Rhizobium Inoculation Improved the Rhizosphere P Dynamics and P Uptake Capacity of Pigeon Pea Plants Grown in Strongly Weathered Soil Only under P Fertilized Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils
2.2. Plant Growth Conditions
2.3. Experimental Design
2.4. Plant and Soil Sampling
2.5. Phosphorus Fractionation of Soil
2.6. Evaluation of Exudation from Root
2.6.1. Evaluation of Organic Acid from Root
2.6.2. Evaluation of Acid Phosphatase Activity of Root
2.7. Data Analysis
= ((Up − U0)/Fp) × 100
3. Results
3.1. Plant Biomass, Plant P and N Uptake, and PUE
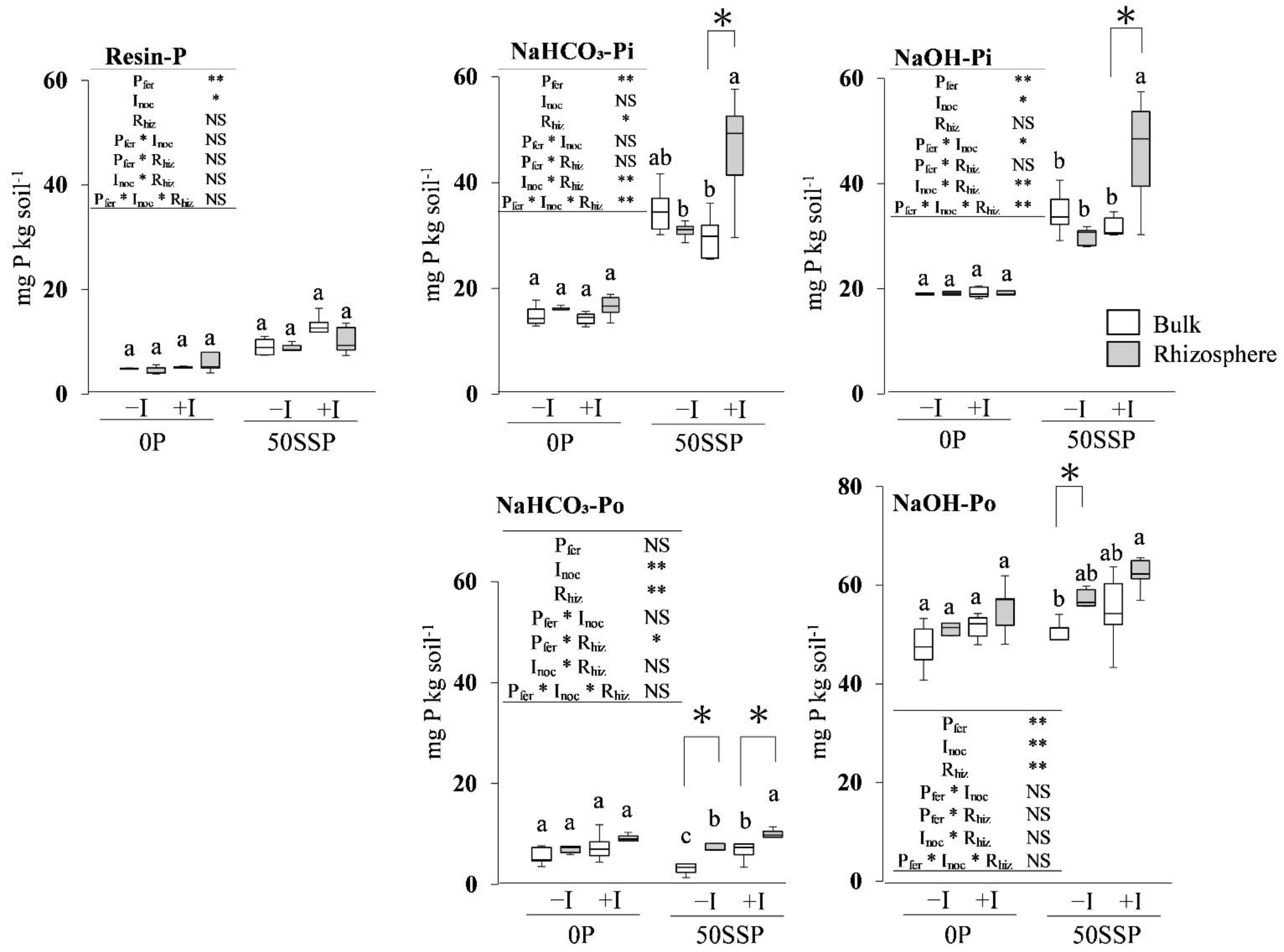
3.2. Fractionated P of Rhizosphere and Bulk Soil
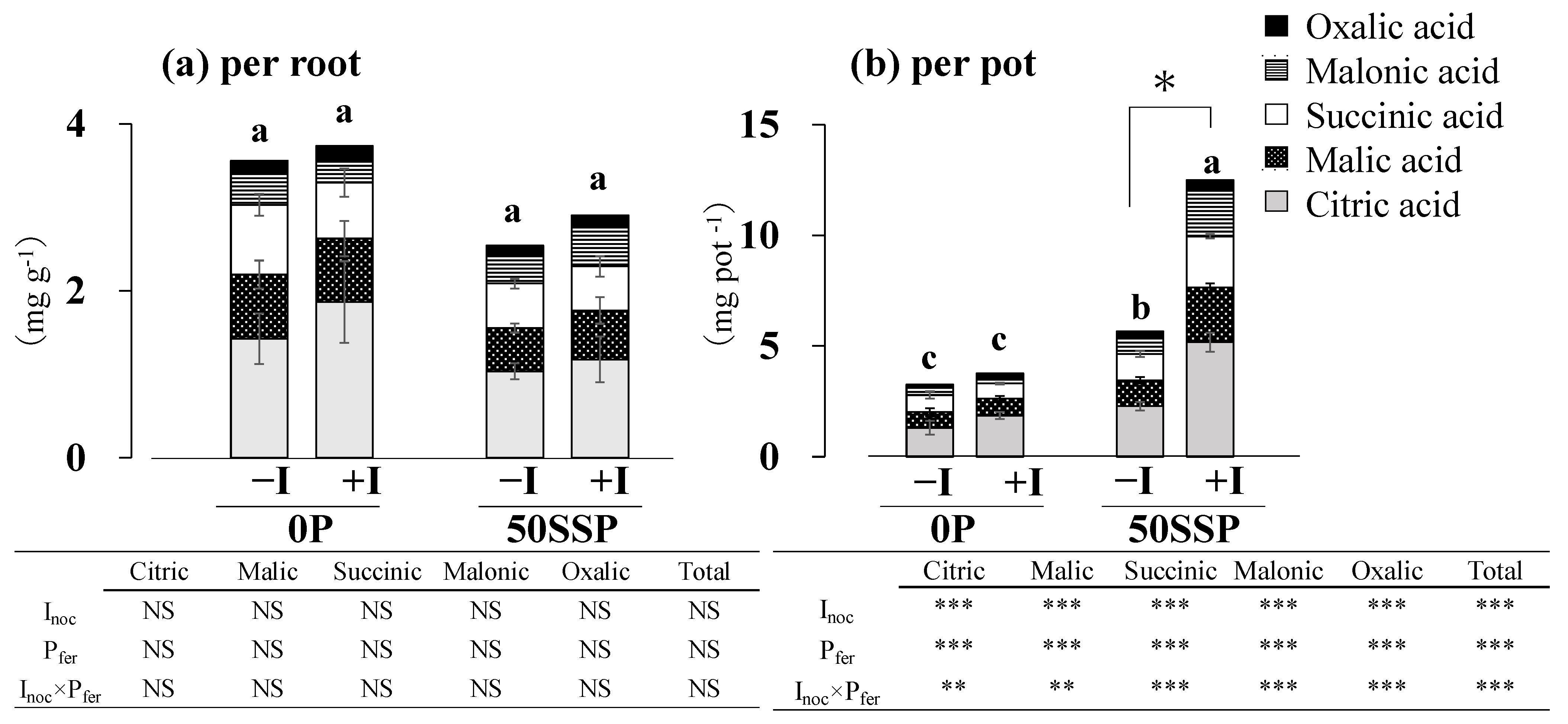
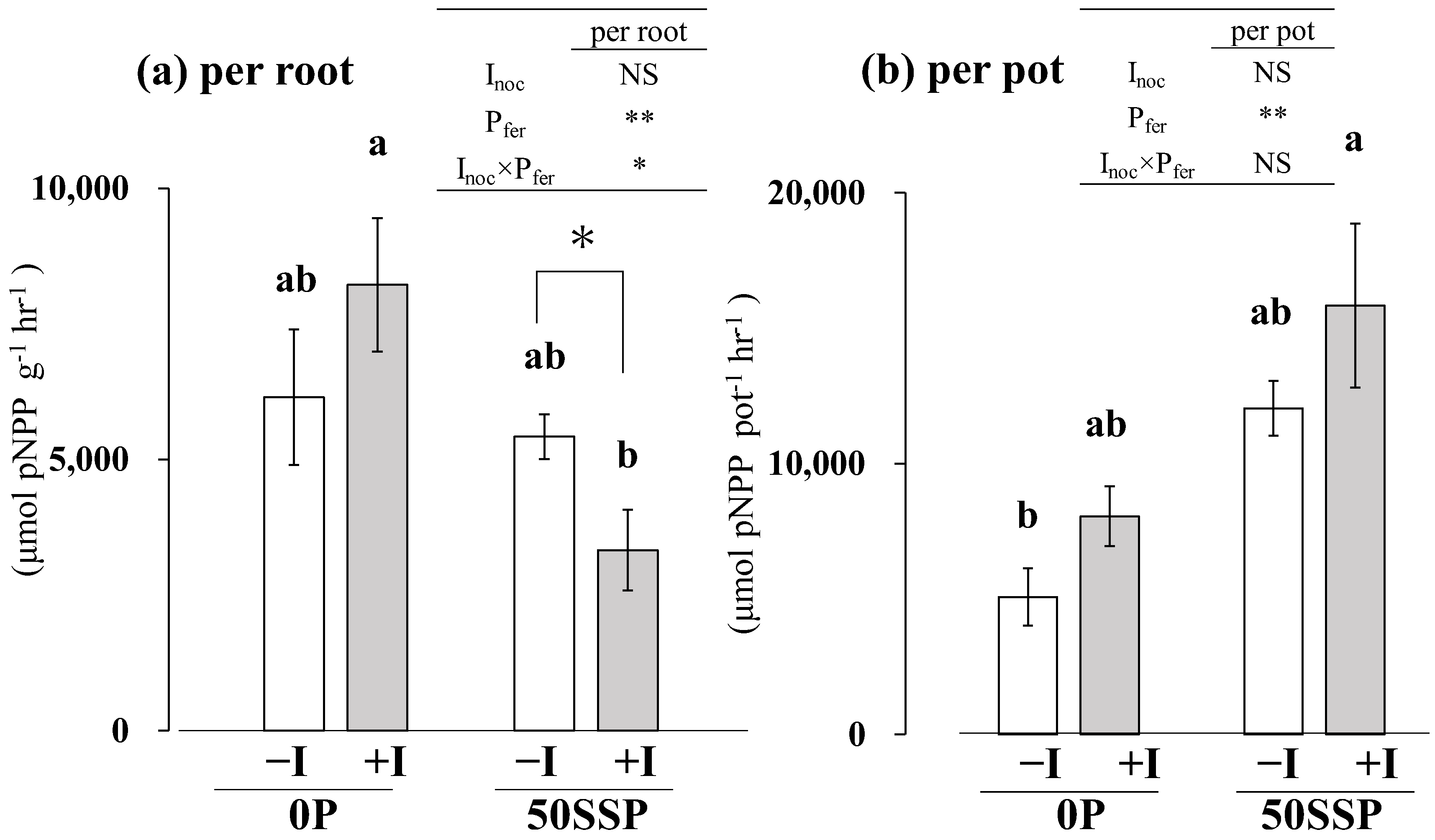
3.3. Plant Root P Acquisition Characteristics
3.4. Rhizosphere and Bulk Soil pH
4. Discussion
4.1. Effect of the Rhizobium Inoculation with P Fertilization on the Rhizosphere Soil P Dynamics
4.2. Effect of the Rhizobium Inoculation with P Fertilization on Plant P Acquisition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Negassa, W.; Leinweber, P. How does the Hedley sequential phosphorus fractionation reflect impacts of land use and management on soil phosphorus: A review. Plant Nutr. Soil Sci. 2009, 172, 305–325. [Google Scholar] [CrossRef]
- Van Vuuren, D.P.; Bouwman, A.F.; Beusen, A.H.W. Phosphorus demand for the 1970–2100 period: A scenario analysis of resource depletion. Glob. Environ. Chang. 2010, 20, 428–439. [Google Scholar] [CrossRef]
- Du, E.; Terrer, C.; Pellegrini, A.; Ahlström, A.; van Lissa, C.J.; Zhao, X.; Xia, N.; Wu, X.; Jackson, R.B. Global patterns of terrestrial nitrogen and phosphorus limitation. Nat. Geosci. 2020, 13, 221–226. [Google Scholar] [CrossRef]
- Hedley, M.J.; Kirk, G.J.R.; Santos, M.B. Phosphorus efficiency and the forms of soil phosphorus utilized by upland rice cultivars. Plant Soil 1994, 158, 53–62. [Google Scholar] [CrossRef]
- Nziguheba, G.; Merckx, R.; Palm, C.A. Soil phosphorus dynamics and maize response to different rates of phosphorus fertilizer applied to an Acrisol in western Kenya. Plant Soil 2002, 243, 1–10. [Google Scholar] [CrossRef]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar] [CrossRef] [Green Version]
