Chemical Profile, Bioactivity, and Biosafety Evaluations of Essential Oils and Main Terpenes of Two Plant Species against Trogoderma granarium
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Insect Species
2.2. Chemicals
2.3. Tested Plants and EO Extraction
2.4. Chemical Analysis of EOs
2.5. Identification of the EO Components
2.6. Isolation and Purification of Main Terpenes
2.7. Contact Toxicity
2.8. Fumigation
2.9. Acetylcholinesterase (AChE) Inhibition
2.10. Toxicity against a Non-Target Terrestrial Organism
2.11. Phytotoxicity Study
2.12. Data Analysis
3. Results
3.1. Aroma Profile of EOs
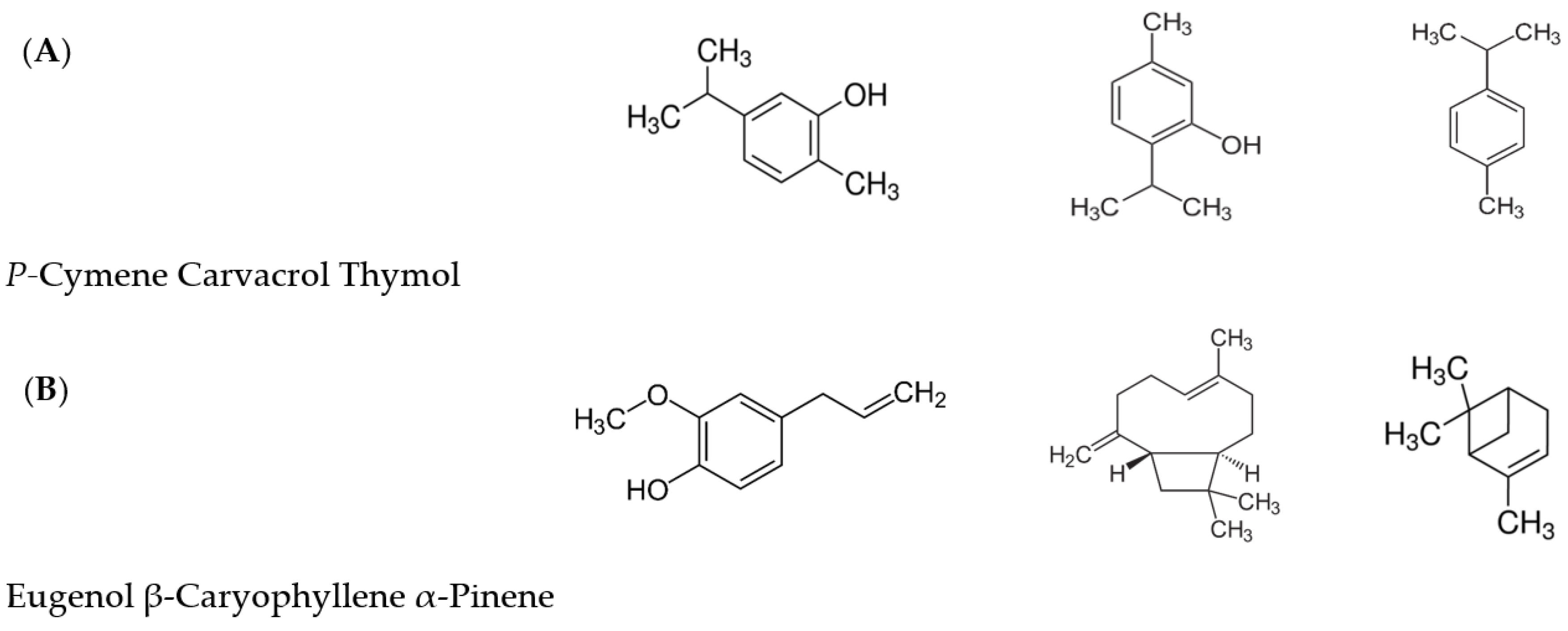
3.1.1. Eugenol
3.1.2. β-Caryophyllene
3.1.3. α-Pinene
3.1.4. Carvacrol
3.1.5. Thymol
3.1.6. p-Cymene
3.2. Insecticidal Activity
3.3. AChE Inhibition
3.4. Toxicity against Earthworm
3.5. Phytotoxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banga, K.S.; Kumar, S.; Kotwaliwale, N.; Mohapatra, D. Major insects of stored food grains. Int. J. Chem. Stud. 2020, 8, 2380–2384. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, insecticidal and repellence activities of essential oils of three Achillea species against the Khapra beetle (Coleoptera: Dermestidae). J. Pest Sci. 2014, 87, 273–283. [Google Scholar] [CrossRef]
- Nenaah, G. Bioactivity of powders and essential oils of three Asteraceae plants as post-harvest grain protectants against three major coleopteran pests. J. Asia-Pac. Entomol. 2014, 17, 701–709. [Google Scholar] [CrossRef]
- Eliopoulos, P.A. New Approaches for Tackling the Khapra Beetle. CAB Int. Rev. 2013, 1–13. Available online: https://www.cabi.org/cabreviews/review/20133064214 (accessed on 14 August 2022).
