Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Biometric and Agroproductivity Characteristics Determination
2.4. Physiological Parameters Determination
2.4.1. Total Chlorophyll Content Determination
2.4.2. Photosynthesis Determination
2.4.3. The Color of Leaves
2.5. Extraction and Determination of Phenolic Compounds
2.6. Extraction and Analysis of the Essential Oil Composition
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dou, H.J.; Niu, G.H.; Gu, M.M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, A.; Karik, U. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Walters, K.J.; Currey, C.J. Hydroponic Greenhouse Basil Production: Comparing Systems and Cultivars. HortTechnology 2015, 25, 645–650. [Google Scholar] [CrossRef] [Green Version]
- Attia, H.; Rebah, F.; Ouhibi, C.; Saleh, M.A.; Althobaiti, A.T.; Alamer, K.H.; Ben Nasri, M.; Lachaal, M. Effect of Potassium Deficiency on Physiological Responses and Anatomical Structure of Basil, Ocimum basilicum L. Biology 2022, 11, 1557. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Ciriello, M.; El-Nakhel, C.; Kyriacou, M.C.; Rouphael, Y. Successive Harvests Modulate the Productive and Physiological Behavior of Three Genovese Pesto Basil Cultivars. Agronomy 2021, 11, 560. [Google Scholar] [CrossRef]
- Barickman, T.C.; Olorunwa, O.J.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Yield, Physiological Performance, and Phytochemistry of Basil (Ocimum basilicum L.) under Temperature Stress and Elevated CO2 Concentrations. Plants 2021, 10, 1072. [Google Scholar] [CrossRef]
- Lazarevic, B.; Carovic-Stanko, K.; Satovic, Z. Physiological Responses of Basil (Ocimum Basilicum L.) Cultivars to Rhizophagus Irregularis Inoculation under Low Phosphorus Availability. Plants 2020, 9, 14. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.E.; Morales, M.R.; Phippen, W.B.; Vieira, R.F.; Hao, Z. Basil: A source of aroma compounds and a popular culinary and ornamental herb. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 499–505. [Google Scholar]
- Carovic-Stanko, K.; Liber, Z.; Politeo, O.; Strikic, F.; Kolak, I.; Milos, M.; Satovic, Z. Molecular and chemical characterization of the most widespread Ocimum species. Plant Syst. Evol. 2011, 294, 253–262. [Google Scholar] [CrossRef]
- Varga, F.; Carovic-Stanko, K.; Ristic, M.; Grdisa, M.; Liber, Z.; Satovic, Z. Morphological and biochemical intraspecific characterization of Ocimum basilicum L. Ind. Crops Prod. 2017, 109, 611–618. [Google Scholar] [CrossRef]
- Marotti, M.; Piccaglia, R.; Giovanelli, E. Differences in essential oil composition of basil (Ocimum basilicum L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem. 1996, 44, 3926–3929. [Google Scholar] [CrossRef]
- Onofrei, V.; Burducea, M.; Lobiuc, A.; Teliban, G.-C.; Ranghiuc, G.; Robu, T. Influence of organic foliar fertilization on antioxidant activity and content of polyphenols in Ocimum basilicum L. Acta Pol. Pharm. 2017, 74, 611–615. [Google Scholar] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Evans, W.B.; Ebelhar, M.W. Yield and composition of Ocimum basilicum L. and Ocimum sanctum L. grown at four locations. Hortscience 2008, 43, 737–741. [Google Scholar] [CrossRef] [Green Version]
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet Basil (Ocimum basilicum L.) Productivity and Raw Material Quality from Organic Cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Scagel, C.F.; Lee, J. Phenolic Composition of Basil Plants Is Differentially Altered by Plant Nutrient Status and Inoculation with Mycorrhizal Fungi. HortScience 2012, 47, 660–671. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/ddn-20220222-1#:~:text=The%20area%20used%20for%20organic,utilised%20agricultural%20area%20(UAA) (accessed on 7 November 2022).
- Teliban, G.-C.; Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Munteanu, N.; Mihalache, G.; Cojocaru, A.; Popa, L.-D.; Stoleru, V. The Effect of Myco-Biocontrol Based Formulates on Yield, Physiology and Secondary Products of Organically Grown Basil. Agriculture 2021, 11, 180. [Google Scholar] [CrossRef]
- Inculet, C.-S.; Mihalache, G.; Sellitto, V.M.; Hlihor, R.-M.; Stoleru, V. The Effects of a Microorganisms-Based Commercial Product on the Morphological, Biochemical and Yield of Tomato Plants under Two Different Water Regimes. Microorganisms 2019, 7, 706. [Google Scholar] [CrossRef] [Green Version]
- Mihalache, G.; Zamfirache, M.M.; Mihasan, M.; Ivanov, I.; Stefan, M.; Raus, L. Phosphate-Solubilizing Bacteria Associated with Runner Bean Rhizosphere. Arch. Biol. Sci. 2015, 67, 793–800. [Google Scholar] [CrossRef]
