Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Culture Conditions
2.2. Dry Weight
2.3. Relative Water Content
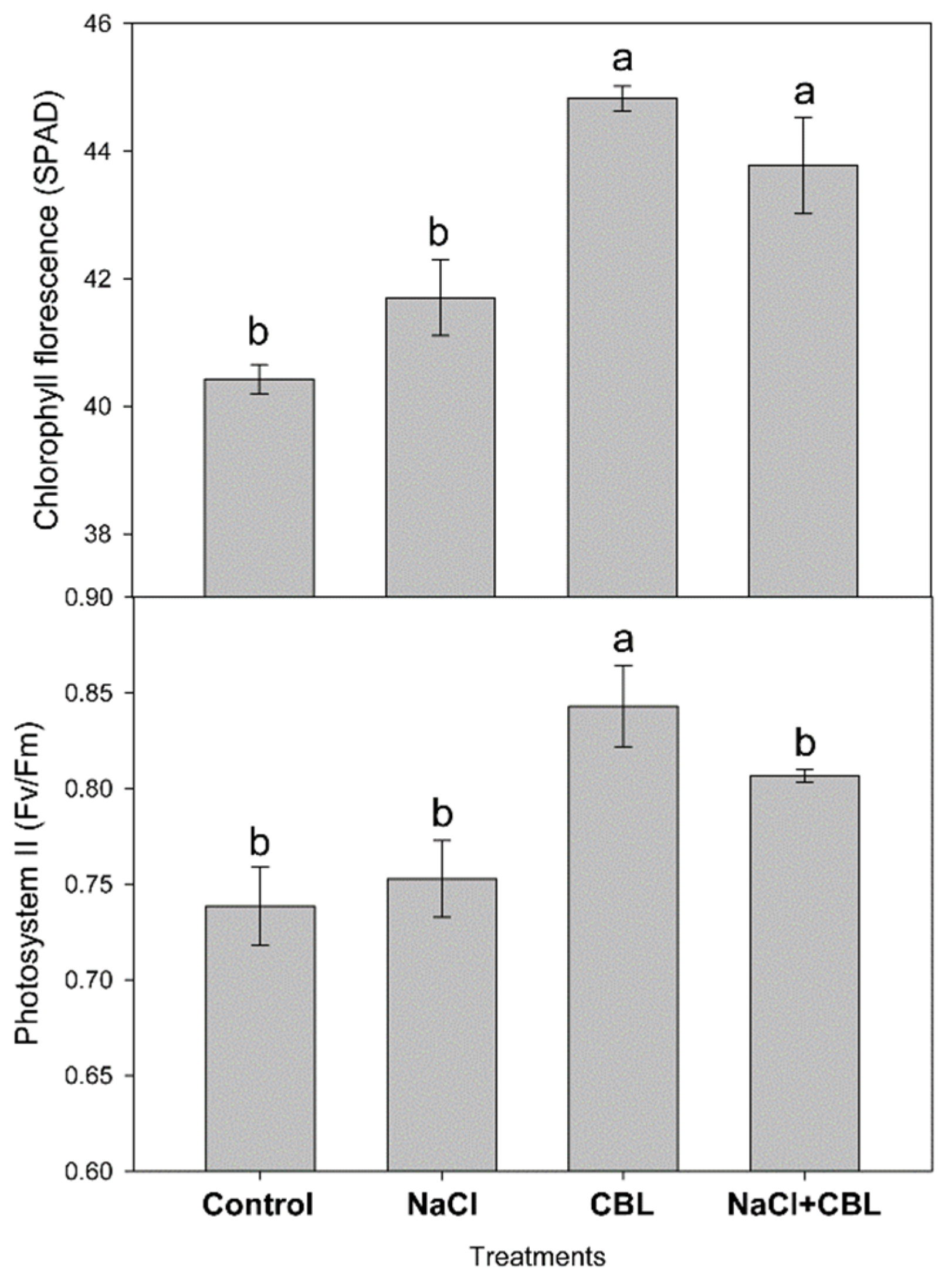
2.4. Chlorophylls and Fluorescence of Photosystem II
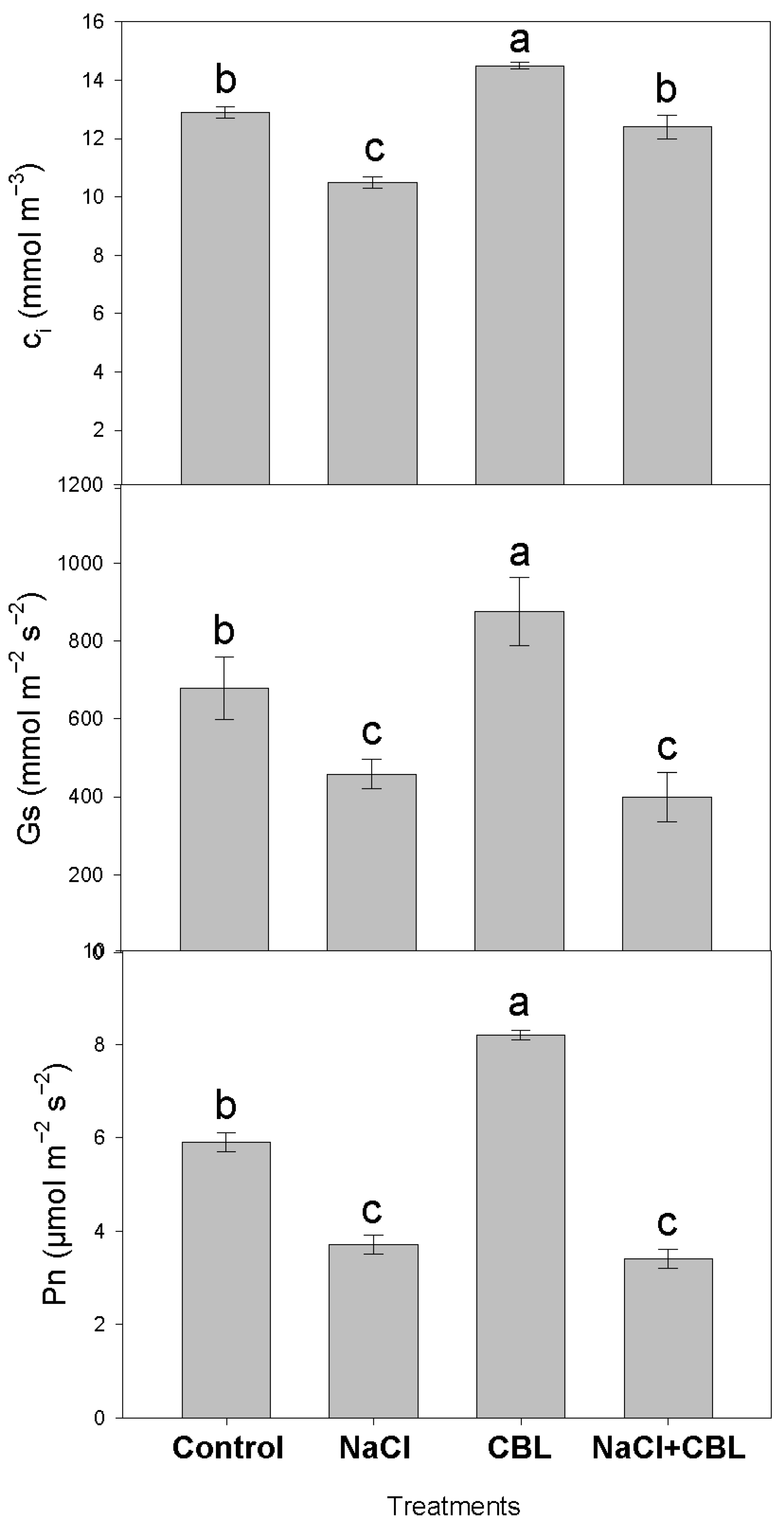
2.5. Gas Exchange Parameters
2.6. Leaf Osmotic Adjustment
2.7. Number of Stomata
2.8. Ion Analysis in Leaf Dry Matter
2.9. Phenolic Extraction and Analysis
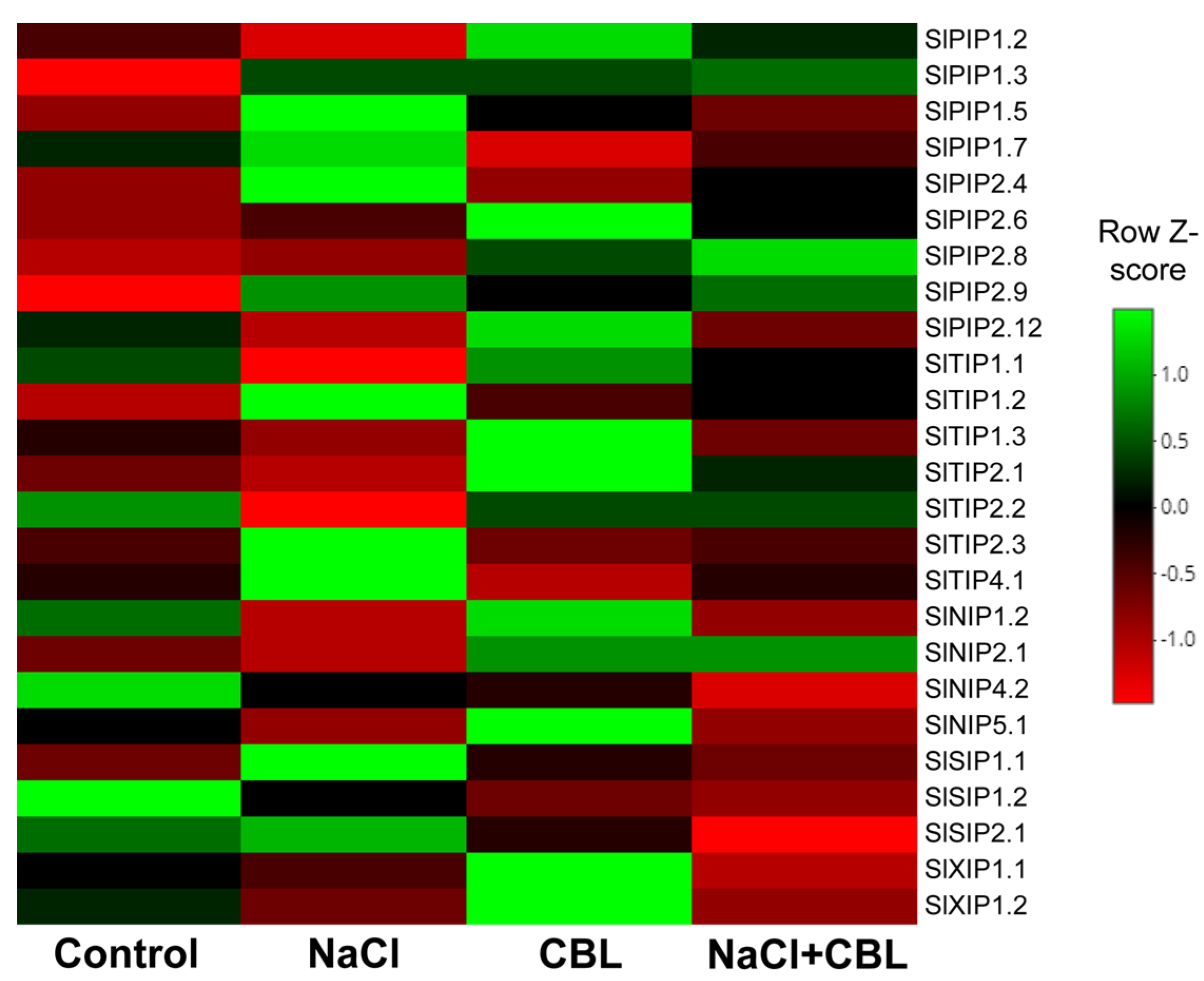
2.10. Aquaporins Expression
2.10.1. RNA Extraction and Reverse Transcription
2.10.2. RNA-Seq Analysis and Differential Expression
2.11. Statistical Analysis
3. Results
3.1. Shoot and Root Dry Weight (DW)
3.2. Relative Water Content (RWC)
3.3. Chlorophylls and Fluorescence of Photosystem II
3.4. Gas Exchange
3.5. Leaf Osmotic Adjustment
3.6. Number of Stomata
3.7. Mineral Content
3.8. Phenolic Compounds Analysis
3.9. Gene Expression of Aquaporins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Chen, S.; Yu, O. Metabolic engineering of flavonoids in plants and microorganisms. Appl. Microbiol. Biotechnol. 2011, 91, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Chen, S.; Wu, F.; Li, Y.; Qian, Y.; Pan, X.; Li, F.; Wang, Y.; Wu, Z.; Fu, C.; Lin, H.; et al. NtMYB4 and NtCHS1 Are Critical Factors in the Regulation of Flavonoid Biosynthesis and Are Involved in Salinity Responsiveness. Front. Plant Sci. 2019, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2013, 77, 367–379. [Google Scholar] [CrossRef]
- Pollastri, S.; Tattini, M. Flavonols: Old compounds for old roles. Ann. Bot. 2011, 108, 1225–1233. [Google Scholar] [CrossRef]
- An, Y.; Feng, X.; Liu, L.; Xiong, L.; Wang, L. ALA-Induced Flavonols Accumulation in Guard Cells Is Involved in Scavenging H2O2 and Inhibiting Stomatal Closure in Arabidopsis Cotyledons. Front. Plant Sci. 2016, 7, 1713. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.F.R.; Alnuaimi, A.K.H.; Askri, A.; Tzortzakis, N. Evaluation of Lettuce (Lactuca sativa L.) Production under Hydroponic System: Nutrient Solution Derived from Fish Waste VS. Inorganic Nutrient Solution. Horticulturae 2021, 7, 292. [Google Scholar] [CrossRef]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar] [CrossRef]