- Islam, W.; Noman, A.; Akutse, K.S.; Qasim, M.; Ali, H.; Haider, I.; Hashem, M.; Alamri, S.; Al Zoubi, O.M.; Khan, K.A. Phyto-derivatives: An efficient eco-friendly way to manage Trogoderma granarium (Everts) (Coleoptera: Dermestidae). Int. J. Trop. Insect Sci. 2021, 41, 915–926. [Google Scholar] [CrossRef]
- Singh, K.D.; Mobolade, A.J.; Bharali, R.; Sahoo, D.; Rajashekar, Y. Main plant volatiles as stored grain pest management approach: A review. J. Agric. Food Res. 2021, 4, 100127. [Google Scholar] [CrossRef]
- Amjad, F.; Hasan, M.U.; Ahmad, F.; Sahi, S.T. Pyrethroid resistance and selection against Trogoderma granarium (Everts) in Punjab, Pakistan. Int. J. Trop. Insect Sci. 2022, 42, 191–202. [Google Scholar] [CrossRef]
- Azhar, H.; Khan, A. Variation in susceptibility to insecticides and synergistic effect of enzyme inhibitors in Pakistani strains of Trogoderma granarium. J. Stored Prod. Res. 2021, 91, 101775. [Google Scholar] [CrossRef]
- Ersino, W.; Teressa, H.; Alemayo, T. Larvicidal activity of Juniperus procera extract against Anopheles mosquito in vitro, North Western Ethiopia. J. Med. Plants Res. 2020, 14, 445–450. Available online: http://www.academicjournals.org/JMPR (accessed on 11 August 2022).
- Isman, M.B. Bioinsecticides based on plant essential oils: A short overview. Z. Für Nat. C 2020, 75, 179–182. [Google Scholar] [CrossRef]
- Prakash, B.; Kumar, A.; Singh, P.; Das, S.; Dubey, N.K. Prospects of plant products in the management of insect pests of food grains: Current status and future perspectives. In Natural Bioactive Compounds; Sinha, R., Häder, D.-P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 317–335. [Google Scholar] [CrossRef]
- Reis, S.L.; Mantello, A.G.; Macedo, J.M.; Gelfuso, E.A.; da Silva, C.P.; Fachin, A.L.; Cardoso, A.M.; Beleboni, R.O. Typical monoterpenes as insecticides and repellents against stored grain pests. Molecules 2016, 21, 258. [Google Scholar] [CrossRef]
- Nenaah, G.E. Chemical composition, toxicity and growth inhibitory activities of essential oils of three Achillea species and their nano-emulsions against Tribolium castaneum (Herbst). Ind. Crop. Prod. 2014, 53, 252–260. [Google Scholar] [CrossRef]
- Adak, T.; Barik, N.; Patil, N.B.; Govindharaj, G.-P.-P.; Gadratagi, B.G.; Annamalai, M.; Mukherjee, A.K.; Rath, P.C. Nanoemulsion of eucalyptus oil: An alternative to synthetic pesticides against two major storage insects (Sitophilus oryzae (L.) and Tribolium castaneum (Herbst)) of rice. Ind. Crop. Prod. 2020, 143, 111849. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Ibrahim, S.I. Chemical composition and the insecticidal activity of certain plants applied as powders and essential oils against two stored-products coleopteran beetles. J. Pest Sci. 2011, 87, 393–402. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Almadiy, A.A.; Al-Assiuty, B.A.; Mahnashi, M.H. The essential oil of Schinus terebinthifolius and its nanoemulsion and isolated monoterpenes: Investigation of their activity against Culex pipiens with insights into the adverse effects on non-target organisms. Pest Manag. Sci. 2022, 78, 1035–1047. [Google Scholar] [CrossRef]
- De Oliveira, A.A.; França, L.P.; Ramos, A.D.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.; Araújo, E.S., Jr.; da Silva, J.N.; Branches, A.D.; Barros, G.D.A.; et al. Larvicidal, adulticidal and repellent activities against Aedes aegypti L. of two commonly used spices, Origanum vulgare L. and Thymus vulgaris L. S. Afr. J. Bot. 2021, 140, 17–24. [Google Scholar] [CrossRef]