- Caruso, G.; Stoleru, V.V.; Munteanu, N.; Sellitto, V.M.; Teliban, G.C.; Burducea, M.; Tenu, I.; Morano, G.; Butnariu, M. Quality Performances of Sweet Pepper under Farming Management. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 47, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Teliban, G.-C.; Stoleru, V.; Burducea, M.; Lobiuc, A.; Munteanu, N.; Popa, L.-D.; Caruso, G. Biochemical, Physiological and Yield Characteristics of Red Basil as Affected by Cultivar and Fertilization. Agriculture 2020, 10, 48. [Google Scholar] [CrossRef]
- Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.-C.; Burducea, M.; Stoleru, V. Dynamic of Phenolic Compounds, Antioxidant Activity, and Yield of Rhubarb under Chemical, Organic and Biological Fertilization. Plants 2020, 9, 355. [Google Scholar] [CrossRef] [PubMed]
- Le Bot, J.; Bernard, C.; Robin, C.; Bourgaud, F.; Adamowicz, S. The ‘trade-off’ between synthesis of primary and secondary compounds in young tomato leaves is altered by nitrate nutrition: Experimental evidence and model consistency. J. Exp. Bot. 2009, 60, 4301–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bufalo, J.; Cantrell, C.L.; Astatkie, T.; Zheljazkov, V.D.; Gawde, A.; Boaro, C.S.F. Organic versus conventional fertilization effects on sweet basil (Ocimum basilicum L.) growth in a greenhouse system. Ind. Crops Prod. 2015, 74, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Stoleru, V.; Munteanu, N.; Hura, C. Organophosphorus pesticide residues in soil and vegetable, through different growing systems. EEMJ 2015, 14, 1465–1473. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018; ISBN 978-3-95547-071-5. [Google Scholar]
- Burducea, M.; Lobiuc, A.; Asandulesa, M.; Zaltariov, M.-F.; Burducea, I.; Popescu, S.M.; Zheljazkov, V.D. Effects of sewage sludge amendments on the growth and physiology of sweet basil. Agronomy 2019, 9, 548. [Google Scholar] [CrossRef] [Green Version]
- Ekren, S.; Sonmez, C.; Ozcakal, E.; Kurttas, Y.S.K.; Bayram, E.; Gurgulu, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Schwieterman, M.L.; Abrahan, C.E.; Colquhoun, T.A.; Folta, K.M. Light Quality Dependent Changes in Morphology, Antioxidant Capacity, and Volatile Production in Sweet Basil (Ocimum basilicum). Front. Plant Sci. 2016, 7, 1328. [Google Scholar] [CrossRef] [Green Version]
- Bowes, K.M.; Zheljazkov, V.D. Factors affecting yields and essential oil quality of Ocimum sanctum L. and Ocimum basilicum L. cultivars. J. Am. Soc. Hortic. 2004, 129, 789–794. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.J.; Niu, G.H.; Gu, M.M.; Masabni, J.G. Responses of Sweet Basil to Different Daily Light Integrals in Photosynthesis, Morphology, Yield, and Nutritional Quality. Hort. Sci. 2018, 53, 496–503. [Google Scholar] [CrossRef]
- Burducea, M.; Lobiuc, A.; Dirvariu, L.; Oprea, E.; Olaru, S.M.; Teliban, G.-C.; Stoleru, V.; Poghirc, V.A.; Cara, I.G.; Filip, M.; et al. Assessment of the Fertilization Capacity of the Aquaculture Sediment for Wheat Grass as Sustainable Alternative Use. Plants 2022, 11, 634. [Google Scholar] [CrossRef]
- Mocan, A.; Vodnar, D.C.; Vlase, L.; Crișan, O.; Gheldiu, A.-M.; Crișan, G. Phytochemical Characterization of Veronica officinalis L., V. teucrium L. and V. orchidea Crantz from Romania and Their Antioxidant and Antimicrobial Properties. Int. J. Mol. Sci. 2015, 16, 21109–21127. [Google Scholar] [CrossRef] [PubMed]
- Burducea, M.; Zheljazkov, V.D.; Dincheva, I.; Lobiuc, A.; Teliban, G.-C.; Stoleru, V.; Zamfirache, M.-M. Fertilization modifies the essential oil and physiology of basil varieties. Ind. Crops Prod. 2018, 121, 282–293. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/ Mass Spectrometry, 4th ed.; Allured Publ.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Butnariu, M.; Sarac, I.; Samfira, I. Spectrophotometric and chromatographic strategies for exploring of the nanostructure pharmaceutical formulations which contains testosterone undecanoate. Sci. Rep. 2020, 10, 3569. [Google Scholar] [CrossRef] [Green Version]
- Dou, H.J.; Niu, G.H.; Gu, M.M. Photosynthesis, Morphology, Yield, and Phytochemical Accumulation in Basil Plants Influenced by Substituting Green Light for Partial Red and/or Blue Light. Hort. Sci. 2019, 54, 1766–1776. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk-Szabela, D.; Wolf, W.M. The Impact of Soil pH on Heavy Metals Uptake and Photosynthesis Efficiency in Melissa officinalis, Taraxacum officinalis, Ocimum basilicum. Molecules 2022, 27, 4671. [Google Scholar] [CrossRef] [PubMed]
