Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Characterization of Plant Extracts
2.1.1. Plant Material
2.1.2. Extract Preparation
2.1.3. Total Polyphenol Analysis
2.1.4. Flavonoid Analysis
2.1.5. Assessment of Extract Antioxidant Activities
2.2. Biological Assay
2.2.1. Isolation of Fungal Cultures
2.2.2. In Vitro Evaluation of Antifungal Potential of Plant Extracts
2.3. Statistical Analysis
3. Results
3.1. Content of Polyphenols, Flavonoids and Antioxidant Activity of Extracts
3.2. Inhibition of Fungal Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Agrios, G.N. Losses caused by plant diseases. In Plant Pathology; Elsevier: Oxford, UK, 2004; pp. 29–45. [Google Scholar]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Bottallico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mule, G.; Morietti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some miediterranean crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Edwards, S.G. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Backhouse, D.; Burgess, L.W.; Summerell, B.A. BiogeoFigurey of Fusarium. In Fusarium Nelson Memorial Symposium; Summerell, B.A., Leslie, J.F., Backhouse, D., Bryden, W.L., Burgess, L.W., Eds.; APS Press: St Paul, MN, USA, 2012; pp. 122–137. [Google Scholar]
- Tekauz, A.; Mitchell Fetch, J.W.; Rossnagel, B.G.; Savard, M.E. Progress in assessing the impact of Fusarium head blight on oat in western Canada and screening of Avena germplasm for resistance. Cereal Res. Commun. 2008, 36, 49–56. [Google Scholar] [CrossRef]
- Hietaniemi, V.; Rämö, S.; Yli-Mattila, T.; Jestoi, M.; Peltonen, S.; Kartio, M.; Sieviläinen, E.; Koivisto, T.; Parikka, P. Updated survey of Fusarium species and toxins in Finnish cereal grains. Food Addit. Contam. Part A 2016, 33, 831–848. [Google Scholar] [CrossRef]
- Tekle, S.; Skinnes, H.; Bjørnstad, A. The germination problem of oat seed lots affected by Fusarium head blight. Eur. J. Plant Pathol. 2013, 135, 147–158. [Google Scholar] [CrossRef]
- Köhl, J.; de Haas, B.H.; Kastelein, P.; Burgers, S.L.G.E.; Waalwijk, C. Population dynamics of Fusarium spp. and Microdochium nivale in crops and crop residues of winter wheat. Phytopathology 2007, 97, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Desjardins, A.E. Fusarium-Mycotoxins Chemistry Genetics and Biology; APS Press: St. Paul, MN, USA, 2006. [Google Scholar]
- Stenglein, S.A.; Dinolfo, M.I.; Barros, G.; Bongiorno, F.; Chulze, S.N.; Moreno, M.V. Fusarium poae pathogenicity and mycotoxin accumulation on selected wheat and barley genotypes at a single location in Argentina. Plant Dis. 2014, 98, 1733–1738. [Google Scholar] [CrossRef] [Green Version]
- Goliński, P.; Waśkiewicz, A.; Wiśniewska, H.; Kiecana, I.; Mielniczuk, E.; Gromadzka, M.; Kostecki, M.; Bocianowski, J.; Rymaniak, E. Reaction of winter wheat (Triticum aestivum L.) cultivars to infection with Fusarium spp. mycotoxins contamination in grain and chaff. Food Addit. Contam. Part A 2010, 27, 1015–1024. [Google Scholar] [CrossRef]
- Yli-Mattila, T. Ecology and evolution of toxigenic Fusarium species in cereals in Northern Europe and Asia. J. Plant Pathol. 2010, 92, 7–18. Available online: https://www.jstor.org/stable/41998764 (accessed on 1 January 2020).
- Beccari, G.; Covarelli, L.; Nicholson, P. Infection processes and soft wheat response to root rot and crown rot caused by Fusarium culmorum. Plant Pathol. 2011, 60, 671–684. [Google Scholar] [CrossRef]
- Hellin, P.; Dedeurwaerder, G.; Duvivier, M.; Scauflaire, J.; Huybrechts, B.; Callebaut, A.; Munaut, F.; Legrève, A. Relationship between Fusarium spp. diversity and mycotoxin contents of mature grains in southern Belgium. Food Addit. Contam. Part A 2016, 33, 1228–1240. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Presence of Enniatins and Beauvericin in Romanian Wheat Samples: From Raw Material to Products for Direct Human Consumption. Toxins 2017, 9, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 establishing a framework for Community action to achieve the sustainable use of pesticides. Off. J. Eur. Union L 2009, 309, 71–86.
