Greenhouse Gas Emissions with Low Disturbance Liquid Dairy Manure Incorporation into a Live Winter Cereal Cover Crop-Corn System
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Weather
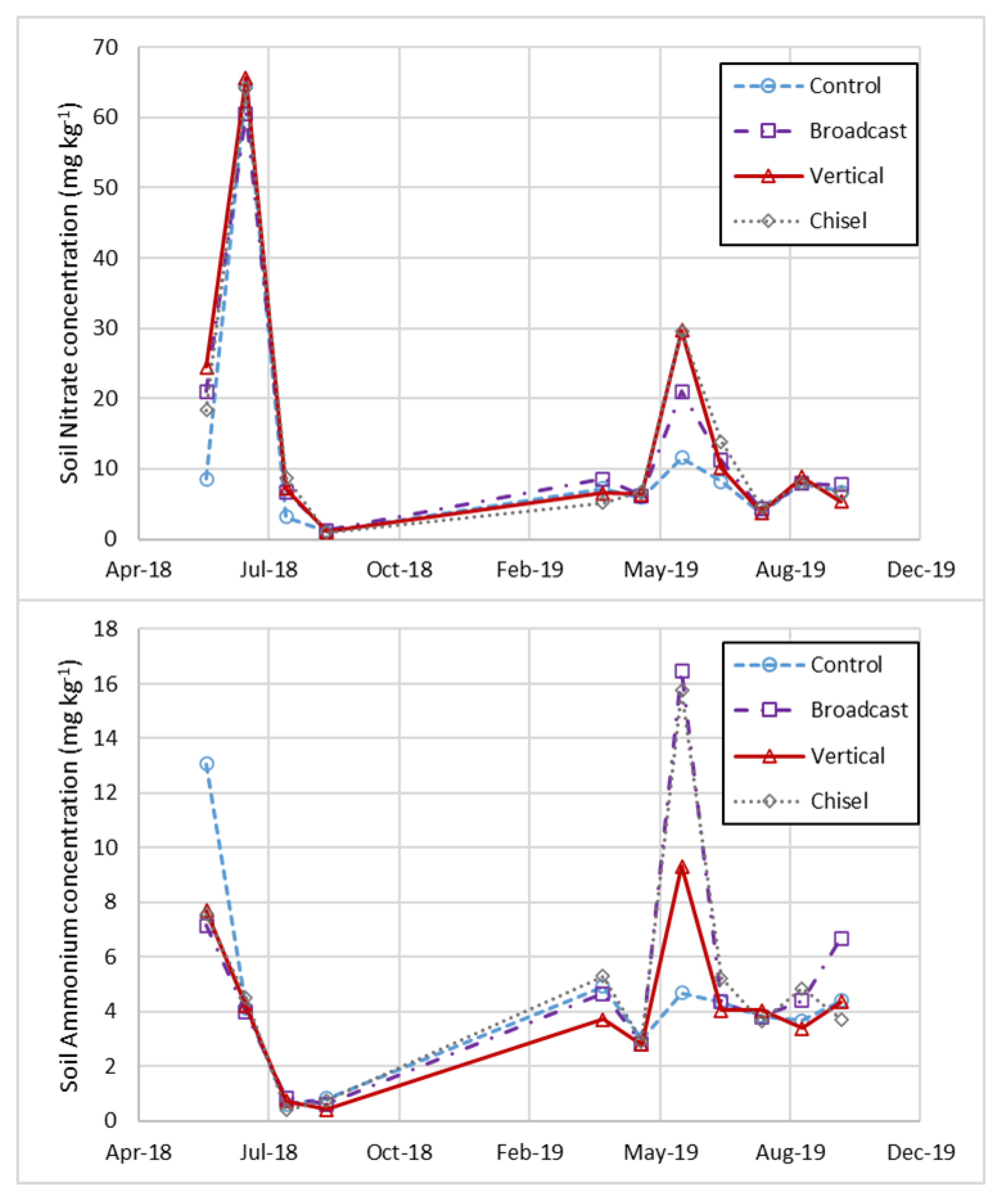
3.2. Plant Yields and Soil Nutrients
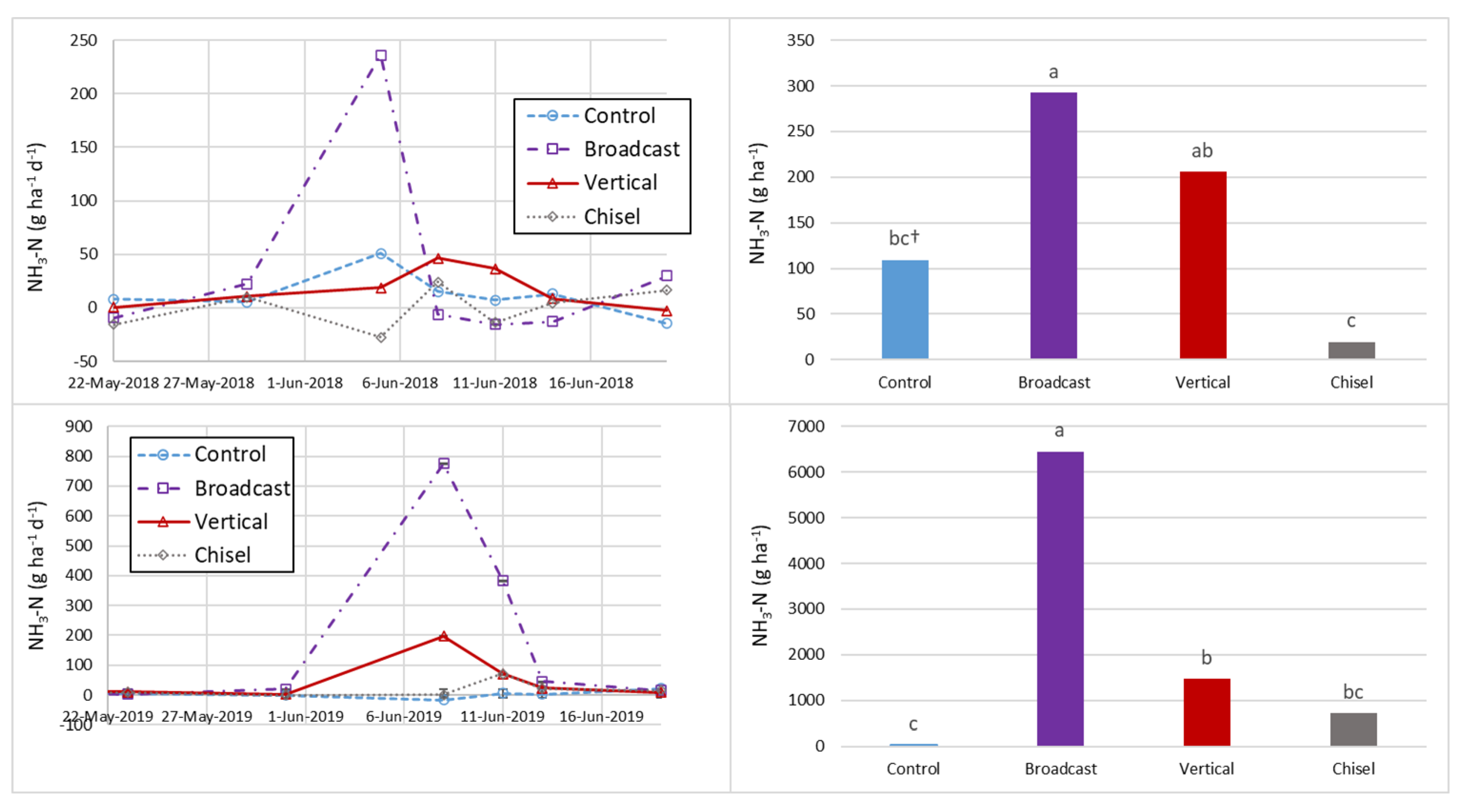
3.3. Ammonia Fluxes
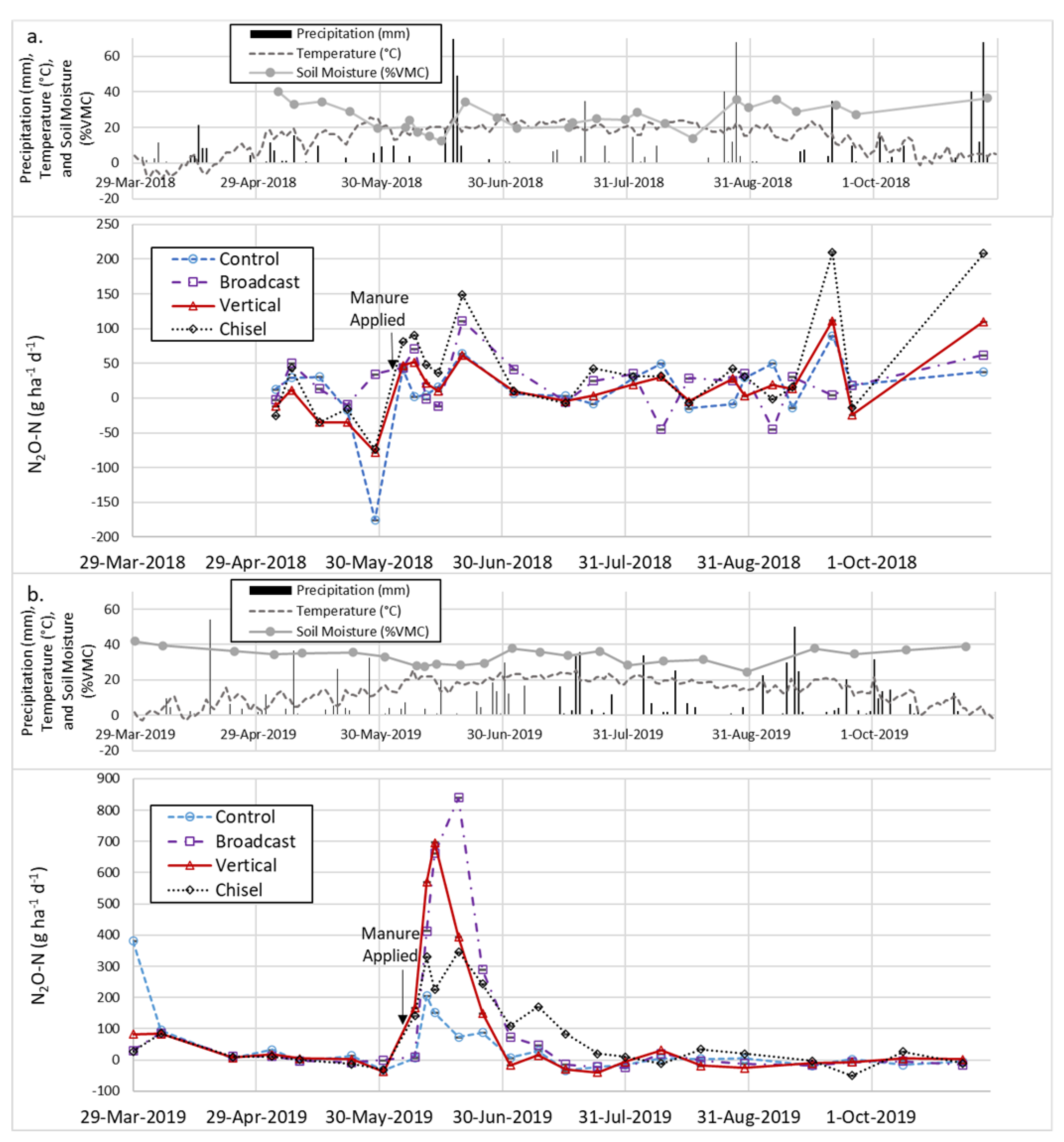
3.4. Nitrous Oxide Fluxes
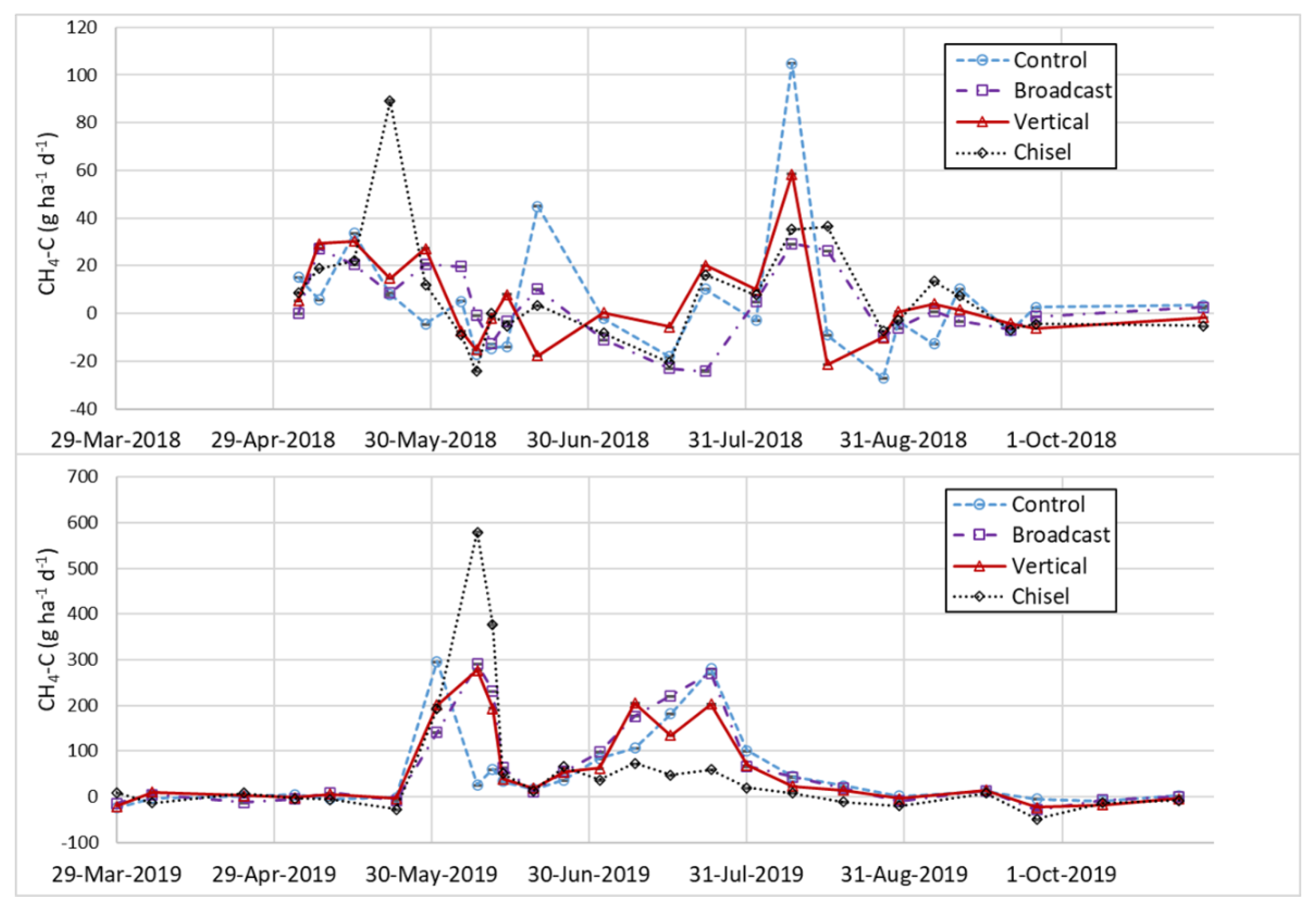
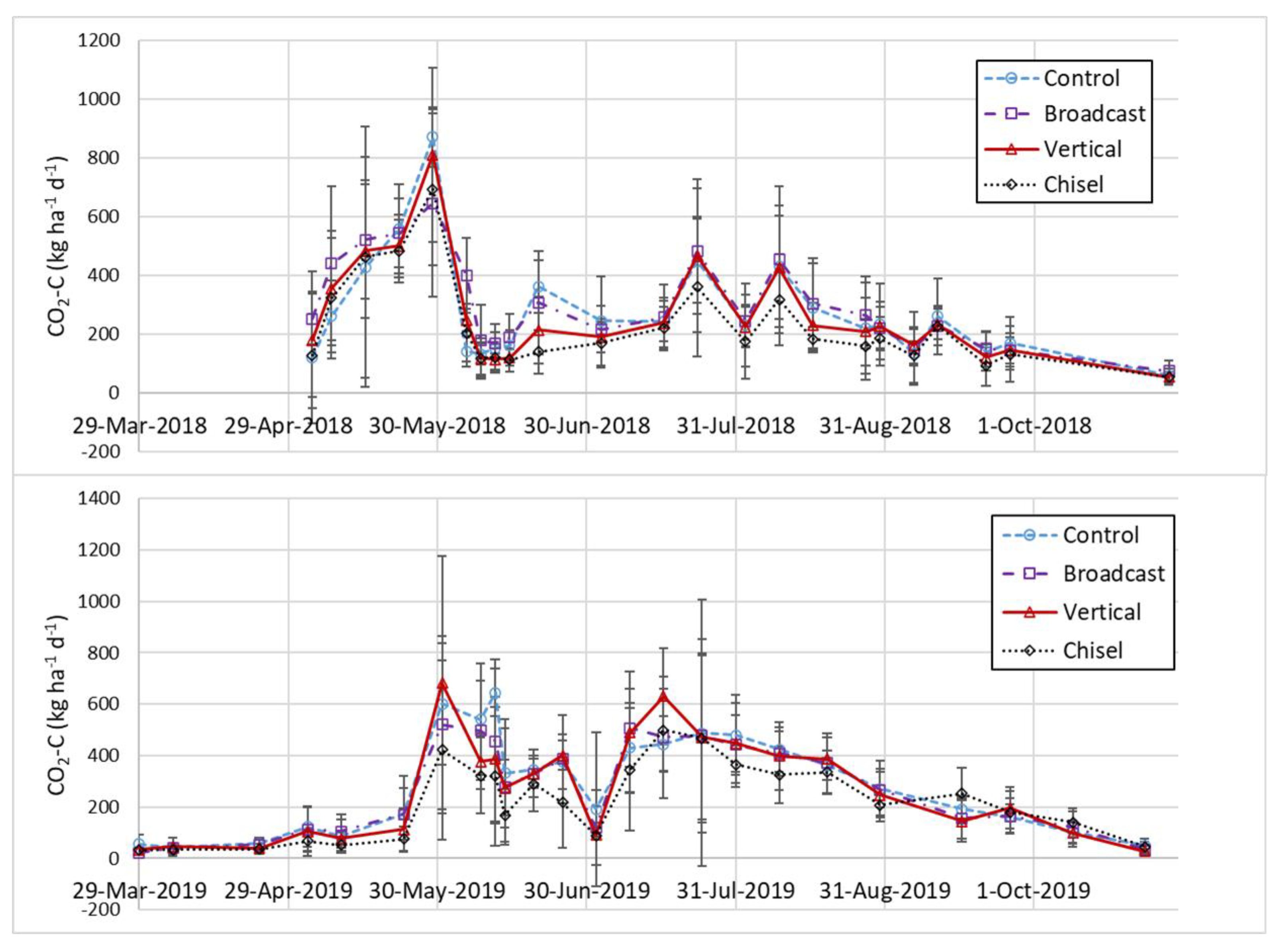
3.5. Carbon Fluxes
3.6. Global Warming Potential
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dittmer, K.M.; Darby, H.M.; Goeschel, T.R.; Adair, E.C. Benefits and tradeoffs of reduced tillage and manure application in a Zea mays silage system. J. Environ. Qual. 2020, 49, 1236–1250. [Google Scholar] [CrossRef] [PubMed]
- Dungan, R.S.; Leytem, A.B.; Tarkalson, D.D.; Ippolito, J.A.; Bjorneberg, D.L. Greenhouse gas emissions from an irrigated dairy forage rotation as influenced by fertilizer and manure applications. Soil Sci. Soc. Am. J. 2017, 81, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Leytem, A.B.; Moore, A.D.; Dungan, R.S. Greenhouse gas emissions from an irrigated crop rotation utilizing dairy manure. Soil Sci. Soc. Am. J. 2019, 83, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Zhu, B.; Wang, S.; Zhu, X.; Vereecken, H.; Bruggemann, N. Stimulation of N2O emission by manure application to agricultural soils may largely offset carbon benefits: A global meta-analysis. Glob. Chang. Biol. 2017, 23, 4068–4083. [Google Scholar] [CrossRef] [PubMed]
- Fargione, J.E.; Bassett, T.; Boucher, S.D.; Bridgham, R.T.; Conant, S.C.; Cook-Patton, S.C.; Ellis, P.W.; Falcucci, A.; Fourqurean, J.W.; Griscom, B.W.; et al. Natural climate solutions for the United States. Sci. Adv. 2018, 4, eaat1869. [Google Scholar]
- Dell, C.J.; Kleinman, P.J.A.; Schmidt, J.P.; Beegle, D.B. Low-disturbance manure incorporation effects on ammonia and nitrate loss. J. Environ. Qual. 2012, 41, 928–937. [Google Scholar] [CrossRef]
- Dell, C.J.; Meisinger, J.J.; Beegle, D.B. Subsurface application of manures slurries for conservation tillage and pasture soils and thier impact on the nitrogen balance. J. Environ. Qual. 2011, 40, 352–361. [Google Scholar] [CrossRef]
- Duncan, E.W.; Dell, C.J.; Kleinman, P.J.; Beegle, D.B. Nitrous oxide and ammonia emissions from injected and broadcast-applied dairy slurry. J. Environ. Qual. 2017, 46, 36–44. [Google Scholar] [CrossRef]