- Nitz, G.M.; Schnitzler, W.H. Effect of PAR and UV-B radiation on the quality and quantity of the essential oil in sweet basil (Ocimum basilicum L.). In Proceedings of the VII International Symposium on Protected Cultivation in Mild Winter Climates: Production, Pest Management and Global Competition, Vols 1 and 2, Kissimmee, FL, USA, 23–27 March 2004; Volume 659, pp. 375–381. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padalia, R.C.; Verma, R.S.; Upadhyay, R.K.; Chauhan, A.; Singh, V.R. Productivity and essential oil quality assessment of promising accessions of Ocimum basilicum L. from north India. Ind. Crops Prod. 2017, 97, 79–86. [Google Scholar] [CrossRef]
- Svecova, E.; Neugebauerová, J. A study of 34 cultivars of basil (Ocimum L.) and their morphological, economic and biochemical characteristics, using standardized descriptors. Acta Univ. Sapientiae Alimentaria 2010, 3, 118–135. [Google Scholar]
- Juskeviciene, D.; Radzevicius, A.; Viskelis, P.; Marockiene, N.; Karkleliene, R. Estimation of Morphological Features and Essential Oil Content of Basils (Ocimum basilicum L.) Grown under Different Conditions. Plants 2022, 11, 1896. [Google Scholar] [CrossRef]
- Matlok, N.; Gorzelany, J.; Stepien, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Burducea, M.; Zheljazkov, V.D.; Lobiuc, A.; Pintilie, C.A.; Virgolici, M.; Silion, M.; Asandulesa, M.; Burducea, I.; Zamfirache, M.M. Biosolids application improves mineral composition and phenolic profile of basil cultivated on eroded soil. Sci. Hortic. 2019, 249, 407–418. [Google Scholar] [CrossRef]
- Rostamikia, Y.; Tabari-Kouchaksaraei, M.; Asgharzadeh, A.; Rahmani, A. Biomass allocation, leaf gas exchange and nutrient uptake of hazelnut seedlings in response to Trichoderma harzianum and Glomus intraradices inoculation. J. Forest Sci. 2017, 63, 219–226. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Cai, P.; Cheng, G.H.; Zhang, Y.Q. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Comite, E.; El-Nakhel, C.; Rouphael, Y.; Ventorino, V.; Pepe, O.; Borzacchiello, A.; Vinale, F.; Rigano, D.; Staropoli, A.; Lorito, M.; et al. Bioformulations with Beneficial Microbial Consortia, a Bioactive Compound and Plant Biopolymers Modulate Sweet Basil Productivity, Photosynthetic Activity and Metabolites. Pathogens 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Sęczyk, L.; Ozdemir, F.A.; Kolodziej, B. In vitro bioaccessibility and activity of basil (Ocimum basilicum L.) phytochemicals as affected by cultivar and postharvest preservation method—Convection drying, freezing, and freeze-drying. Food Chem. 2022, 382, 132363. [Google Scholar] [CrossRef]
- Jakovljevic, D.; Stankovic, M.; Warchol, M.; Skrzypek, E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: A review. Plant Cell Tiss. Organ Cult. 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Menexes, G.; Georgiou, P.; Dordas, C. Effect of Water Stress on the Physiological Characteristics of Five Basil (Ocimum basilicum L.) Cultivars. Agronomy 2020, 10, 1029. [Google Scholar] [CrossRef]
- Sutuliene, R.; Lauzike, K.; Pukas, T.; Samuoliene, G. Effect of Light Intensity on the Growth and Antioxidant Activity of Sweet Basil and Lettuce. Plants 2022, 11, 1709. [Google Scholar] [CrossRef]
- De la Portilla, N.; Vaca, R.; Mora-Herrera, M.E.; Salinas, L.; del Aguila, P.; Yanez-Ocampo, G.; Lugo, J. Soil Amendment with Biosolids and Inorganic Fertilizers: Effects on Biochemical Properties and Oxidative Stress in Basil (Ocimum basilicum L.). Agronomy 2020, 10, 1117. [Google Scholar] [CrossRef]
- Gavric, T.; Jurkovic, J.; Gadzo, D.; Cengic, L.; Sijahovic, E.; Basic, F. Fertilizer effect on some basil bioactive compounds and yield. Cienc. Agrotec. 2021, 45, 1–9. [Google Scholar] [CrossRef]
- Cruz, L.R.O.; Fernandes, A.; Di Gioia, F.; Petropoulos, S.A.; Polyzos, N.; Dias, M.I.; Pinela, J.; Kostic, M.; Sokovic, M.D.; Ferreira, I.C.F.R.; et al. The Effect of Nitrogen Input on Chemical Profile and Bioactive Properties of Green- and Red-Colored Basil Cultivars. Antioxidants 2020, 9, 1036. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.C.S.; Pires, A.S.; Yamamoto, C.H.; Couri, M.R.C.; Taranto, A.G.; Alves, M.S.; Araujo, A.L.D.D.; de Sousa, O.V. Methyl Chavicol and Its Synthetic Analogue as Possible Antioxidant and Antilipase Agents Based on the In Vitro and In Silico Assays. Oxid. Med. Cell. Longev. 2018, 2018, 2189348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool—A Review of a Biologically Active Compound of Commercial Importance. Nat. Prod. Commun. 2008, 3, 1183–1192. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Lv, J.; Xie, J.M.; Yu, J.H.; Li, J.; Zhang, J.; Tang, C.N.; Niu, T.H.; Patience, B.E. Effect of slow-release fertilizer on soil fertility and growth and quality of wintering Chinese chives (Allium tuberm Rottler ex Spreng.) in greenhouses. Sci. Rep. 2021, 11, 8070. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.A.; Gao, W.; Huang, S.W.; Tang, J.W.; Li, M.Y.; Zhang, H.Z.; Chen, X.P.; Masiliunas, D. Substitution of manure for chemical fertilizer affects soil microbial community diversity, structure and function in greenhouse vegetable production systems. PLoS ONE 2020, 15, e0214041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samec, D.; Maretic, M.; Lugaric, I.; Mesic, A.; Salopek-Sondi, B.; Duralija, B. Assessment of the differences in the physical, chemical and phytochemical properties of four strawberry cultivars using principal component analysis. Food Chem. 2015, 194, 828–834. [Google Scholar] [CrossRef]

| Month | Average Temperature (°C) | Atmospheric Humidity (%) | Rainfall (mm) | |||
|---|---|---|---|---|---|---|
| 2019 | 2020 | 2019 | 2020 | 2019 | 2020 | |
| April | 10.7 | 11.1 | 66 | 42 | 6.9 | 1.6 |
| May | 16.6 | 14.4 | 77 | 67 | 74.9 | 130.5 |
| June | 22.7 | 21.3 | 59 | 71 | 8.4 | 99.0 |
| July | 22.0 | 22.1 | 67 | 61 | 3.8 | 7.9 |
| August | 22.1 | 23.6 | 67 | 54 | 35.1 | 8.8 |
| Average/Sum | 18.8 | 18.5 | 67.2 | 59.0 | 129.1 | 247.8 |
| Treatment | Plant Height (cm) | Ramifications (No. per Plant) | No. of Leaves per Plant | Leaf Area Index (LAI) (cm2 per Plant) |
|---|---|---|---|---|
| Cultivar | ||||
| ‘Aromat de Buzau’ | 53.05 ± 1.45 a | 13.22 ± 0.58 b | 473.61 ± 18.70 b | 3387.95 ± 174.59 b |
| ‘Macedon’ | 64.61 ± 4.63 a | 20.55 ± 1.45 a | 618.17 ± 32.69 a | 3886.50 ± 167.79 b |
| ‘Cuisoare’ | 58.61 ± 6.00 a | 14.06 ± 1.00 b | 315.22 ± 8.37 c | 3962.94 ± 98.77 b |
| ‘Serafim’ | 38.89 ± 2.00 b | 14.61 ± 0.58 b | 457.69 ± 34.49 b | 4901.61 ± 307.60 a |
| Fertilization type | ||||
| Chemical | 54.63 ± 2.33 | 15.42 ± 0.67 | 471.28 ± 20.08 | 4319.39 ± 87.72 |
| Organic | 53.79 ± 2.33 | 15.67 ± 1.20 | 460.59 ± 41.09 | 4064.51 ± 353.93 |
| Microorganisms | 52.96 ± 1.76 | 15.75 ± 0.58 | 466.65 ± 38.02 | 3720.35 ± 196.91 |
| n.s. | n.s. | n.s. | n.s. | |
| Treatment | Fresh Yield (g per Plant) | Dry Yield (g per Plant) | ||||
|---|---|---|---|---|---|---|
| Leaves Weight | Stem Weight | Total Weight | Leaves Weight | Stem Weight | Total Weight | |
| Cultivar | ||||||
| ‘Aromat de Buzau’ | 116.17 ± 2.68 b | 208.76 ± 11.03 a | 324.93 ± 10.82 | 15.42 ± 0.19 c | 31.96 ± 1.48 b | 47.38 ± 1.58 b |
| ‘Macedon’ | 133.71 ± 5.32 b | 201.15 ± 17.67 a | 334.86 ± 22.78 | 18.43 ± 0.85 b | 37.94 ± 2.68 a | 56.36 ± 3.35 a |
| ‘Cuisoare’ | 156.59 ± 6.45 a | 172.15 ± 5.83 a | 328.74 ± 6.14 | 22.67 ± 0.63 a | 25.09 ± 0.94 c | 47.77 ± 0.98 b |
| ‘Serafim’ | 169.45 ± 9.82 a | 123.85 ± 5.30 b | 293.31 ± 12.67 | 20.62 ± 1.06 ab | 14.45 ± 0.43 d | 35.07 ± 1.37 c |
| n.s. | ||||||
| Fertilization type | ||||||
| Chemical | 153.87 ± 5.11 | 193.43 ± 10.28 a | 347.30 ± 14.12 a | 20.55 ± 0.74 | 29.49 ± 1.49 a | 50.04 ± 1.97 a |
| Organic | 141.27 ± 9.17 | 178.66 ± 3.61 ab | 319.93 ± 5.60 ab | 18.67 ± 1.00 | 28.40 ± 0.26 a | 47.07 ± 0.84 ab |
| Microorganisms | 136.81 ± 3.21 | 157.34 ± 1.92 b | 294.15 ± 4.54 b | 18.64 ± 0.35 | 24.18 ± 0.46 b | 42.82 ± 0.11 b |
| n.s. | n.s. | |||||
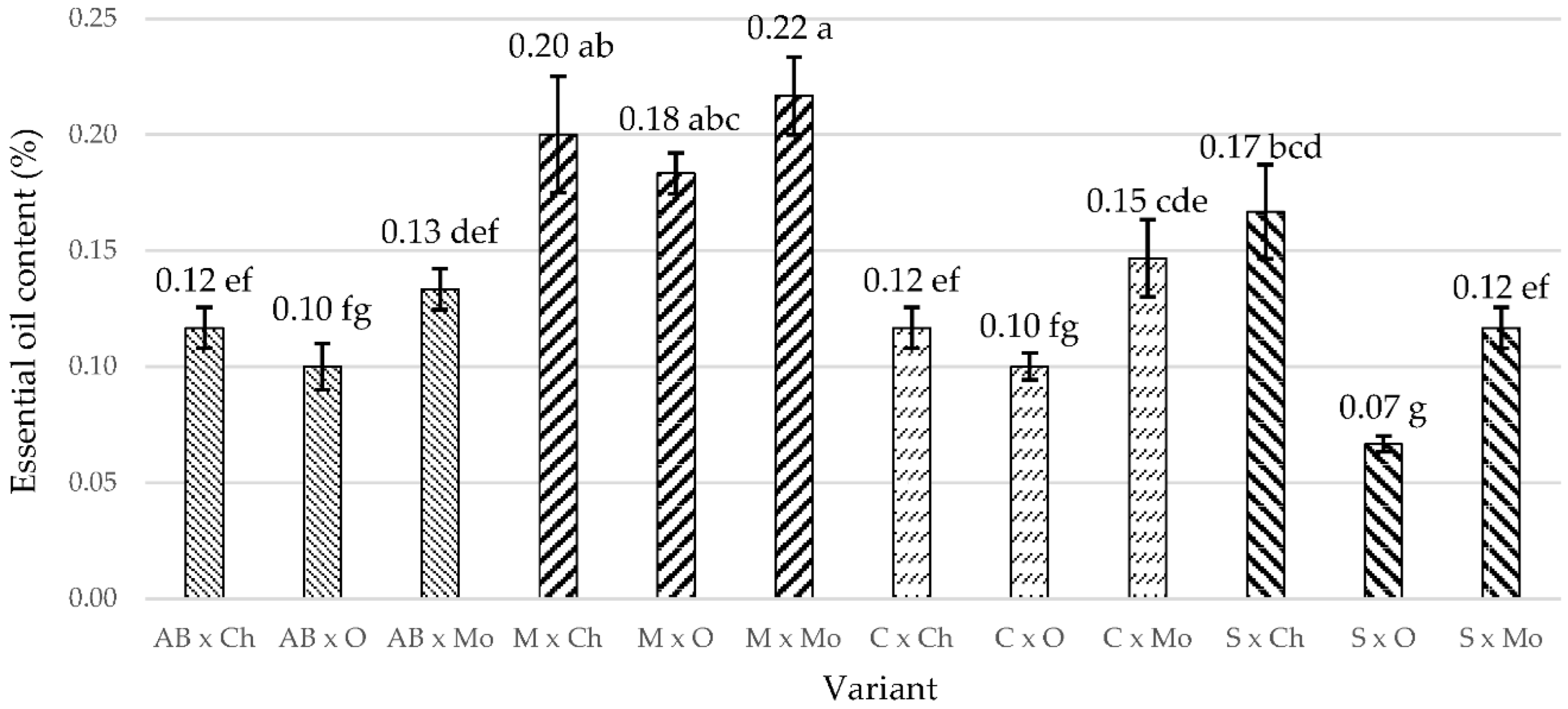
| Treatment | Plant Height (cm) | Ramifications (No. per Plant) | No. of Leaves per Plant | Leaf Area Index (LAI, cm2 per Plant) |
|---|---|---|---|---|
| AB × Ch | 50.83 ± 2.17 bcde | 12.83 ± 0.17 d | 408.67 ± 18.89 cdef | 3332.17 ± 148.13 d |
| AB × O | 48.83 ± 4.51 bcde | 11.33 ± 1.74 d | 480.33 ± 36.32 bcd | 3258.00 ± 217.59 d |
| AB × Mo | 59.50 ± 6.45 ab | 15.50 ± 2.18 bcd | 531.83 ± 77.30 abc | 3573.67 ± 310.89 cd |
| M × Ch | 69.67 ± 4.92 a | 20.33 ± 1.17 ab | 676.33 ± 34.51 a | 4529.17 ± 9.68 abc |
| M × O | 66.33 ± 4.21 ab | 22.50 ± 1.32 a | 606.00 ± 44.26 ab | 3765.67 ± 275.66 cd |
| M × Mo | 57.83 ± 5.93 abc | 18.83 ± 2.73 abc | 572.17 ± 59.09 abc | 3364.67 ± 494.36 cd |
| C × Ch | 60.67 ± 10.54 ab | 14.50 ± 1.32 cd | 353.17 ± 13.17 def | 4238.83 ± 87.13 bcd |
| C × O | 59.33 ± 2.62 ab | 14.50 ± 2.00 cd | 302.33 ± 28.99 ef | 3855.50 ± 281.15 cd |
| C × Mo | 55.83 ± 5.42 abcd | 13.17 ± 0.33 d | 290.17 ± 20.46 f | 3794.50 ± 35.22 cd |
| S × Ch | 37.33 ± 2.46 e | 14.00 ± 1.89 cd | 446.94 ± 34.47 bcdef | 5177.38 ± 261.39 ab |
| S × O | 40.67 ± 6.65 cde | 14.33 ± 1.09 cd | 453.70 ± 123.28 bcdef | 5378.88 ± 909.63 a |