- Orsoni, N.; Degola, F.; Nerva, L.; Bisceglie, F.; Spadola, G.; Chitarra, W.; Terzi, V.; Delbono, S.; Ghizzoni, R.; Morcia, C.; et al. Double Gamers—Can Modified Natural Regulators of Higher Plants Act as Antagonists against Phytopathogens? The Case of Jasmonic Acid Derivatives. Int. J. Mol. Sci. 2020, 21, 8681. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Skwaryło-Bednarz, B.; Mielniczuk, E.; Bisceglie, F.; Pelosi, G.; Degola, F.; Gałązka, A.; Grzęda, E. Effect of Thiosemicarbazone Derivatives and Fusarium culmorum (Wm.G. Sm.) Sacc. Infection of Winter Wheat Seedlings on Their Health Status and Soil Biological Activity. Agronomy 2022, 12, 116. [Google Scholar] [CrossRef]
- Jamiołkowska, A. Preparaty biotechniczne i biologiczne w ochronie papryki słodkiej (Capsicum annuum L.) przed grzybami chorobotwórczymi i indukowaniu reakcji obronnych roślin. Rozpr. Nauk. UP W Lub. 2013, 379, 117. [Google Scholar]
- Kawka, M.; Pilarek, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. Roślinne metabolity jako kluczowy bioprodukt biotechnologii roślin. Biul. Wydz. Farm. WUM 2017, 8, 68–79. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crop. Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Canadanovic-Brunet, J.M.; Djilas, S.M.; Cetkovic, G.S.; Tumbas, V.T. Free-radical scavenging activity of wormwood (Artemisia absinthium L) extracts. J. Sci. Food Agric. 2005, 85, 265–272. [Google Scholar] [CrossRef]
- Šernaitė, L. Plant extracts: Antimicrobial and antifungal activity and appliance in plant protection. Sodininkystė Ir Daržininkystė 2017, 36, 58–68. [Google Scholar]
- Shabana, Y.M.; Abdalla, M.E.; Shanin, A.A.; El-Sawy, M.M.; Draz, I.S.; Youssif, A.W. Efficacy of plant extracts in controlling wheat leaf rust disease caused by Puccinia triticina. Egypt. J. Basic Appl. Sci. 2017, 4, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Jamiołkowska, A. Natural compounds as elicitors of plant resistance against diseases and new biocontrol strategies. Agronomy 2020, 10, 173. [Google Scholar] [CrossRef] [Green Version]
- Jamiołkowska, A.; Kowalski, R. Laboratory effect of Silphium perfoliatum L. on the growth of tested fungi. Acta Sci. Pol. Hortorum Cultus 2012, 11, 43–55. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. Lebensm.-Wiss. Und-Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Wyrostek, J.; Kowalski, R. Effect of ultrasound and fragmentation of the raw material on the extraction of phenolic compounds from peppermint leaves and black tea. Przemysł Chem. 2022, 101, 928–933. [Google Scholar]
- Alsirrag, M.; Ali, R. Determination of antimicrobial and antioxidants activity of Salvia przewalskii seed oil against pathogenic bacteria and fungi. J. Phys. Conf. Ser. 2018, 1032, 012070. [Google Scholar] [CrossRef]
- Dulger, G.; Dulger, B. Antifungal activity of Salvia verticillata subsp. verticillata against fungal pathogens. Düzce Üniversitesi Sağlık Bilimleri Enstitüsü Derg. 2021, 11, 305–307. [Google Scholar] [CrossRef]