- Jokela, W.E.; Coblentz, W.K.; Hoffman, P.C. Dairy heifer manure management, dietary phosphorus and soil test P effects on runoff phosphorus. J. Environ. Qual. 2012, 41, 1600–1611. [Google Scholar] [CrossRef] [Green Version]
- Jokela, W.E.; Sherman, J.; Cavadini, J. Nutrient runoff losses from liquid dairy manure applied with low-disturbance methods. J. Environ. Qual. 2016, 45, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Kovar, J.L.; Moorman, T.B.; Singer, J.W.; Cambardella, C.A.; Tomer, M.D. Swine manure injection with low-disturbance applicator and cover crops reduce phosphorus losses. J. Environ. Qual. 2011, 40, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Omonode, R.A.; Vyn, T.J. Tillage and nitrogen source impacts on relationships between nitrous oxide emission and nitrogen recovery efficiency in corn. J. Environ. Qual. 2019, 48, 421–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, B.C.; Scott, A.; Parker, J.P. Field N2O, CO2, and CH4 fluxes in relation to tillage, compaction and soil quality in Scotland. Soil Till. Res. 1999, 53, 29–39. [Google Scholar] [CrossRef]
- Gagnon, B.; Ziadi, N.; Rochette, P.; Chantigny, M.H.; Angers, D.A. Fertilizer source influenced nitrous oxide emissions from a clay soil under corn. Soil Sci. Soc. Am. J. 2011, 75, 595–604. [Google Scholar] [CrossRef]
- Kool, D.M.; Dolfing, J.; Wrage, N.; Van Groenigen, J.W. Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol. Biochem. 2011, 43, 174–178. [Google Scholar] [CrossRef]
- Thangarajan, R.; Bolan, N.S.; Tian, G.; Naidu, R.; Kunhikrishnan, A. Role of organic amendment application on greenhouse gas emission. Sci. Tot. Environ. 2013, 465, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Lognoul, M.; Theodorakopoulos, N.; Hiel, M.-P.; Regaert, D.; Broux, F.; Heinesch, B.; Bodson, B.; Vandenbol, M.; Aubinet, M. Impact of tillage on greenhouse gas emissions by an agricultural crop and dynamics of N2O fluxes: Insights from automated closed chamber measurements. Soil Till. Res. 2017, 167, 80–89. [Google Scholar] [CrossRef]