- Nenaah, G.E.; Ibrahim, S.; Al-Assiuty, B. Chemical composition, insecticidal activity and persistence of three Asteraceae essential oils and their nanoemulsions against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2015, 61, 9–16. [Google Scholar] [CrossRef]
- Papanikolaou, N.E.; Kavallieratos, N.G.; Iliopoulos, V.; Evergetis, E.; Skourti, A.; Nika, E.P.; Haroutounian, S.A. Essential oil coating: Mediterranean culinary plants as grain protectants against larvae and adults of Tribolium castaneum and Trogoderma granarium. Insects 2022, 13, 165. [Google Scholar] [CrossRef]
- Song, C.; Zhao, J.; Zheng, R.; Hao, C.; Yan, X. Chemical composition and bioactivities of thirteen non-host plant essential oils against Plutella xylostella L. (Lepidoptera: Plutellidae). J. Asia-Pac. Entomol. 2022, 25, 101881. [Google Scholar] [CrossRef]
- Gupta, P.; Preet, S.; Singh, A.N. Preparation of Thymus vulgaris (L.) essential oil nanoemulsion and its chitosan encapsulation for controlling mosquito vectors. Sci. Rep. 2022, 12, 4335. [Google Scholar] [CrossRef]
- Wiese, N.; Fischer, J.; Heidler, J.; Lewkowski, O.; Degenhardt, J.; Erler, S. The terpenes of leaves, pollen, and nectar of thyme (Thymus vulgaris) inhibit growth of bee disease-associated microbes. Sci. Rep. 2018, 8, 14634. [Google Scholar] [CrossRef]
- Salih, A.M.; Al-Qurainy, F.; Nadeem, M.; Tarroum, M.; Khan, S.; Shaikhaldein, H.O.; Al-Hashimi, A.; Alfagham, A.; Alkahtani, J. Optimization Method for Phenolic Compounds Extraction from Medicinal Plant (Juniperus procera) and Phytochemicals Screening. Molecules 2021, 26, 7454. [Google Scholar] [CrossRef] [PubMed]
- NIST. NIST Chemistry WebBook: NIST Standard Reference Database Number 69. 2017. Available online: http://webbook.nist.gov/chemistry/ (accessed on 16 January 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Almadiy, A.A.; Nenaah, G.E. Essential oil of Origanum vulgare, its nanoemulsion and bioactive monoterpenes as eco-friendly novel green pesticides for controlling Aedes aegypti, the common vector of Dengue virus. J. Essent. Oil Res. 2022, 34, 424–438. [Google Scholar] [CrossRef]
- OECD (Organization for Economic Co-operation and Development). Guideline for Testing of Chemicals no. 207. Earthworm, Acute Toxicity Tests. In OECD-Guideline for Testing Chemicals; OECD: Paris, France, 1984. [Google Scholar] [CrossRef]
- Pavela, R. Essential oils from Foeniculum vulgare Miller as a safe environmental insecticide against the aphid Myzus persicae Sulzer. Environ. Sci. Pollut. R. 2018, 25, 10904–10910. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: London, UK, 1971. [Google Scholar]
- del Fierro, R.S.; Maquilang, Q.M.A.; Sanjorjo, R.A.S.; Tradio, M.D.; Shen, C.-C.; Ragasa, C.Y. Secondary metabolites from Cinnamomum cebuense. J. Med. Plants Res. 2012, 6, 2146–2149. [Google Scholar]
- Ragasa, C.Y.; Espineli, D.L.; Agoo, E.M.G.; del Fierro, R.S. Chemical constituents of Cinnamomum cebuense. Chin. J. Nat. Med. 2013, 11, 0264–0268. [Google Scholar] [CrossRef]
- Lee, S.-G. Alpha-pinene and myrtenol: Complete H-1 NMR assignment. Magn. Res. Chem. 2002, 40, 311–312. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Scutti, J.A.B.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Khan, M.; Khan, S.T.; Khan, N.A.; Mahmood, A.; Al-Kedhairy, A.A.; Alkhathlan, H.Z. The composition of the essential oil and aqueous distillate of Origanum vulgare L. growing in Saudi Arabia and evaluation of their antibacterial activity. Arab. J. Chem. 2018, 11, 1189–1200. [Google Scholar] [CrossRef]
