Dose–Response Curves of Pelargonic Acid against Summer and Winter Weeds in Central Italy
Abstract
:1. Introduction
1.1. Sustainable Weed Management
1.2. Natural Herbicides and Pelargonic Acid
1.3. Objective, Novelty and Importance of the Study
2. Materials and Methods
2.1. Field Experiments: Site, Description and Treatments
2.2. Measurements
2.3. Statistical Analysis
3. Results and Discussion
3.1. Weed Species and Dose–Response Curves in the Two Experiments
3.2. Winter Weeds
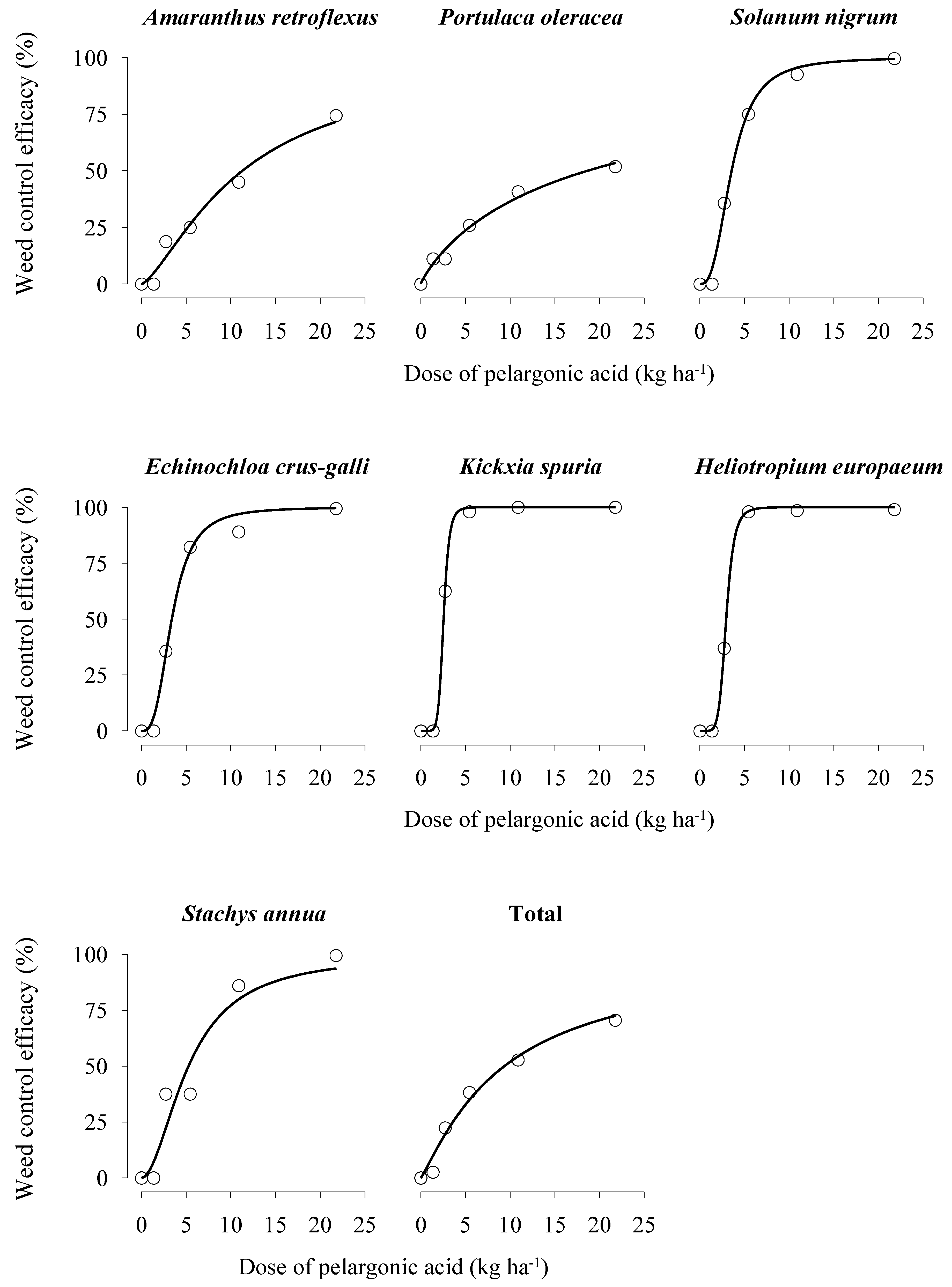
3.3. Summer Weeds
3.4. Total Weeds and Pelargonic Acid Considerations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giannini, V.; Loddo, D.; McElroy, J.S. Integrated weed management: Tools and strategies in a world of pesticide restriction. Ital. J. Agron. 2021, 16, 1981. [Google Scholar] [CrossRef]
- Crmaric, I.; Keller, M.; Krauss, J.; Delabays, N. Efficacy of natural fatty acid based herbicides on mixed weed stands. Jul. Kühn Arch. 2018, 458, 327–332. [Google Scholar] [CrossRef]
- Loddo, D.; McElroy, J.S.; Giannini, V. Problems and perspectives in weed management. Ital. J. Agron. 2021, 16, 1854. [Google Scholar] [CrossRef]
- Pannacci, E.; Lattanzi, B.; Tei, F. Non-chemical weed management strategies in minor crops: A review. Crop Prot. 2017, 96, 44–58. [Google Scholar] [CrossRef]
- Pannacci, E.; Tei, F.; Guiducci, M. Mechanical weed control in organic winter wheat. Ital. J. Agron. 2017, 12, 336–342. [Google Scholar] [CrossRef] [Green Version]
- Pannacci, E.; Tei, F.; Guiducci, M. Evaluation of mechanical weed control in legume crops. Crop Prot. 2018, 104, 52–59. [Google Scholar] [CrossRef]
- Pannacci, E.; Pettorossi, D.; Tei, F. Phytotoxic effects of aqueous extracts of sunflower on seed germination and growth of Sinapis alba L., Triticum aestivum L. and Lolium multiflorum Lam. Allelopath. J. 2013, 32, 23–36. [Google Scholar]
- Pannacci, E.; Pettorossi, D.; Regni, L.; Tei, F. Allelopathic potential of mugwort (Artemisia vulgaris L.) to control the Italian ryegrass (Lolium multiflorum Lam.) in winter wheat. Allelopath. J. 2015, 36, 257–272. [Google Scholar]