- Barzana, G.; Rios, J.J.; Lopez-Zaplana, A.; Nicolas-Espinosa, J.; Yepes-Molina, L.; Garcia-Ibañez, P.; Carvajal, M. Interrelations of nutrient and water transporters in plants under abiotic stress. Physiol. Plant 2020, 171, 595–619. [Google Scholar] [CrossRef]
- Navarro, J.M.; Martínez, V.; Carvajal, M. Ammonium, bicarbonate and calcium effects on tomato plants grown under saline conditions. Plant Sci. 2000, 157, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, N.; Martínez, V.; Carvajal, M. Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J. Plant Nutr. Soil Sci. 2004, 167, 616–622. [Google Scholar] [CrossRef]
- Bernstein, N. Plants and salt: Plant response and adaptations to salinity. In Model Ecosystems in Extreme Environments; Seckbach, J., Rampelotto, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 101–112. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Zhang, P.; Senge, M.; Dai, Y. Effects of salinity stress on growth, yield, fruit quality and water use efficiency of tomato under hydroponics system. Rev. Agric. Sci. 2016, 4, 46–55. [Google Scholar] [CrossRef]
- Singh, H.; Kumar, P.; Kumar, A.; Kyriacou, M.; Colla, G.; Rouphael, Y. Grafting Tomato as a Tool to Improve Salt Tolerance. Agronomy 2020, 10, 263. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Di Stasio, E.; Van Oosten, M.J.; Silletti, S.; Raimondi, G.; Dell’Aversana, E.; Carillo, P.; Maggio, A. Ascophyllum nodosum-based algal extracts act as enhancers of growth, fruit quality, and adaptation to stress in salinized tomato plants. J. Appl. Phycol. 2018, 30, 2675–2686. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar Applications of Protein Hydrolysate, Plant and Seaweed Extracts Increase Yield but Differentially Modulate Fruit Quality of Greenhouse Tomato. Hortscience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Shad, M.I.; Ashraf, M.A.; Rasheed, R.; Hussain, I.; Ali, S. Exogenous Coumarin Decreases Phytotoxic Effects of Manganese by Regulating Ascorbate–Glutathione Cycle and Glyoxalase System to Improve Photosynthesis and Nutrient Acquisition in Sesame (Sesamum indicum L.). J. Soil Sci. Plant Nutr. 2022. [Google Scholar] [CrossRef]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic Acid and Salicylic Acid Foliar Treatments Reduce Short-Term Salt Stress in Chinese Cabbage by Increasing Phenolic Compounds Accumulation and Photosynthetic Performance. Plants 2021, 10, 2346. [Google Scholar] [CrossRef] [PubMed]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; Ruiz, J.M.; Ferreres, F.; Moreno, D.A. Phenolic profiles of cherry tomatoes as influenced by hydric stress and rootstock technique. Food Chem. 2012, 134, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Burguieres, E.; McCue, P.; Kwon, Y.-I.; Shetty, K. Effect of vitamin C and folic acid on seed vigour response and phenolic-linked antioxidant activity. Bioresour. Technol. 2007, 98, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Yangui, T.; Sayadi, S.; Chakroun, H.; Dhouib, A. Effect of hydroxytyrosol-rich preparations on phenolic-linked antioxidant activity of seeds. Eng. Life Sci. 2011, 11, 511–516. [Google Scholar] [CrossRef]
- Del Amor, F.M.; Martinez, V.; Cerdá, A. Salt Tolerance of Tomato Plants as Affected by Stage of Plant Development. HortScience 2001, 36, 1260–1263. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Hand, M.J.; Taffouo, V.D.; Nouck, A.E.; Nyemene, K.P.; Tonfack, B.; Meguekam, T.L.; Youmbi, E. Effects of Salt Stress on Plant Growth, Nutrient Partitioning, Chlorophyll Content, Leaf Relative Water Content, Accumulation of Osmolytes and Antioxidant Compounds in Pepper (Capsicum annuum L.) Cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 481–490. [Google Scholar] [CrossRef]
- Türkan, I.; Bor, M.; Özdemir, F.; Koca, H. Differential responses of lipid peroxidation and antioxidants in the leaves of drought-tolerant P. acutifolius Gray and drought-sensitive P. vulgaris L. subjected to polyethylene glycol mediated water stress. Plant Sci. 2005, 168, 223–231. [Google Scholar] [CrossRef]
