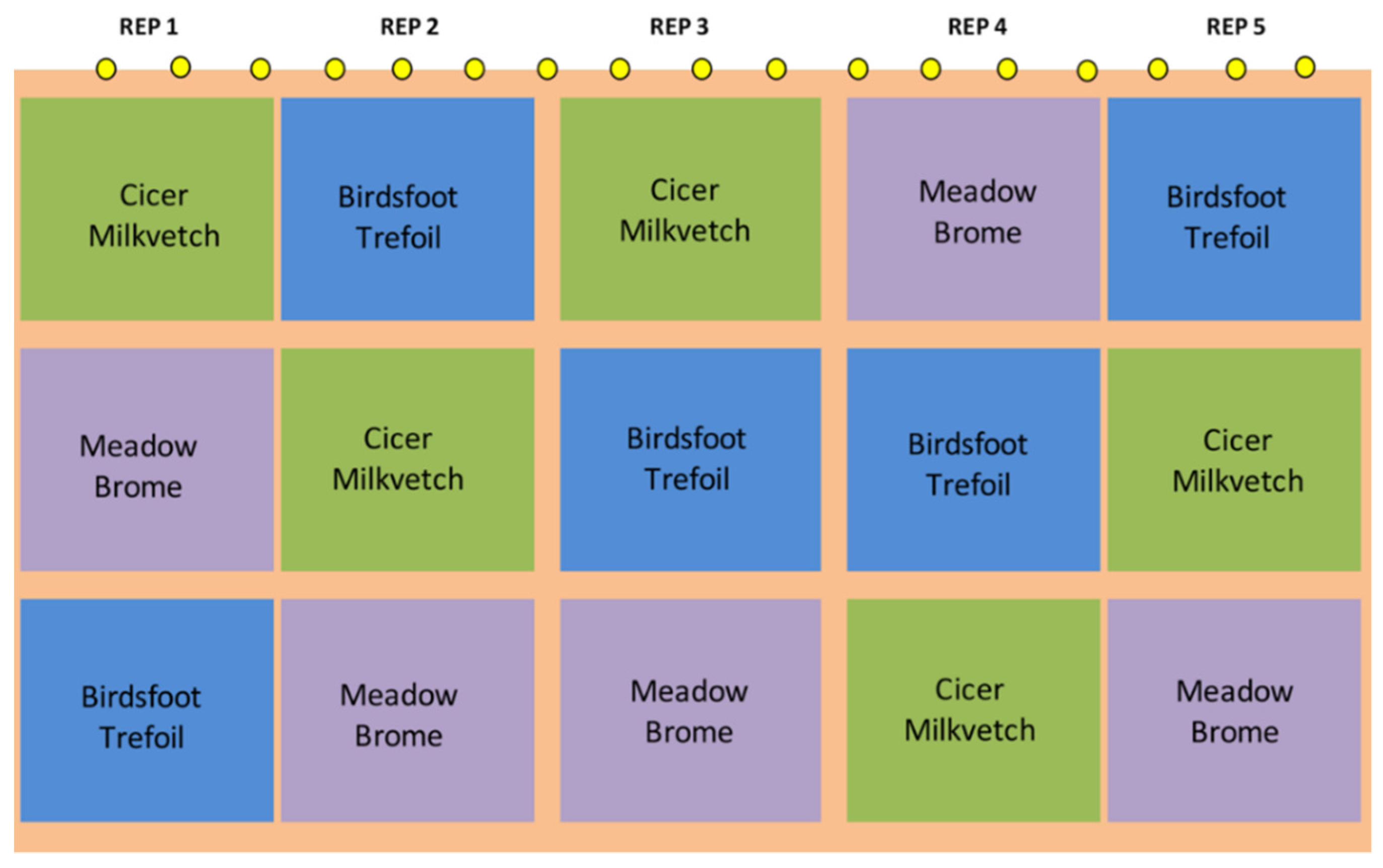
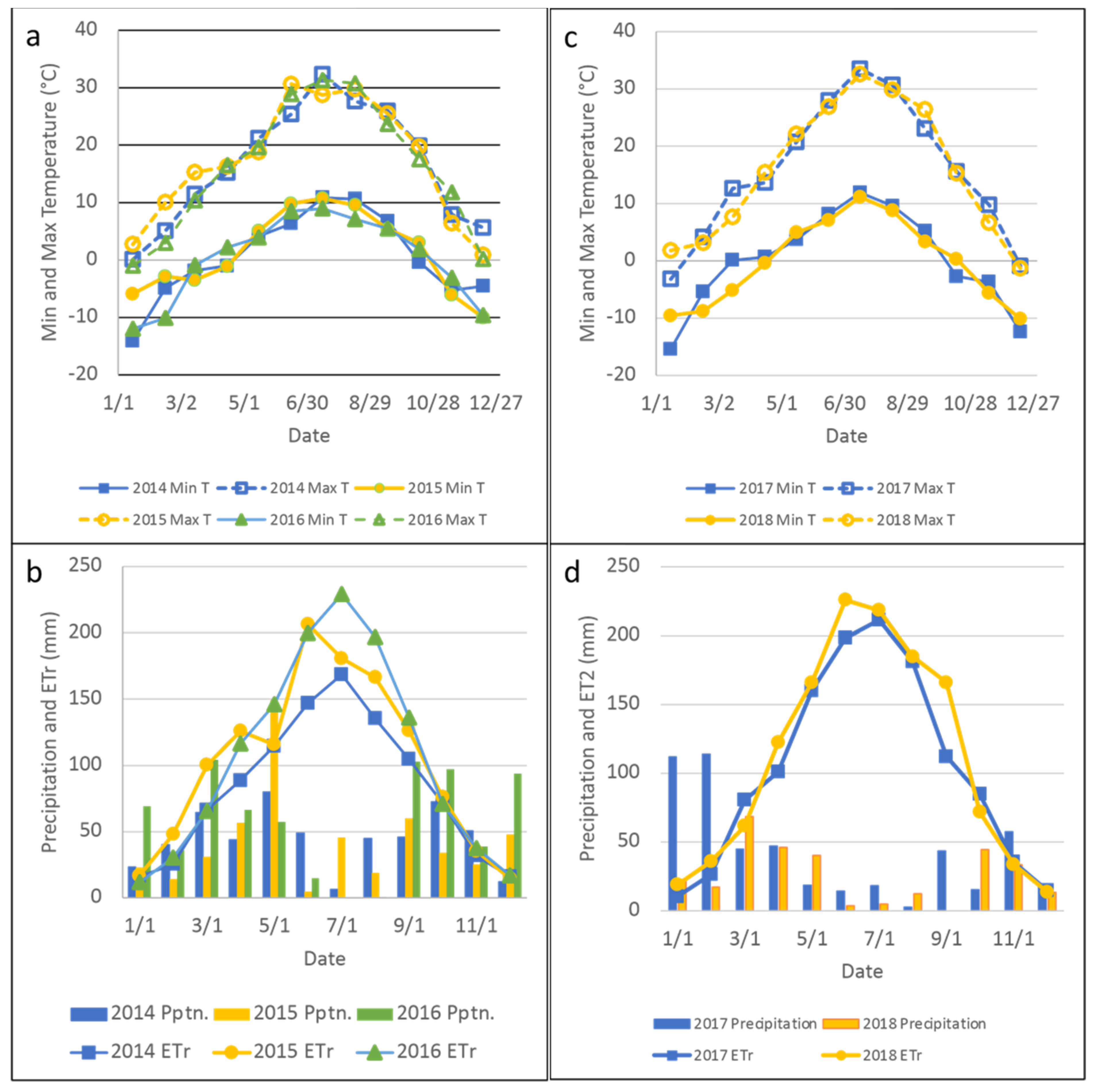
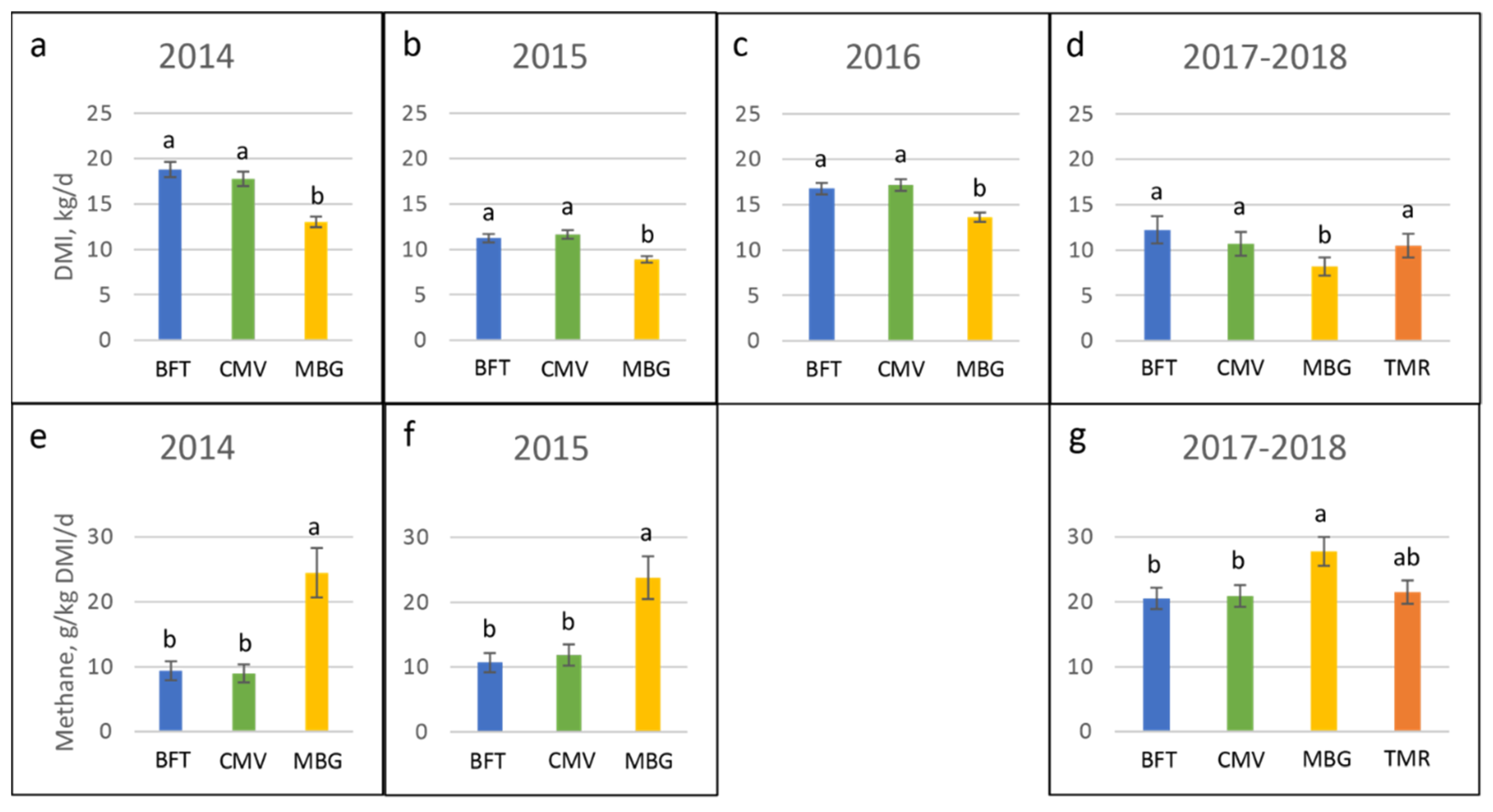
| Year | Breed | Status | n Per Spatial Replication | Initial and Final Body Weight, kg | Start-End Date |
|---|---|---|---|---|---|
| 2014 | Angus | Pregnant cows | 1 | 617 (±8.4) to 689 (±15.9) | 9 June–22 August |
| 2015 | Angus | Yearlings | 2, 2 reps | 414 (±9.7) to 413 (±9.3) | 6 July–21 August |
| 2015 | Charolais | Yearlings | 2, 3 reps | 462 (±9.3) to 450 (±9.7) | 6 July–21 August |
| 2016 | Angus | Dry cows | 1 | 654 (±19.2) to 664 (±17.4) | 31 May–12 August |
| 2017 | Angus | 2-year-old heifers | 1 | 504 (±6.7) to 542 (±7.8) | 22 May–17 August |
| 2018 | Angus | 2-year-old heifers | 1 | 587 (±12.9) to 614 (±13.4) | 29 May–22 August |
| CP | aNDF | ADF | Fat | Lignin | Ash | IVTDMD | NFC | TDN | CT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Species 2 | g kg−1 DM | |||||||||
| 2014 | ||||||||||
| BFT | 271 a | 188 b | 188 b | 24 b | 62 a | 85 c | 942 a | 432 a | 778 a | 22.14 a |
| CMV | 254 b | 186 b | 173 c | 23 c | 60 a | 89 b | 924 b | 444 a | 754 b | 2.84 b |
| MBG | 178 c | 492 a | 311 a | 40 a | 35 b | 126 a | 862 c | 185 b | 572 c | 3.10 b |
| 2015 | ||||||||||
