Effect of Hyacinth Treatment by Hydrogen Peroxide Stabilized with Silver and Some Fungicides on the Fungal Infection of Substrate and Bulbs and on Plant Growth and Development
Abstract
1. Introduction
1.1. Influence of Treatment of Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides on the Coverage of Bulbs and Substrate with Mycelium
1.2. Influence of the Treatment of Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides on Plant Growth and Quality
2. Materials and Methods
2.1. Growing Media Preparation and Their Physical and Chemical Properties
2.2. Experimental Design
- Control
- Bisterane (hydrogen peroxide stabilized with silver—H2O2-Ag+) 1%.
- Bisterane (hydrogen peroxide stabilized with silver—H2O2-Ag+) 2%.
- Bisterane (hydrogen peroxide stabilized with silver—H2O2-Ag+) 3%.
- Bisterane (hydrogen peroxide stabilized with silver—H2O2-Ag+) 5%.
- Bisterane (hydrogen peroxide stabilized with silver—H2O2-Ag+) 10%.
- Yamato 303 SE 0.5%.
- Signum 33 WG 0.5%.
- Biszop 80 WG 1%.
2.3. Measurements and Observations
2.4. Statistical Analysis
2.4.1. Statistical Calculations for Mycelial Coverage of Bulbs and Substrate
2.4.2. Statistical Calculations for Plant Growth and Quality Traits
3. Results
3.1. Coverage of Bulbs and Substrate with Mycelium after the Treatment of Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides
3.2. Plant Growth and Quality after the Treatment of Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides
4. Discussion
4.1. Effect of the Treatment of Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides on the Mycelial Coverage of Bulbs and Substrate
4.2. Effects of Treating Hyacinth Bulbs by Hydrogen Peroxide Stabilized with Silver and Fungicides on Plant Growth and Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horst, R.K. Westcott’s Plant Disease Handbook, 7th ed.; Springer: Ithaca, NY, USA, 2008; p. 1349. [Google Scholar]
- Miyamoto, K.; Kotake, T.; Boncela, A.J.; Saniewski, M.; Ueda, J. Hormonal regulation of gummosis and composition of gums from bulbs of hyacinth (Hyacinthus orientalis). J. Plant Physiol. 2015, 174, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Bayer SeedGrowth. 2020. Available online: https://www.agro.bayer.com.pl/-/media/website/files/technologia-zaprawiania-roslin/wsparcie-w-zaprawianiu-nasion.pdf?download=true (accessed on 17 November 2022).
- Wojdyła, A.T.; Orlikowski, L.B.; Wiśniewski, J.; Waszkiewicz, E. In vitro effect of plant protection products on saprotrophic fungi developing on bulbs during their forcing. In Zeszyty Naukowe Instytutu Ogrodnictwa; Institute of Horticulture—National Research Institute: Skierniewice, Poland, 2022. (In Polish) [Google Scholar]