- Tanase, C.; Boz, I.; Stingu, A.; Volf, I.; Popa, V.I. Physiological and biochemical responses induced by spruce bark aqueous extract and deuterium depleted water with synergistic action in sunflower (Helianthus annuus L.) plants. Ind. Crop. Prod. 2014, 60, 160–167. [Google Scholar] [CrossRef]
- Suksungworn, R.; Roytrakul, S.; Gomes, N.G.; Duangsrisai, S. A shotgun proteomic approach reveals protein expression in morphological changes and programmed cell death in Mimosa pigra seedlings after treatment with coumarins. S. Afr. J. Bot. 2021, 142, 370–379. [Google Scholar] [CrossRef]
- Noor, I.; Sohail, H.; Hasanuzzaman, M.; Hussain, S.; Li, G.; Liu, J. Phosphorus confers tolerance against manganese toxicity in Prunus persica by reducing oxidative stress and improving chloroplast ultrastructure. Chemosphere 2021, 291, 132999. [Google Scholar] [CrossRef] [PubMed]
- Mota-Cadenas, C.; Alcaraz-López, C.; Martínez-Ballesta, M.C.; Carvajal, M. How Salinity Affects Co2 Fixation by Horticultural Crops. HortScience 2010, 45, 1798–1803. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Tsai, T.-T.; Tsai, H.-C.; Shih, C.-N. Response of three Glycine species to salt stress. Environ. Exp. Bot. 2006, 56, 120–125. [Google Scholar] [CrossRef]
- Moradi, F.; Ismail, A.M. Responses of Photosynthesis, Chlorophyll Fluorescence and ROS-Scavenging Systems to Salt Stress During Seedling and Reproductive Stages in Rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Martens, S.; Preuß, A.; Matern, U. Multifunctional flavonoid dioxygenases: Flavonol and anthocyanin biosynthesis in Arabidopsis thaliana L. Phytochemistry 2010, 71, 1040–1049. [Google Scholar] [CrossRef]
- Watkins, J.M.; Hechler, P.J.; Muday, G.K. Ethylene-Induced Flavonol Accumulation in Guard Cells Suppresses Reactive Oxygen Species and Moderates Stomatal Aperture. Plant Physiol. 2014, 164, 1707–1717. [Google Scholar] [CrossRef]