- Medjahed, F.; Merouane, A.; Saadi, A.; Bader, A.; Cioni, P.L.; Flamini, G. Chemical profile and antifungal potential of essential oils from leaves and flowers of Salvia algeriensis (Desf.): A comparative study. Chil. J. Agric. Res. 2016, 76, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahman, A.; Alkhail, A. Antifungal activity of some extract against some plant pathogenic fungi. Pak. J. Biol. Sci. 2005, 8, 413–417. [Google Scholar] [CrossRef] [Green Version]
- Sebeşan, M.; Cărăban, A. Analysis of the Essential Oils from Thyme (Thymus vulgaris L.) and from Peppermint (Mentha piperita L.). Chem. Bull. Politeh. Univ. Timis. 2008, 53, 1–2. [Google Scholar]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Ganie, A.A.; Pala, S.A.; Mir, R.A. Antifungal activity of some plant extracts on some pathogenic fungi. Arch. Phytopathol. Plant Prot. 2014, 47, 279–284. [Google Scholar] [CrossRef]
- Mutlu-Ingok, A.; Devecioglu, D.; Dikmetas, D.N.; Karbancioglu-Guler, F.; Capanoglu, E. Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules 2020, 25, 4711. [Google Scholar] [CrossRef]
- Poulios, E.; Giaginis, C.; Vasios, G.K. Current advances on the extraction and identification of bioactive components of sage (Salvia spp.). Curr Pharm Biotechnol. 2019, 20, 845–857. [Google Scholar] [CrossRef]
- Topçu, G. Bioactive triterpenoids from Salvia species. J. Nat. Prod. 2006, 69, 482–487. [Google Scholar] [CrossRef]
- Redondo-Blanco, S.; Fernandez, J.; Lopez-Ibanez, S.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Plant phytochemicals in food preservation: Antifungal bioactivity: A review. J. Food Protect. 2020, 83, 163–171. [Google Scholar] [CrossRef] [Green Version]
- Dammaka, I.; Hamdi, Z.; Kammun, S.; Euch, E.; Zemni, H.; Mliki, A.; Hassouna, M.; Lasram, S. Evaluation of antifungal and anti-ochratoxigenic activities of Salvia officinalis, Lavandula dentata and Laurus nobilis essential oils and a major monoterpene constituent 1,8-cineole against Aspergillus carbonarius. Ind. Crop Prod. 2019, 128, 85–93. [Google Scholar] [CrossRef]
- Džamič, A.; Sokovič, M.; Ristič, M.; Grujič-Jovanovič, S.; Vukojevič, J.; Marin, P.D. Chemical composition and antifungal activity of Salvia sclarea (Lamiaceae) essential oil. Arch. Biol. Sci. 2008, 60, 233–237. [Google Scholar] [CrossRef]
- Yilar, M.; Kadioǧlu, I. Antifungal Activities of some Salvia Species Extracts on Fusarium oxysporum f. sp. radicis- lycopersici (Forl) Mycelium Growth In-Vitro. Egypt. J. Pest Cont. 2016, 26, 115–118. [Google Scholar]
- Rowshan, V.; Najafian, S. Polyphenolic contents and antioxidant activities of aerial parts of Salvia multicaulis from the Iran flora. Nat. Prod. Res. 2020, 34, 2351–2353. [Google Scholar] [CrossRef]
- Benedec, D.; Hanganu, D.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Raita, O.; Bischin, C.; Silaghi-Dumitrescu, R.; Vlase, L. Assessment of rosmarinic acid content in six Lamiaceae species extracts and their antioxidant and antimicrobial potential. Pak. J. Pharm. Sci. 2015, 28, 2297–2303. [Google Scholar]