- Good, L.W.; Carvin, R.; Lamba, J.; Fitzpatrick, F.A. Seasonal variation in sediment and phosphorus yields in four Wisconsin agricultural watersheds. J. Environ. Qual. 2018, 48, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Griffith, K.E.; Young, E.O.; Klaiber, L.B.; Kramer, S.R. Winter rye cover crop impacts on runoff water quality in a northern New York (USA) tile-drained maize agroecosystem. Water Air Soil Pollut. 2020, 231, 84. [Google Scholar] [CrossRef]
- Liu, J.; Baulch, H.M.; Macrae, M.L.; Wilson, H.F.; Elliot, J.A.; Bergström, L.; Glenn, A.J.; Vadas, P.A. Agricultural water quality in cold climates: Processes, drivers, management options, and research needs. J. Environ. Qual. 2019, 48, 792–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Ayuso, M.; Gabriel, J.L.; Quemada, M. The kill date as amanagement tool for cover cropping success. PLoS ONE 2014, 9, e109587. [Google Scholar] [CrossRef] [PubMed]
- Thapa, R.; Mirsky, S.B.; Tully, K.L. Cover crops reduce nitrate leaching in agroecosystems: A global meta-analysis. J. Environ. Qual. 2018, 47, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.C.; Castellano, M.J.; Sawyer, J.E.; Pantoja, J.L. Cover crop effects on nitrous oxide emissions: Role of mineralizable carbon. Soil Sci. Soc. Am. J. 2013, 77, 1765–1773. [Google Scholar] [CrossRef] [Green Version]
- Peterson, S.O.; Mutegi, J.K.; Hansen, E.M.; Munkholm, L.J. Tillage effects on N2O emissions as influenced by a winter cover crop. Soil Biol. Biochem. 2011, 43, 1509–1517. [Google Scholar] [CrossRef]
- Mirsky, S.B.; Curran, W.S.; Mortensen, D.A.; Ryan, M.R.; Shumway, D. Timing of cover-crop management effects on weed suppression in no-till planted soybean using a roller-crimper. Weed Sci. 2011, 59, 380–389. [Google Scholar] [CrossRef]
- Reed, H.K.; Karsten, H.D.; Curran, W.S.; Tooker, J.F.; Duiker, S.W. Planting green effects on corn and soybean production. Agron. J. 2019, 111, 2314–2325. [Google Scholar] [CrossRef] [Green Version]
- Dobbie, K.E.; Smith, K.A. Nitrous oxide emission factors for agricultural soils Great Britain: The impact of soil water-filled pore space and other controlling variables. Glob. Chang. Biol. 2003, 9, 204e218. [Google Scholar]
- Linn, D.M.; Doran, J.W. Aerobic and anaerobic microbial populations in no-till and plowed soils. Soil Sci. Soc. Am. J. 1984, 48, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Otte, B.; Mirsky, S.B.; Schomberg, H.; Davis, B.; Tully, K. Effect of cover crop termination timing on pools and fluxes of inorganic nitrogen in no-till corn. Agron. J. 2019, 111, 2832–2842. [Google Scholar] [CrossRef]
