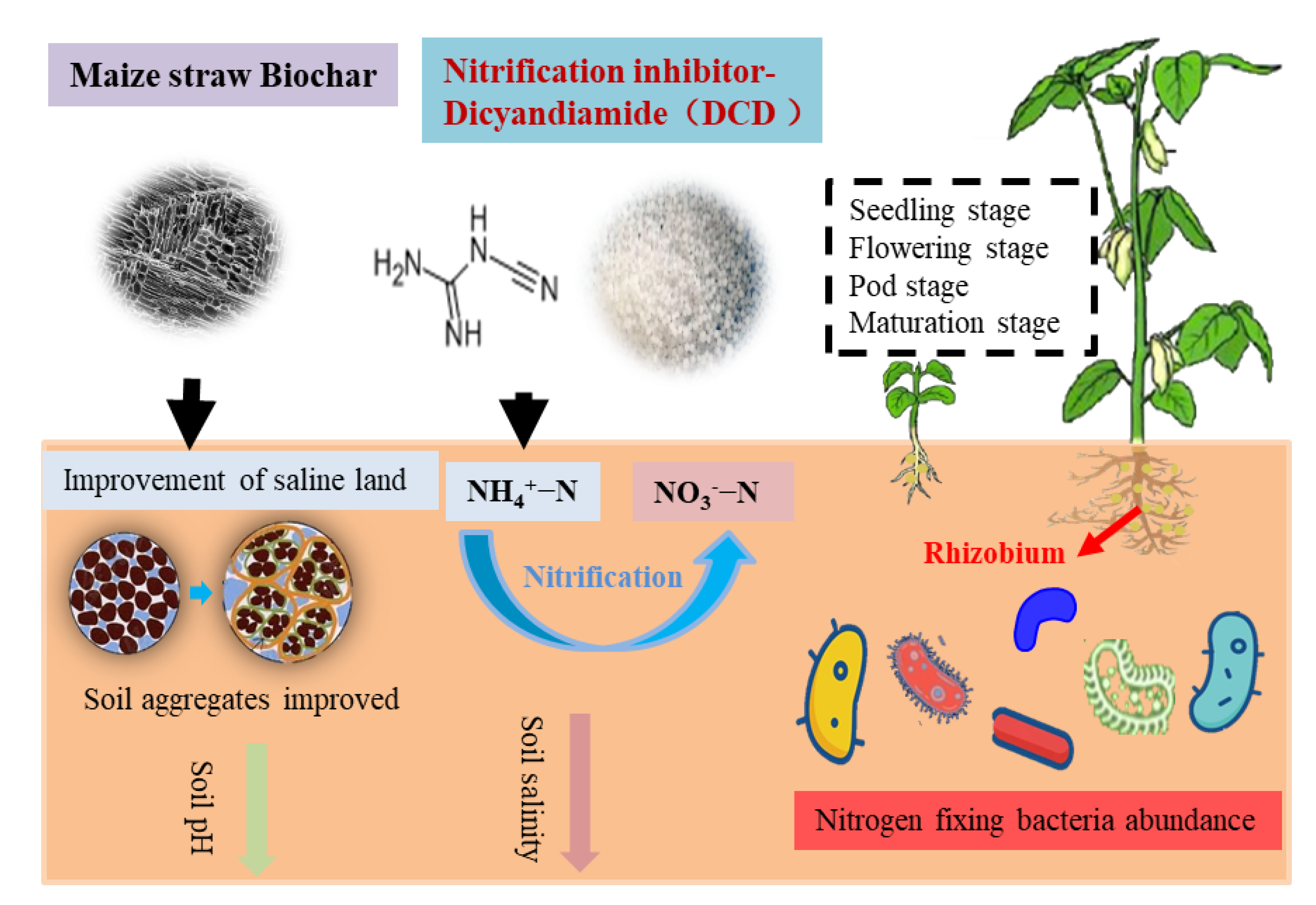
Biochar and Nitrification Inhibitor (Dicyandiamide) Combination Had a Double-Win Effect on Saline-Alkali Soil Improvement and Soybean Production in the Yellow River Delta, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site
2.2. Experiment Design
2.3. Soil Sampling and Chemical Analysis
2.4. Soil Basial Properties and Soybean Yield
2.5. 16S RNA Analysis
2.6. Calculations and Statistical Analysis
control)/Yield of fertilizer area × 100%
3. Results
3.1. Effects of Biochar and Inhibitor Addition on Ammonium and Nitrate Nitrogen Contents in the Soil
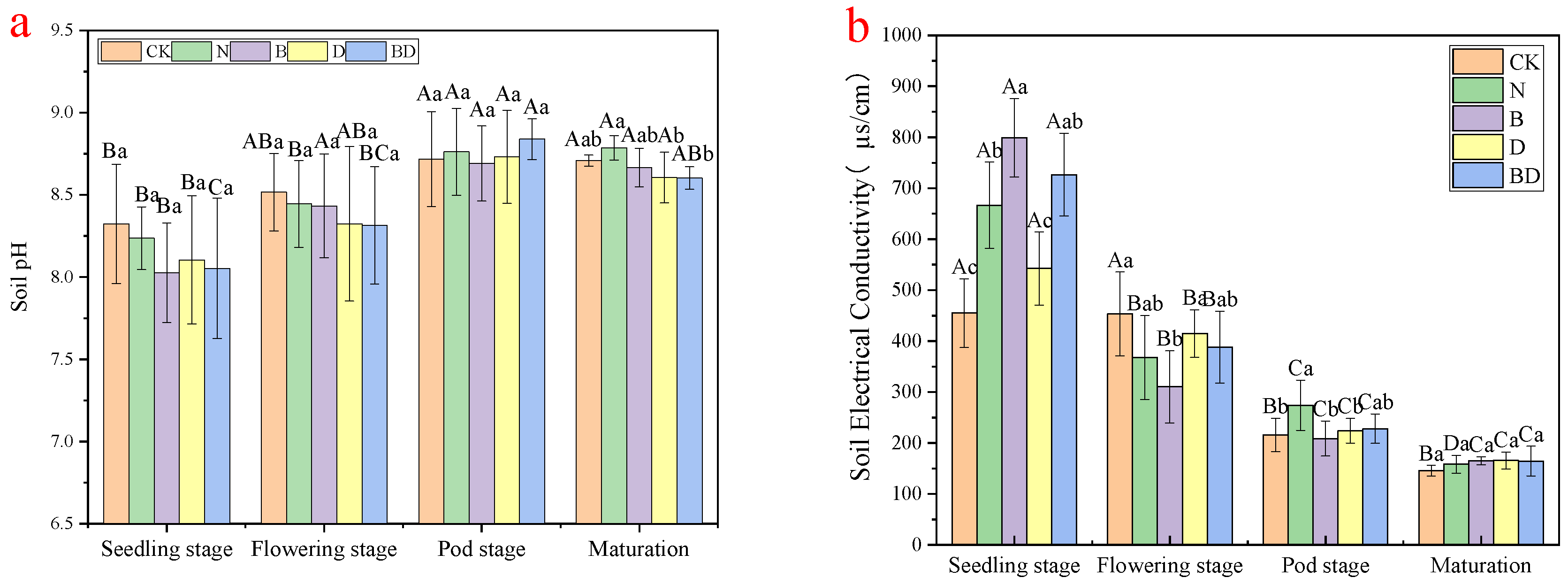
3.2. Effects of Biochar and Inhibitor Addition on the Soil’s pH and the Soil’s Electrical Conductivity
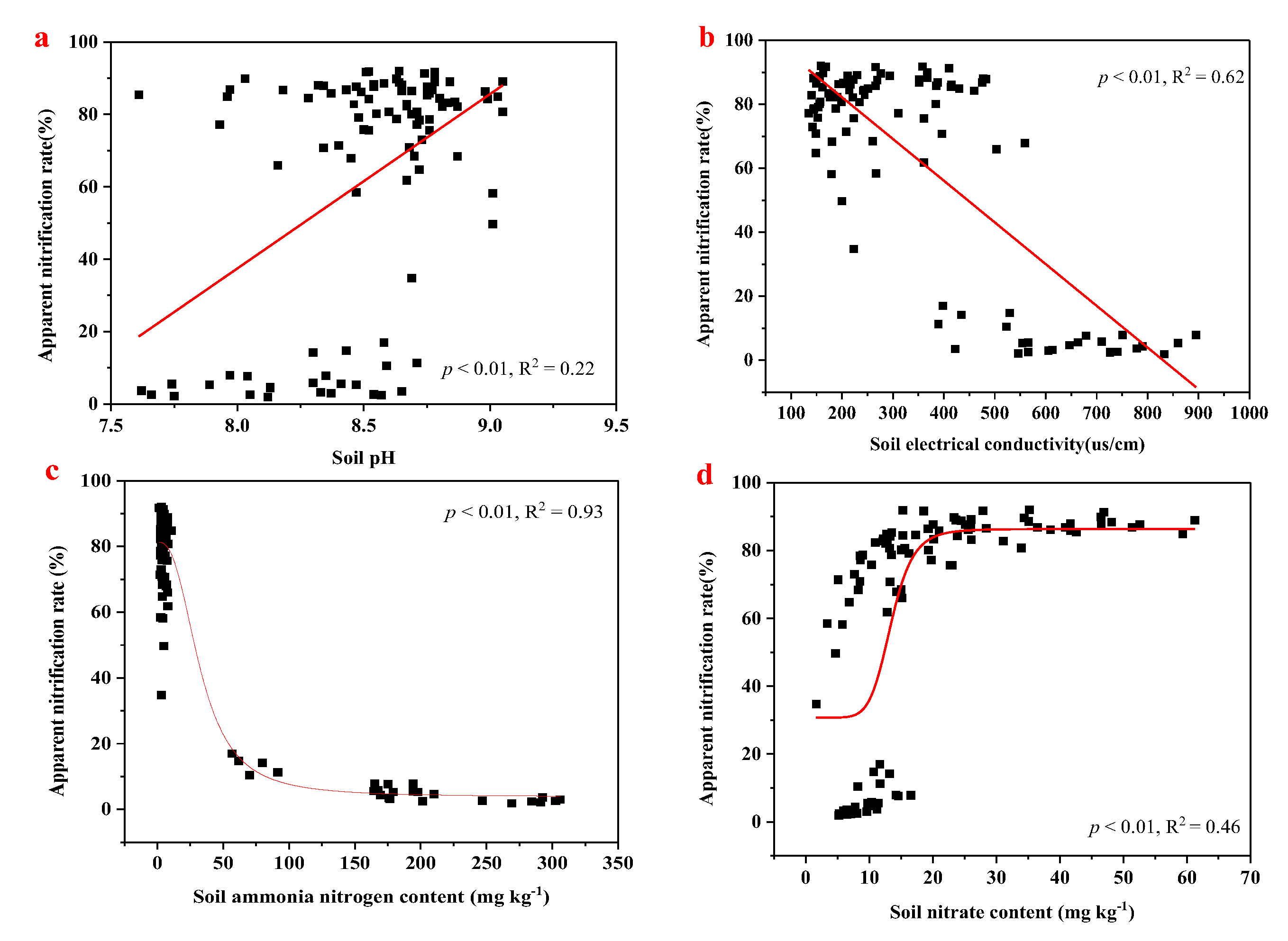
3.3. Effects of the Soil’s Apparent Nitrification Rate on the Soil’s pH, Electrical Conductivity, Ammonia Content, and Nitrite
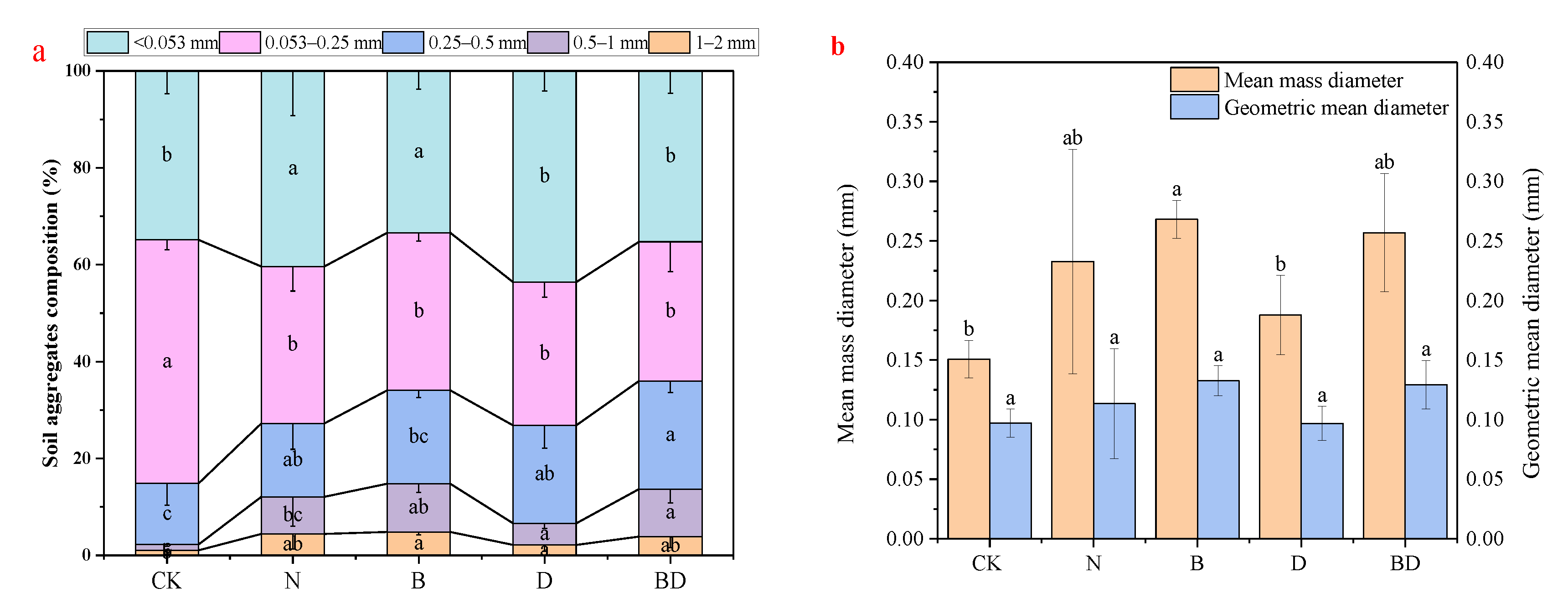
3.4. Effects of Biochar and Inhibitor Addition on the Soil’s Aggregates and Structural Stability
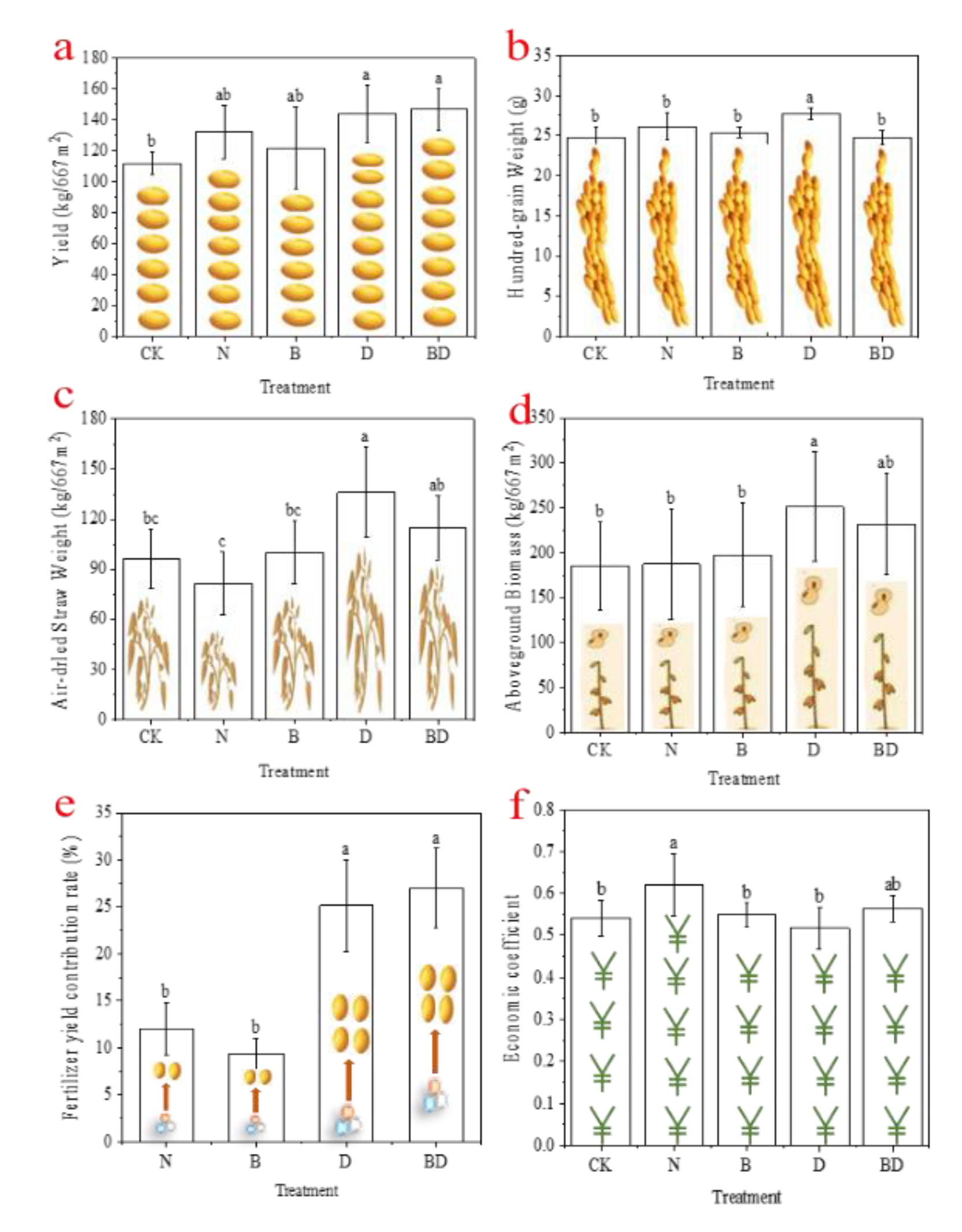
3.5. Effects of Biochar and Inhibitors on Soybean Yield and Economic Indicators
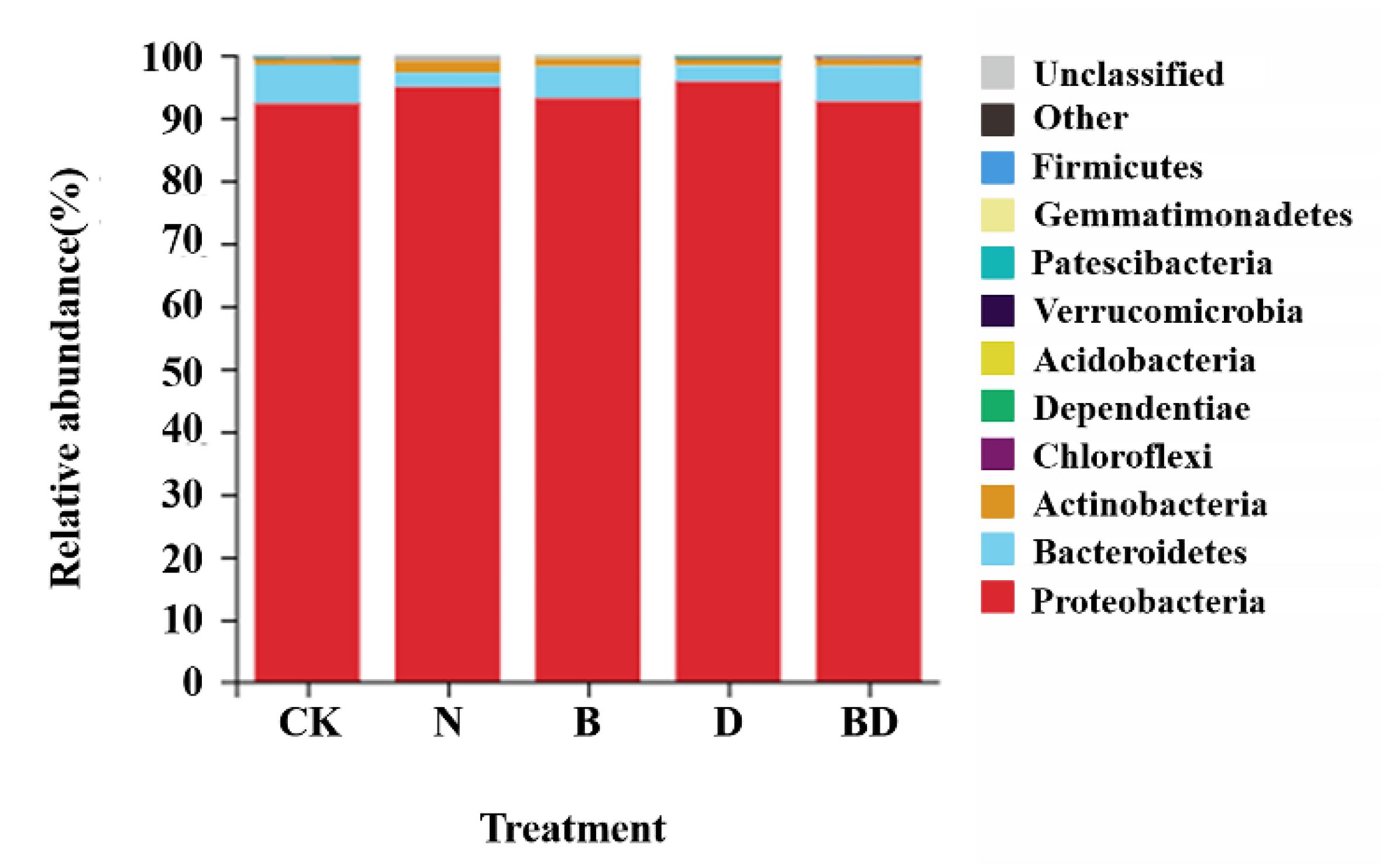
3.6. 16S rRNA for Bacteria Community in Soybean Root and Rhizosphere Soil
3.6.1. Bacterial Diversity (OTU, ACE, Chao1, and Shannon Index)
3.6.2. Bacterial Composition
4. Discussion
4.1. Effect of Biochar and Nitrification Inhibitor on Valid Nitrogen
4.2. Effect of Biochar and Nitrification Inhibitor on the Soil’s Basial Properties
4.3. Effect of Biochar and Nitrification Inhibitor on Bacteria Abundance
4.4. Relationships between Bacterial Community and Soil Properties
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Setia, R.; Marschner, P. Carbon mineralization in saline soils as affected by residue composition and water potential. Biol. Fert. Soils 2013, 49, 71–77. [Google Scholar] [CrossRef]