- Żegliński, J. Stabilization of Hydrogen Peroxide in Silica Xerogel—Study of the Interaction of Composite Components and Its Characterization. Ph.D. Thesis, Medical Academy in Gdańsk, Gdańsk, Poland, 2006. (In Polish). [Google Scholar]
- Anzai, K.; Kunitaka, O.; Goto, Y.; Yamamoto, H.; Ozawa, T. Oxidation dependent changes in the stability and permeability of lipid bilayers. Antioxid. Redox Signal. 1999, 1, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.V.; Cancro, L.P.; Fischman, S.L. Hydrogen Peroxide: A Review of Its Use in Dentistry. J. Periodontol. 1995, 66, 786–796. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, K.; Genco, R.; Wilson, M. Antimicrobial Properties of Hydrogen Peroxide and Sodium Bicarbonate Individually and in Combination Against Selected Oral, Gram-negative, Facultative Bacteria. J. Dent. Res. 1986, 65, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Nurnaeimah, N.; Mat, N.; Mohd, K.S.; Badaluddin, N.A.; Yusoff, N.; Sajili, M.H.; Mahmud, K.; Adnan, A.F.M.; Khandaker, M.M. The Effects of Hydrogen Peroxide on Plant Growth, Mineral Accumulation, as Well as Biological and Chemical Properties of Ficus deltoidea. Agronomy 2020, 10, 599. [Google Scholar] [CrossRef]
- Fallik, E.; Aharoni, Y.; Grinberg, S.; Copel, A.; Klein, J.D. Postharvest hydrogen peroxide treatment inhibits decay in eggplant and sweet red pepper. Crop Prot. 1994, 13, 451–454. [Google Scholar] [CrossRef]
- Mani, F.; Bettaieb, T.; Zheni, K. Effect of hydrogen peroxide and thiurea on fluorescence and tuberization of potato (So-lanum tuberosum L.). J. Stress Physiol. Biochem. 2012, 3, 62–71. [Google Scholar]
- Kortekamp, A. Effectiveness of calcium salts, hydrogen peroxide, azoxystrobin, and antagonistic bacteria to control post-harvest rot on tobacco caused byRhizopus oryzae. Int. J. Pest Manag. 2006, 52, 109–115. [Google Scholar] [CrossRef]
- Cerioni, L.; Lazarte, M.D.L.; Villegas, J.M.; Rodríguez-Montelongo, L.; Volentini, S.I. Inhibition of Penicillium expansum by an oxidative treatment. Food Microbiol. 2013, 33, 298–301. [Google Scholar] [CrossRef] [PubMed]
- El-Mougy, N.; El-Gamal, N.; Abdalla, M. The Use of Fungicide Alternatives for Controlling Postharvest Decay of Strawberry and Orange Fruits. J. Plant Prot. Res. 2008, 48, 385–396. [Google Scholar] [CrossRef]
- Youssef, K.Y.A.; Mustafa, Z.M.M.; Gehan, A.; Mounir, G.A.; Abo Rehab, M.E.A. Preliminary Studies on Fungal Species Associated with Guava Fruit Drop Disease and Possible Management. Egypt. J. Phytopathol. 2015, 43, 11–23. [Google Scholar] [CrossRef]