- Molnar, M.; Jerković, I.; Suknović, D.; Rajs, B.B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of six medicinal plant extracts obtained by two conventional methods and supercritical CO- extraction targeted on coumarin content, 2, 2-diphenyl-1-picrylhydrazyl radical scavenging capacity and total phenols content. Molecules 2017, 22, 348. [Google Scholar] [CrossRef] [Green Version]
- Neagu, E.; Roman, G.P.; Radu, G.L. Antioxidant capacity of some Salvia officinalis concentrated extracts. Rev. Roum. Chim. 2011, 56, 777–782. [Google Scholar]
- Cieśla, Ł.; Staszek, D.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Development of chromatographic and free radical scavenging activity fingerprints by thin-layer chromatography for selected Salvia species. Phytochem. Anal. 2011, 22, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Safari, M.R.; Azizi, O.; Heidary, S.S.; Kheiripour, N.; Ravan, A.P. Antiglycation and antioxidant activity of four Iranian medical plant extracts. J. Pharmacopuncture 2018, 21, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Korpinen, R.I.; Välimaa, A.L.; Liimatainen, J.; Kunnas, S. Essential Oils and Supercritical CO2 Extracts of Arctic Angelica (Angelica archangelica L.), Marsh Labrador Tea (Rhododendron tomentosum) and Common Tansy (Tanacetum vulgare)—Chemical Compositions and Antimicrobial Activities. Molecules 2021, 26, 7121. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska-Wierdak, R.; Sałata, A.; Kniaziewicz, M. Tansy (Tanacetum vulgare L.)-A Wild-Growing Aromatic Medicinal Plant with a Variable Essential Oil Composition. Agronomy 2022, 12, 277. [Google Scholar] [CrossRef]
- Keskitalo, M.; Pehu, E.; Simon, J.E. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem. Syst. Ecol. 2001, 29, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Bączek, K.; Kosakowska, O.; Przybył, J.; Kuźma, P.; Ejdys, M.; Obiedziński, M.; Węglarz, Z. Intraspecific variability of yarrow (Achillea millefolium L.) in respect of developmental and chemical traits. Herba Pol. 2015, 61, 7–52. [Google Scholar] [CrossRef] [Green Version]
- Ivănescu, B.; Tuchiluș, C.; Corciovă, A.; Lungu, C.; Mihai, C.T.; Gheldiu, A.M.; Vlase, L. Antioxidant, antimicrobial and cytotoxic activity of Tanacetum vulgare, Tanacetum corymbosum and Tanacetum macrophyllum extracts. Farmacia 2018, 66, 282–288. [Google Scholar]
- Wens, A.; Geuens, J. In vitro and in vivo antifungal activity of plant extracts against common phytopathogenic fungi. J. BioSci. Biotechnol. 2022, 11, 15–21. [Google Scholar] [CrossRef]