- Gunarathne, V.; Senadeera, A.; Gunarathne, U.; Biswas, J.K.; Almaroai, Y.A.; Vithanage, M. Potential of biochar and organic amendments for reclamation of coastal acidic-salt affected soil. Biochar 2020, 2, 107–120. [Google Scholar] [CrossRef] [Green Version]
- You, X.; Yin, S.; Suo, F.; Xu, Z.; Chu, D.; Kong, Q.; Zhang, C.; Li, Y.; Liu, L. Biochar and fertilizer improved the growth and quality of the ice plant (Mesembryanthemum crystallinum L.) shoots in a coastal soil of Yellow River Delta, China. Sci. Total Environ. 2021, 775, 144893. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Sun, J. Soil Salinity Drives the Distribution Patterns and Ecological Functions of Fungi in Saline-Alkali Land in the Yellow River Delta, China. Front. Microbiol. 2020, 11, 594284. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Berlin/Heidelberg, Germany, 2018; pp. 45–50. [Google Scholar]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Zhang, L.; Song, Y.; Li, Y.; Zheng, Y.; Zhang, J.; et al. Effects of Nitrification Inhibitors on Soil Nitrification and Ammonia Volatilization in Three Soils with Different pH. Agronomy 2021, 11, 1674. [Google Scholar] [CrossRef]
- Luo, X.; Wang, L.; Liu, G.; Wang, X.; Wang, Z.; Zheng, H. Effects of biochar on carbon mineralization of coastal wetland soils in the Yellow River Delta, China. Ecol. Eng. 2016, 94, 329–336. [Google Scholar] [CrossRef]
- Sun, H.; Lu, H.; Chu, L.; Shao, H.; Shi, W. Biochar applied with appropriate rates can reduce N leaching, keep N retention and not increase NH3 volatilization in a coastal saline soil. Sci. Total Environ. 2017, 575, 820–825. [Google Scholar] [CrossRef]