- Aziz, N.; Faraz, M.; Pandey, R.; Shakir, M.; Fatma, T.; Varma, A.; Barman, I.; Prasad, R. Facile Algae-Derived Route to Biogenic Silver Nanoparticles: Synthesis, Antibacterial, and Photocatalytic Properties. Langmuir 2015, 31, 11605–11612. [Google Scholar] [CrossRef] [PubMed]
- Lamsal, K.; Kim, S.W.; Jung, J.H.; Kim, Y.S.; Kim, K.S.; Lee, Y.S. Application of Silver Nanoparticles for the Control of Colletotrichum Species In Vitro and Pepper Anthracnose Disease in Field. Mycobiology 2011, 39, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Salaheldin, T. Silver Nanoparticles as a Potent Fungicide for Citrus Phytopathogenic Fungi. J. Nanomed. Res. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Serey, R.A.; Torres, R.; Latorre, B.A. Pre- and post-infection activity of new fungicides against Botrytis cinerea and other fungi causing decay of table grapes. Cienc. Investig. Agrar. 2007, 34, 215–224. [Google Scholar] [CrossRef]
- Sallato, B.V.; Torres, R.; Zoffoli, J.P.; Latorre, B.A. Effect of boscalid on postharvest decay of strawberry caused by Botrytis cinerea and Rhizopus stolonifera. Span. J. Agric. Res. 2007, 5, 67–78. [Google Scholar] [CrossRef]
- Hauke, K.; Creemers, P.; Brugmans, W.; Van Laer, S. Signum, a new fungicide with interesting properties in resistance management of fungal diseases in strawberries. Commun. Agric. Appl. Biol. Sci. 2004, 69, 743–755. [Google Scholar] [PubMed]
- Amadioha, A.C. Control of storage rot of potato caused by Rhizopus oryzae. Int. J. Pest Manag. 1996, 42, 311–314. [Google Scholar] [CrossRef]
- Bhale, U.N.; Rajkonda, J.N. Compatibility of Fungicides and Antagonistic Activity of Trichoderma spp. against Plant Pathogens. Biosci. Methods 2015, 6, 1–9. Available online: http://bm.biopublisher.ca (accessed on 17 November 2022). [CrossRef]
- Ray, M.K.; Mishra, P.K.; Baruah, P.K. Control of Fungal Pathogen Pestalotiopsis disseminata Causing Grey Blight Disease in Som (Persea bombycina Kost.): An In Vitro Study. Int. J. Pure Appl. Biosci. 2016, 4, 180–185. [Google Scholar] [CrossRef]
- Jafariyan, T.; Zarea, M.J. Hydrogen peroxide affects plant growth promoting effects of Azospirillum. J. Crop Sci. Biotechnol. 2016, 19, 167–175. [Google Scholar] [CrossRef]
- Lopez-Delgado, H.; Zawaleta-Mancera, H.A.; Mora-Herrera, M.E.; Vasquez-Rivera, M.; Flores-Gutierrez, F.X.; Scott, I.M. Hydrogen peroxide increases potato tubers and stem starch content, stem diameter, and stem lignin content. Am. J. Potato Res. 2005, 4, 279–285. [Google Scholar] [CrossRef]