- Raimbault, B.A.; Vyn, T.J.; Tollenaar, M. Corn response to rye cover crop, tillage methods, and planter options. Agron. J. 1991, 83, 287–290. [Google Scholar] [CrossRef]
- Ewing, R.P.; Wagger, M.G.; Denton, H.P. Tillage and cover crop management effects on soil water and corn yield. Soil Sci. Soc. Am. J. 1991, 55, 1081–1085. [Google Scholar] [CrossRef]
- Duiker, S.W.; Curran, W.S. Rye cover crop mananagement for corn production in the northern Mid-Atlantic region. Agron. J. 2005, 97, 1413–1418. [Google Scholar] [CrossRef]
- Sherman, J.F.; Young, E.O.; Cavadini, J. Tillage and liquid dairy manure effects on overland flow nitrogen and phosphorus loss potential in an upper Midwest corn silage-winter triticale cropping system. Agronomy 2021, 11, 1775. [Google Scholar] [CrossRef]
- Peters, J. Wisconsin Procedures for Soil Testing, Plant Analysis and Feed and Forage Analysis. 2013. Available online: https://uwlab.soils.wisc.edu/lab-procedures// (accessed on 11 November 2019).
- Parkin, T.B.; Venterea, R.T. Chamber-based trace gas flux measurements. In Sampling Protocols; Follett, R.F., Ed.; USDA-ARS: Beltsville, MD, USA, 2010. Available online: www.ars.usda.gov/research/GRACEnet (accessed on 7 March 2018).
- Venterea, R.T. Simplified Method for Quantifying Theoretical Underestimation of Chamber-Based Trace Gas Fluxes. J. Environ. Qual. 2010, 39, 126–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egan, L. Determination of Ammonia by Flow Injection Analysis (High Throughput) (Salicylate Method/DCIC) (Multi Matrix Method); QuikChem Method 90-107-06-3-A; Lachat Instruments: Loveland, CO, USA, 2011. [Google Scholar]
- Harbridge, J. Determination of Nitrate in 2M KCl Soil Extracts by Flow Injection Analysis; Quikchem Method 12-107-04-1-J; Lachat Instruments: Loveland, CO, USA, 2007. [Google Scholar]
- Schulte, E.E.; Hopkins, B.G. Estimation of soil organic matter by weight 3. Organic matter (LOI) loss-on-ignition. In Soil Organic Matter: Analysis and Interpretation; Magdoff, F.R., Tabatabai, M.A., Hanlon, E.A., Jr., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 21–31. [Google Scholar]
- Laboski, C.A.M.; Peters, J.B. Nutrient Application Guidelines for Field, Vegetable, and Fruit Crops in Wisconsin; University of Wisconsin–Extension, Ed.; Cooperative Extension: Madison, WI, USA, 2012. [Google Scholar]