- He, K.; He, G.; Wang, C.; Zhang, H.; Xu, Y.; Wang, S.; Kong, Y.; Zhou, G.; Hu, R. Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl. Soil Ecol. 2020, 155, 103674. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Dong, G.; Du, Z.; Wu, W.; Chadwick, D.; Bol, R. The importance of ammonia volatilization in estimating the efficacy of nitrification inhibitors to reduce N2O emissions: A global meta-analysis. Environ. Pollut. 2021, 271, 116365. [Google Scholar] [CrossRef]
- Robinson, A.; Di, H.J.; Cameron, K.C.; Podolyan, A.; He, J. The effect of soil pH and dicyandiamide (DCD) on N2O emissions and ammonia oxidiser abundance in a stimulated grazed pasture soil. J. Soil Sediment 2014, 14, 1434–1444. [Google Scholar] [CrossRef]
- Yuan, L.; Chen, X.; Jia, J.; Chen, H.; Shi, Y.; Ma, J.; Liang, C.; Liu, Y.; Xie, H.; He, H.; et al. Stover mulching and inhibitor application maintain crop yield and decrease fertilizer N input and losses in no-till cropping systems in Northeast China. Agric. Ecosyst. Environ. 2021, 312, 107360. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Müller, C.; Cai, Z. Ecological and practical significances of crop species preferential N uptake matching with soil N dynamics. Soil Boil. Biochem. 2016, 103, 63–70. [Google Scholar] [CrossRef]
- Xie, W.; Wu, L.; Zhang, Y.; Wu, T.; Li, X.; Ouyang, Z. Effects of straw application on coastal saline topsoil salinity and wheat yield trend. Soil Tillage Res. 2017, 169, 1–6. [Google Scholar] [CrossRef]
- Kumar, V.; Wagenet, R.J. Salt effects on urea hydrolysis and nitrification during leaching through laboratory soil columns. Plant Soil 1985, 85, 219–227. [Google Scholar] [CrossRef]
- Rao, D.L.N.; Batra, L. Ammonia volatilization from applied nitrogen in alkali soils. Plant Soil 1983, 70, 219–228. [Google Scholar] [CrossRef]