- Jung, J.; Kim, S.; Min, J.; Kim, Y.; Lamsal, K.; Kim, K.S.; Lee, Y.S. The effect of nano-silver liquid against the white rot of the green onion caused by Sclerotium cepivorum. Mycobiology 2010, 38, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Tort, N.; Turkyilmaz, B. Physiological Effects of Captan Fungicide on Pepper (Capsicum annuum L.) Plant. Pak. J. Biol. Sci. 2003, 6, 2026–2029. [Google Scholar] [CrossRef]
- Seyhan, M.; Yüzbaşioğlu, E.; Dalyan, E.; Akpinar, I.; Unal, M. Genotoxicity and antioxidant enzyme activities induced by the captan fungicide in the root of bell pepper (Capsicum annuum L. var. grossum L. cv. Kandil). Trak. Univ. J. Nat. Sci. 2019, 20, 97–103. [Google Scholar] [CrossRef]
- Manjunath, A.; Bagyaraj, D.J. Effect of fungicides on mycorrhizal colonization and growth of onion. In Plant and Soil; Springer: Berlin/Heidelberg, Germany, 1984; Volume 80, pp. 147–150. Available online: https://www.jstor.org/stable/42934673 (accessed on 3 August 2022).
- Joshi, J.; Sharma, S.; Guruprasad, K. Foliar application of pyraclostrobin fungicide enhances the growth, rhizobial-nodule formation and nitrogenase activity in soybean (var. JS-335). Pestic. Biochem. Physiol. 2014, 114, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zawadzińska, A.; Salachna, P.; Nowak, J.S.; Kowalczyk, W. Response of Interspecific Geraniums to Waste Wood Fiber Sub-strates and Additional Fertilization. Agriculture 2021, 11, 119. [Google Scholar] [CrossRef]
- Breś, W.; Komosa, A. Controlled nutrition of horticultural plants. In Nutrition of Horticultural Plants. Fundamentals and Perspectives; Komosa, A., Ed.; PWRiL: Poznań, Poland, 2012. (In Polish) [Google Scholar]
- Skomra, U.; Bocianowski, J.; Agacka, M. Agro-morphological differentiation between European hop (Humulus lupulus L.) cultivars in relation to their origin. J. Food Agric. Environ. 2013, 11, 1123–1128. [Google Scholar]
- Chaurasia, S.; Chaurasia, S.; Chaurasia, A.K.; Chaurasia, S.; Chaurasia, R.K. In vitro inhibitory effect of fungicides on mycelial growth of Rhizopus oryzae (Went & Prins Geerl.). Int. J. Adv. Res. Biol. Sci. 2017, 4, 176–183. [Google Scholar]
- Alka, P.G.; Patil, R.K.; Prajapati, B.K. Evaluation of fungicides against Rhizopus fruit rot of tomato. J. Plant Dis. Sci. 2017, 12, 139–143. [Google Scholar]
- Meng, X.; Huang, Z.; Fan, C. Postharvest Treatment with Hydrogen Peroxide to Control Orange Fruit Decay Caused by Penicillum digitatum and Penicillum italicum. Int. J. Appl. Agric. Sci. 2019, 5, 114. [Google Scholar] [CrossRef]