- Cambardella, C.A.; Elliot, E.T. Carbon and nitrogen distribution in aggregates from cutivated and native grassland soils. Soil Sci. Soc. Am. J. 1993, 57, 1071–1076. [Google Scholar] [CrossRef] [Green Version]
- Booth, D.T.; Cox, S.E.; Berryman, R.D. Point sampling digital imagery with SamplePoint. Environ. Monit. Assess. 2006, 123, 97–108. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute Inc. SAS 9.4 Guide to Software Updates; SAS Institute Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Blackmer, A.M.; Pottker, D.; Cerrato, M.E.; Webb, J. Correlations between soil nitrate concentrations in late spring and corn yields in Iowa. J. Prod. Agric. 1989, 2, 103–109. [Google Scholar] [CrossRef]
- Fox, R.H.; Roth, G.W.; Iversen, K.V.; Piekielek, W.P. Soil and tissue nitrate tests compared for predicting soil nitrogen availability to corn. Agron. J. 1989, 81, 971–974. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Wienhold, B.J.; Black, A.L. Tillage, nitrogen, and cropping system effects on soil carbon sequestration. Soil Sci. Soc. Am. J. 2002, 66, 906–912. [Google Scholar] [CrossRef]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils. 2003, 37, 221–230. [Google Scholar] [CrossRef]
- Abdalla, M.; Osborne, B.; Lanigan, G.; Forristal, D.; Williams, M.; Smith, P.; Jones, M.B. Conservation tillage systems: A review of its consequences for greenhouse gas emissions. Soil Use Manag. 2013, 29, 199–209. [Google Scholar] [CrossRef]
- Meisinger, J.J.; Jokela, W.E. Ammonia volatilization from dairy and poultry manure. In Managing Nutrients and Pathogens from Animal Agriculture (NRAES-130); Natural Resource, Agriculture, & Engineering Service: Ithaca, NY, USA, 2000. [Google Scholar]
- Sommer, S.G.; Genermont, S.; Cellier, P.; Hutchings, N.J.; Olesen, J.E.; Morvan, T. Process controlling ammonia emission from livestock slurry in the field. Eur. J. Agron. 2003, 19, 465–486. [Google Scholar] [CrossRef]
- Powell, J.M.; Jokela, W.E.; Misselbrook, T.H. Dairy slurry application method impacts ammonia emission and nitrate leaching in no-till corn silage. J. Environ. Qual. 2011, 40, 383–392. [Google Scholar] [CrossRef]
- Sherman, J.F.; Young, E.O.; Jokela, W.E.; Cavadini, J. Impacts of low-disturbance dairy manure incorporation on ammonia and greenhouse gas fluxes in a corn silage-winter rye cover crop system. J. Environ. Qual. 2021, 50, 836–846. [Google Scholar] [CrossRef]