- de-Bashan, L.E.; Magallon-Servin, P.; Lopez, B.R.; Nannipieri, P. Biological activities affect the dynamic of P in dryland soils. Biol. Fertil. Soils 2022, 58, 105–119. [Google Scholar] [CrossRef]
- Gerke, J.; Römer, W.; Jungk, A. The excretion of citric and malic acid by proteoid roots of Lupinus albus L.; effects on soil solution concentrations of phosphate, iron, and aluminum in the proteoid rhizosphere in samples of an oxisol and a luvisol. Plant Nutr. Soil Sci. 1994, 157, 289–294. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Shen, W.; Kuzyakov, Y. Phosphatase activity and acidification in lupine and maize rhizosphere depend on phosphorus availability and root properties: Coupling zymography with planar optodes. Appl. Soil Ecol. 2021, 167, 104029. [Google Scholar] [CrossRef]
- Li, H.; Shen, J.; Zhang, F.; Marschner, P.; Cawthray, G.; Rengel, Z. Phosphorus uptake and rhizosphere properties of intercropped and monocropped maize, faba bean, and white lupin in acidic soil. Biol. Fertil. Soils 2010, 46, 79–91. [Google Scholar] [CrossRef]
- Otani, T.; Ae, N.; Tanaka, H. Phosphorus (P) uptake mechanisms of crops grown in soils with low P status: II. significance of organic acids in root exudates of pigeonpea. Soil Sci. Plant Nutr. 1996, 42, 553–560. [Google Scholar] [CrossRef]
- Ae, N.; Otani, T.; Makino, T.; Tazawa, J. Role of cell wall of groundnut roots in solubilizing sparingly soluble phosphorus in soil. Plant Soil 1996, 186, 197–204. [Google Scholar] [CrossRef]
- Wasaki, J.; Yamamura, T.; Shinano, T.; Osaki, M. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 2003, 248, 129–136. [Google Scholar] [CrossRef]
- Imai, K.; Sugihara, S.; Wasaki, J.; Tanaka, H. Effects of White Lupin and Groundnut on Fractionated Rhizosphere Soil P of Different P-Limited Soil Types in Japan. Agronomy 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Sugihara, S.; Kawashita, T.; Shitindi, M.; Massawe, B.; Tanaka, H. Soil Science and Plant Nutrition Dynamics of fractionated rhizosphere soil P and plant P uptake under maize/P-mobilizing legumes intercropping in strongly weathered soil of Tanzania. Soil Sci. Plant Nutr. 2021, 67, 312–322. [Google Scholar] [CrossRef]
