Effects of Phenological Changes on Plant Production—From the View of Stipa krylovii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Soil Temperature and Water Content Measurements
2.4. Phenology Observation
2.5. Leaf Gas Exchange Parameters and Dry Mass Measurements
2.6. Statistical Analysis
3. Results
3.1. Changes in Soil Water Content and Temperature
3.2. Changes in the Phenology of Stipa krylovii
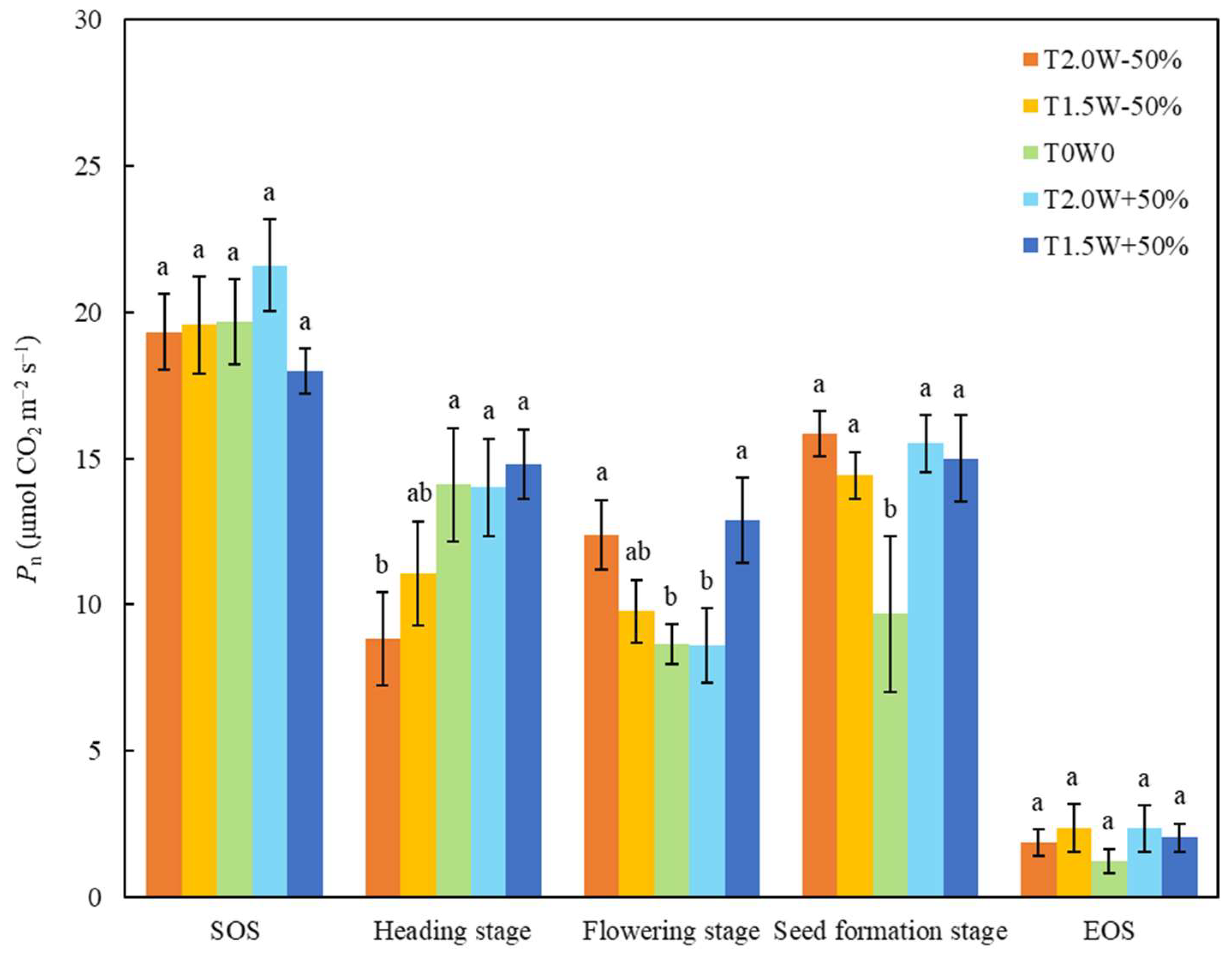
3.3. Responses of the Net CO2 Assimilation Rate at Different Stages
3.4. Changes in the Above-Ground Dry Mass of Stipa krylovii
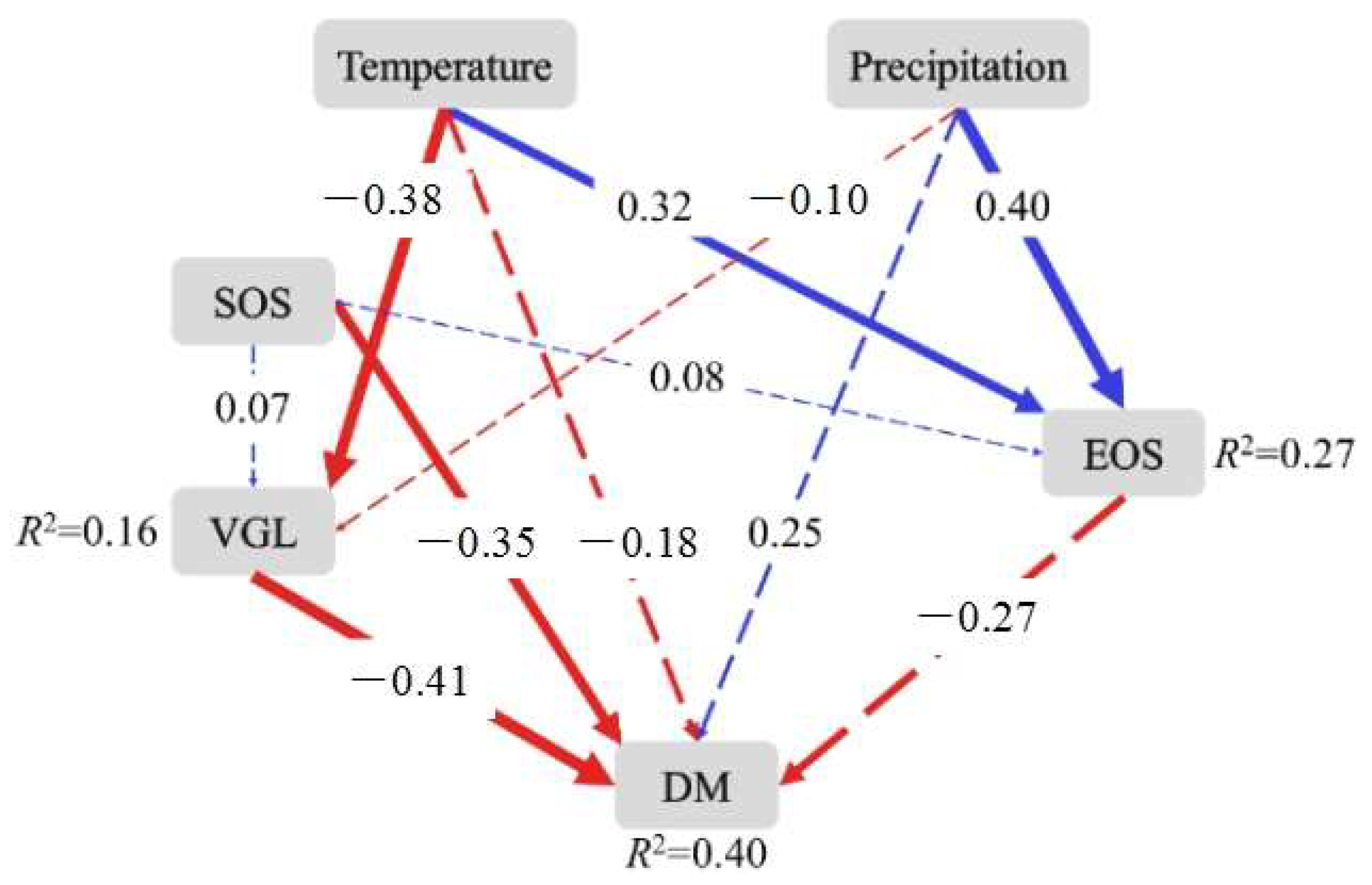
3.5. Factors Affecting Plant Production of Stipa krylovii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azizan, F.A.; Astuti, I.S.; Aditya, M.I.; Febbiyanti, T.R.; Williams, A.; Young, A.; Abdul Aziz, A. Using multi-temporal satellite data to analyse phenological responses of rubber (Hevea brasiliensis) to climatic variations in South Sumatra, Indonesia. Remote Sens. 2021, 13, 2932. [Google Scholar] [CrossRef]
- Lang, W.; Chen, X.; Qian, S.; Liu, G.; Piao, S. A new process-based model for predicting autumn phenology: How is leaf senescence controlled by photoperiod and temperature coupling? Agric. For. Meteorol. 2019, 268, 124–135. [Google Scholar] [CrossRef]
- Lieth, H. Phenology and Seasonality Modeling; Springer: Berlin/Heidelberg, Germany, 1974; pp. 3–19. [Google Scholar]
- Ren, S.; Chen, X.; Pan, C. Temperature-precipitation background affects spatial heterogeneity of spring phenology responses to climate change in northern grasslands (30°N–55°N). Agric. For. Meteorol. 2022, 315, 108816. [Google Scholar] [CrossRef]
- Fan, D.; Zhao, X.; Zhu, W.; Sun, W.; Qiu, Y. An improved phenology model for monitoring green-up date variation in Leymus chinensis steppe in Inner Mongolia during 1962–2017. Agric. For. Meteorol. 2020, 291, 108091. [Google Scholar] [CrossRef]
- Fu, Y.H.; Piao, S.; Zhou, X.; Geng, X.; Hao, F.; Vitasse, Y.; Janssens, I.A. Short photoperiod reduces the temperature sensitivity of leaf-out in saplings of Fagus sylvatica but not in horse chestnut. Glob. Change Biol. 2019, 25, 1696–1703. [Google Scholar] [CrossRef]