- Moghaddam, S.S.; Ibrahim, R.; Damalas, C.A.; Noorhosseini, S.A. Effects of Gamma Stress and Carbon Dioxide on Eight Bioactive Flavonoids and Photosynthetic Efficiency in Centella asiatica. J. Plant Growth Regul. 2017, 36, 957–969. [Google Scholar] [CrossRef]
- Perera, T.; Tirimanne, S. Role of Microbial Communities in Sustainable Rice Cultivation. In Role of Microbial Communities for Sustainability; Seneviratne, G., Zavahir, J., Eds.; Springer: Singapore, 2021; Volume 29, pp. 189–223. [Google Scholar]
- Rehman, A.; Farooq, M.; Naveed, M.; Nawaz, A.; Shahzad, B. Seed priming of Zn with endophytic bacteria improves the productivity and grain biofortification of bread wheat. Eur. J. Agron. 2018, 94, 98–107. [Google Scholar] [CrossRef]
- Carvajal, M.; Cooke, D.; Clarkson, D. Water transport across plant plasma membrane. Plant Growth Regul. 1998, 25, 89–95. [Google Scholar] [CrossRef]
- Cabañero, F.; Martinez-Ballesta, M.; Teruel, J.; Carvajal, M. New evidences about the relationship between aquaporin and calcium in salinity-stressed pepper plants. Plant Cell Physiol. 2006, 47, 224–233. [Google Scholar] [CrossRef]
- Diaz, M.; Bastias, E.; Pacheco, P.; Tapia, L.; Martínez-Ballesta, M.; Carvajal, M. Characterization of the physiological response of the highly-tolerant tomato cv. ‘poncho negro’ to salinity and excess boron. J. Plant Nutr. 2011, 34, 1254–1267. [Google Scholar] [CrossRef]
- Wimmer, M.; Goldbach, H. Boron in the Apoplast of Higher Plants. In The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions: The Significance of the Apoplast for the Mineral Nutrition of Higher Plants; Sattelmacher, B., Horst, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 19–32. [Google Scholar] [CrossRef]
- Sun, Y.; Kong, X.; Li, C.; Liu, Y.; Ding, Z. Potassium Retention under Salt Stress Is Associated with Natural Variation in Salinity Tolerance among Arabidopsis Accessions. PLoS ONE 2015, 10, e0124032. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gamboa, G.; Garde-Cerdán, T.; Martínez-Lapuente, L.; Costa, B.S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P. Phenolic composition of Tempranillo Blanco (Vitis vinifera L.) grapes and wines after biostimulation via a foliar seaweed application. J. Sci. Food Agric. 2019, 100, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Zamljen, T.; Hudina, M.; Veberič, R.; Slatnar, A. Biostimulative effect of amino acids and green algae extract on capsaicinoid and other metabolite contents in fruits of Capsicum spp. Chem. Biol. Technol. Agric. 2021, 8, 63. [Google Scholar] [CrossRef]
- Lefevere, H.; Bauters, L.; Gheysen, G. Salicylic Acid Biosynthesis in Plants. Front. Plant Sci. 2020, 11, 338. [Google Scholar] [CrossRef]
- Abd-Elkader, D.Y.; Mohamed, A.A.; Feleafel, M.N.; Al-Huqail, A.A.; Salem, M.Z.M.; Ali, H.M.; Hassan, H.S. Photosynthetic Pigments and Biochemical Response of Zucchini (Cucurbita pepo L.) to Plant-Derived Extracts, Microbial, and Potassium Silicate as Biostimulants Under Greenhouse Conditions. Front. Plant Sci. 2022, 13, 879545. [Google Scholar] [CrossRef]
- Bárzana, G.; Carvajal, M. Genetic regulation of water and nutrient transport in water stress tolerance in roots. J. Biotechnol. 2020, 324, 134–142. [Google Scholar] [CrossRef]
- Alleva, K.; Niemietz, C.M.; Sutka, M.; Maurel, C.; Parisi, M.; Tyerman, S.D.; Amodeo, G. Plasma membrane of Beta vulgaris storage root shows high water channel activity regulated by cytoplasmic pH and a dual range of calcium concentrations. J. Exp. Bot. 2006, 57, 609–621. [Google Scholar] [CrossRef]
- Singh, R.K.; Deshmukh, R.; Muthamilarasan, M.; Rani, R.; Prasad, M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol. Biochem. 2020, 149, 178–189. [Google Scholar] [CrossRef]
- Hanba, Y.; Shibasaka, M.; Hayashi, Y.; Hayakawa, T.; Kasamo, K.; Terashima, I.; Katuhara, M. PCP Award—Overexpression of the barley aquaporin HvPIP2; 1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol. 2006, 47, S24. [Google Scholar]