- Kamara, A.Y.; Abaidoo, R.; Kwari, J.; Omoigui, L. Influence of phosphorus application on growth and yield of soybean genotypes in the tropical savannas of northeast Nigeria. Arch. Agron. Soil Sci. 2007, 53, 539–552. [Google Scholar] [CrossRef]
- Kolawole, G.O. Effect of phosphorus fertilizer application on the performance of maize/soybean intercrop in the southern Guinea savanna of Nigeria. Arch. Agron. Soil Sci. 2011, 58, 189–198. [Google Scholar] [CrossRef]
- Sanginga, N.; Thottappilly, G.; Dashiell, K. Effectiveness of rhizobia nodulating recent promiscuous soyabean selections in the moist savanna of Nigeria. Soil Biol. Biochem. 2000, 32, 127–133. [Google Scholar] [CrossRef]
- Osunde, A.O.; Gwam, S.; Bala, A.; Sanginga, N.; Okogun, J.A. Responses to rhizobial inoculation by two promiscuous soybean cultivars in soils of the Southern Guinea savanna zone of Nigeria. Biol. Fertil. Soils 2003, 37, 274–279. [Google Scholar] [CrossRef]
- Thuita, M.; Pypers, P.; Herrmann, L.; Okalebo, R.J.; Othieno, C.; Muema, E.; Lesueur, D. Commercial rhizobial inoculants significantly enhance growth and nitrogen fixation of a promiscuous soybean variety in Kenyan soils. Biol. Fertil. Soils 2012, 48, 87–96. [Google Scholar] [CrossRef]
- Ronner, E.; Franke, A.C.; Vanlauwe, B.; Dianda, M.; Edeh, E.; Ukem, B.; Bala, A.; van Heerwaarden, J.; Giller, K.E. Understanding variability in soybean yield and response to P-fertilizer and rhizobium inoculants on farmers’ fields in northern Nigeria. Field Crop. Res. 2016, 186, 133–145. [Google Scholar] [CrossRef]
- Kyei-Boahen, S.; Savala, C.E.N.; Chikoye, D.; Abaidoo, R. Growth and Yield Responses of Cowpea to Inoculation and Phosphorus Fertilization in Different Environments. Front. Plant Sci. 2017, 8, 646. [Google Scholar] [CrossRef] [Green Version]
- Ulzen, J.; Abaidoo, R.C.; Ewusi-Mensah, N.; Masso, C. On-farm evaluation and determination of sources of variability of soybean response to Bradyrhizobium inoculation and phosphorus fertilizer in northern Ghana. Agric. Ecosyst. Environ. 2018, 267, 23–32. [Google Scholar] [CrossRef]
- Wolde-meskel, E.; Van Heerwaarden, J.; Abdulkadir, B.; Kassa, S.; Aliyi, I.; Degefu, T.; Wakweya, K.; Kanampiu, F.; Giller, K.E. Additive yield response of chickpea (Cicer arietinum L.) to rhizobium inoculation and phosphorus fertilizer across smallholder farms in Ethiopia. Agric. Ecosyst. Environ. 2018, 261, 144–152. [Google Scholar] [CrossRef]