- Pfluke, P.D.; Jokela, W.E.; Bosworth, S.C. Ammonia volatilization from surface-banded and broadcast application of liquid dairy manure on grass forage. J. Environ. Qual. 2011, 40, 374–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, L. A New Dynamic Chamber Technique for Measuring Ammonia Emissions from Land-Spread Manure and Fertilizers. Acta Agric. Scand. Sect. B-Soil Plant Sci. 1994, 44, 35–46. [Google Scholar] [CrossRef]
- Webb, J.; Pain, B.F.; Bittman, S.; Morgan, J. The impacts of manure application methods on emissions of ammonia, nitrous oxide and on crop response—A review. Agric. Ecosyst. Environ. 2010, 137, 39–46. [Google Scholar] [CrossRef]
- van Kessel, C.; Venterea, R.T.; Six, J.; Adviento-Borbe, M.A.; Linquist, B.; van Groenigen, K.J. Climate, duration, and N placement determine N2O emissions in reduced tillage systems: A meta-analysis. Glob. Change Biol. 2013, 19, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Mkhabela, M.S.; Madani, A.; Gordon, R.; Burton, D.; Cudmore, D.; Elmi, A.; Hart, W. Gaseous and leaching nitrogen losses from no-tillage and conventional tillage systems following surface application of cattle manure. Soil Till. Res. 2008, 98, 187–199. [Google Scholar] [CrossRef]
- Dalal, R.C.; Gibson, I.; Allen, D.E.; Menzies, N.W. Green waste compost reduces nitrous oxide emissions from feedlot manure applied to soil. Agric. Ecosyst. Environ. 2010, 136, 273–281. [Google Scholar] [CrossRef]
- Flessa, H.; Beese, F. Laboratory estimates of trace gas emissions following surface application and injection of cattle slurry. J. Environ. Qual. 2000, 29, 262–268. [Google Scholar] [CrossRef]
- Perala, P.; Kapuinen, P.; Esala, M.; Tyynela, S.; Regina, K. Influence of slurry and mineral fertiliser application techniques on N2O and CH4 fluxes from a barley field in southern Finland. Agric. Ecosyst. Environ. 2006, 117, 71–78. [Google Scholar] [CrossRef]
- Ullah, S.; Moore, T.R. Biogeochemical controls on methane, nitrous oxide, and carbon dioxide fluxes from deciduous forest soils in eastern Canada. J. Geophys. Res. 2011, 116, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Halvorson, A.D.; Del Grosso, S.J.; Stewart, C.E. Manure and inorganic nitrogen affect trace gas emissions under semi-arid irrigated corn. J. Environ. Qual. 2016, 45, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Drury, C.F.; Reynolds, W.D.; Tan, C.S.; Welacky, T.W.; Calder, W.; McLaughlin, N.B. Emissions of nitrous oxide and carbon dioxide: Influence of tillage type and nitrogen placement depth. Soil Sci. Soc. Am. J. 2006, 70, 570–581. [Google Scholar] [CrossRef]