- Venturini, M.E.; Blanco, D.; Oria, R. In vitro antifungal activity of several antimicrobial compounds against Penicillium expansum. J. Food Prot. 2002, 65, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, S.; Karabulut, O.; Ilhan, K.; Sehirli, A. Use and Efficiency of Disinfectants within a Hydrocooler System for Postharvest Disease Control in Sweet Cherry. Int. J. Fruit Sci. 2020, 20, S1590–S1606. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Akram, M.; Wasaya, A.; Ansar, M.; Hussain, S.; Iqbal, A.; Hussan, S.A. Improvement of Antioxidant Activities and Yield of Spring Maize through Seed Priming and Foliar Application of Plant Growth Regulators under Heat Stress Conditions; Semina: Ciências Agrárias: Londrina, Brazil, 2017; Volume 38, pp. 47–56. [Google Scholar] [CrossRef]
- Jira-Anunkul, W.; Pattanagul, W. Effects of hydrogen peroxide application on agronomic traits of rice (Oryza sativa L.) under drought stress. Plant Soil Environ. 2021, 67, 221–229. [Google Scholar] [CrossRef]
- Kanungo, M.; Joshi, J. Impact of Pyraclostrobin (F-500) on Crop Plants. Plant Sci. Today 2014, 1, 174–178. [Google Scholar] [CrossRef]
- Köhle, H.; Grossmann, K.; Jabs, T.; Gerhard, M.; Kaiser, W.; Glaab, J.; Herms, S. Physiological effects of the strobilurin fungicide F 500 on plants. In Modern Fungicides and Antifungal Compounds III; Dehne, H.W., Gisi, U., Juck, K.H., Russel, P.E., Lyr, H., Eds.; Agroconcept GmbH: Bonn, Germany, 2002; pp. 61–74. [Google Scholar]
- Swoboda, C.; Pedersen, P. Effect of Fungicide on Soybean Growth and Yield. Agron. J. 2009, 101, 352–356. [Google Scholar] [CrossRef]
- Amaro, A.C.E.; Ramos, A.R.P.; Macedo, A.C.; Ono, E.O.; Rodrigues, J.D. Effects of the fungicides azoxystrobin, pyra-clostrobin and boscalid on the physiology of Japanese cucumber. Sci. Hortic. 2018, 228, 66–75. [Google Scholar] [CrossRef]
- Grossmann, K.; Kwiatkowski, J.; Caspar, G. Regulation of Phytohormone Levels, Leaf Senescence and Transpiration by the Strobilurin Kresoxim-methyl in Wheat (Triticum aestivum). J. Plant Physiol. 1999, 154, 805–808. [Google Scholar] [CrossRef]
- Ypema, H.L.; Gold, R.E. Kresoxim—methyl: Modification of a Naturally Occurring Compound to Produce a New Fungicide. Plant Dis. 1999, 83, 4–19. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Value |
|---|---|
| Total pore space (%) | 92.1 |
| Bulk density (g·cm−3) | 0.15 |
| Shrinkage (%) | 21.2 |
| Water volume at −10 cm H2O (%) | 75.0 |
| Air volume at −10 cm H2O (%) | 17.1 |
| Organic matter (%) | 59.5 |
| Ash content (%) | 40.5 |
| pH (H2O, 1:3) | 6.5 |
| EC (mS·cm−1) | 0.14 |
| N-NO3 (mg·dm−3) | 6.0 |
| P (mg·dm−3) | 23.0 |
| K (mg·dm−3) | 14.0 |
| Mg (mg·dm−3) | 259.0 |
| Ca (mg·dm−3) | 719.0 |
| Na (mg·dm−3) | 32.5 |
| S (mg·dm−3) | 16.0 |
| Cl (mg·dm−3) | 15.2 |
| Fe (mg·dm−3) | 28.1 |
| Mn (mg·dm−3) | 3.74 |
| Cu (mg·dm−3) | 0.32 |
| Zn (mg·dm−3) | 3.61 |