- Van Hoorna, J.W.; Katerji, N.; Hamdy, A.; Mastrorilli, M. Effect of salinity on yield and nitrogen uptake of four grain legumes and on biological nitrogen contribution from the soil. Agric. Water Manag. 2001, 51, 87–98. [Google Scholar] [CrossRef]
- Nan, M.; Xiuzhi, Z.; Xinqi, W.; Chang, P.; Hongjun, G.; Ping, Z.; Yan, G. Effects of 40 years applications of inorganic and organic fertilization on soil bacterial community in a maize agroecosystem in northeast China. Eur. J. Agron. 2021, 130, 126332. [Google Scholar]
- Kumar, A.; Bhattacharya, T.; Mukherjee, S.; Sarkar, B. A perspective on biochar for repairing damages in the soil–plant system caused by climate change-driven extreme weather events. Biochar 2022, 4, 22. [Google Scholar] [CrossRef]
- Tauqeer, H.M.; Basharat, Z.; Adnan Ramzani, P.M.; Farhad, M.; Lewinska, K.; Turan, V.; Karczewska, A.; Khan, S.A.; Faran, G.E.; Iqbal, M. Aspergillus niger-mediated release of phosphates from fish bone char reduces Pb phytoavailability in Pb-acid batteries polluted soil, and accumulation in fenugreek. Environ. Pollut. 2022, 313, 120064. [Google Scholar] [CrossRef]
- Rasool, B.; Mahmood-ur, R.; Zubair, M.; Khan, M.A.; Ramzani, P.M.A.; Dradrach, A.; Turan, V.; Iqbal, M.; Khan, S.A.; Tauqeer, H.M.; et al. Synergetic Efficacy of Amending Pb-Polluted Soil with P-Loaded Jujube (Ziziphus mauritiana) Twigs Biochar and Foliar Chitosan Application for Reducing Pb Distribution in Moringa Leaf Extract and Improving Its Anti-cancer Potential. Water Air Soil Pollut. 2022, 233, 344. [Google Scholar] [CrossRef]
- Beusch, C.; Melzer, D.; Cierjacks, A.; Kaupenjohann, M. Amending a tropical Arenosol: Increasing shares of biochar and clay improve the nutrient sorption capacity. Biochar 2022, 4, 16. [Google Scholar] [CrossRef]