- Hao, X. Nitrous oxide and carbon dioxide emissions during the nongrowing season from manured soils under rainfed and irrigated conditions. Geomicrobiol. J. 2015, 32, 648–654. [Google Scholar] [CrossRef]
- Reicosky, D.C.; Archer, D.W. Moldboard plow tillage depth and short-term carbon dioxide release. Soil Till. Res. 2007, 94, 109–121. [Google Scholar] [CrossRef]
- Kainiemi, V.; Arvidsson, J.; Kätterer, T. Effects of autumn tillage and residue management on soil respiration in a long-term field experiment in Sweden. J. Plant Nutr. Soil Sci. 2015, 178, 189–198. [Google Scholar] [CrossRef]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories; Calvo Buendia, E., Tanabe, K., Kranjc, A., Baasansuren, J., Fukuda, M., Ngarize, S., Osako, A., Pyrozhenko, Y., Shermanau, P., Federici, S., Eds.; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
| Depth | Treatment | OM † | P | K | N | C |
|---|---|---|---|---|---|---|
| (cm) | (%) | (mg kg−1) | (mg kg−1) | (g kg−1) | (g kg−1) | |
| May 2018 | ||||||
| 0–5 | Control | 4.79 | 30.3 | 218 | 2.41 | 24.4 |
| Broadcast | 4.88 | 31.0 | 220 | 2.40 | 25.8 | |
| Vertical Till | 4.78 | 31.8 | 223 | 2.46 | 25.3 | |
| Chisel Plow | 4.93 | 30.0 | 212 | 2.31 | 24.5 | |
| CV | 9.4 | 13.9 | 8.1 | 6.8 | 6.5 | |
| p-value | NS ‡ | NS | NS | NS | NS | |
| 5–10 | Control | 3.69 | 18.0 | 108 | 1.85 | 18.8 |
| Broadcast | 3.72 | 18.8 | 121 | 1.90 | 19.1 | |
| Vertical Till | 3.68 | 18.8 | 117 | 1.85 | 18.6 | |
| Chisel Plow | 3.66 | 18.8 | 119 | 1.80 | 18.0 | |
| CV | 9.1 | 9.4 | 16.5 | 7.6 | 7.2 | |
| p-value | NS | NS | NS | NS | NS | |
| 10–20 | Control | 3.14 | 14.5 | 43.3 | 1.51 | 14.6 |
| Broadcast | 2.96 | 15.0 | 50.3 | 1.49 | 14.2 | |
| Vertical Till | 3.03 | 14.3 | 48.0 | 1.48 | 14.4 | |
| Chisel Plow | 3.13 | 14.3 | 51.8 | 1.40 | 14.2 | |
| CV | 10.4 | 7.1 | 16.5 | 8.8 | 7.9 | |
| p-value | NS | NS | NS | NS | NS | |
| 20–30 | Control | 2.46 | 13.0 | 33.3 | 0.96 | 9.25 |
| Broadcast | 2.17 | 11.5 | 34.3 | 0.76 | 7.95 | |
| Vertical Till | 2.00 | 10.5 | 33.5 | 0.70 | 7.16 | |
| Chisel Plow | 1.86 | 10.0 | 34.3 | 0.48 | 5.88 | |
| CV | 25.2 | 20.4 | 9.0 | 48.4 | 39.0 | |