- Xin, S.; Yu, G.; Sun, L.; Qiang, X.; Xu, N.; Cheng, X. Expression of tomato SlTIP2;2 enhances the tolerance to salt stress in the transgenic Arabidopsis and interacts with target proteins. J. Plant Res. 2014, 127, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Pou, A.; Medrano, H.; Flexas, J.; Tyerman, S.D. A putative role for TIP and PIP aquaporins in dynamics of leaf hydraulic and stomatal conductances in grapevine under water stress and re-watering. Plant Cell Environ. 2012, 36, 828–843. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, G.; Erice, G.; Aroca, R.; Zamarreño, M.; García-Mina, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis and salicylic acid regulate aquaporins and root hydraulic properties in maize plants subjected to drought. Agric. Water Manag. 2017, 202, 271–284. [Google Scholar] [CrossRef]
- Hu, J.; Li, Y.; Jeong, B. Silicon Alleviates Temperature Stresses in Poinsettia by Regulating Stomata, Photosynthesis and Oxidative Damages. Agronomy 2020, 10, 1419. [Google Scholar] [CrossRef]


| Treatment | Ψw (MPa) | Ψπ (MPa) | Ψp (MPa) |
|---|---|---|---|
| Control | −0.22 ± 0.10 b | −1.01 ± 0.05 b | 0.81 ± 0.01 b |
| NaCl | −0.27 ± 0.07 b | −1.09 ± 0.04 b | 0.82 ± 0.01 b |
| CBL | −0.23 ± 0.02 b | −1.22 ± 0.18 b | 1.02 ± 0.00 a |
| NaCl + CBL | −1.36 ± 0.25 a | −2.02 ± 0.18 a | 0.64 ± 0.05 c |
| Mean Stomata in 0.048 mm2 | |||
|---|---|---|---|
| Treatments | Total Stomata | Stomata Open | Stomata Closed |
| Control | 13.8 ± 0.60 b | 5.5 ± 1.12 bc | 8.3 ± 1.23 a |
| NaCl | 11.3 ± 0.49 b | 3.8 ± 0.54 c | 7.5 ± 0.96 a |
| CBL | 20.5 ± 1.65 a | 12.3 ± 1.82 a | 8.2 ± 1.19 a |
| NaCl + CBL | 12.2 ± 0.79 b | 6.3 ± 0.49 b | 5.8 ± 1.01 a |
| Control | NaCl | CBL | NaCl + CBL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Macronutrients (mmol kg−1 D.W.) | ||||||||||||
| Ca | 716.21 | ± | 7.18 a | 504.31 | ± | 24.21 b | 713.67 | ± | 49.56 a | 782.37 | ± | 54.75 a |
| K | 998.13 | ± | 19.43 a | 618.93 | ± | 14.27 b | 966.92 | ± | 9.78 a | 798.44 | ± | 18.25 ab |
| Mg | 94.27 | ± | 5.18 a | 70.53 | ± | 2.46 b | 97.27 | ± | 9.64 a | 63.06 | ± | 7.25 b |