- Menzel, A. Trends in phenological phases in Europe between 1951 and 1996. Int. J. Biometeorol. 2000, 44, 76–81. [Google Scholar] [CrossRef]
- Cong, N.; Huang, K.; Zhang, Y. Unsynchronized driving mechanisms of spring and autumn phenology over Northern Hemisphere Grasslands. Front. For. Glob. Change 2021, 3, 610162. [Google Scholar] [CrossRef]
- Caffarra, A.; Donnelly, A.; Chuine, I. Modelling the timing of Betula pubescens budburst. II. Integrating complex effects of photoperiod into process-based models. Clim. Res. 2011, 46, 159–170. [Google Scholar] [CrossRef]
- Peng, J.; Wu, C.; Zhang, X.; Wang, X.; Gonsamo, A. Satellite detection of cumulative and lagged effects of drought on autumn leaf senescence over the Northern Hemisphere. Glob. Change Biol. 2019, 25, 2174–2188. [Google Scholar] [CrossRef]
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Ren, S.; Li, Y.; Peichl, M. Diverse effects of climate at different times on grassland phenology in mid-latitude of the Northern Hemisphere. Ecol. Indic. 2020, 113, 106260. [Google Scholar] [CrossRef]
- Bucher, S.F.; Römermann, C.; Bonser, S. The timing of leaf senescence relates to flowering phenology and functional traits in 17 herbaceous species along elevational gradients. J. Ecol. 2021, 109, 1537–1548. [Google Scholar] [CrossRef]
- Chang, Q.; Xiao, X.; Jiao, W.; Wu, X.; Doughty, R.; Wang, J.; Du, L.; Zou, Z.; Qin, Y. Assessing consistency of spring phenology of snow-covered forests as estimated by vegetation indices, gross primary production, and solar-induced chlorophyll fluorescence. Agric. For. Meteorol. 2019, 275, 305–316. [Google Scholar] [CrossRef]
- Piao, S.; Friedlingstein, P.; Ciais, P.; Viovy, N.; Demarty, J. Growing season extension and its impact on terrestrial carbon cycle in the Northern Hemisphere over the past 2 decades. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Churkina, G.; Schimel, D.; Braswell, B.H.; Xiao, X. Spatial analysis of growing season length control over net ecosystem exchange. Glob. Change Biol. 2005, 11, 1777–1787. [Google Scholar] [CrossRef]