- Cayuela, M.L.; van Zwieten, L.; Singh, B.P.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M.A. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- Cui, Q.; Xia, J.B.; Yang, H.J.; Liu, J.T.; Shao, P.S. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.; Geisseler, D.; Wu, Z.; Gong, P.; Xue, Y.; Yu, C.; Juan, Y.; Horwath, W.R. Available C and N affect the utilization of glycine by soil microorganisms. Geoderma 2016, 283, 32–38. [Google Scholar] [CrossRef]
- Bilen, S.; Turan, V. Enzymatic Analyses in Soils. In Practical Handbook on Agricultural Microbiology; Amaresan, N., Patel, P., Amin, D., Eds.; Humana: New York, NY, USA, 2022; pp. 377–385. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Boil. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Turan, V.; Aydın, S.; Sönmez, O. Production, Cost Analysis, and Marketing of Bioorganic Liquid Fertilizers and Plant Nutrition Enhancers. In Industrial Microbiology Based Entrepreneurship. Microorganisms for Sustainability; Amaresan, N., Dharumadurai, D., Cundell, D.R., Eds.; Springer: Singapore, 2022; Volume 42, pp. 193–198. [Google Scholar]
- Ma, Q.; Wu, L.; Wang, J.; Ma, J.; Zheng, N.; Hill, P.W.; Chadwick, D.R.; Jones, D.L. Fertilizer regime changes the competitive uptake of organic nitrogen by wheat and soil microorganisms: An in-situ uptake test using 13C, 15N labelling, and 13C-PLFA analysis. Soil Boil. Biochem. 2018, 125, 319–327. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.; Wang, H.; Gai, X. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, H.; Wang, F.; Sun, C.; Zhang, X. Effects of biochar incorporation on soil viable and necromass carbon in the luvisol soil. Soil Use Manag. 2021, 38, 318–330. [Google Scholar] [CrossRef]