- Pannacci, E.; Masi, M.; Farneselli, M.; Tei, F. Evaluation of Mugwort (Artemisia vulgaris L.) Aqueous Extract as a Potential Bioherbicide to Control Amaranthus retroflexus L. in Maize. Agriculture 2020, 10, 642. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16, 149–171. [Google Scholar] [CrossRef]
- Vurro, M.; Miguel-Rojas, C.; Perez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest. Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, S.F.; Holser, R.A. Evaluation of biodiesels from several oilseed sources as environmental friendly contact herbicides. Ind. Crops Prod. 2007, 26, 63–68. [Google Scholar] [CrossRef]
- Fukuda, M.; Tsujino, Y.; Fujimori, K.; Wakabayashi, K.; Böger, P. Phytotoxic activity of middle-chain fatty acids I: Effects on cell constituents. Pestic. Biochem. Physiol. 2004, 80, 143–150. [Google Scholar] [CrossRef]
- Cordeau, S.; Triolet, M.; Wayman, S.; Steinberg, C.; Guillemin, J.P. Bioherbicides: Dead in the water? A review of the existing products for integrated weed management. Crop Prot. 2016, 87, 44–49. [Google Scholar] [CrossRef]
- Korres, N.E.; Burgos, N.R.; Travlos, I.; Vurro, M.; Gitsopoulos, T.K.; Varanasi, V.K.; Duke, S.O.; Kudsk, P.; Brabham, C.; Rouse, C.E.; et al. New directions for integrated weed management: Modern technologies, tools and knowledge discovery. Adv. Agron. 2019, 55, 243–319. [Google Scholar]
- Belz, R.G.; Piepho, H.P. Predicting biphasic responses in binary mixtures: Pelargonic acid versus glyphosate. Chemosphere 2017, 178, 88–98. [Google Scholar] [CrossRef]
- Pannacci, E. Optimization of foramsulfuron doses for post-emergence weed control in maize (Zea mays L.). Span. J. Agric. Res. 2016, 14, e1005. [Google Scholar] [CrossRef] [Green Version]
- Pannacci, E.; Covarelli, G. Efficacy of mesotrione used at reduced doses for post-emergence weed control in maize (Zea mays L.). Crop Prot. 2009, 28, 57–61. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Julius Kühn-Institut: Quedlinburg, Germany, 2018. [Google Scholar]
- Braun-Blanquet, J. Pflanzensoziologie, Grundzüge der Vegetationskunde, 3rd ed.; Springer: Wien, Austria, 1964; p. 866. [Google Scholar] [CrossRef]
- Pannacci, E.; Farneselli, M.; Guiducci, M.; Tei, F. Mechanical weed control in onion seed production. Crop Prot. 2020, 135, 105221. [Google Scholar] [CrossRef]