- De Mesquita, B.M.; do Nascimento, P.G.G.; Souza, L.G.S.; de Farias, I.F.; Romézio, A.C. Synthesis, larvicidal and acetylcholinesterase inhibitory activities of carvacrol/thymol and derivatives. Quim. Nova 2018, 41, 412–416. [Google Scholar] [CrossRef]
- Farias, A.L.F.; Rodrigues, A.B.L.; Martins, R.L.; Rabelo, D.M.; Farias, C.W.F.; Almeida, S.S.M.D.S.D. Chemical characterization, antioxidant, cytotoxic and microbiological activities of the essential oil of leaf of Tithonia Diversifolia (Hemsl) A. Gray (Asteraceae). Pharmaceuticals 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, I.; Mossa, J.S.; El-Feraly, F.S. Additional antibacterial diterpenes from the bark of Juniperus procera. Phytother. Res. 1996, 10, 604–607. [Google Scholar] [CrossRef]
- Kinyanjui, T.; Gitu, P.M.; Kamau, G.N. Potential antitermite compounds from Juniperus procera extracts. Chemosphere 2000, 41, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Porte, A.; Godoy, R. Chemical composition of Thymus vulgaris L. (Thyme) essential oil from the Rio de Janeiro state, Brazil. J. Serb. Chem. Soc. 2008, 73, 307–310. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef]
- Zawiślak, G.; Walasek-Janusz, M.; Zalewska, E.D.; Gruszecki, R. Studies on the yield and chemical composition of the herb of plants of the genus Ocimum depending on the development stage of the plant. Agronomy 2022, 12, 2710. [Google Scholar] [CrossRef]
- Bouguerra, N.; Djebbar, F.T.; Soltani, N. Algerian Thymus vulgaris essential oil: Chemical composition and larvicidal activity against the mosquito Culex pipiens. Int. J. Mosq. Res. 2017, 4, 37–42. [Google Scholar]
- Rocha, B.C.A.; Kawase, K.Y.F.; Coelho, G.L.V. Characterization of the essential oil of Thymus vulgaris by supercritical fluid extraction. Acta. Hortic. 2018, 1198, 17–22. [Google Scholar] [CrossRef]
- El-Said, H.; Ashgar, S.S.; Bader, A.; AlQathama, A.; Halwani, M.; Ascrizzi, R.; Flamini, G. Essential oil analysis and antimicrobial evaluation of three aromatic plant species growing in Saudi Arabia. Molecules 2021, 26, 959. [Google Scholar] [CrossRef]
- Saleem, S.; ul Hasan, M.; Sagheer, M.; Sahi, S.T. Insecticidal activity of essential oils of four medicinal plants against different stored grain insect pests. Pak. J. Zool. 2014, 46, 1407–1414. [Google Scholar]
- Printrakoon, C.; Bullangpoti, V. Efficiency of monoterpene compounds for control of rice pest Pomacea canaliculata. Agric. Nat. Resour. 2021, 55, 7–14. [Google Scholar] [CrossRef]
- Finetti, L.; Civolani, S.; Bernacchia, G. Monoterpenes-induced toxicity in nymphal stages of Halyomorpha halys. J. Plant Dis. Prot. 2021, 128, 1371–1375. [Google Scholar] [CrossRef]
- De Medeiros, M.D.; da Silva, A.C.; Citó, A.M.; Borges, A.R.; de Lima, S.G.; Lopes, J.A.; Figueiredo, R.C. In vitro antileishmanial activity and cytotoxicity of essential oil from Lippia sidoides Cham. Parasitol. Int. 2011, 60, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Escobar, A.; Pérez, M.; Romanelli, G.; Blustein, G. Thymol bioactivity: A review focusing on practical applications. Arab. J. Chem. 2020, 13, 9243–9269. [Google Scholar] [CrossRef]
- Navrátilová, B.; Švécarová, M.; Bednář, J.; Ondřej, V. In Vitro Polyploidization of Thymus vulgaris L. and its effect on composition of essential oils. Agronomy 2021, 11, 596. [Google Scholar] [CrossRef]