- Israel, D.W. Investigation of the Role of Phosphorus in Symbiotic Dinitrogen Fixation. Plant Physiol. 1987, 84, 835–840. [Google Scholar] [CrossRef]
- O’Hara, G.W. Nutritional constraints on root nodule bacteria affecting symbiotic nitrogen fixation: A review. Aust. J. Exp. Agric. 2001, 41, 417–433. [Google Scholar] [CrossRef]
- Rondina, A.B.L.; dos Santos Sanzovo, A.W.; Guimarães, G.S.; Wendling, J.R.; Nogueira, M.A.; Hungria, M. Changes in root morphological traits in soybean co-inoculated with Bradyrhizobium spp. and Azospirillum brasilense or treated with A. brasilense exudates. Biol. Fertil. Soils 2020, 56, 537–549. [Google Scholar] [CrossRef]
- Umali-Garcia, M.; Libuit, J.S.; Baggayan, R.L. Effects of Rhizobium inoculation on growth and nodulation of Albizia falcataria (L.) Fosh. and Acacia mangium Willd. in the nursery. Plant Soil 1988, 108, 71–78. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B. Method to measure microbial phosphate in soils. Soil Biol. Biochem. 1982, 14, 377–385. [Google Scholar] [CrossRef]
- Truog, E. The determination of readily available phosphorus in soils. J. Am. Soc. Agron. 1930, 22, 874–882. [Google Scholar] [CrossRef] [Green Version]
- Vanlauwe, B.; Hungria, M.; Kanampiu, F.; Giller, K.E. The role of legumes in the sustainable intensification of African smallholder agriculture: Lessons learnt and challenges for the future. Agric. Ecosyst. Environ. 2019, 284, 106583. [Google Scholar] [CrossRef]
- Fossou, R.K.; Ziegler, D.; Zézé, A.; Barja, F.; Perret, X. Two major clades of bradyrhizobia dominate symbiotic interactions with pigeonpea in fields of Côte d’Ivoire. Front. Microbiol. 2016, 7, 1793. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.P.; Miwa, H.; Obirih-Opareh, J.; Suzaki, T.; Yasuda, M.; Okazaki, S. Novel rhizobia exhibit superior nodulation and biological nitrogen fixation even under high nitrate concentrations. FEMS Microbiol. Ecol. 2020, 96, fiz184. [Google Scholar] [CrossRef]