- Streibig, J.C.; Rudemo, M.; Jensen, J.E. Dose-response curves and statistical models. In Herbicide Bioassay; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 29–55. [Google Scholar]
- Seefeldt, S.S.; Jensen, J.E.; Furst, E.P. Log-logistic analysis of dose-response relationships. Weed Tech. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Onofri, A.; Pannacci, E. Spreadsheet tools for biometry classes in crop science programmes. Commun. Biom. Crop. Sci. 2014, 9, 3–13. [Google Scholar]
- Ritz, C.; Streibig, J.C. Bioassay Analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Kudsk, P.; Streibig, J.C. Adjuvant and formulations. In Herbicide Bioassay; Streibig, J.C., Kudsk, P., Eds.; CRC Press: Boca Raton, FL, USA, 1993; pp. 99–116. [Google Scholar]
- Pannacci, E.; Mathiassen, S.K.; Kudsk, P. The effect of adjuvants on the rainfastness and performance of tribenuron-methyl on broadleaf weeds. Weed Biol. Manag. 2010, 10, 126–131. [Google Scholar] [CrossRef]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Van Der Maarel, E. Transformation of cover-abundance values in phytosociology and its effects on community similarity. Vegetatio 1979, 39, 97–114. [Google Scholar] [CrossRef]
- Ogbangwor, N.; Söchting., H.P. Studies on the efficacy of pelargonic acid for weed control. In Proceedings of the 30th German Conference on Weed Biology and Weed Control, Online, 22–24 February 2022; Volume 468, pp. 424–431. [Google Scholar] [CrossRef]
- Travlos, I.; Kanatas, P.; Tsekoura, A.; Gazoulis, I.; Papastylianou, P.; Kakabouki, I.; Antonopoulos, N. Efficacy of different herbicides on Echinochloa colona (L.) Link control and the first case of its glyphosate resistance in Greece. Agronomy 2020, 10, 1056. [Google Scholar] [CrossRef]
- Covarelli, L.; Contemori, R. Efficacia Erbicida di un Nuovo Disseccante ad Azione di Contatto. Proceedings Giornate Fitopatologiche. Ragusa, Italy, 3–7 May 1998. pp. 423–428. Available online: http://www.giornatefitopatologiche.it/it/elenco/24/1998/efficacia-erbicida-di-un-nuovo-disseccante-ad-azione-di-contatto/1888 (accessed on 23 November 2022).