- Stavropoulou, E.; Nasiou, E.; Skiada, P.; Giannakou, I.O. Effects of four terpenes on the mortality of Ditylenchus dipsaci (Kühn) Filipjev. Eur. J. Plant Pathol. 2021, 160, 137–146. [Google Scholar] [CrossRef]
- Vasilatis, A.A.; Gianfagna, T.; Simon, J.E. Investigation of growth inhibition by thymol and carvacrol from Thymus spp. and Origanum vulgare on Botrytis cinerea. J. Med. Act. Plants 2021, 10, 24–31. [Google Scholar]
- Hou, H.; Zhang, X.; Zhao, T.; Zhou, L. Effects of Origanum vulgare essential oil and its two main components, carvacrol and thymol, on the plant pathogen Botrytis cinerea. PeerJ 2020, 8, e9626. [Google Scholar] [CrossRef]
- Fernandes, M.; Pereira, R.B.; Pereira, D.M.; Fortes, A.G.; Castanheira, E.; Gonçalves, M. New eugenol derivatives with enhanced insecticidal activity. Int. J. Mol. Sci. 2020, 21, 9257. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological properties and prospects for the application of eugenol—A review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- Santana, A.S.; Baldin, E.L.L.; dos Santos, T.L.B.; Baptista, Y.A.; dos Santos, M.C.; Lima, A.P.S.; Tanajura, L.S.; Vieira, T.M.; Crotti, A.E.M. Synergism between essential oils: A promising alternative to control Sitophilus zeamais (Coleoptera: Curculionidae). Crop. Prot. 2022, 153, 105882. [Google Scholar] [CrossRef]
- Cao, A.; Yan, D.; Ouyang, C.; Wang, Q.; Li, Y. Overview of mechanisms and uses of biopesticides. Int. J. Pest Manag. 2021, 6, 65–72. [Google Scholar]
- Zhou, L.; Li, C.; Zhang, Z.; Li, X.; Dong, Y.; Cao, H. Biological activity and safety evaluation of monoterpenes against the peach aphid (Myzus persicae Sulzer) (Hemiptera: Aphididae). Int. J. Trop. Insect Sci. 2021, 41, 2747–2754. [Google Scholar] [CrossRef]
- Liaquat, F.; Qunlu, L.; Arif, S.; Haroon, U.; Saqib, S.; Zaman, W.; Jianxin, S.; Shengquan, C.; Li, L.X.; Akbar, M.; et al. Isolation and characterization of pathogen causing brown rot in lemon and its control by using ecofriendly botanicals. Physiol. Mol. Plant Pathol. 2021, 114, 101639. [Google Scholar] [CrossRef]
- Asghar, M.; Younas, M.; Arshad, B.; Zaman, W.; Ayaz, A.; Rasheed, S.; Shah, A.H.; Ullah, F.; Saqib, S. Bioactive potential of cultivated Mentha arvensis L. for preservation and production of health-oriented food. J. Anim. Plant Sci. 2022, 32, 835–844. [Google Scholar] [CrossRef]
- Sigma-Aldrich. Material Safety Data Sheet (MSDS): Toxicological Information, Section 11; Sigma-Aldrich: St. Louis, MI, USA, 2010. [Google Scholar]
| a,b Components | c RI Exp. | d RI Lit. | Concentration (%) | |
|---|---|---|---|---|
| T. vulgaris | J. procera | |||
| 2-Hexanal | 856 | 855 | 1.1 | 0.1 |
| Heptanal | 902 | 901 | - | 0.1 |
| a-Thujene | 921 | 924 | 1.3 | tr |
| a-Pinene | 930 | 932 | 1.1 | 6.1 |
| Camphene | 944 | 946 | 0.4 | – |
| Sabinene | 966 | 969 | tr | 0.1 |
| 1-Octen-3-ol | 976 | 973 | 0.1 | 0.2 |
| β-Myrcene | 986 | 988 | 1.1 | 0.3 |
| p-Cymene | 1020 | 1021 | 10.3 | – |
| 1,8-Cineole | 1028 | 1027 | 0.2 | tr |
| (4E,6Z)-allo-Ocimene | 1036 | 1037 | – | 0.4 |
| γ-Terpinene | 1052 | 1054 | 3.4 | – |
| Terpinolene | 1085 | 1086 | 0.2 | 0.3 |
| Linalool | 1096 | 1100 | 1.2 | – |
| Camphor | 1140 | 1141 | 0.2 | 0.2 |
| Pinocarvone | 1158 | 1160 | 0.2 | 0.2 |
| Borneol | 1164 | 1165 | 0.1 | 0.4 |
| Terpinen-4-ol | 1172 | 1174 | 1.1 | 2.7 |
| a-Terpineol | 1190 | 1188 | 0.1 | 0.4 |
| Piperitone | 1252 | 1249 | 0.1 | 0.6 |
| Carvone | 1254 | 1252 | 0.3 | - |
| Thymoquinone | 1255 | 1254 | 0.4 | 0.2 |
| Thymol | 1288 | 1289 | 58.1 | – |
| Carvacrol | 1296 | 1298 | 8.3 | – |
| Eugenol | 1355 | 1356 | 0.2 | 71.3 |