- Cui, L.; Li, D.; Wu, Z.; Zhang, L.; Xue, Y.; Gong, P.; Song, Y.; Li, X.; Xiao, F.; Yang, L.; et al. Effects of Combined Nitrification Inhibitors on Nitrogen Transformation, Maize Yield and Nitrogen Uptake in Two Different Soils. Commun. Soil Sci. Plant Anal. 2022, 53, 1039–1049. [Google Scholar] [CrossRef]
- Du, Z.-L.; Zhao, J.-K.; Wang, Y.-D.; Zhang, Q.-Z. Biochar addition drives soil aggregation and carbon sequestration in aggregate fractions from an intensive agricultural system. J. Soil Sediment 2016, 17, 581–589. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Luo, J.; Zaman, M.; Houlbrooke, D.; Ding, W.; Ledgard, S.; Ghani, A. Use of nitrogen process inhibitors for reducing gaseous nitrogen losses from land-applied farm effluents. Biol. Fert. Soils 2013, 50, 133–145. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fert. Soils 2006, 43, 699–708. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Boil. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Zhang, A.; Liu, Y.; Pan, G.; Hussain, Q.; Li, L.; Zheng, J.; Zhang, X. Effect of biochar amendment on maize yield and greenhouse gas emissions from a soil organic carbon poor calcareous loamy soil from Central China Plain. Plant Soil 2011, 351, 263–275. [Google Scholar] [CrossRef]
- Rasul, M.; Cho, J.; Shin, H.S.; Hur, J. Biochar-induced priming effects in soil via modifying the status of soil organic matter and microflora: A review. Sci. Total Environ. 2022, 805, 150304. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Ma, B.; Chang, S.X.; Gong, J. Biochar addition affected the dynamics of ammonia oxidizers and nitrification in microcosms of a coastal alkaline soil. Biol. Fert. Soils 2013, 50, 321–332. [Google Scholar] [CrossRef]