- Muñoz, M.; Torres-Pagán, N.; Peiró, R.; Guijarro, R.; Sánchez-Moreiras, A.M.; Verdeguer, M. Phytotoxic effects of three natural compounds: Pelargonic acid, carvacrol, and cinnamic aldehyde, against problematic weeds in Mediterranean crops. Agronomy 2020, 10, 791. [Google Scholar] [CrossRef]
- Kanatas, P.; Zavra, S.-M.; Tataridas, A.; Gazoulis, I.; Antonopoulos, N.; Synowiec, A.; Travlos, I. Pelargonic Acid and Caraway Essential Oil Efficacy on Barnyardgrass (Echinochloa crus-galli (L.) P. Beauv.) and Johnsongrass (Sorghum halepense (L.) Pers.). Agronomy 2022, 12, 1755. [Google Scholar] [CrossRef]
- Webber, C.L., III; Taylor, M.J.; Shrefler, J.W. Weed control in yellow squash using sequential postdirected applications of pelargonic acid. HortTechnology 2014, 24, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Kanatas, P.; Antonopoulos, N.; Gazoulis, I.; Travlos, I.S. Screening glyphosate-alternative weed control options in important perennial crops. Weed Sci. 2021, 69, 704–718. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.; Papastylianou, P.; Gazoulis, I.; Kakabouki, I.; Tsekoura, A. Yield, quality and weed control in soybean crop as affected by several cultural and weed management practices. Not. Bot. Hort. Agrobot. 2020, 48, 329–341. [Google Scholar] [CrossRef] [Green Version]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable crop and weed management in the era of the EU Green Deal: A survival guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The herbicidal potential of different pelargonic acid products and essential oils against several important weed species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Coleman, R.; Penner, D. Organic acid enhancement of pelargonic acid. Weed Technol. 2008, 22, 38–41. [Google Scholar] [CrossRef]
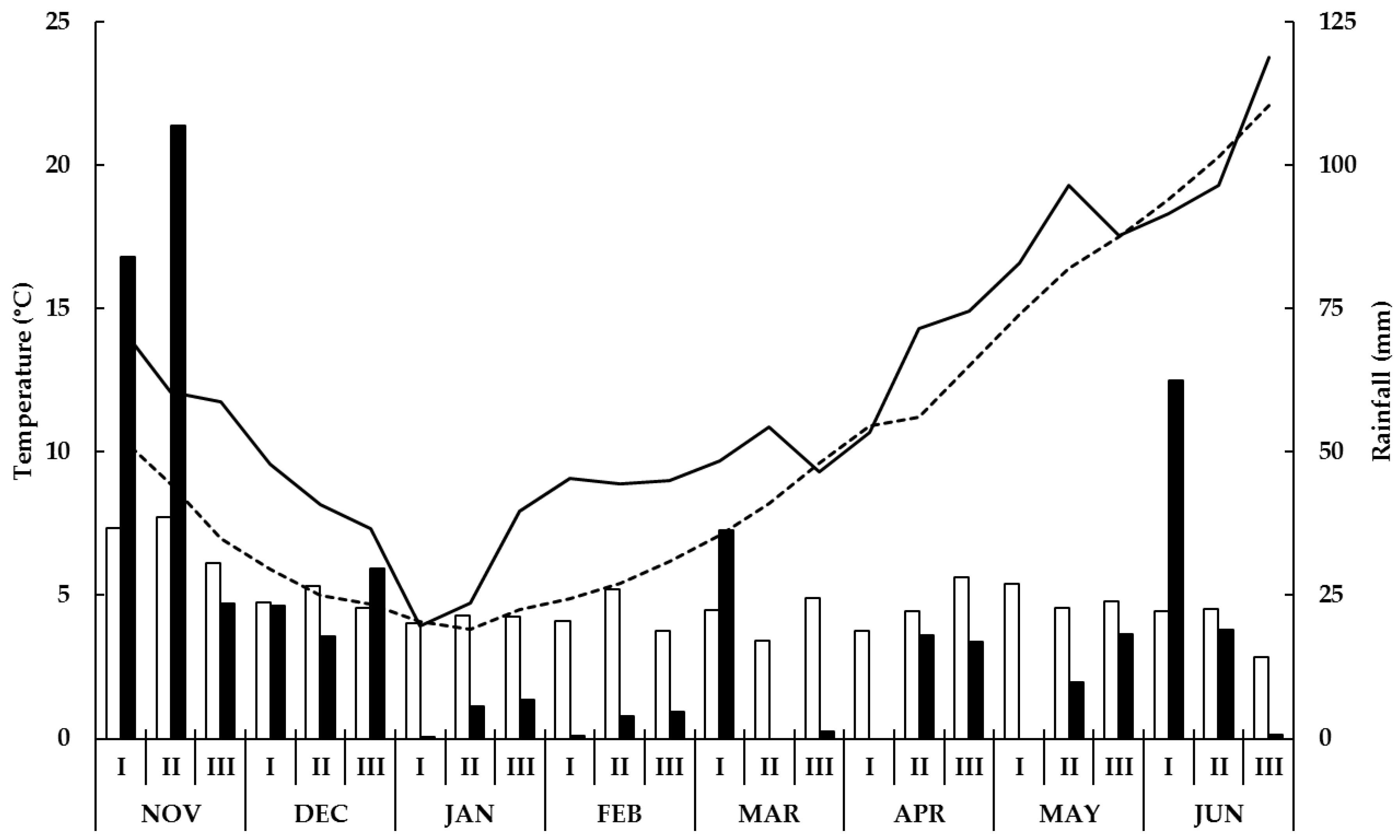



| Weeds Species | BBCH-Scale (at Treatments Time) | 4 WAT | |||||
|---|---|---|---|---|---|---|---|
| Ground Cover (%) | Density (n. Plants m−2) | Dry Biomass (g m−2) | |||||
| Winter experiment | |||||||
| Papaver rhoeas | 14–18 | 17.5 | (0.2) | 155.3 | (17.5) | 14.1 | (1.6) |