| a-Copaene | 1371 | 1374 | 0.1 | tr |
| β-Elemene | 1390 | 1389 | – | 0.2 |
| β-Caryophyllene | 1415 | 1417 | 5.2 | 11.8 |
| α-Humulene | 1451 | 1452 | – | 0.4 |
| Acetovanillone | 1462 | 1460 | 0.3 | – |
| Germacrene D | 1480 | 1484 | tr | 0.1 |
| β-Bisabolene | 1508 | 1505 | 0.9 | 0.2 |
| d-Cadinene | 1520 | 1522 | 0.2 | – |
| Caryophyllene oxide | 1580 | 1582 | 0.4 | – |
| γ-Cadinol | 1656 | 1655 | 0.5 | – |
| Grouped compounds (%) | - | - | ||
| Monoterpene hydrocarbons | - | - | 20.4% | 7.9% |
| Oxygenated monoterpenes | - | - | 70.3% | 75.7% |
| Sesquiterpene hydrocarbons | - | - | 7.6% | 15.3% |
| Others | - | - | 0.9% | 0% |
| Total | - | - | 99.2% | 98.9% |
| % Yield (mL/100 g dry wt.) | - | - | 1.43 | 0.92 |
| Plant Oil (Fractions) | 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | p | ||
| T. vulgaris | EO | 21.4 (17.1–27.5) | 53.3 (48.3–64.9) | 2.0 ± 0.26 | 0.47 | 15.1 (12.6–21.8) | 39.3 (33.5–47.4) | 1.8 ± 0.21 | 2.20 | 0.345 |
| Carvacrol | 26.1 (22.3–32.5) | 58.2 (52.2–70.8) | 2.1 ± 0.22 | 2.03 | 21.2 (18.1–33.5) | 48.7 (41.0–60.4) | 2.0 ± 0.20 | 1.70 | 0.408 | |
| Thymol | 55.7 (46.8–64.1) | 127.9 (118.2–143.1) | 2.2 ± 0.21 | 2.44 | 46.4 (40.3–55.8) | 109.1 (97.0–123.3) | 2.4 ± 0.18 | 2.06 | 0.349 | |
| P-Cymene | 81.7 (75.6–93.3) | 188.5 (175.7–204.1) | 3.0 ± 0.38 | 3.26 | 70.6 (65.6–82.5) | 164.8 (151.3–181.5) | 2.7 ± 0.28 | 3.08 | 0.538 | |
| J. procera | EO | 33.7 (28.1–39.0) | 70.3 (60.0–82.7) | 2.5 ± 0.30 | 1.34 | 24.5 (20.7–30.2) | 61.2 (53.3–71.1) | 2.6 ± 0.28 | 2.11 | 0.302 |
| Eugenol | 37.5 (33.9–42.7) | 83.5 (78.2–94.4) | 3.4 ± 0.40 | 2.36 | 30.6 (25.3–36.7) | 70.0 (65.8–82.2) | 2.1 ± 0.24 | 2.21 | 0651 | |
| α-Pinene | 60.6 (53.9–68.2) | 147.2 (134.0–163.1) | 2.4 ± 0.37 | 3.22 | 54.1 (47.6–62.3) | 128.3 (116.7–152.9) | 2.8 ± 0.22 | 3.13 | 0.493 | |
| β-Caryophyllene | 77.0 (69.3–91.9) | 162.4 (146.5–177.3) | 2.2 ± 0.35 | 1.81 | 62.4 (53.7–73.6) | 133.3 (121.4–150.1) | 2.7 ± 0.17 | 3.62 | 0.611 | |
| Plant Oil (Fractions) | 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | p | ||
| T. vulgaris | EO | 16.1 (13.0–21.8) | 42.4 (34.3–48.2) | 2.1 ± 0.42 | 1.32 | 11.5 (9.1–14.2) | 30.1 (23.1–35.5) | 2.1 ± 0.32 | 1.38 | 0.399 |
| Carvacrol | 18.8 (16.0–22.6) | 43.4 (39.1–51.5) | 1.6 ± 0.48 | 0.76 | 14.6 (12.2–18.3) | 34.8 (30.3–42.2) | 1.6 ± 0.30 | 0.55 | 0.502 | |
| Thymol | 42.5 (35.3–50.1) | 95.2 (82.2–114.9) | 2.4 ± 0.51 | 1.84 | 37.3 (30.8–42.5) | 80.4 (73.2–93.5) | 2.5 ± 0.48 | 1.42 | 0.411 | |
| P-Cymene | 70.6 (62.8–84.1) | 161.4 (149.6–178.0) | 3.7 ± 0.24 | 3.15 | 58.3 (49.6–71.4) | 135.5 (124.7–150.9) | 2.5 ± 0.36 | 1.15 | 0.713 | |
| J. procera | EO | 25.5 (19.7–32.6) | 64.9 (56.4–74.3) | 2.3 ± 0.47 | 1.22 | 19.5 (17.3–23.2) | 49.0 (43.7–58.1) | 2.6 ± 0.62 | 1.15 | 0.420 |
| Eugenol | 28.6 (24.3–33.1) | 67.1 (61.4–77.8) | 2.1 ± 0.26 | 1.12 | 22.4 (19.6–26.7) | 57.7 (52.3–68.2) | 2.0 ± 0.21 | 1.24 | 0.581 | |
| α-Pinene | 50.4 (45.3–63.5) | 121.8 (113.0–134.4) | 2.4 ± 0.18 | 2.87 | 44.9 (38.1–54.3) | 110.6 (101.3–121.6) | 2.2 ± 0.22 | 2.35 | 0.403 | |
| β-Caryophyllene | 69.6 (63.7–77.8) | 151.1 (136.1–170.1) | 2.9 ± 0.37 | 3.15 | 51.6 (45.3–63.8) | 122.5 (113.9–136.7) | 3.1 ± 0.28 | 3.11 | 0.719 | |
| Plant Oil (Fractions) | 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | p | ||
| T. vulgaris | EO | 30.4 (27.1–34.5) | 58.3 (49.3–66.9) | 2.0 ± 0.26 | 0.47 | 23.0 (18.6–27.8) | 47.3 (41.5–56.4) | 1.04 | 1.04 | 0.567 |
| Carvacrol | 37.7 (31.1–45.0) | 80.3 (70.0–89.7) | 2.5 ± 0.30 | 1.34 | 29.5 (24.7–34.2) | 69.2 (64.3–77.1) | 2.11 | 2.11 | 0.409 | |