- El-Kalamouni, C.; Venskutonis, P.R.; Zebib, B.; Merah, O.; Raynaud, C.; Talou, T. Antioxidant and Antimicrobial Activities of the Essential Oil of Achillea millefolium L. Grown in France. Medicines 2017, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Ghanati, F.; Bakhtiarian, S.; Parast, B.M.; Behrooz, M.K. Production of new active phytocompounds by Achillea millefolium L. after elicitation with silver nanoparticles and methyl jasmonate. Biosci. Biotechnol. Res. Asia 2014, 11, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.; Santos-Buelga, C.; Ferreira, I.C. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef] [PubMed]
- Bimbiraitė, K.; Ragažinskienė, O.; Maruška, A.; Kornyšova, O. Comparison of the chemical composition of four yarrow (Achilea millefolium L.) morphotypes. Biologija 2008, 54, 208–212. [Google Scholar] [CrossRef]
- Georgieva, L.; Gadjalova, A.; Mihaylova, D.; Pavlov, A. Achillea millefolium L.-phytochemical profile and in vitro antioxidant activity. Int. Food Res. J. 2015, 22, 1347–1352. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Fierascu, R.C.; Ortan, A.; Soare, L.C.; Paunescu, A. In vitro antioxidant and antifungal properties of Achillea millefolium L. Rom Biotechnol. Lett. 2015, 20, 10626–10636. [Google Scholar]
- Kordali, S.; Kotan, R.; Mavi, A.; Cakir, A.; Ala, A.; Yildirim, A. Determination of the chemical composition and antioxidant activity of the essential oil of Artemisia dracunculus and of the antifungal and antibacterial activities of Turkish Artemisia absinthium, A. dracunculus, Artemisia santonicum, and Artemisia spicigera essential oils. J. Agric. Food Chem. 2005, 53, 9452–9458. [Google Scholar]
- Umpiérrez, M.L.; Lagreca, M.E.; Cabrera, R.; Grille, G.; Rossini, C. Essential oils from Asteraceae as potential biocontrol tools for tomato pests and diseases. Phytochem. Rev. 2012, 11, 339–350. [Google Scholar] [CrossRef]
- Andreu, V.; Levert, A.; Amiot, A.; Cousin, A.; Aveline, N.; Bertrand, C. Chemical composition and antifungal activity of plant extracts traditionally used in organic and biodynamic farming. Environ. Sci. Pollut. Res. Int. 2018, 25, 29971–29982. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Al Sane, K.; Ben Ammar, W.; Azeiz, S.; Brahim, A.H.; et al. Chemical composition and antioxidant and antimicrobial activities of wormwood (Artemisia absinthium L.) essential oils and phenolics. J. Chem. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuniga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind. Crop. Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Aberham, A.; Cicek, S.S.; Schneider, P.; Stuppner, H. Analysis of sesquiterpene lactones, lignans, and flavonoids in wormwood (Artemisia absinthium L.) using high-performance liquid chromatography (HPLC)− mass spectrometry, reversed phase HPLC, and HPLC− solid phase extraction− nuclear magnetic resonance. J. Agric. Food Chem. 2010, 58, 10817–10823. [Google Scholar] [CrossRef] [PubMed]
- Orlikowski, L.B.; Skrzypczak, C.; Harnaj, I. Biological activity of grapefruit extract in the control of forme speciales of Fusarium oxysporum. J. Plant Prot. Res. 2001, 41, 104–111. [Google Scholar]