| S × Mo | 38.67 ± 1.48 de | 15.50 ± 1.04 bcd | 472.42 ± 26.63 bcde | 4148.56 ± 117.69 bcd |
| Treatment | Fresh Yield (g per Plant) | Dry Yield (g per Plant) | ||||
|---|---|---|---|---|---|---|
| Leaves Weight | Stem Weight | Total Weight | Leaves Weight | Stem Weight | Total Weight | |
| AB × Ch | 118.76 ± 3.96 cd | 218.90 ± 10.41 ab | 337.65 ± 7.85 ab | 16.15 ± 0.25 de | 33.62 ± 2.79 cd | 49.77 ± 2.74 b |
| AB × O | 108.72 ± 1.67 d | 222.14 ± 12.12 ab | 330.85 ± 13.79 abc | 14.89 ± 0.46 e | 35.13 ± 1.19 bc | 50.01 ± 1.57 b |
| AB × Mo | 121.05 ± 3.68 cd | 185.23 ± 18.81 abcd | 306.28 ± 16.16 bc | 15.23 ± 0.14 e | 27.13 ± 3.17 de | 42.36 ± 3.07 bc |
| M × Ch | 155.58 ± 2.86 ab | 228.78 ± 20.24 a | 384.36 ± 22.51 a | 21.58 ± 0.91 abc | 41.95 ± 1.19 a | 63.52 ± 1.59 a |
| M × O | 130.13 ± 8.53 bcd | 208.96 ± 19.29 abc | 339.09 ± 27.19 ab | 17.90 ± 1.24 cde | 40.87 ± 3.86 ab | 58.77 ± 5.01 a |
| M × Mo | 115.43 ± 16.16 d | 165.69 ± 21.94 cd | 281.13 ± 37.64 bc | 15.80 ± 1.75 e | 30.99 ± 3.26 cde | 46.79 ± 4.55 b |
| C × Ch | 163.87 ± 5.27 a | 177.49 ± 1.29 bcd | 341.37 ± 6.51 ab | 24.63 ± 1.06 a | 25.74 ± 0.90 e | 50.38 ± 1.93 b |
| C × O | 155.67 ± 12.72 ab | 169.73 ± 5.38 cd | 325.40 ± 7.41 abc | 21.01 ± 1.07 abc | 24.56 ± 1.75 e | 45.58 ± 1.64 b |
| C × Mo | 150.23 ± 8.94 abc | 169.23 ± 15.34 cd | 319.47 ± 22.13 abc | 22.36 ± 0.59 ab | 24.98 ± 1.94 e | 47.34 ± 2.44 b |
| S × Ch | 177.29 ± 16.84 a | 148.54 ± 14.10 de | 325.83 ± 29.22 abc | 19.82 ± 1.75 bcd | 16.67 ± 1.28 f | 36.49 ± 2.73 c |
| S × O | 170.55 ± 18.10 a | 113.82 ± 17.77 e | 284.37 ± 6.53 bc | 20.87 ± 2.45 abc | 13.05 ± 1.41 f | 33.92 ± 1.41 c |
| S × Mo | 160.52 ± 3.60 ab | 109.21 ± 7.39 e | 269.72 ± 8.97 c | 21.17 ± 0.74 abc | 13.62 ± 0.75 f | 34.79 ± 0.79 c |
| Treatment | CCI | Photosynthesis µmol m−2 s−1 | L | a | b |
|---|---|---|---|---|---|
| Cultivar | |||||
| ‘Aromat de Buzau’ | 13.60 ± 0.06 d | 2.69 ± 0.23 a | 35.50 ± 0.31 a | −6.21 ± 0.09 c | 13.79 ± 1.44 a |
| ‘Macedon’ | 22.97 ± 0.35 b | 1.30 ± 0.04 c | 33.83 ± 0.05 b | −5.72 ± 0.06 b | 11.52 ± 0.06 a |
| ‘Cuisoare’ | 17.53 ± 0.33 c | 1.81 ± 0.04 b | 34.32 ± 0.44 b | −6.40 ± 0.12 c | 12.75 ± 0.20 a |
| ‘Serafim’ | 35.63 ± 0.66 a | 0.89 ± 0.01 d | 22.83 ± 0.06 c | 1.75 ± 0.08 a | −0.15 ± 0.01 b |
| Fertilization type | |||||
| Chemical | 23.73 ± 0.03 a | 1.47 ± 0.06 b | 31.55 ± 0.12 ab | −4.14 ± 0.05 ab | 8.95 ± 0.10 |
| Organic | 21.93 ± 0.50 b | 1.61 ± 0.13 ab | 31.33 ± 0.16 b | −4.03 ± 0.06 a | 10.02 ± 1.11 |
| Microorganisms | 21.57 ± 0.18 b | 1.93 ± 0.11 a | 31.99 ± 0.16 a | −4.27 ± 0.05 b | 9.46 ± 0.14 |
| n.s. | |||||
| Treatment | CCI | Photosynthesis (µmol m−2 s−1) | L | a | b |
|---|---|---|---|---|---|
| AB × Ch | 14.26 ± 0.09 e | 2.68 ± 0.18 b | 35.04 ± 0.44 ab | −6.20 ± 0.08 d | 11.84 ± 0.35 b |
| AB × O | 13.45 ± 0.32 e | 1.96 ± 0.17 cd | 35.74 ± 0.49 a | −6.19 ± 0.17 d | 16.77 ± 4.02 a |
| AB × Mo | 13.10 ± 0.44 e | 3.43 ± 0.34 a | 35.72 ± 0.25 a | −6.24 ± 0.07 de | 12.76 ± 0.08 b |
| M × Ch | 26.16 ± 1.41 b | 0.95 ± 0.12 fg | 33.95 ± 0.30 b | −5.74 ± 0.13 c | 11.54 ± 0.32 b |
| M × O | 21.14 ± 0.50 c | 1.15 ± 0.36 efg | 32.78 ± 0.33 c | −5.39 ± 0.02 b | 10.96 ± 0.59 b |
| M × Mo | 21.58 ± 0.27 c | 1.79 ± 0.14 cd | 34.78 ± 0.12 ab | −6.04 ± 0.10 cd | 12.06 ± 0.14 b |
| C × Ch | 18.39 ± 0.35 d | 1.50 ± 0.01 def | 33.97 ± 0.75 b | −6.28 ± 0.14 de | 12.43 ± 0.31 b |
| C × O | 17.40 ± 0.49 d | 2.28 ± 0.06 bc | 34.16 ± 0.31 b | −6.33 ± 0.12 de | 12.69 ± 0.09 b |
| C × Mo | 16.81 ± 0.62 d | 1.64 ± 0.08 de | 34.85 ± 0.53 ab | −6.60 ± 0.16 e | 13.15 ± 0.36 b |
| S × Ch | 36.16 ± 0.89 a | 0.75 ± 0.07 g | 23.22 ± 0.20 d | 1.63 ± 0.02 a | 0.01 ± 0.14 c |
| S × O | 35.84 ± 1.81 a | 1.05 ± 0.07 fg | 22.64 ± 0.19 d | 1.78 ± 0.08 a | −0.33 ± 0.09 c |
| S × Mo | 34.84 ± 0.94 a | 0.88 ± 0.03 g | 22.63 ± 0.17 d | 1.83 ± 0.17 a | −0.14 ± 0.08 c |
| Treatment | Caffeic Acid | Hyperoside | Isoquercitrin | Rutin | Quercitrin |
|---|---|---|---|---|---|
| AB × Ch | 5.23 ± 0.46 a | tr | 24.39 ± 2.03 c | 41.53 ± 4.82 de | 3.54 ± 0.48 bc |
| AB × O | 4.97 ± 0.39 ab | tr | 28.86 ± 2.54 bc | 44.95 ± 5.45 d | 4.11 ± 0.23 b |
| AB × Mo | 4.97 ± 0.67 ab | tr | 28.86 ± 2.26 bc | 42.12 ± 2.39 de | 5.79 ± 0.49 a |
| M × Ch | 2.22 ± 0.13 d | 7.04 ± 0.06 b | 35.18 ± 4.77 ab | 38.71 ± 2.28 def | 2.05 ± 0.14 efg |
| M × O | 3.40 ± 0.28 c | 6.62 ± 0.11 c | 34.10 ± 1.91 ab | 32.78 ± 0.63 f | 2.05 ± 0.10 efg |
| M × Mo | 3.41 ± 0.23 c | 7.87 ± 0.09 a | 37.34 ± 3.15 a | 36.93 ± 0.23 ef | 3.35 ± 0.39 bc |
| C × Ch | 3.53 ± 0.17 c | tr | 23.62 ± 1.61 c | 95.27 ± 0.69 c | 1.68 ± 0.20 fg |
| C × O | 3.75 ± 0.43 bc | tr | 34.25 ± 1.68 ab | 116.06 ± 0.23 b | 2.80± 0.16 cde |