| Thymol | 58.2 (50.6–67.9) | 132.1 (124.1–143.5) | 2.4 ± 0.24 | 2.60 | 47.6 (39.4–55.5) | 99.3 (88.1–112.3) | 2.43 | 2.43 | 0.456 | |
| P-Cymene | 73.6 (65.7–82.8) | 162.1 (146.1–183.1) | 2.9 ± 0.37 | 3.15 | 63.6 (56.3–71.8) | 141.5 (130.9–162.7) | 3.11 | 3.11 | 0.381 | |
| J. procera | EO | 39.5 (34.4–46.2) | 82.6 (76.8–91.2) | 2.2 ± 0.22 | 1.65 | 31.3 (27.3–35.7) | 63.1 (57.6–71.8) | 1.25 | 1.25 | 0.623 |
| Eugenol | 49.2 (42.6–52.9) | 110.1 (97.1–127.5) | 2.4 ± 0.24 | 2.60 | 40.6 (36.4–47.5) | 88.7 (80.1–100.5) | 2.17 | 2.17 | 0.461 | |
| α-Pinene | 61.0 (55.2–66.1) | 150.8 (138.6–174.0) | 2.4 ± 0.22 | 2.63 | 51.8 (42.3–57.4) | 134.1 (122.6–144.1) | 3.73 | 3.73 | 0.388 | |
| β-Caryophyllene | 77.0 (70.3–84.5) | 213.4 (194.7–236.6) | 2.2 ± 0.20 | 5.66 | 65.3 (60.2–76.6) | 172.3 (158.5–187.3) | 2.9 ± 0.41 | 3.74 | 0.644 | |
| Plant Oil (Fractions) | 24 h | 48 h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | LC50 (95% fl) | LC95 (95% fl) | Slope (± S.E.) | Chi2 (df = 7) | p | ||
| T. vulgaris | EO | 21.6 (17.1–26.5) | 50.3 (43.3–60.9) | 2.0 ± 0.26 | 0.47 | 14.2 (11.6–20.8) | 40.3 (34.5–49.4) | 1.8 ± 0.21 | 1.66 | 0.643 |
| Carvacrol | 29.2 (28.1–38.0) | 73.1 (65.0–83.7) | 2.5 ± 0.30 | 1.34 | 22.4 (17.7–29.2) | 59.2 (51.3–74.1) | 2.6 ± 0.28 | 2.02 | 0.498 | |
| Thymol | 50.8 (46.6–62.9) | 118.1 (119.1–147.5) | 2.4 ± 0.24 | 2.60 | 2.6 (31.4–43.5) | 90.3 (84.1–104.3) | 2.6 ± 0.24 | 2.43 | 0.823 | |
| P-Cymene | 65.6 (58.7–74.8) | 150.1 (139.1–169.1) | 2.9 ± 0.37 | 3.15 | 54.6 (47.3–60.8) | 127.5 (118.9–143.7) | 3.1 ± 0.28 | 3.11 | 0.754 | |
| J. procera | EO | 26.5 (23.2–32.5) | 70.4 (64.4–74.4) | 1.8 ± 0.20 | 0.35 | 21.8 (22.2–31.2) | 54.3 (48.2–63.3) | 2.1 ± 0.19 | 1.68 | 0.532 |
| Eugenol | 41.4 (36.5–50.6) | 91.6 (84.6–108.5) | 2.2 ± 0.30 | 1.86 | 30.2 (26.5–37.0) | 80.1 (72.5–93.2) | 2.0 ± 0.30 | 1.43 | 0.672 | |
| α–Pinene | 55.6 (47.2–64.7) | 139.3 (128.0–151.4) | 3.0 ± 0.31 | 3.88 | 42.5 (36.0–51.4) | 118.5 (109.4–130.5) | 2.0 ± 0.25 | 2.34 | 0.896 | |
| β–Caryophyllene | 70.1 (65.8–78.9) | 182.5 (171.6–201.0) | 2.0 ± 0.26 | 3.42 | 56.4 (50.2–68.6) | 137.6 (126.7–153.3) | 3.1 ± 0.33 | 2.78 | 0.322 | |
| Plant Oil (Fractions) | a IC50 (mg/L) | (95% Fiducial Limits) | Slope (±S.E.) | Chi 2 (df = 8) | p | |
|---|---|---|---|---|---|---|
| T. vulgaris | EO | 18.23 | (15.08–22.44) | 2.06 ± 0.33 | 2.22 | 0.733 |
| Carvacrol | 19.08 | (17.21–23.50) | 1.08 ± 0.19 | 1.21 | 0.614 | |
| Thymol | 30.14 | (24.08–37.44) | 2.26 ± 0.33 | 3.35 | 0.723 | |
| P-Cymene | 42.00 | (37.08–53.21) | 2.26 ± 0.33 | 3.22 | 0.523 | |
| J. procera | EO | 23.22 | (20.33–27.91) | 1.16 ± 0.23 | 2.63 | 0.526 |
| Eugenol | 26.16 | (23.84–31.12) | 1.12 ± 0.27 | 2.33 | 0.565 | |
| a-Pinene | 34.93 | (31.14–41.82) | 1.18 ± 0.19 | 3.25 | 0.245 | |
| β-Caryophyllene | 59.78 | (54.21–72.50) | 2.08 ± 0.19 | 3.21 | 0.544 | |
| Methomyl | 2.24 × 10−3 | (1.66 × 10−3–3.30 × 10−3) | 2.02 ± 0.12 | 2.33 | 0.423 | |
| Plant Material | % Mortality (Mean ± S.E.) at Different Concentrations (mg kg−1) | ||||||
|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | |||||
| 5 Days | 10 Days | 5 Days | 10 Days | 5 Days | 10 Days | ||
| T. vulgaris | EO | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| Carvacrol | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| Thymol | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| P-Cymene | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| J. procera | EO | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c |