- Orlikowski, L.B.; Skrzypczak, C.; Jaworska-Marosz, A. Influence of grapefruit extract on the growth and development of Botrytis spp. and grey mold development on lily and peony. Bull. Pol. Acad. Sci. Biol. Sci. 2001, 49, 373–378. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]






| Plant Extract | Flavonoids (mg/mL) ± SD | Polyphenols (mg/mL) ± SD | Antioxidant Activity, Free Radical-Scavenging Ability | |
|---|---|---|---|---|
| % Inhibition ± SD | mM Trolox ± SD | |||
| yarrow (Y) | 5.05 c ± 0.130 | 59.56 b ± 4.080 | 56.13 a ± 0.386 | 84.40 a ± 0.608 |
| tansy (T) | 7.82 b ± 0.069 | 77.12 a ± 3.075 | 57.79 a ± 6.383 | 87.02 a ± 10.065 |
| sage (S) | 21.12 a ± 0.904 | 81.95 a ± 8.117 | 67.58 a ± 2.855 | 102.44 a ± 4.502 |
| wormwood (W) | 5.30 c ± 0.218 | 43.83 c ± 3.280 | 62.92 a ± 5.857 | 95.10 a ± 9.235 |
| Experimental Combination | Number of Days ± SD | |||||
|---|---|---|---|---|---|---|
| 2nd | 4th | 6th | 8th | 10th | ||
| Y | 5% concentration | 34.09 ef ± 3.94 | 16.43 e ± 10.68 | 7.96 e ± 8.33 | −9.43 e ± 6.38 | −23.7 e ± 3.33 |
| T | 38.64 de ± 13.64 | 23.19 e ± 12.88 | 11.07 e ± 8.45 | −10.03 e ± 8.42 | −19.4 e ± 3.03 | |
| S | 29.55 ef ± 14.19 | 54.11 bc ± 14.22 | 41.87 e ± 9.51 | 27.05 c ± 9.86 | 13.6 cd ± 10.49 | |
| W | 19.32 f ± 5.21 | 19.32 e ± 7.29 | 9.34 e ± 9.07 | −9.73 e ± 7.76 | −21.6 e ± 6.84 | |
| Y | 10% concentration | 42.55 cde ± 12.77 | 20.93 e ± 13.32 | 10.83 e ± 9.38 | 8.26 d ± 7.34 | 2.77 d ± 6.43 |
| T | 51.06 bcd ± 3.69 | 29.07 de ± 2.01 | 17.5 e ± 2.50 | 15.29 cd ± 1.06 | 7.56 d ± 3.05 | |
| S | 35.11 de ± 14.39 | 40.12 cd ± 13.09 | 31.25 d ± 8.20 | 22.94 c ± 8.75 | 19.65 c ± 7.87 | |
| W | 38.30 de ± 3.69 | 20.35 e ± 5.04 | 10.42 e ± 3.15 | 9.48 d ± 2.65 | 3.78 d ± 4.43 | |
| Y | 20% concentration | 67.57 a ± 0.00 | 59.35 ab ± 3.71 | 50.3 bc ± 10.80 | 47.05 b ± 8.74 | 36.25 b ± 7.24 |
| T | 67.57 a ± 5.41 | 65.42 ab ± 3.53 | 56.81 b ± 7.17 | 53.61 b ± 6.20 | 44.81 b ± 6.53 | |
| S | 63.96 ab ± 3.12 | 73.83 a ± 1.62 | 76.33 a ± 1.02 | 72.43 a ± 3.99 | 61.30 a ± 8.90 | |
| W | 56.76 abc ± 5.41 | 52.80 bc ± 3.53 | 51.19 bc ± 1.54 | 47.26 b ± 1.00 | 39.51 b ±0.61 | |
| Experimental Combination | Number of Days ± SD | |||||
|---|---|---|---|---|---|---|
| 2nd | 4th | 6th | 8th | 10th | ||
| Y | 5% concentration | 26.98 ef ± 2.75 | 2.14 f ± 18.33 | 10.2 h ± 9.33 | 16.48 d ± 5.56 | 6.48 e ± 2.85 |
| T | 34.92 de ± 15.31 | 11.54 f ± 23.54 | 27.86 g ± 15.30 | 28.52 c ± 14.92 | 16.30 d ± 11.98 | |
| S | 57.14 b ± 4.76 | 46.58 cde ± 1.96 | 47.01 def ± 2.69 | 45.19 b ± 1.95 | 29.07 c ± 4.72 | |
| W | 42.06 cd ± 4.96 | 35.47 e ± 7.06 | 38.31 fg ± 2.83 | 38.52 bc ± 2.10 | 23.33 cd ± 1.11 | |
| Y | 10% concentration | 15.46 f ± 3.57 | 42.67 de ± 1.53 | 45.61 ef ± 2.50 | 30.93 c ± 3.16 | 16.67 d ± 2.22 |
| T | 50.52 bc ± 12.37 | 59.67 bcd ± 13.50 | 50.76 cde ± 5.15 | 37.96 bc ± 10.28 | 23.89 cd ± 7.78 | |
| S | 54.64 bc ± 9.45 | 71.33 ab ± 4.16 | 67.56 ab ± 7.45 | 45.00 b ± 5.47 | 26.85 c ± 5.89 | |