| B (mg·dm−3) | 0.36 |
| Combinat. | Bulbs Covered with Mycelium | Substrate Mycelium Coverage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After 4 Weeks | After 8 Weeks | After 12 Weeks | After 4 Weeks | After 8 Weeks | After 12 Weeks | |||||||
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| 1 | 29.75 a | 18.47 | 26 a | 11.997 | 5.813a | 6.082 | 1.4375 a | 4.149 | 3 a | 5.372 | 0.875 a | 2.843 |
| 2 | 18.04 b | 14.46 | 11.312 b | 7.784 | 1.5 cde | 3.593 | 1.125 ab | 3.18 | 1.9375 b | 3.937 | 0 b | 0 |
| 3 | 8.5 de | 9.39 | 4.562 c | 5.857 | 0.75 def | 2.651 | 0.125 cd | 1.118 | 0.25 c | 1.355 | 0.125 b | 1.118 |
| 4 | 5.69 ef | 9.77 | 5.125 c | 6.161 | 1.5 cde | 3.68 | 0 d | 0 | 0.0625 c | 0.559 | 0 b | 0 |
| 5 | 2.87 f | 5.14 | 3.812 c | 5.75 | 0.438 ef | 1.629 | 0.125 cd | 1.118 | 0.3125 c | 1.658 | 0 b | 0 |
| 6 | 3.37 f | 6.74 | 0.875 d | 3.058 | 0.25 f | 1.097 | 0.5 bcd | 2.333 | 0.375 c | 1.546 | 0.125 b | 1.118 |
| 7 | 11.28 cd | 7.59 | 8.875 b | 7.247 | 1.75 cd | 4.141 | 0.75 bc | 2.767 | 0.6875 c | 2.214 | 0.3125 b | 1.658 |
| 8 | 29.13 a | 16.03 | 25.062 a | 16.506 | 4 b | 5.921 | 0 d | 0 | 0.6875 c | 2.066 | 0.375 b | 1.738 |
| 9 | 12.13 c | 8.34 | 3.75 c | 4.673 | 2.062 c | 4.262 | 0.125 cd | 1.118 | 0.1875 c | 1.244 | 0.25 b | 1.097 |
| LSD0.05 | 3.57 | 2.67 | 1.245 | 0.688 | 0.817 | 0.434 | ||||||
| F-ANOVA | 63.32 *** | 94.56 *** | 16.28 *** | 4.57 *** | 11.24 *** | 3.20 ** | ||||||
| Trait 1 | CPG 4 w | CPG 8 w | CPG 12 w | PPG 4 w | PPG 8 w | PPG 12 w |
|---|---|---|---|---|---|---|
| CPG 4 w | 1 | |||||
| CPG 8 w | 0.961 *** | 1 | ||||
| CPG 12 w | 0.918 *** | 0.926 *** | 1 | |||
| PPG 4 w | 0.461 | 0.432 | 0.455 | 1 | ||
| PPG 8 w | 0.722 * | 0.711 * | 0.711 * | 0.909 *** | 1 | |
| PPG 12 w | 0.757 * | 0.768 * | 0.899 *** | 0.542 | 0.682 * | 1 |
| Trait (Short Key) | Source of Variation | Combinations | Residual |
|---|---|---|---|
| Petal length (PL) | d.f. | 8 | 710 |
| m.s. | 4.028 * | 1.991 | |
| Petal width (PW) | d.f. | 8 | 710 |
| m.s. | 28.24 | 15.6 | |
| Flower diameter (FD) | d.f. | 8 | 710 |
| m.s. | 1.4476 *** | 0.3172 | |
| Inflorescence height (IH) | d.f. | 8 | 710 |
| m.s. | 0.1942 * | 0.09716 | |
| Inflorescence width (IW) | d.f. | 8 | 710 |
| m.s. | 0.188537 *** | 0.008382 | |
| Flower number (FN) | d.f. | 8 | 351 |
| m.s. | 63.74 *** | 17.46 | |
| Fresh mass of flowers (FMF) | d.f. | 8 | 351 |
| m.s. | 25.249 *** | 4.321 | |
| Dry mass of flowers (DMF) | d.f. | 8 | 670 |
| m.s. | 0.08985 | 0.0598 | |
| Total height (TH) | d.f. | 8 | 668 |
| m.s. | 43.054 *** | 6.996 | |
| Leaf number (LN) | d.f. | 8 | 710 |
| m.s. | 0.5359 | 0.3553 | |