| Matricaria chamomilla | 14–18 | 19.4 | (1.9) | 109.3 | (18.4) | 3.2 | (1.1) |
| Lolium multiflorum | 13–21 | 9.7 | (3.0) | 35.3 | (15.7) | 4.0 | (2.4) |
| Veronica hederifolia | 14–22 | 21.3 | (2.2) | 93.3 | (32.3) | 10.3 | (0.6) |
| Total | 67.8 | (6.0) | 393.3 | (50.7) | 31.6 | (1.9) | |
| Summer experiment | |||||||
| Stachys annua | 18–22 | 5 | (0.1) | - | - | ||
| Amaranthus retroflexus | 14–22 | 50 | (0.1) | - | - | ||
| Portulaca oleracea | 14–24 | 84 | (3.1) | - | - | ||
| Echinochloa crus-galli | 21–22 | 18 | (0.1) | - | - | ||
| Kickxia spuria | 16–18 | 5 | (0.1) | - | - | ||
| Solanum nigrum | 12–14 | 18 | (0.1) | - | - | ||
| Heliotropium europaeum | 12–14 | 5 | (0.1) | - | - | ||
| Total | 184.4 | (3.3) | - | - | |||
| Weeds | Weed Ground Cover (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | ED50 (kg ha−1) | ED70 (kg ha−1) | ED90 (kg ha−1) | ED95 (kg ha−1) | ||||||
| Papaver rhoeas | 4.30 | (0.32) | 6.5 | (0.1) | 7.9 | (0.2) | 10.9 | (0.52) | 12.9 | (0.8) |
| Matricaria chamomilla | 2.29 | (0.21) | 11.6 | (0.5) | 16.8 | (1.0) | >21.8 | >21.8 | ||
| Lolium multiflorum | 1.54 | (0.14) | >21.8 | >21.8 | >21.8 | >21.8 | ||||
| Veronica hederifolia | 2.34 | (0.27) | 10.3 | (0.5) | 14.8 | (1.0) | >21.8 | >21.8 | ||
| Total | 2.39 | (0.20) | 9.3 | (0.4) | 13.3 | (0.6) | >21.8 | >21.8 | ||
| Weeds | Weed density (n. plants m−2) | |||||||||
| b | ED50 (kg ha−1) | ED70 (kg ha−1) | ED90 (kg ha−1) | ED95 (kg ha−1) | ||||||
| Papaver rhoeas | 2.36 | (0.32) | 4.2 | (0.1) | 6.1 | (0.2) | 10.8 | (0.8) | 14.8 | (1.4) |
| Matricaria chamomilla | 2.10 | (0.06) | 19.4 | (0.3) | >21.8 | >21.8 | >21.8 | |||
| Lolium multiflorum | 1.01 | (0.21) | >21.8 | >21.8 | >21.8 | >21.8 | ||||
| Veronica hederifolia | 2.47 | (0.22) | 9.2 | (0.4) | 13.0 | (0.6) | >21.8 | >21.8 | ||
| Total | 2.11 | (0.32) | 12.2 | (0.9) | 18.2 | (1.8) | >21.8 | >21.8 | ||
| Weeds | Weed biomass (g m−2) | |||||||||
| b | ED50 (kg ha−1) | ED70 (kg ha−1) | ED90 (kg ha−1) | ED95 (kg ha−1) | ||||||
| Papaver rhoeas | 2.50 | (0.15) | 3.3 | (0.1) | 4.6 | (0.1) | 7.9 | (0.4) | 10.7 | (0.8) |