- Angert, A.; Biraud, S.; Bonfils, C.; Henning, C.C.; Buermann, W.; Pinzon, J.; Tucker, C.J.; Fung, I. Drier summers cancel out the CO2 uptake enhancement induced by warmer springs. Proc. Natl. Acad. Sci. USA 2005, 102, 10823–10827. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.D.; Black, T.A.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S.; et al. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A.; et al. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Xu, L.; Liu, L.; Ding, D. Modeling greenup date of dominant grass species in the Inner Mongolian Grassland using air temperature and precipitation data. Int. J. Biometeorol. 2013, 58, 463–471. [Google Scholar] [CrossRef]
- Ren, S.; Yi, S.; Peichl, M.; Wang, X. Diverse responses of vegetation phenology to climate change in different grasslands in Inner Mongolia during 2000–2016. Remote Sens. 2017, 10, 17. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; in press; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., Eds.; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Yuan, W.; Zhou, G.; Wang, Y.; Han, X.; Wang, Y. Simulating phenological characteristics of two dominant grass species in a semi-arid steppe ecosystem. Ecol. Res. 2007, 22, 784–791. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Lv, X.; He, Q.; Zhou, M. Environmental factors rather than productivity drive autumn leaf senescence: Evidence from a grassland in situ simulation experiment. Agric. For. Meteorol. 2022, 327, 109221. [Google Scholar] [CrossRef]
- Yahdjian, L.; Sala, O.E. A rainout shelter design for intercepting different amounts of rainfall. Oecologia 2002, 133, 95–101. [Google Scholar] [CrossRef] [PubMed]
- National Meteorological Administration. Agrometeorological Observation Criterion; Meteorological Press: Beijing, China, 1993. [Google Scholar]
- Frechette, E.; Chang, C.Y.; Ensminger, I. Variation in the phenology of photosynthesis among eastern white pine provenances in response to warming. Glob. Change Biol. 2020, 26, 5217–5234. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G.; Shimizu, H. Are plant growth and photosynthesis limited by pre-drought following rewatering in grass? J. Exp. Bot. 2009, 60, 3737–3749. [Google Scholar] [CrossRef] [Green Version]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Change Biol. 2019, 25, 1922–1940. [Google Scholar] [CrossRef]