| W | 24.74 ef ± 9.94 | 52.33 b–e ± 6.35 | 57.44 bcd ± 2.82 | 46.11 b ± 5.64 | 30.74 c ± 3.78 | |
| Y | 20% concentration | 48.39 bcd ± 7.39 | 59.41 bcd ± 4.67 | 56.53 b–e ± 1.80 | 38.89 bc ± 5.47 | 24.07 cd ± 3.90 |
| T | 72.58 a ± 2.79 | 71.47 ab ± 6.85 | 67.54 ab ± 4.58 | 56.48 a ± 2.85 | 41.85 b ± 4.98 | |
| S | 74.19 a ± 2.79 | 83.53 a ± 1.02 | 75.00 a ± 1.71 | 65.37 a ± 2.74 | 52.59 a ± 1.95 | |
| W | 61.29 ab ± 4.84 | 63.82 bc ± 6.18 | 59.7 bc ± 5.92 | 44.63 b ± 5.04 | 28.89 c ± 4.19 | |
| Experimental Combination | Number of Days ± SD | |||||
|---|---|---|---|---|---|---|
| 2nd | 4th | 6th | 8th | 10th | ||
| Y | 5% concentration | 28.57 a ± 0.00 | 13.01 g ± 15.42 | 8.05 d ± 4.09 | −9.09 c ± 5.21 | −22.41 c ± 3.33 |
| T | 28.57 a ± 0.00 | 36.99 b–e ± 4.75 | 22.88 bcd ± 31.66 | 4.17 bc ± 44.25 | −2.41 bc ± 56.23 | |
| S | 16.67 abc ± 4.12 | 23.97 efg ± 5.44 | 22.03 bcd ± 7.77 | 4.17 bc ± 11.50 | −20.00 c ± 23.29 | |
| W | 21.40 ab ± 7.14 | 21.23 fg ± 5.17 | 11.00 cd ± 5.83 | −9.84 c ± 4.59 | −27.93 c ± 6.57 | |
| Y | 10% concentration | 16.67 abc ± 8.33 | 41.24 a–d ± 3.09 | 33.10 ab ± 6.37 | 30.52 ab± 6.54 | 22.32 ab ± 7.09 |
| T | 4.17 c ± 12.50 | 53.61 a ± 0.00 | 46.13 a ± 1.06 | 39.78 a ± 2.06 | 35.94 a ± 1.02 | |
| S | 16.67 abc ± 8.33 | 42.27 a–d ± 12.50 | 42.96 ab ± 9.21 | 41.96 a ± 10.04 | 38.39 a ± 10.46 | |
| W | 11.08 bc ± 4.81 | 51.03 ab ± 3.89 | 48.93 a ± 11.20 | 43.87 a ± 13.32 | 35.27 a ± 21.29 | |
| Y | 20% concentration | 25.71 ab ± 9.90 | 32.00 de ± 4.00 | 37.75 ab ± 1.84 | 30.60 ab ± 0.55 | 29.95 a ± 2.74 |
| T | 28.57 a ± 4.95 | 48.00 abc ± 5.29 | 49.40 a ± 3.19 | 42.90 a ± 4.47 | 39.06 a ± 2.07 | |
| S | 22.86 ab ± 13.09 | 54.00 a ± 7.21 | 42.17 ab ± 3.19 | 39.75 a ± 5.78 | 36.98 a ± 3.16 | |
| W | 14.31 bc ± 4.95 | 34.68 c–f ± 13.32 | 30.12 abc ± 4.34 | 19.55 ab ± 5.27 | 17.96 ab ± 1.35 | |
| Experimental Combination | Number of Days ± SD | |||||
|---|---|---|---|---|---|---|
| 2nd | 4th | 6th | 8th | 10th | ||
| Y | 5% concentration | 3.64 e ± 15.75 | 13.67 f ± 6.01 | 21.09 f ± 2.73 | 9.63 d ± 3.57 | 0.93 d ± 1.60 |
| T | 23.64 cde ± 11.89 | 24.82 def ± 3.47 | 28.18 ef ± 0.36 | 27.59 bc ± 16.52 | 5.37 cd ± 4.85 | |
| S | 37.27 bcd ± 4.72 | 37.41 cde ± 9.22 | 41.96 cd ± 7.12 | 25.93 bc ± 6.51 | 6.11 cd ± 3.89 | |
| W | 25.45 cd ± 7.87 | 32.73 cde ± 7.19 | 37.58 de ± 5.06 | 26.85 bc ± 5.04 | 7.96 cd ± 2.80 | |
| Y | 10% concentration | 2.63 e ± 12.06 | 23.47 ef ± 3.68 | 29.01 ef ± 2.88 | 14.44 cd ± 5.36 | 4.44 cd ± 2.94 |
| T | 31.58 cd ± 15.79 | 42.86 bc ± 10.75 | 43.41 cd ± 5.80 | 29.81 bc ± 5.34 | 12.96 bc ± 3.21 | |
| S | 43.86 abc ± 6.08 | 46.94 abc ± 6.12 | 48.68 bc ± 4.40 | 30.93 abc ± 4.10 | 7.04 cd ± 5.16 | |
| W | 17.54 de ± 20.44 | 38.44 cd ± 12.17 | 44.02 cd ± 9.09 | 29.26 bc ± 13.22 | 13.52 bc ± 8.36 | |
| Y | 20% concentration | 60.28 a ± 2.46 | 55.21 ab ± 6.44 | 46.36 cd ± 9.68 | 35.37 ab ± 10.40 | 20.19 ab ± 13.38 |