| Matricaria chamomilla | 2.00 | (0.07) | 14.2 | (0.2) | 21.7 | (0.5) | >21.8 | >21.8 | ||
| Lolium multiflorum | 1.17 | (0.13) | >21.8 | >21.8 | >21.8 | >21.8 | ||||
| Veronica hederifolia | 3.11 | (0.18) | 8.4 | (0.2) | 11.1 | (0.3) | 17.1 | (0.8) | 21.7 | (1.3) |
| Total | 2.16 | (0.20) | 9.8 | (0.4) | 14.5 | (0.9) | >21.8 | >21.8 | ||
| Weeds | Weed Ground Cover (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| b | ED50 (kg ha−1) | ED70 (kg ha−1) | ED90 (kg ha−1) | ED95 (kg ha−1) | ||||||
| Stachys annua | 1.90 | (0.46) | 5.3 | (0.7) | 8.2 | (1.4) | 16.8 | (5.2) | >21.8 | |
| Amaranthus retroflexus | 1.41 | (0.19) | 11.4 | (1.0) | 20.7 | (2.7) | >21.8 | >21.8 | ||
| Portulaca oleracea | 0.89 | (0.06) | 18.7 | (1.2) | >21.8 | >21.8 | >21.8 | |||
| Echinochloa crus-galli | 2.97 | (0.28) | 3.4 | (0.1) | 4.5 | (0.2) | 7.1 | (0.6) | 9.2 | (0.9) |
| Kickxia spuria | 8.01 | (1.99) | 2.6 | (0.04) | 2.8 | (0.03) | 3.4 | (0.2) | 3.7 | (0.3) |
| Solanum nigrum | 2.73 | (0.21) | 3.6 | (0.1) | 4.9 | (0.2) | 8.0 | (0.6) | 10.5 | (0.9) |
| Heliotropium europaeum | 6.53 | (0.73) | 3.0 | (0.03) | 3.4 | (0.1) | 4.1 | (0.2) | 4.6 | (0.3) |
| Total | 1.18 | (0.08) | 9.0 | (0.5) | 18.5 | (1.4) | >21.8 | >21.8 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannacci, E.; Ottavini, D.; Onofri, A.; Tei, F. Dose–Response Curves of Pelargonic Acid against Summer and Winter Weeds in Central Italy. Agronomy 2022, 12, 3229. https://doi.org/10.3390/agronomy12123229
Pannacci E, Ottavini D, Onofri A, Tei F. Dose–Response Curves of Pelargonic Acid against Summer and Winter Weeds in Central Italy. Agronomy. 2022; 12(12):3229. https://doi.org/10.3390/agronomy12123229
Chicago/Turabian StylePannacci, Euro, Daniele Ottavini, Andrea Onofri, and Francesco Tei. 2022. "Dose–Response Curves of Pelargonic Acid against Summer and Winter Weeds in Central Italy" Agronomy 12, no. 12: 3229. https://doi.org/10.3390/agronomy12123229
APA StylePannacci, E., Ottavini, D., Onofri, A., & Tei, F. (2022). Dose–Response Curves of Pelargonic Acid against Summer and Winter Weeds in Central Italy. Agronomy, 12(12), 3229. https://doi.org/10.3390/agronomy12123229