- Sadowsky, M.J.; Tully, R.E.; Cregan, P.B.; Keyser, H.H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl. Environ. Microbiol. 1987, 53, 2624–2630. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Marschner, P.; Zhang, F. Phosphorus pools and other soil properties in the rhizosphere of wheat and legumes growing in three soils in monoculture or as a mixture of wheat and legume. Plant Soil 2012, 354, 283–298. [Google Scholar] [CrossRef]
- Hanson, W.C. The photometric determination of phosphorus in fertilizers using the phosphovanado-molybdate complex. J. Sci. Food Agric. 1950, 1, 172–173. [Google Scholar] [CrossRef]
- Tiessen, H.; Moir, J.O. Characterization of available P by sequential extraction. In Soil Sampling and Methods of Analysis; Carter, M.R., Gregorich, E.G., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 75–86. [Google Scholar]
- Sugihara, S.; Funakawa, S.; Nishigaki, T.; Kilasara, M.; Kosaki, T. Dynamics of fractionated P and P budget in soil under different land management in two Tanzanian croplands with contrasting soil textures. Agric. Ecosyst. Environ. 2012, 162, 101–107. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Lipton, D.S.; Blanchar, R.W.; Blevins, D.G. Citrate, Malate, and Succinate Concentration in Exudates from P-Sufficient and P-Stressed Medicago sativa L. Seedlings. Plant Physiol. 1987, 85, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Von Wirén, N.; Mori, S.; Marschner, H.; Römheld, V. Iron Inefficiency in Maize Mutant ys1 (Zea mays L. cv Yellow-Stripe) Is Caused by a Defect in Uptake of Iron Phytosiderophores. Plant Physiol. 1994, 106, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura, H.; Sugihara, S.; Kamiya, T.; Artigas Ramirez, M.D.; Miyatake, M.; Fujiwara, T.; Takuji, O.; Motobayashi, T.; Yokoyama, T.; Bellingrath-Kimura, S.D.; et al. Sulfur application enhances secretion of organic acids by soybean roots and solubilization of phosphorus in rhizosphere. Soil Sci. Plant Nutr. 2021, 67, 400–407. [Google Scholar] [CrossRef]
- Ishidzuka, S. Soil enzymes. In The Japanese Society of Forest Environment the Methods of Forest-Environment Studies; Hakuyusha Publishers: Tokyo, Japan, 1999; pp. 219–222. [Google Scholar]