| BFT | 219 b | 324 b | 271 b | 14 b | 67 a | 59 c | 790 b | 382 b | 708 b | 15.12 a |
| CMV | 258 a | 244 c | 220 c | 14 b | 59 b | 76 b | 912 a | 415 a | 773 a | 2.44 b |
| MBG | 199 c | 531 a | 317 a | 24 a | 31 c | 92 a | 805 b | 169 c | 663 c | 3.32 b |
| 2016 | ||||||||||
| BFT | 201 b | 325 b | 251 b | 16 b | 60 a | 52 b | 803 b | 406 a | 735 a | 16.41 a |
| CMV | 228 a | 289 c | 243 b | 14 c | 57 b | 68 a | 867 a | 400 a | 744 a | 1.91 b |
| MBG | 139 c | 583 a | 354 a | 25 a | 39 c | 70 a | 749 c | 194 b | 619 b | 1.73 b |
| 2017 | ||||||||||
| BFT | 206 b | 333 b | 237 b | 20 b | 49 ab | 62 ab | 863 b | 387 ab | 745 a | 19.29 a |
| CMV | 253 a | 250 c | 211 c | 18 c | 45 b | 78 a | 915 b | 408 a | 775 a | 1.89 b |
| MBG | 129 c | 568 a | 348 a | 27 a | 51 a | 40 b | 766 c | 232 b | 618 b | 1.25 b |
| Confinement 1 | 147 | 329 | 206 | 24 | 39 | 85 | 435 | 724 | 0.71 | |
| 2018 | ||||||||||
| BFT | 200 a | 310 b | 233 b | 23 b | 57 a | 60 b | 827 b | 401 a | 754 a | 12.94 a |
| CMV | 226 a | 261 c | 225 b | 15 c | 64 a | 73 a | 870 a | 429 a | 765 a | 0.85 b |
| MBG | 73 b | 639 a | 406 a | 37 a | 49 b | 66 ab | 713 c | 201 b | 557 b | −0.62 b 2 |
| Confinement 1 | 121 | 359 | 248 | 24 | 41 | 85 | 431 | 680 | 0.68 | |
| Means | ||||||||||