| C × Mo | 5.19 ± 0.63 a | tr | 39.49 ± 4.58 a | 130.90 ± 0.61 a | 2.98 ± 0.17 cd |
| S × Ch | 4.97 ± 0.28 ab | tr | 6.52 ± 0.79 d | 10.36 ± 0.09 h | 2.05 ± 0.04 efg |
| S × O | 4.75 ± 0.28 ab | tr | 8.05 ± 0.46 d | 14.51 ± 0.24 gh | 1.30 ± 0.01 g |
| S × Mo | 4.75 ± 0.09 ab | tr | 10.36 ± 0.61 d | 20.30 ± 0.23 g | 2.42 ± 0.02 def |
| No | Name | Class | RIcalc | RIlit | Chemical | Organic | Microorganisms |
|---|---|---|---|---|---|---|---|
| 1 | Eucalyptol (Cineole) | Oxygenated monoterpenes | 1031 | 1030 | tr | 0.25 | 0.21 |
| 2 | cis-β-Ocimene | Monoterpene hydrocarbons | 1040 | 1037 | tr | 0.24 | 0.11 |
| 3 | β-Linalool | Oxygenated monoterpenes | 1095 | 1096 | 13.07 | 25.16 | 22.84 |
| 4 | Cis-thujone | Oxygenated monoterpenes | 1101 | 1102 | 0.19 | 0.12 | 0.11 |
| 5 | Trans-thujone | Oxygenated monoterpenes | 1112 | 1114 | tr | 0.09 | 0.18 |
| 6 | (Z)-β-Ocimene oxide | Oxygenated monoterpenes | 1128 | 1132 | tr | 0.35 | 0.15 |
| 7 | Camphor | Oxygenated monoterpenes | 1141 | 1145 | 0.45 | 1.01 | 0.70 |
| 8 | Methyl chavicol | Phenylpropanoids | 1195 | 1196 | 42.95 | 47.57 | 49.29 |
| 9 | Bornyl acetate | Oxygenated monoterpenes | 1284 | 1285 | 1.36 | 0.70 | 0.58 |
| 10 | Trans-linalool oxide acetate | Oxygenated monoterpenes | 1287 | 1288 | 0.73 | 0.20 | 0.34 |
| 11 | Neryl acetate | Oxygenated monoterpenes | 1359 | 1361 | 0.27 | 0.14 | tr |
| 12 | Geranyl acetate | Oxygenated monoterpenes | 1379 | 1381 | tr | 0.11 | tr |
| 13 | β-Elemene | Sesquiterpene hydrocarbons | 1389 | 1390 | 7.31 | 3.47 | 2.89 |
| 14 | Methyl eugenol | Phenylpropanoids | 1402 | 1403 | 2.19 | 0.44 | 0.64 |
| 15 | β-Caryophyllene | Sesquiterpene hydrocarbons | 1417 | 1419 | 0.48 | 0.40 | 0.35 |
| 16 | α-Guaiene | Sesquiterpene hydrocarbons | 1436 | 1439 | 1.62 | 0.72 | 0.71 |
| 17 | cis-Muurola-3,5-diene | Sesquiterpene hydrocarbons | 1448 | 1450 | 0.36 | tr | tr |
| 18 | trans-Muurola-3,5-diene | Sesquiterpene hydrocarbons | 1451 | 1453 | 0.20 | tr | tr |
| 19 | Humulene (α-Caryophyllene) | Sesquiterpene hydrocarbons | 1454 | 1454 | 1.22 | 0.34 | 0.35 |
| 20 | trans-Muurola-4(14),5-diene | Sesquiterpene hydrocarbons | 1465 | 1466 | 0.60 | 0.12 | 0.21 |
| 21 | Germacrene D | Sesquiterpene hydrocarbons | 1481 | 1481 | 5.87 | 3.60 | 3.16 |
| 22 | Bicyclogermacrene | Sesquiterpene hydrocarbons | 1500 | 1501 | 1.81 | 0.48 | 0.57 |
| 23 | α-Bulnesene | Oxygenated sesquiterpenes | 1510 | 1509 | 3.26 | 1.38 | 1.10 |
| 24 | γ-Cadinene | Sesquiterpene hydrocarbons | 1513 | 1513 | 2.21 | 1.35 | 1.47 |
| 25 | cis-Muurol-5-en-4-β-ol | Oxygenated sesquiterpenes | 1551 | 1552 | 0.57 | 0.23 | 0.12 |
| 26 | Elemicin | Phenylpropanoids | 1555 | 1557 | 2.14 | 0.71 | 0.79 |
| 27 | cis-Muurol-5-en-4-α-ol | Oxygenated sesquiterpenes | 1559 | 1561 | 3.06 | 4.76 | 6.58 |
| 28 | 1,10-di-epi-Cubenol | Oxygenated sesquiterpenes | 1618 | 1619 | 0.91 | 0.40 | 0.35 |
| 29 | 1-epi-Cubenol | Oxygenated sesquiterpenes | 1627 | 1628 | 1.76 | 1.38 | 0.81 |
| 30 | epi-α-Cadinol | Oxygenated sesquiterpenes | 1638 | 1640 | 4.40 | 3.27 | 4.39 |
| tr ≥ 0.03 | |||||||
| Monoterpene hydrocarbons | tr | 0.24 | 0.11 | ||||
| Oxygenated monoterpenes | 16.06 | 28.13 | 25.10 | ||||
| Phenylpropanoids | 47.27 | 48.72 | 50.72 | ||||
| Sesquiterpene hydrocarbons | 21.67 | 10.48 | 9.72 | ||||
| Oxygenated sesquiterpenes | 13.96 | 11.43 | 13.35 | ||||
| No | Name | Class | RIcalc | RIlit | Chemical | Organic | Microorganisms |
|---|---|---|---|---|---|---|---|
| 1 | cis-β-Ocimene | Monoterpene hydrocarbons | 1041 | 1037 | 0.18 | 0.33 | 0.29 |
| 2 | β-Linalool | Oxygenated monoterpenes | 1095 | 1096 | 1.16 | 0.80 | 1.90 |
| 3 | cis-Thujone | Oxygenated monoterpenes | 1101 | 1102 | tr | 0.15 | tr |
| 4 | trans-Thujone | Oxygenated monoterpenes | 1112 | 1114 | 0.17 | 0.17 | 0.40 |
| 5 | Camphor | Oxygenated monoterpenes | 1141 | 1145 | 0.35 | tr | 0.29 |
| 6 | (Z)-Isocitral | Oxygenated monoterpenes | 1163 | 1164 | 0.94 | 1.09 | 0.91 |
| 7 | (E)- Isocitral | Oxygenated monoterpenes | 1179 | 1180 | 1.29 | 1.45 | 1.20 |
| 8 | Methyl chavicol | Phenylpropanoids | 1195 | 1196 | 0.56 | 0.42 | 1.07 |
| 9 | Nerol | Oxygenated monoterpenes | 1227 | 1229 | 12.19 | 11.27 | 8.86 |
| 10 | Neral | Oxygenated monoterpenes | 1235 | 1238 | 20.52 | 25.94 | 24.34 |
| 11 | Geraniol | Oxygenated monoterpenes | 1251 | 1252 | 3.18 | 2.86 | 2.31 |
| 12 | Geranial | Oxygenated monoterpenes | 1265 | 1267 | 26.19 | 32.20 | 29.36 |
| 13 | Neryl acetate | Oxygenated monoterpenes | 1359 | 1361 | 1.71 | 1.28 | 1.19 |