- Nguyen, T.T.N.; Xu, C.-Y.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.M.; Bai, S.H. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply biochar to ameliorate soda saline-alkali land, improve soil function and increase corn nutrient availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Liu, G.; Xia, Y.; Chen, L.; Jiang, Z.; Zheng, H.; Wang, Z. Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J. Soil Sediments 2016, 17, 780–789. [Google Scholar] [CrossRef]
- Liu, G.M.; Zhang, X.C.; Wang, X.P.; Shao, H.B.; Yang, J.S.; Wang, X.P. Soil enzymes as indicators of saline soil fertility under various soil amendments. Agric. Ecosyst. Environ. 2017, 237, 274–279. [Google Scholar]
- Kim, J.S.; Sparovek, G.; Longo, R.M.; De Melo, W.J.; Crowley, D. Bacterial diversity of terra preta and pristine forest soil from the Western Amazon. Soil Boil. Biochem. 2007, 39, 684–690. [Google Scholar] [CrossRef]
- Dempster, D.N.; Gleeson, D.B.; Solaiman, Z.M.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2011, 354, 311–324. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, J.; Gao, Y.; Zhao, H.; Zhang, G.; Cui, B. Shifts in the soil bacterial community along a salinity gradient in the Yellow River Delta. Land Degrad. Dev. 2020, 31, 2255–2267. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Rousk, J. Linking Microbial Community Structure to Trait Distributions and Functions Using Salinity as an Environmental Filter. mBio 2019, 10, e01607-19. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Liu, J.J.; Banerjee, S.; Zhou, N.; Zhao, Z.Y.; Zhang, K.; Tian, C.Y. Soil pH is equally important as salinity in shaping bacterial communities in saline soils under halophytic vegetation. Sci. Rep. 2018, 8, 4550. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Chen, C.; Xiang, Y.; Rezaei Rashti, M.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. GCB Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]


| Treatment | Read Numbers (×104) | OTU | Shannon | Simpson | Chao | Ace |
|---|---|---|---|---|---|---|