| BFT | 219 | 296 | 236 | 19 | 59 | 64 | 845 | 402 | 744 | 17.18 |
| CMV | 244 | 246 | 214 | 17 | 57 | 77 | 898 | 419 | 762 | 1.99 |
| MBG | 144 | 563 | 347 | 31 | 41 | 79 | 779 | 196 | 606 | 1.76 |
| Confinement 1 | 134 | 344 | 227 | 24 | 40 | 85 | 433 | 702 | 0.70 | |
| Species | Pre-Grazing | Post-Grazing | Forage Disappearance | Utilization |
|---|---|---|---|---|
| kg DM/ha | % | |||
| 2014 | ||||
| BFT | 4089 (178) a | 2479 (132) a | 1113 (124) b | 27 |
| CMV | 3781 (165) a | 2031 (105) b | 1528 (120) a | 40 |
| MBG | 2741 (119) b | 2038 (148) b | 1485 (174) a | 54 |
| 2015 | ||||
| BFT | 4687 (132) b | 2719 (98) a | 1998 (139) b | 43 |
| CMV | 5345 (132) a | 2652 (98) a | 2733 (139) a | 51 |
| MBG | 3060 (132) c | 1396 (98) b | 1664 (136) b | 54 |
| 2016 | ||||
| BFT | 5641 (168) b | 3057 (77) b | 2584 (140) a | 46 |
| CMV | 6398 (168) a | 3713 (77) a | 2685 (140) a | 42 |
| MBG | 4862 (168) c | 2805 (77) b | 2047 (140) b | 42 |
| 2017 and 2018 | ||||
| Period 1 | ||||
| BFT | 4568 (684) c | 2547 (402) b | 2365 (412) b | 52 |
| CMV | 7810 (1170) a | 4242 (670) a | 3886 (412) a | 50 |
| MBG | 6232 (933) b | 4653 (735) a | 1705 (412) b | 27 |
| Period 2 | ||||
| BFT | 4030 (603) b | 2821 (446) b | 1035 (412) b | 26 |
| CMV | 6316 (946) a | 3263 (516) b | 3012 (412) a | 48 |