| 14 | α-Copaene | Sesquiterpene hydrocarbons | 1375 | 1376 | 0.48 | 0.42 | 0.39 |
| 15 | Geranyl acetate | Oxygenated monoterpenes | 1379 | 1381 | tr | 0.27 | tr |
| 16 | β-Elemene | Sesquiterpene hydrocarbons | 1389 | 1390 | 0.00 | 0.32 | tr |
| 17 | Methyl eugenol | Phenylpropanoids | 1403 | 1403 | 0.73 | 0.50 | 0.61 |
| 18 | β-Caryophyllene | Sesquiterpene hydrocarbons | 1417 | 1419 | 10.03 | 6.16 | 8.73 |
| 19 | α-trans-Bergamotene | Sesquiterpene hydrocarbons | 1433 | 1434 | 3.02 | 1.83 | 2.52 |
| 20 | Humulene (α-Caryophyllene) | Sesquiterpene hydrocarbons | 1453 | 1454 | 1.69 | 0.97 | 1.42 |
| 21 | (E)-β-Farnesene | Sesquiterpene hydrocarbons | 1455 | 1456 | 1.46 | 0.98 | 1.33 |
| 22 | Sesquisabinene | Sesquiterpene hydrocarbons | 1457 | 1459 | 0.23 | 0.20 | tr |
| 23 | Germacrene D | Sesquiterpene hydrocarbons | 1481 | 1481 | 2.20 | 1.35 | 2.18 |
| 24 | (Z)-γ-Bisabolene | Sesquiterpene hydrocarbons | 1514 | 1515 | 0.43 | 0.42 | 0.37 |
| 25 | (E)-γ-Bisabolene | Sesquiterpene hydrocarbons | 1528 | 1530 | 9.28 | 6.65 | 8.34 |
| 26 | epi-α-Cadinol | Oxygenated sesquiterpenes | 1638 | 1640 | tr | 0.21 | tr |
| tr ≥ 0.03 | |||||||
| Monoterpene hydrocarbons | 0.18 | 0.33 | 0.29 | ||||
| Oxygenated monoterpenes | 67.71 | 77.47 | 70.75 | ||||
| Phenylpropanoids | 1.29 | 0.92 | 1.68 | ||||
| Sesquiterpene hydrocarbons | 28.82 | 19.29 | 25.28 | ||||
| Oxygenated sesquiterpenes | tr | 0.21 | tr | ||||
| No | Name | Class | RIcalc | RIlit | Chemical | Organic | Microorganisms |
|---|---|---|---|---|---|---|---|
| 1 | Sabinene | Monoterpene hydrocarbons | 969 | 974 | 0.09 | 0.06 | tr |
| 2 | Sylvestrene | Monoterpene hydrocarbons | 1026 | 1030 | 0.12 | 0.11 | 0.14 |
| 3 | Eucalyptol (1,8-Cineole) | Oxygenated monoterpenes | 1031 | 1030 | 2.62 | 2.32 | 1.28 |
| 4 | cis-β-Ocimene | Monoterpene hydrocarbons | 1041 | 1037 | 0.47 | 0.69 | 0.42 |
| 5 | Terpinolene | Monoterpene hydrocarbons | 1086 | 1088 | 0.20 | 0.09 | 0.14 |
| 6 | β-Linalool | Oxygenated monoterpenes | 1095 | 1096 | 40.17 | 37.52 | 30.42 |
| 7 | cis-Thujone | Oxygenated monoterpenes | 1101 | 1102 | tr | 0.16 | 0.18 |
| 8 | trans-Thujone | Oxygenated monoterpenes | 1112 | 1114 | tr | 0.08 | 0.11 |
| 9 | (Z)-β-Ocimene oxide | Oxygenated monoterpenes | 1128 | 1132 | 0.63 | 0.58 | 0.32 |
| 10 | Camphor | Oxygenated monoterpenes | 1141 | 1145 | 0.54 | 0.43 | 0.46 |
| 11 | α-Terpineol | Oxygenated monoterpenes | 1188 | 1188 | 0.97 | 1.07 | tr |
| 12 | Methyl chavicol | Phenylpropanoids | 1195 | 1196 | tr | 1.05 | 1.14 |
| 13 | cis-Carveol | Oxygenated monoterpenes | 1229 | 1229 | 0.14 | 0.33 | 0.29 |
| 14 | Geranial | Oxygenated monoterpenes | 1266 | 1267 | 0.18 | 0.42 | 0.38 |
| 15 | Bornyl acetate | Oxygenated monoterpenes | 1284 | 1285 | 3.63 | 1.59 | 2.23 |
| 16 | trans-Linalool oxide acetate | Oxygenated monoterpenes | 1287 | 1288 | 0.12 | 0.19 | 0.24 |
| 17 | Eugenol | Phenylpropanoids | 1356 | 1358 | 9.93 | 11.06 | 8.88 |
| 18 | α-Copaene | Sesquiterpene hydrocarbons | 1375 | 1376 | tr | 0.21 | 0.16 |
| 19 | β-Elemene | Sesquiterpene hydrocarbons | 1389 | 1390 | 4.38 | 5.37 | 6.10 |
| 20 | Methyl eugenol | Phenylpropanoids | 1403 | 1403 | 0.30 | 0.50 | 0.65 |
| 21 | β-Caryophyllene | Sesquiterpene hydrocarbons | 1417 | 1419 | 0.29 | 0.40 | 0.36 |
| 22 | α-trans-Bergamotene | Sesquiterpene hydrocarbons | 1433 | 1434 | 5.03 | 5.33 | 8.12 |
| 23 | α-Guaiene | Sesquiterpene hydrocarbons | 1436 | 1439 | 1.29 | 1.16 | 1.42 |
| 24 | cis-Muurola-3,5-diene | Sesquiterpene hydrocarbons | 1448 | 1450 | 0.36 | 0.40 | tr |
| 25 | trans-Muurola-3,5-diene | Sesquiterpene hydrocarbons | 1451 | 1452 | tr | tr | 0.48 |
| 26 | Humulene (α-Caryophyllene) | Sesquiterpene hydrocarbons | 1453 | 1454 | 1.02 | 0.96 | 1.25 |
| 27 | trans-Muurola-4(14),5-diene | Sesquiterpene hydrocarbons | 1466 | 1466 | 0.59 | 0.64 | 0.79 |
| 28 | Germacrene D | Sesquiterpene hydrocarbons | 1481 | 1481 | 5.94 | 6.26 | 6.85 |
| 29 | Bicyclogermacrene | Sesquiterpene hydrocarbons | 1500 | 1501 | 0.70 | 0.82 | 1.01 |
| 30 | α-Bulnesene | Sesquiterpene hydrocarbons | 1509 | 1509 | 1.99 | 2.09 | 2.68 |
| 31 | γ-Cadinene | Sesquiterpene hydrocarbons | 1513 | 1513 | 3.50 | 3.48 | 4.48 |
| 32 | β-Sesquiphellandrene | Sesquiterpene hydrocarbons | 1522 | 1522 | 0.25 | 0.25 | 0.41 |
| 33 | trans-Nerolidol | Sesquiterpene hydrocarbons | 1561 | 1563 | tr | 0.20 | 0.18 |
| 34 | 5-epi-7-epi-α-Eudesmol | Oxygenated sesquiterpenes | 1605 | 1607 | 1.97 | 0.98 | 1.58 |
| 35 | 1,10-di-epi-Cubenol | Oxygenated sesquiterpenes | 1618 | 1628 | 1.34 | 1.44 | 1.75 |
| 36 | epi-α-Cadinol | Oxygenated sesquiterpenes | 1638 | 1640 | 9.92 | 10.51 | 13.52 |