| Eugenol | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| a-Pinene | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| β-Caryophyllene | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| Negative control (water) | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | 0.0 ± 0.0 c | |
| α-Cypermethrin 10.0 mg kg−1 | 30.8 ± 2.4 b | 36.2 ± 2.3 b | 54.9± 1.9 b | 68.3 ± 2.6 b | 82.4 ± 2.2 b | 90.1 ± 1.6 a | |
| α-Cypermethrin 20.0 mg kg−1 | 64.2 ± 3.3 a | 70.7 ± 2.1 a | 88.1 ± 2.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| * F-value | 1219.1 | 902.1 | 1973.9 | 2001.8 | 5765.9 | 8810.7 | |
| p-value | 0.004 | 0.006 | 0.002 | 0.007 | 0.004 | 0.003 | |
| Test Material | Concentration (µg/mL) | Germination (%) | RL | SL | |
|---|---|---|---|---|---|
| T. vulgaris | EO | 50 | 90.7 ± 1.3 a | 8.98 ± 0.38 a | 3.35 ± 0.13 a |
| 100 | 83.3 ± 1.4 ab | 8.35 ± 0.20 a | 3.24 ± 0.12 a | ||
| 200 | 74.4 ± 1.3b | 8.02 ± 0.19 a | 2.48 ± 0.12 ab | ||
| Carvacrol | 50 | 89.9 ± 2.3 a | 9.04 ± 0.22 a | 3.30 ± 0.12 a | |
| 100 | 80.8 ± 1.2 ab | 8.85 ± 0.21 a | 2.91 ± 0.14 a | ||
| 200 | 71.3 ± 1.2b | 8.06 ± 0.18 a | 2.21 ± 0.10 c | ||
| Thymol | 50 | 90.2 ± 1.4 a | 9.07 ± 0.36 a | 3.36 ± 0.16 a | |
| 100 | 89.6 ± 2.3 a | 9.04 ± 0.22 a | 3.32 ± 0.12 a | ||
| 200 | 82.7 ± 1.1 ab | 8.95 ± 0.17 a | 3.18 ± 0.15 a | ||
| p-Cymene | 50 | 91.4 ± 2.7 a | 9.09 ± 0.20 a | 3.37 ± 0.15 a | |
| 100 | 91.3 ± 1.4 a | 9.05 ± 0.22 a | 3.37 ± 0.09 a | ||
| 200 | 89.8 ± 1.8 a | 9.04 ± 0.16 a | 3.36 ± 0.15 a | ||
| J. procera | EO | 50 | 91.4 ± 1.4 a | 9.02 ± 0.38 a | 3.26 ± 0.16 a |
| 100 | 90.8 ± 1.9 a | 8.96 ± 0.24 a | 3.13 ± 0.12 a | ||
| 200 | 88.3 ± 2.1 a | 8.63 ± 0.19 a | 2.69 ± 0.11 ab | ||
| Eugenol | 50 | 91.9 ± 2.9 a | 9.03 ± 0.38 a | 3.30 ± 0.16 a | |
| 100 | 89.6 ± 1.6 a | 8.99 ± 0.24 a | 3.25 ± 0.12 a | ||
| 200 | 84.1 ± 2.3 ab | 8.94 ± 0.19 a | 3.08 ± 0.11 a | ||
| β-Caryophyllene | 50 | 91.6 ± 1.5 a | 9.08 ± 0.18 a | 3.31 ± 0.15 a | |
| 100 | 91.1 ± 1.6 a | 9.07 ± 0.00 a | 3.24 ± 0.12 a | ||
| 200 | 90.4 ± 1.4 a | 9.04 ± 0.33 a | 3.11 ± 0.11 a | ||
| α-Pinene | 50 | 91.3 ± 1.4 a | 9.09 ± 0.18 a | 3.33 ± 0.15 a | |
| 100 | 91.1 ± 1.6 a | 9.07 ± 0.00 a | 3.21 ± 0.12 a | ||
| 200 | 90.7 ± 1.6 a | 9.05 ± 0.33 a | 3.06 ± 0.11 a | ||
| Control | 91.3 ± 1.4 a | 9.08 ± 0.28 a | 3.37 ± 0.15 a | ||
| F value | - | 8.2 | 2.4 | 2.2 | |
| P value | - | 0.0072 | 0.0034 | 0.008 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almadiy, A.; Nenaah, G. Chemical Profile, Bioactivity, and Biosafety Evaluations of Essential Oils and Main Terpenes of Two Plant Species against Trogoderma granarium. Agronomy 2022, 12, 3112. https://doi.org/10.3390/agronomy12123112
Almadiy A, Nenaah G. Chemical Profile, Bioactivity, and Biosafety Evaluations of Essential Oils and Main Terpenes of Two Plant Species against Trogoderma granarium. Agronomy. 2022; 12(12):3112. https://doi.org/10.3390/agronomy12123112
Chicago/Turabian StyleAlmadiy, Abdulrhman, and Gomah Nenaah. 2022. "Chemical Profile, Bioactivity, and Biosafety Evaluations of Essential Oils and Main Terpenes of Two Plant Species against Trogoderma granarium" Agronomy 12, no. 12: 3112. https://doi.org/10.3390/agronomy12123112
APA StyleAlmadiy, A., & Nenaah, G. (2022). Chemical Profile, Bioactivity, and Biosafety Evaluations of Essential Oils and Main Terpenes of Two Plant Species against Trogoderma granarium. Agronomy, 12(12), 3112. https://doi.org/10.3390/agronomy12123112