| CK | 8.56 ± 0.78a | 501.5 ± 157.83a | 2.78 ± 0.32a | 0.66 ± 0.04a | 507.02 ± 102.89a | 514.12 ± 87.56a |
| N | 8.46 ± 0.15a | 528 ± 87.9a | 2.4 ± 0.56a | 0.57 ± 0.07a | 578.43 ± 83.03a | 592.58 ± 79.17a |
| B | 9.03 ± 0.76a | 449.25 ± 68.78a | 2.45 ± 0.31a | 0.61 ± 0.04a | 505.71 ± 71.43a | 513.63 ± 70.54a |
| D | 8.56 ± 0.45a | 491.5 ± 72.8a | 2.38 ± 0.36a | 0.57 ± 0.04a | 557.3 ± 80.06a | 563.77 ± 81.64a |
| BD | 8.45 ± 0.35a | 451.25 ± 69.07a | 2.5 ± 0.25a | 0.6 ± 0.07a | 521.16 ± 53.21a | 525.09 ± 61.65a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Wang, G.; Zhang, H.; Chen, H.; Ma, Q. Biochar and Nitrification Inhibitor (Dicyandiamide) Combination Had a Double-Win Effect on Saline-Alkali Soil Improvement and Soybean Production in the Yellow River Delta, China. Agronomy 2022, 12, 3154. https://doi.org/10.3390/agronomy12123154
Yu C, Wang G, Zhang H, Chen H, Ma Q. Biochar and Nitrification Inhibitor (Dicyandiamide) Combination Had a Double-Win Effect on Saline-Alkali Soil Improvement and Soybean Production in the Yellow River Delta, China. Agronomy. 2022; 12(12):3154. https://doi.org/10.3390/agronomy12123154
Chicago/Turabian StyleYu, Chunxiao, Guangmei Wang, Haibo Zhang, Hongpeng Chen, and Qian Ma. 2022. "Biochar and Nitrification Inhibitor (Dicyandiamide) Combination Had a Double-Win Effect on Saline-Alkali Soil Improvement and Soybean Production in the Yellow River Delta, China" Agronomy 12, no. 12: 3154. https://doi.org/10.3390/agronomy12123154
APA StyleYu, C., Wang, G., Zhang, H., Chen, H., & Ma, Q. (2022). Biochar and Nitrification Inhibitor (Dicyandiamide) Combination Had a Double-Win Effect on Saline-Alkali Soil Improvement and Soybean Production in the Yellow River Delta, China. Agronomy, 12(12), 3154. https://doi.org/10.3390/agronomy12123154