- Chien, S.H.; Sikora, F.J.; Gilkes, R.J.; Mclaughlin, M.J. Comparing of the difference and balance methods to calculate percent recovery of fertilizer phosphorus applied to soils: A critical discussion. Nutr. Cycl. Agroecosyst. 2012, 92, 1–8. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhang, W.; Wang, L.; Tian, X.; Yang, S.; Jiang, W.; Ruijven, J.; Li, L. The role of complementarity and selection effects in P acquisition of intercropping systems. Plant Soil 2018, 422, 479–493. [Google Scholar] [CrossRef]
- Wang, Y.; Lambers, H. Root-released organic anions in response to low phosphorus availability: Recent progress, challenges and future perspectives. Plant Soil 2020, 447, 135–156. [Google Scholar] [CrossRef]
- Li, L.; Tilman, D.; Lambers, H.; Zhang, F.S. Plant diversity and overyielding: Insights from belowground facilitation of intercropping in agriculture. New Phytol. 2014, 203, 63–69. [Google Scholar] [CrossRef]
- Qiao, X.; Bei, S.; Li, C.; Dong, Y.; Li, H.; Christie, P.; Zhang, F.; Zhang, J. Enhancement of faba bean competitive ability by arbuscular mycorrhizal fungi is highly correlated with dynamic nutrient acquisition by competing wheat. Sci. Rep. 2015, 5, 8122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, S.H.; Prochnow, L.I.; Tu, S.; Snyder, C.S. Agronomic and environmental aspects of phosphate fertilizers varying in surce and solubility: An update review. Nutr. Cycl. Agroecosyst. 2011, 89, 229–255. [Google Scholar] [CrossRef]
- Martins, D.; Macovei, A.; Leonetti, P.; Balestrazzi, A.; Araujo, S. The influence of phosphate deficiency on legume symbiotic N2 fixation. In Legumes Nitrogen Fixation in Soils with Low Phosphorus Availability; Sulieman, S., Tran, L.S.P., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 41–75. [Google Scholar]
- Ma, Y.; Chen, R. Nitrogen and Phosphorus Signaling and Transport During Legume–Rhizobium Symbiosis. Front. Plant Sci. 2021, 12, 683601. [Google Scholar] [CrossRef] [PubMed]
- Masso, C.; Mukhongo, R.W.; Thuita, M.; Abaidoo, R.; Ulzen, J.; Kariuki, G.; Kalumuna, M. Biological inoculants for sustainable intensification of agriculture in sub-saharan africa smallholder farming systems. In Climate Change and Multi-Dimensional Sustainability in African Agriculture; Lal, R., Kraybill, D., Hansen, D., Singh, B.R., Msogoya, T., Eik, L., Eds.; Springer International Publishing: New York, NY, USA, 2016; pp. 639–658. [Google Scholar]