| Leaf length (LL) | d.f. | 8 | 710 |
| m.s. | 34.383 *** | 4.638 | |
| Leaf width (LW) | d.f. | 8 | 710 |
| m.s. | 0.32609 *** | 0.06746 | |
| Quality evaluation of plant (QEP) | d.f. | 8 | 709 |
| m.s. | 3.862 | 2.496 | |
| Fresh mass of the above-ground part (without flowers) (FMAP) | d.f. | 8 | 710 |
| m.s. | 74.5 *** | 11.92 | |
| Dry weight of the above-ground part (without flowers) (DWAP) | d.f. | 8 | 710 |
| m.s. | 0.26687 *** | 0.06831 | |
| Starting height (SH) | d.f. | 6 | 133 |
| m.s. | 2.217 *** | 0.1337 | |
| Chlorophyll Index DUALEX (CLD) | d.f. | 8 | 351 |
| m.s. | 76.82 *** | 17.63 | |
| Flavonoid Index DUALEX (FLD) | d.f. | 8 | 351 |
| m.s. | 0.05422 * | 0.02188 | |
| Nitrogen Balance Index (NBI) | d.f. | 8 | 351 |
| m.s. | 90.76 *** | 13.7 |
| Trait | Combination | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | LSD0.05 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| EN | Mean | 12.21 a | 12.21 a | 11.66 c | 11.97 abc | 11.98 abc | 12.08 abc | 11.73 bc | 12.16 ab | 12.32 a | 0.44 |
| s.d. | 1.131 | 1.466 | 1.234 | 1.387 | 1.519 | 1.813 | 1.214 | 1.389 | 1.429 | ||
| PW | Mean | 7.337 b | 7.338 b | 9.012 a | 7.4 b | 7.406 b | 7.494 b | 7.38 b | 7.175 b | 6.912 b | 1.23 |
| s.d. | 0.61 | 0.482 | 10.323 | 0.536 | 0.552 | 0.542 | 5.649 | 0.516 | 0.605 | ||
| FD | Mean | 4.362 cd | 4.216 d | 4.395 bcd | 4.432 bc | 4.462 abc | 4.509 abc | 4.624 a | 4.559 ab | 4.639 a | 0.18 |
| s.d. | 0.7667 | 0.4799 | 0.456 | 0.6607 | 0.573 | 0.5039 | 0.6033 | 0.511 | 0.4305 | ||
| IH | Mean | 3.704 bc | 3.697 bc | 3.697 bc | 3.665 c | 3.682 bc | 3.761 abc | 3.722 abc | 3.769 ab | 3.818 a | 0.1 |
| s.d. | 0.5638 | 0.2625 | 0.2598 | 0.2921 | 0.2333 | 0.2905 | 0.2535 | 0.2519 | 0.2605 | ||
| IW | Mean | 0.6576 f | 0.7363 bc | 0.6988 de | 0.6779 ef | 0.6756 ef | 0.7546 b | 0.7146 cd | 0.79 a | 0.7863 a | 0.03 |
| s.d. | 0.09829 | 0.1043 | 0.07919 | 0.09318 | 0.07291 | 0.09642 | 0.09227 | 0.09284 | 0.09054 | ||
| FN | Mean | 19.62 cd | 19.67 cd | 19.12 d | 20.37 bcd | 19.93 bcd | 21.05 ab | 21.95 a | 19.42 cd | 20.65 abc | 1.3 |
| s.d. | 5.559 | 4.066 | 3.131 | 4.106 | 3.198 | 4.397 | 5.038 | 4.42 | 2.923 | ||
| FMF | Mean | 12.33 cd | 11.82 cde | 11.61 e | 12.42 bc | 11.81 cde | 12.38 bc | 13.19 a | 11.71 de | 12.99 ab | 0.65 |
| s.d. | 2.421 | 2.247 | 1.995 | 2.017 | 2.2 | 1.947 | 1.984 | 1.887 | 1.95 | ||
| DMF | Mean | 1.221 a | 1.218 a | 1.226 a | 1.214 a | 1.174 a | 1.205 a | 1.241 a | 1.137 a | 1.242 a | 0.77 |
| s.d. | 0.3283 | 0.1982 | 0.2823 | 0.2339 | 0.182 | 0.2426 | 0.2214 | 0.227 | 0.2618 | ||
| TH | Mean | 22.09 c | 22.17 bc | 21.26 d | 21.56 cd | 21.83 cd | 21.69 cd | 23.14 a | 22.93 ab | 23.31 a | 0.82 |