| MBG | 6126 (917) a | 4365 (690) a | 1861 (412) ab | 30 |
| Period 3 | ||||
| BFT | 4665 (698) a | 3022 (478) a | 1698 (412) a | 36 |
| CMV | 5200 (779) a | 3191 (504) a | 2004 (412) a | 39 |
| MBG | 4747 (711) a | 3558 (562) a | 1302 (412) a | 27 |
| BFT | CMV | MBG | Confinement | |
|---|---|---|---|---|
| Dietary N intake, g/d | 425.57 a | 449.40 a | 162.91 b | 201.60 b |
| Urinary N output, g/d | 133.75 a | 131.38 a | 54.07 b | 66.77 b |
| Fecal N output, g/d | 97.72 a | 70.49 b | 68.15 b | 101.73 a |
| Total N excretion, g/d | 221.89 a | 200.66 a | 128.73 b | 157.93 ab |
| N retention, g/d | 202.99 a | 263.57 a | 44.30 b | 42.72 a |
| Urinary N, % of intake | 34.15 ab | 31.87 b | 42.75 a | 22.12 c |
| Fecal N, % of intake | 25.81 b | 17.05 c | 47.98 a | 41.94 a |
| Total N excretion, % of intake | 55.85 bc | 45.87 c | 86.82 a | 64.10 b |
| N retention, % of intake | 42.58 a | 53.65 a | 27.31 b | 39.51 ab |
| Urinary: Fecal N | 1.21 b | 1.70 a | 0.71 c | 0.53 c |
| Blood urea N, mg/dL | 18.24 a | 19.22 a | 7.68 b | 8.96 b |
| Urinary N, g/L | 5.5 a | 4.8 a | 2.7 b | 4.0 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MacAdam, J.W.; Pitcher, L.R.; Bolletta, A.I.; Guevara Ballesteros, R.D.; Beauchemin, K.A.; Dai, X.; Villalba, J.J. Increased