- Ström, L.; Owen, A.G.; Godbold, D.L.; Jones, D.L. Organic acid behaviour in a calcareous soil implications for rhizosphere nutrient cycling. Soil Biol. Biochem. 2005, 37, 2046–2054. [Google Scholar] [CrossRef]
- Jones, D.L.; Darrah, P.R. Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 1994, 166, 247–257. [Google Scholar] [CrossRef]
- Spohn, M.; Zeißig, I.; Brucker, E.; Widdig, M.; Lacher, U.; Aburto, F. Phosphorus solubilization in the rhizosphere in two saprolites with contrasting phosphorus fractions. Geoderma 2020, 366, 114245. [Google Scholar] [CrossRef]
- Oburger, E.; Kirk, G.J.D.; Wenzel, W.W.; Puschenreiter, M.; Jones, D.L. Interactive effects of organic acids in the rhizosphere. Soil Biol. Biochem. 2009, 41, 449–457. [Google Scholar] [CrossRef]
- Sulieman, S.; Schulze, J.; Tran, L.S.P. Comparative analysis of the symbiotic efficiency of Medicago truncatula and Medicago sativa under phosphorus deficiency. Int. J. Mol. Sci. 2013, 14, 5198–5213. [Google Scholar] [CrossRef] [Green Version]
- Sanz-Saez, A.; Morales, F.; Arrese-Igor, C.; Aranjuelo, I. P deficiency: A major limiting factor for rhizobial symbiosis. In Legumes Nitrogen Fixation in Soils with Low Phosphorus Availability; Sulieman, S., Tran, L.S.P., Eds.; Springer International Publishing: New York, NY, USA, 2017; pp. 21–39. [Google Scholar]
- Rejmánková, E.; Macek, P. Response of root and sediment phosphatase activity to increased nutrients and salinity. Biogeochemistry 2008, 90, 159–169. [Google Scholar] [CrossRef]
- Krishnappa, R.; Aftab Hussain, I.S. Phosphorus acquisition from deficient soil: Involvement of organic acids and acid phosphatase in pigeon pea (Cajanus cajan L. mills sp). Indian J. Plant Physiol. 2014, 19, 197–204. [Google Scholar] [CrossRef]
- Ae, N.; Arihara, J.; Okada, K.; Yoshihara, T.; Johansen, C. Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 1990, 24, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Phosphorus benefits of different legume crops to subsequent wheat grown in different soils of Western Australia. Plant Soil 2005, 271, 175–187. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. Soil Sci. Plant Nutr. 2012, 12, 547–561. [Google Scholar] [CrossRef]