| T | 64.54 a ± 6.50 | 54.57 ab ± 13.65 | 49.80 abc ± 6.14 | 29.26 bc ± 12.35 | 20.00 ab ± 4.34 | |
| S | 63.12 a ± 8.86 | 59.31 a ± 8.09 | 58.10 ab ± 2.65 | 39.07 ab ± 6.24 | 14.26 bc ± 6.86 | |
| W | 56.03 ab ± 2.46 | 58.99 a ± 3.94 | 58.50 a ± 3.56 | 46.11 a ± 2.94 | 26.11 a ± 5.47 | |
| Fungus Species | Experimental Combination | Mycelium Surface and Structure | Obverse | Reverse |
|---|---|---|---|---|
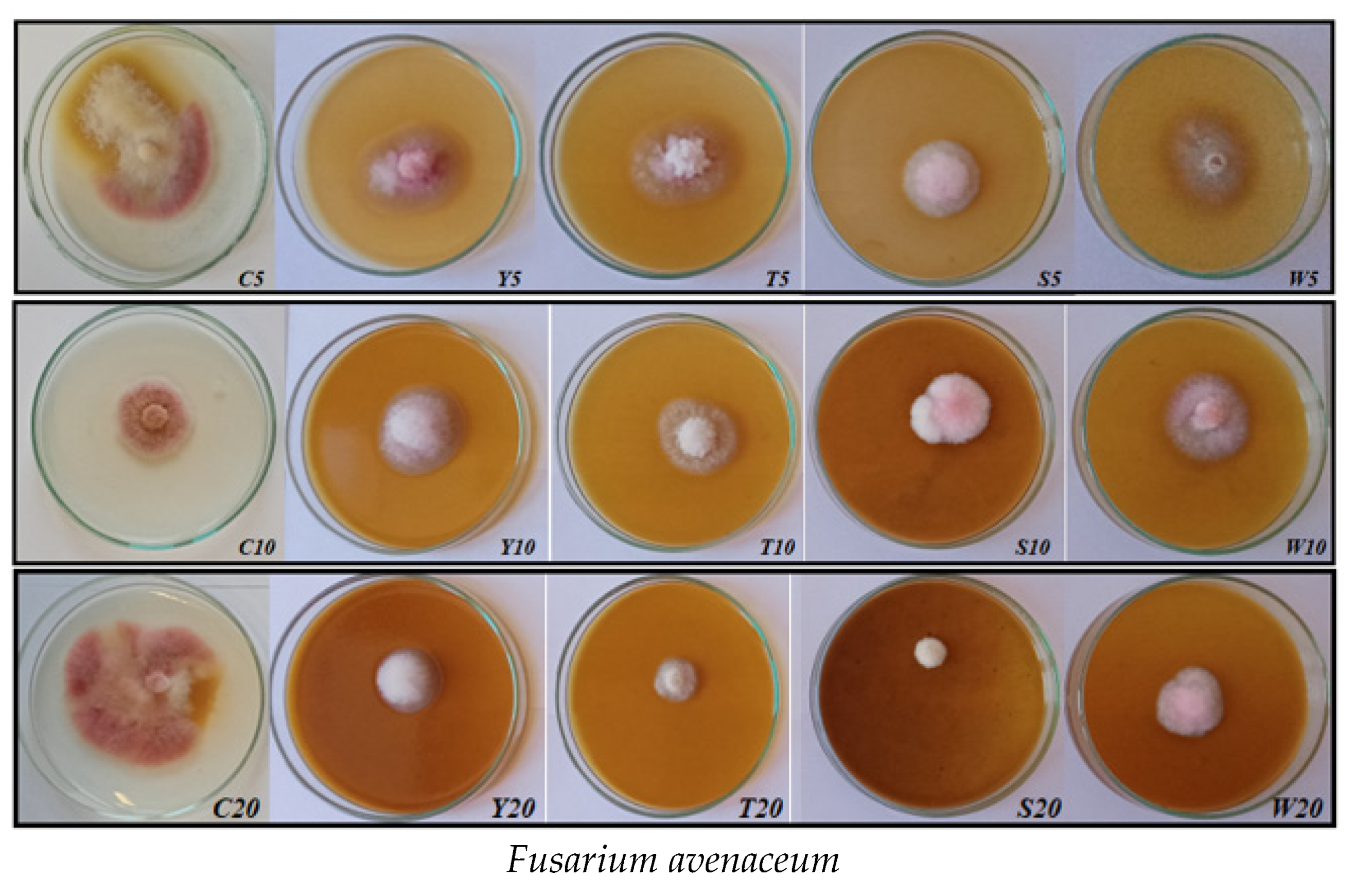
| F. avenaceum | C5, C10, C20 | fluffy, slightly elevated | pink-white; pink | maroon |
| Y5, Y10, Y20 | substrate, slightly compact, elevated in the center | white-purple | purple, light purple | |
| T5, T10, T20 | slightly compact; elevated | white | slightly pink; colorless | |
| S5, S10, S20 | fluffy, slightly elevated | pink-white; white-cream | slightly pink; colorless | |
| W5, W10, W20 | flat, centrally slightly elevated | white-gray-pink | pink-white | |
| F. culmorum | C5, C10, C20 | fluffy, regular, even growth | white-pink | purple |
| Y5, Y10, Y20 | fluffy, slightly elevated in the center | purple | maroon | |
| T5, T10, T20 | fluffy, slightly raised | purple; pink-white | purple; maroon | |
| S5, S10, S20 | compact; elevated | white-pink | light pink | |
| W5, W10, W20 | fluffy, slightly elevated in the center | gray-pink-white | maroon | |
| F. graminearum | C5, C10, C20 | regular growth, elevated in the center | white-pink; pink | maroon |
| Y5, Y10, Y20 | irregular, substrate, elevated in the center | pink-purple-white | brown and maroon | |
| T5, T10, T20 | regular growth, elevated | white-pink | pink | |
| S5, S10, S20 | regular growth, elevated | white-pink-yellow | light brown | |
| W5, W10, W20 | regular growth, elevated in the center | white-purple | gray-purple; light pink | |
| F. sporotrichioides | C5, C10, C20 | fluffy, regular, even growth | pink-white-yellow | maroon |
| Y5, Y10, Y20 | fluffy, slightly raised | pink-white-yellow | maroon | |
| T5, T10, T20 | fluffy, slightly raised | pink-white-yellow | maroon | |
| S5, S10, S20 | compact; elevated | pink-white-yellow | maroon | |
| W5, W10, W20 | fluffy, elevated and compact | pink-white-yellow; white-yellow | maroon |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study. Agronomy 2022, 12, 3204. https://doi.org/10.3390/agronomy12123204
Kursa W, Jamiołkowska A, Wyrostek J, Kowalski R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study. Agronomy. 2022; 12(12):3204. https://doi.org/10.3390/agronomy12123204
Chicago/Turabian StyleKursa, Weronika, Agnieszka Jamiołkowska, Jakub Wyrostek, and Radosław Kowalski. 2022. "Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study" Agronomy 12, no. 12: 3204. https://doi.org/10.3390/agronomy12123204
APA StyleKursa, W., Jamiołkowska, A., Wyrostek, J., & Kowalski, R. (2022). Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium spp.—An In Vitro Study. Agronomy, 12(12), 3204. https://doi.org/10.3390/agronomy12123204