Nitrogen Retention and Reduced Methane Emissions of Beef Cattle Grazing Legume vs. Grass Irrigated Pastures in the Mountain West USA. Agronomy 2022, 12, 304. https://doi.org/10.3390/agronomy12020304
MacAdam JW, Pitcher LR, Bolletta AI, Guevara Ballesteros RD, Beauchemin KA, Dai X, Villalba JJ. Increased Nitrogen Retention and Reduced Methane Emissions of Beef Cattle Grazing Legume vs. Grass Irrigated Pastures in the Mountain West USA. Agronomy. 2022; 12(2):304. https://doi.org/10.3390/agronomy12020304
Chicago/Turabian StyleMacAdam, Jennifer W., Lance R. Pitcher, Andrea I. Bolletta, Raul D. Guevara Ballesteros, Karen A. Beauchemin, Xin Dai, and Juan J. Villalba. 2022. "Increased Nitrogen Retention and Reduced Methane Emissions of Beef Cattle Grazing Legume vs. Grass Irrigated Pastures in the Mountain West USA" Agronomy 12, no. 2: 304. https://doi.org/10.3390/agronomy12020304
APA StyleMacAdam, J. W., Pitcher, L. R., Bolletta, A. I., Guevara Ballesteros, R. D., Beauchemin, K. A., Dai, X., & Villalba, J. J. (2022). Increased Nitrogen Retention and Reduced Methane Emissions of Beef Cattle Grazing Legume vs. Grass Irrigated Pastures in the Mountain West USA. Agronomy, 12(2), 304. https://doi.org/10.3390/agronomy12020304