| Soil pH | Sand | Silt | Clay | TC | TN | Total-P | Truog-P | P Ads. Cap. | Alo | Feo | Ald | Fed | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (H2O) | (KCl) | (%) | (g kg−1) | (mg kg−1) | (g P2O5 kg−1) | (g kg−1) | ||||||||
| Ultisols | 4.2 | 3.3 | 40 | 36 | 24 | 25.1 | 1.9 | 349.1 | 10.4 | 6.6 | 1.2 | 1.3 | 2.9 | 29.2 |
| Plant Biomass | P Uptake | N Uptake | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Total | Leaf | Stem | Root | Total | Leaf | Stem | Root | Total | |||
| Inoc | ** | * | *** | *** | * | NS | ** | * | ** | ** | *** | ** | ||
| 8.2 | 17.1 | 22.8 | 17.0 | 5.6 | 16.0 | 8.0 | 9.3 | 7.3 | 9.2 | 45.4 | 17.7 | |||
| Pfer | *** | *** | *** | *** | *** | NS | *** | *** | *** | *** | *** | *** | ||
| 90.7 | 96.3 | 64.6 | 127.6 | 146.0 | 0.1 | 81.8 | 167.1 | 46.0 | 21.1 | 39.3 | 48.8 | |||
| Inoc × Pfer | ** | ** | * | ** | ** | NS | ** | ** | ** | NS | NS | * | ||
| 10.7 | 12.6 | 9.0 | 12.9 | 11.7 | 1.0 | 8.6 | 14.8 | 8.5 | 1.1 | 2.1 | 6.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, S.; Okazaki, S.; Monica, N.D.; Ohkama-Ohtsu, N.; Tanaka, H.; Sugihara, S. Rhizobium Inoculation Improved the Rhizosphere P Dynamics and P Uptake Capacity of Pigeon Pea Plants Grown in Strongly Weathered Soil Only under P Fertilized Conditions. Agronomy 2022, 12, 3149. https://doi.org/10.3390/agronomy12123149
Yamamoto S, Okazaki S, Monica ND, Ohkama-Ohtsu N, Tanaka H, Sugihara S. Rhizobium Inoculation Improved the Rhizosphere P Dynamics and P Uptake Capacity of Pigeon Pea Plants Grown in Strongly Weathered Soil Only under P Fertilized Conditions. Agronomy. 2022; 12(12):3149. https://doi.org/10.3390/agronomy12123149
Chicago/Turabian StyleYamamoto, Saki, Shin Okazaki, Nakei D. Monica, Naoko Ohkama-Ohtsu, Haruo Tanaka, and Soh Sugihara. 2022. "Rhizobium Inoculation Improved the Rhizosphere P Dynamics and P Uptake Capacity of Pigeon Pea Plants Grown in Strongly Weathered Soil Only under P Fertilized Conditions" Agronomy 12, no. 12: 3149. https://doi.org/10.3390/agronomy12123149
APA StyleYamamoto, S., Okazaki, S., Monica, N. D., Ohkama-Ohtsu, N., Tanaka, H., & Sugihara, S. (2022). Rhizobium Inoculation Improved the Rhizosphere P Dynamics and P Uptake Capacity of Pigeon Pea Plants Grown in Strongly Weathered Soil Only under P Fertilized Conditions. Agronomy, 12(12), 3149. https://doi.org/10.3390/agronomy12123149