| tr ≥ 0.03 | |||||||
| Monoterpene hydrocarbons | 0.89 | 0.95 | 0.70 | ||||
| Oxygenated monoterpenes | 49.01 | 44.68 | 35.91 | ||||
| Phenylpropanoids | 10.23 | 12.61 | 10.67 | ||||
| Sesquiterpene hydrocarbons | 25.33 | 27.57 | 34.28 | ||||
| Oxygenated sesquiterpenes | 13.23 | 12.92 | 16.85 | ||||
| No | Name | Class | RIcalc | RIlit | Chemical | Organic | Microorganisms |
|---|---|---|---|---|---|---|---|
| 1 | α-Pinene | Monoterpene hydrocarbons | 932 | 939 | 0.19 | 0.21 | 0.07 |
| 2 | Sabinene | Monoterpene hydrocarbons | 969 | 974 | 0.24 | 0.25 | 0.13 |
| 3 | β-Myrcene | Monoterpene hydrocarbons | 988 | 990 | 0.31 | 0.33 | tr |
| 4 | Limonene | Monoterpene hydrocarbons | 1024 | 1028 | 0.34 | 0.35 | 0.21 |
| 5 | Eucalyptol (1,8-Cineole) | Oxygenated monoterpenes | 1031 | 1030 | 0.62 | 0.65 | 3.95 |
| 6 | cis-β-Ocimene | Monoterpene hydrocarbons | 1041 | 1037 | 0.41 | 0.43 | tr |
| 7 | Fenchone | Oxygenated monoterpenes | 1083 | 1085 | 0.34 | 0.35 | 0.18 |
| 8 | Terpinolene | Monoterpene hydrocarbons | 1086 | 1088 | 0.24 | 0.25 | tr |
| 9 | β-Linalool | Monoterpene hydrocarbons | 1095 | 1096 | 57.49 | 60.80 | 49.52 |
| 10 | Camphor | Oxygenated monoterpenes | 1141 | 1145 | 1.77 | 1.87 | 1.19 |
| 11 | α-Terpineol | Oxygenated monoterpenes | 1188 | 1188 | 1.43 | 1.51 | 1.22 |
| 12 | endo-Fenchyl acetate | Oxygenated monoterpenes | 220 | 1221 | 0.33 | 0.35 | 0.37 |
| 13 | cis-Carveol | Oxygenated monoterpenes | 1229 | 1229 | 0.26 | 0.27 | 0.20 |
| 14 | Geranial | Oxygenated monoterpenes | 1266 | 1267 | 0.35 | 0.37 | 0.28 |
| 15 | Bornyl acetate | Oxygenated monoterpenes | 1254 | 1285 | 0.43 | 0.45 | 0.59 |
| 16 | Eugenol | Phenylpropanoids | 1356 | 1358 | 8.34 | 6.81 | 10.37 |
| 17 | α-Copaene | Sesquiterpene hydrocarbons | 1375 | 1376 | 0.20 | 0.21 | 0.24 |
| 18 | β-Elemene | Sesquiterpene hydrocarbons | 1389 | 1390 | 6.55 | 6.92 | 7.78 |
| 19 | Methyl eugenol | Phenylpropanoids | 1403 | 1403 | 0.33 | 0.75 | 0.02 |
| 20 | β-Caryophyllene | Sesquiterpene hydrocarbons | 1417 | 1419 | 1.55 | 1.33 | 1.41 |
| 21 | α-trans-Bergamotene | Sesquiterpene hydrocarbons | 1433 | 1434 | 0.60 | 0.64 | 1.70 |
| 22 | α-Guaiene | Sesquiterpene hydrocarbons | 1436 | 1439 | 1.55 | 1.64 | 1.90 |
| 23 | Humulene (α-Caryophyllene) | Sesquiterpene hydrocarbons | 1454 | 1454 | 0.00 | 0.00 | 0.57 |
| 24 | Germacrene D | Sesquiterpene hydrocarbons | 1481 | 1481 | 5.24 | 4.54 | 6.23 |
| 25 | β-Selinene | Sesquiterpene hydrocarbons | 1489 | 1490 | 0.26 | 0.28 | 0.45 |
| 26 | Bicyclogermacrene | Sesquiterpene hydrocarbons | 1500 | 1501 | tr | 0.08 | 0.68 |
| 27 | α-Bulnesene | Sesquiterpene hydrocarbons | 1509 | 1509 | 2.79 | 1.95 | 3.44 |
| 28 | γ-Cadinene | Sesquiterpene hydrocarbons | 1513 | 1513 | 1.37 | 1.45 | 1.82 |
| 29 | 1,10-di-epi-Cubenol | Oxygenated sesquiterpenes | 1618 | 1628 | 0.50 | 0.53 | 0.72 |
| 30 | epi-α-Cadinol | Oxygenated sesquiterpenes | 1638 | 1640 | 3.81 | 3.02 | 2.99 |
| tr ≥ 0.03 | |||||||
| Monoterpene hydrocarbons | 59.21 | 62.63 | 49.93 | ||||
| Oxygenated monoterpenes | 5.53 | 5.84 | 7.99 | ||||
| Phenylpropanoids | 8.67 | 7.57 | 10.40 | ||||
| Sesquiterpene hydrocarbons | 20.13 | 19.04 | 26.21 | ||||
| Oxygenated sesquiterpenes | 4.31 | 3.55 | 3.72 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teliban, G.-C.; Burducea, M.; Mihalache, G.; Zheljazkov, V.D.; Dincheva, I.; Badjakov, I.; Popa, L.-D.; Bodale, I.; Vlăduț, N.-V.; Cojocaru, A.; et al. Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types. Agronomy 2022, 12, 3219. https://doi.org/10.3390/agronomy12123219
Teliban G-C, Burducea M, Mihalache G, Zheljazkov VD, Dincheva I, Badjakov I, Popa L-D, Bodale I, Vlăduț N-V, Cojocaru A, et al. Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types. Agronomy. 2022; 12(12):3219. https://doi.org/10.3390/agronomy12123219
Chicago/Turabian StyleTeliban, Gabriel-Ciprian, Marian Burducea, Gabriela Mihalache, Valtcho D. Zheljazkov, Ivayla Dincheva, Ilian Badjakov, Lorena-Diana Popa, Ilie Bodale, Nicolae-Valentin Vlăduț, Alexandru Cojocaru, and et al. 2022. "Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types" Agronomy 12, no. 12: 3219. https://doi.org/10.3390/agronomy12123219
APA StyleTeliban, G.-C., Burducea, M., Mihalache, G., Zheljazkov, V. D., Dincheva, I., Badjakov, I., Popa, L.-D., Bodale, I., Vlăduț, N.-V., Cojocaru, A., Munteanu, N., Stan, T., Caruso, G., & Stoleru, V. (2022). Morphological, Physiological and Quality Performances of Basil Cultivars under Different Fertilization Types. Agronomy, 12(12), 3219. https://doi.org/10.3390/agronomy12123219