- Soolanayakanahally, R.Y.; Guy, R.D.; Silim, S.N.; Song, M. Timing of photoperiodic competency causes phenological mismatch in balsam poplar (Populus balsamifera L.). Plant Cell Environ. 2013, 36, 116–127. [Google Scholar] [CrossRef]
- Cho, M.A.; Ramoelo, A.; Dziba, L. Response of land surface phenology to variation in tree cover during green-up and senescence periods in the semi-arid savanna of Southern Africa. Remote Sens. 2017, 9, 689. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Piao, S.; Cong, N.; Zhang, G.; Jassens, I. A Precipitation impacts on vegetation spring phenology on the Tibetan Plateau. Glob. Change Biol. 2015, 21, 3647–3656. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.H.; Piao, S.; Delpierre, N.; Hao, F.; Hanninen, H.; Liu, Y.; Sun, W.; Janssens, I.A.; Campioli, M. Larger temperature response of autumn leaf senescence than spring leaf-out phenology. Glob. Change Biol. 2018, 24, 2159–2168. [Google Scholar] [CrossRef]
- Pudas, E.; Tolvanen, A.; Poikolainen, J.; Sukuvaara, T.; Kubin, E. Timing of plant phenophases in Finnish Lapland in 1997–2006. Boreal Environ. Res. 2008, 13, 31–43. [Google Scholar] [CrossRef] [Green Version]
- Jolly, W.M.; Nemani, R.; Running, S.W. A generalized, bioclimatic index to predict foliar phenology in response to climate. Glob. Change Biol. 2005, 11, 619–632. [Google Scholar] [CrossRef]
- Zhou, J.; Cai, W.; Qin, Y.; Lai, L.; Guan, T.; Zhang, X.; Jiang, L.; Du, H.; Yang, D.; Cong, Z.; et al. Alpine vegetation phenology dynamic over 16 years and its covariation with climate in a semi-arid region of China. Sci. Total Environ. 2016, 572, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, R.A.; Dash, J.; Rodriguez-Galiano, V.F.; Janous, D.; Pavelka, M.; Marek, M.V. Extreme warm temperatures alter forest phenology and productivity in Europe. Sci. Total Environ. 2016, 563, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Ganjurjav, H.; Hu, G.; Gornish, E.; Zhang, Y.; Li, Y.; Yan, Y.; Wu, H.; Yan, J.; He, S.; Danjiu, L.; et al. Warming and spring precipitation addition change plant growth pattern but have minor effects on growing season mean gross ecosystem productivity in an alpine meadow. Sci. Total Environ. 