| Na | 8.01 | ± | 1.36 b | 580.08 | ± | 9.60 a | 4.92 | ± | 0.87 b | 800.48 | ± | 93.52 a |
| Micronutrients (µmol kg−1 D.W.) | ||||||||||||
| Cu | 162.60 | ± | 19.09 a | 195.96 | ± | 19.53 a | 166.25 | ± | 6.66 b | 91.33 | ± | 2.10 b |
| Fe | 1574.36 | ± | 66.69 b | 1757.73 | ± | 161.54 ab | 1881.67 | ± | 61.24 a | 1817.24 | ± | 112.41 a |
| Mo | 146.39 | ± | 2.01 b | 165.39 | ± | 2.93 ab | 149.38 | ± | 3.98 b | 174.32 | ± | 5.41 a |
| Zn | 502.53 | ± | 48.68 a | 508.02 | ± | 32.87 a | 303.14 | ± | 42.03 b | 616.33 | ± | 88.56 a |
| Treatment | Cafeoyl Glucaric Acid (CGA) | Caffeic Acid | Rutin | Total | ||
|---|---|---|---|---|---|---|
| I | II | III | ||||
| Control | 0.93 ± 0.008 b | 1.61 ± 0.02 b | 4 ± 0.03 c | 0.62 ± 0.04 c | 0.52 ± 0.03 c | 7.69 ± 0.12 c |
| NaCl | 1.17 ± 0.05 a | 2 ± 0.06 a | 5.5 ± 0.15 b | 0.8 ± 0.19 b | 0.78 ± 0.03 b | 10.3 ± 0.35 b |
| CBL | 0.83 ± 0.24 b | 2.43 ± 0.13 a | 6.1 ± 0.2 ab | 1.1 ± 0.03 a | 0.85 ± 0.02 a | 11.24 ± 0.5 ab |
| NaCl + CBL | 1.15 ± 0.01 a | 2.72 ± 0.2 a | 6.31 ± 0.15 a | 1.05 ± 0.07 a | 0.82 ± 0.1 a | 12.06 ± 0.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez-Alonso, A.; Garcia-Ibañez, P.; Bárzana, G.; Carvajal, M. Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions. Agronomy 2022, 12, 3230. https://doi.org/10.3390/agronomy12123230
Martinez-Alonso A, Garcia-Ibañez P, Bárzana G, Carvajal M. Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions. Agronomy. 2022; 12(12):3230. https://doi.org/10.3390/agronomy12123230
Chicago/Turabian StyleMartinez-Alonso, Alberto, Paula Garcia-Ibañez, Gloria Bárzana, and Micaela Carvajal. 2022. "Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions" Agronomy 12, no. 12: 3230. https://doi.org/10.3390/agronomy12123230
APA StyleMartinez-Alonso, A., Garcia-Ibañez, P., Bárzana, G., & Carvajal, M. (2022). Leaf Gas Exchange and Growth Responses of Tomato Plants to External Flavonoids Application as Biostimulators under Normal and Salt-Stressed Conditions. Agronomy, 12(12), 3230. https://doi.org/10.3390/agronomy12123230