| p-value | NS | NS | NS | NS | NS | |
| October 2019 | ||||||
| 0–5 | Control | 4.07 a § | 25.8 c | 165 b | 2.44 a | 23.8 b |
| Broadcast | 4.31 a | 36.8 b | 232 a | 2.60 a | 25.2 a | |
| Vertical Till | 4.12 a | 40.6 a | 237 a | 2.45 a | 24.1 b | |
| Chisel Plow | 3.40 b | 28.8 c | 176 b | 2.03 b | 19.8 c | |
| CV | 11.3 | 22.6 | 18.1 | 11.8 | 11.7 | |
| p-value | 0.001 | 0.0002 | 0.0003 | 0.002 | <0.0001 | |
| 5–10 | Control | 3.37 | 15.0 b | 78.1 b | 2.02 | 18.90 |
| Broadcast | 3.47 | 18.3 a | 124 a | 2.05 | 19.60 | |
| Vertical Till | 3.37 | 18.6 a | 115 a | 1.95 | 18.30 | |
| Chisel Plow | 3.13 | 19.6 a | 109 a | 1.82 | 17.30 | |
| CV | 9.0 | 15.1 | 20.2 | 9.2 | 9.5 | |
| p-value | NS | 0.05 | 0.009 | NS | NS | |
| 10–20 | Control | 2.69 b | 12.0 | 38.9 | 1.56 | 14.6 |
| Broadcast | 2.86 a | 13.2 | 49.7 | 1.56 | 14.5 | |
| Vertical Till | 2.76 ab | 13.7 | 50.7 | 1.31 | 12.4 | |
| Chisel Plow | 2.50 c | 12.4 | 48.4 | 1.21 | 12.2 | |
| CV | 9.4 | 11.0 | 20.6 | 20.4 | 17.8 | |
| p-value | 0.003 | NS | NS | NS | NS | |
| 20–30 | Control | 1.89 a | 10.5 | 36.4 | 0.80 a | 8.00 a |
| Broadcast | 2.13 a | 12.2 | 34.8 | 1.00 a | 9.65 a | |
| Vertical Till | 1.81 a | 10.6 | 38.4 | 1.03 a | 9.61 a | |
| Chisel Plow | 1.20 b | 8.74 | 39.8 | 0.34 b | 4.14 b | |
| CV | 34.2 | 21.4 | 12.6 | 66.4 | 52.3 | |
| p-value | 0.01 | NS | NS | 0.03 | 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sherman, J.; Young, E. Greenhouse Gas Emissions with Low Disturbance Liquid Dairy Manure Incorporation into a Live Winter Cereal Cover Crop-Corn System. Agronomy 2022, 12, 2978. https://doi.org/10.3390/agronomy12122978
Sherman J, Young E. Greenhouse Gas Emissions with Low Disturbance Liquid Dairy Manure Incorporation into a Live Winter Cereal Cover Crop-Corn System. Agronomy. 2022; 12(12):2978. https://doi.org/10.3390/agronomy12122978
Chicago/Turabian StyleSherman, Jessica, and Eric Young. 2022. "Greenhouse Gas Emissions with Low Disturbance Liquid Dairy Manure Incorporation into a Live Winter Cereal Cover Crop-Corn System" Agronomy 12, no. 12: 2978. https://doi.org/10.3390/agronomy12122978
APA StyleSherman, J., & Young, E. (2022). Greenhouse Gas Emissions with Low Disturbance Liquid Dairy Manure Incorporation into a Live Winter Cereal Cover Crop-Corn System. Agronomy, 12(12), 2978. https://doi.org/10.3390/agronomy12122978