2022, 841, 156712. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Y.; Jia, Q.; Zhou, G. Increasing temperature shortened the carbon uptake period and decreased the cumulative net ecosystem productivity in a maize cropland in Northeast China. Field Crops Res. 2021, 267, 108150. [Google Scholar] [CrossRef]
- Bao, G.; Chen, J.; Chopping, M.; Bao, Y.; Bayarsaikhan, S.; Dorjsuren, A.; Tuya, A.; Jirigala, B.; Qin, Z. Dynamics of net primary productivity on the Mongolian Plateau: Joint regulations of phenology and drought. Int. J. Appl. Earth Obs. Geoinf. 2019, 81, 85–97. [Google Scholar] [CrossRef]
- Ge, W.; Han, J.; Zhang, D.; Wang, F. Divergent impacts of droughts on vegetation phenology and productivity in the Yungui Plateau, southwest China. Ecol. Indic. 2021, 127, 107743. [Google Scholar] [CrossRef]
- Jeong, S.J.; Ho, C.H.; Gim, H.J.; Brown, M.E. Phenology shifts at start vs. end of growing season in temperate vegetation over the Northern Hemisphere for the period 1982–2008. Glob. Change Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Wu, C.; Gough, C.M.; Chen, J.M.; Gonsamo, A. Evidence of autumn phenology control on annual net ecosystem productivity in two temperate deciduous forests. Ecol. Eng. 2013, 60, 88–95. [Google Scholar] [CrossRef]
- Bauerle, W.L.; Oren, R.; Way, D.A.; Qian, S.S.; Stoy, P.C.; Thornton, P.E.; Bowden, J.D.; Hoffman, F.M.; Reynolds, R.F. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Natl. Acad. Sci. USA 2012, 109, 8612–8617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Hou, X.; Peng, D.; Gonsamo, A.; Xu, S. Land surface phenology of China’s temperate ecosystems over 1999–2013: Spatial–temporal patterns, interaction effects, covariation with climate and implications for productivity. Agric. For. Meteorol. 2016, 216, 177–187. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, B.; Yang, Q.; Chen, G.; Yang, B.; Lu, L.; Shen, M.; Peng, Y. Responses of net primary productivity to phenological dynamics in the Tibetan Plateau, China. Agric. For. Meteorol. 2017, 232, 235–246. [Google Scholar] [CrossRef]


| SOS | EOS | LOS | VGL | RGL | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | P | F | p | F | p | |
| Y | 2 | 64.24 | <0.001 | 22.22 | <0.001 | 20.84 | <0.001 | 4.16 | <0.05 | 19.64 | <0.001 |
| T | 1 | 0.92 | 0.34 | 1.83 | 0.18 | 0.10 | 0.75 | 0.83 | 0.37 | 0.99 | 0.33 |
| Pre | 1 | 0.92 | 0.34 | 49.57 | <0.001 | 29.38 | <0.001 | 1.35 | 0.25 | 12.53 | <0.001 |
| Y × T | 2 | 1.80 | 0.18 | 0.46 | 0.64 | 0.21 | 0.81 | 0.99 | 0.38 | 0.86 | 0.43 |
| Y × Pre | 2 | 0.83 | 0.44 | 36.99 | <0.001 | 16.58 | <0.001 | 1.19 | 0.32 | 6.76 | <0.001 |
| T × Pre | 1 | 0.06 | 0.80 | 0.01 | 0.93 | 0.02 | 0.90 | 2.90 | 0.10 | 0.12 | 0.73 |
| Y × T × Pre | 2 | 0.23 | 0.80 | 0.26 | 0.77 | 0.03 | 0.98 | 2.56 | 0.09 | 2.16 | 0.13 |
| PnSOS | Pnh | Pnf | Pns | PnEOS | DM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | F | p | F | p | F | p | F | P | F | p | F | p | |
| Y | 2 | 6.82 | <0.01 | 13.15 | <0.001 | 1.21 | 0.31 | 17.54 | <0.001 | 7.01 | <0.01 | 7.70 | <0.01 |
| T | 1 | 1.05 | 0.31 | 1.86 | 0.19 | 1.18 | 0.29 | 0.92 | 0.35 | 0.05 | 0.83 | 1.07 | 0.31 |
| Pre | 1 | 0.23 | 0.64 | 10.41 | <0.01 | 0.13 | 0.72 | 0.24 | 0.63 | 0.64 | 0.43 | 1.18 | 0.29 |
| Y × T | 2 | 0.64 | 0.53 | 0.66 | 0.52 | 5.57 | <0.01 | 2.79 | 0.08 | 0.02 | 0.98 | 0.08 | 0.93 |
| Y × Pre | 2 | 0.65 | 0.53 | 1.71 | 0.20 | 3.83 | <0.05 | 3.23 | 0.06 | 0.02 | 0.98 | 0.19 | 0.83 |
| T × Pre | 1 | 2.01 | 0.17 | 0.13 | 0.73 | 18.67 | <0.001 | 0.58 | 0.45 | 0.97 | 0.34 | 1.73 | 0.20 |
| Y × T × Pre | 2 | 0.23 | 0.80 | 0.33 | 0.72 | 10.41 | <0.001 | 5.63 | <0.05 | 1.32 | 0.27 | 1.90 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Zhou, G.; Lv, X.; He, Q.; Zhou, M. Effects of Phenological Changes on Plant Production—From the View of Stipa krylovii. Agronomy 2022, 12, 3208. https://doi.org/10.3390/agronomy12123208
Yu H, Zhou G, Lv X, He Q, Zhou M. Effects of Phenological Changes on Plant Production—From the View of Stipa krylovii. Agronomy. 2022; 12(12):3208. https://doi.org/10.3390/agronomy12123208
Chicago/Turabian StyleYu, Hongying, Guangsheng Zhou, Xiaomin Lv, Qijin He, and Mengzi Zhou. 2022. "Effects of Phenological Changes on Plant Production—From the View of Stipa krylovii" Agronomy 12, no. 12: 3208. https://doi.org/10.3390/agronomy12123208