| s.d. | 3.198 | 2.524 | 2.209 | 2.342 | 2.516 | 2.891 | 2.32 | 3.207 | 2.208 | ||
| LN | Mean | 5.687 a | 5.637 a | 5.75 a | 5.825 a | 5.638 a | 5.638 a | 5.646 a | 5.8 a | 5.813 a | 0.19 |
| s.d. | 0.8656 | 0.5335 | 0.5156 | 0.4975 | 0.6005 | 0.6005 | 0.621 | 0.5372 | 0.5055 | ||
| LL | Mean | 13.23 e | 13.58 e | 13.81 de | 13.81 de | 14.27 cd | 14.41 bcd | 14.52 bc | 15.01 ab | 15.2 a | 0.67 |
| s.d. | 2.374 | 1.887 | 1.714 | 1.806 | 3.714 | 1.956 | 1.78 | 1.452 | 1.854 | ||
| LW | Mean | 2.108 d | 2.226 abc | 2.153 cd | 2.161 cd | 2.176 bcd | 2.228 abc | 2.302 a | 2.283 a | 2.245 ab | 0.08 |
| s.d. | 0.308 | 0.1892 | 0.1783 | 0.1777 | 0.1726 | 0.1768 | 0.5394 | 0.1524 | 0.2018 | ||
| QEP | Mean | 4.481 b | 5.1 a | 4.569 b | 4.588 b | 4.581 b | 4.462 b | 4.52 b | 4.381 b | 4.35 b | 0.49 |
| s.d. | 0.5363 | 4.537 | 0.4411 | 0.4555 | 0.4317 | 0.5017 | 0.4996 | 0.4587 | 0.4865 | ||
| FMAP | Mean | 20.48 d | 21.01 cd | 21.02 cd | 21.44 cd | 21.45 cd | 21.91 bc | 22.6 ab | 22.98 ab | 23.27 a | 1.08 |
| s.d. | 3.781 | 3.924 | 2.83 | 3.538 | 2.961 | 3.826 | 3.122 | 3.338 | 3.563 | ||
| DWAP | Mean | 1.55 d | 1.625 cd | 1.602 cd | 1.657 abc | 1.613 cd | 1.65 bc | 1.659 abc | 1.733 a | 1.724 ab | 0.08 |
| s.d. | 0.2806 | 0.2632 | 0.1978 | 0.3662 | 0.187 | 0.2515 | 0.2102 | 0.3177 | 0.2236 | ||
| SH | Mean | 1.94 a | 1.77 b | 1.46 d | 1.605 c | 1.785 b | 1.91 a | 1.765 b | 0.11 | ||
| s.d. | 0.347 | 0.3672 | 0.2836 | 0.3677 | 0.3233 | 0.4789 | 0.3617 | ||||
| CLD | Mean | 45.69 d | 47.06 cd | 48.1 bc | 49.05 ab | 48.66 bc | 48.37 bc | 47.09 cd | 48.24 bc | 50.58 a | 1.85 |
| s.d. | 4.375 | 4.504 | 4.425 | 3.281 | 3.087 | 3.696 | 5.017 | 4.212 | 4.777 | ||
| FLD | Mean | 1.539 ab | 1.608 a | 1.533 b | 1.513 b | 1.501 b | 1.525 b | 1.524 b | 1.495 b | 1.479 b | 0.07 |
| s.d. | 0.1643 | 0.1539 | 0.1526 | 0.1264 | 0.1385 | 0.1233 | 0.1579 | 0.1448 | 0.1632 | ||
| NBI | Mean | 29.87 cd | 29.45 d | 31.64 b | 32.58 b | 32.7 b | 31.88 b | 31.16 bc | 32.39 b | 34.39 a | 1.63 |
| s.d. | 3.967 | 3.377 | 4.115 | 2.866 | 3.743 | 3.14 | 4.12 | 4.348 | 3.358 |
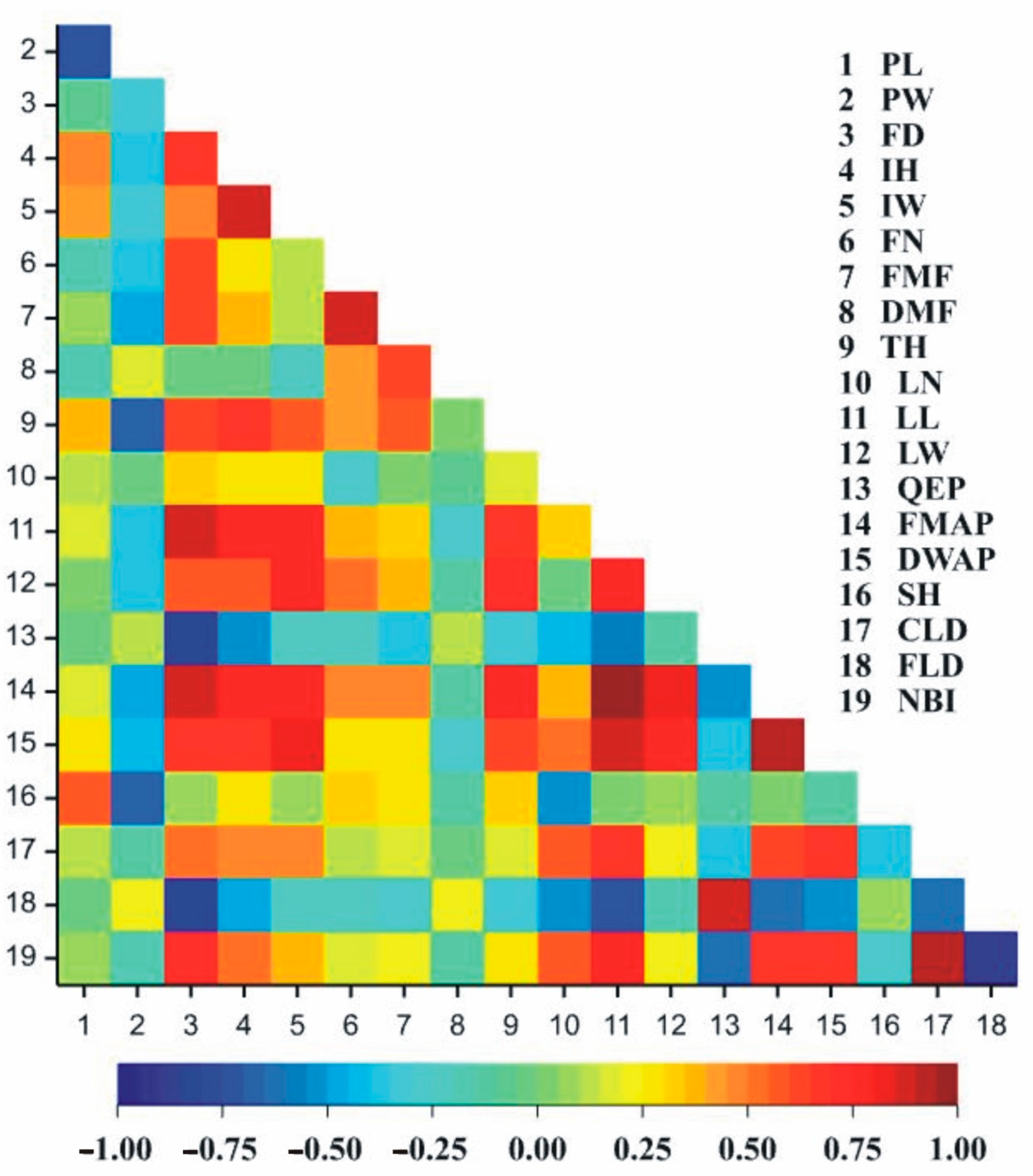
| Trait | EN | PW | FD | IH | IW | FN | FMF | DMF | TH | LN | LL | LW | QEP | FMAP | DWAP | SH | CLD | FLD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PW | −0.74 * | |||||||||||||||||
| FD | −0.08 | −0.31 | ||||||||||||||||
| IH | 0.49 | −0.4 | 0.65 | |||||||||||||||
| IW | 0.41 | −0.34 | 0.46 | 0.86 ** | ||||||||||||||
| FN | −0.16 | −0.39 | 0.6 | 0.26 | 0.14 | |||||||||||||
| FMF | 0.05 | −0.47 | 0.61 | 0.38 | 0.11 | 0.87 ** | ||||||||||||
| DMF | −0.18 | 0.16 | −0.02 | −0.01 | −0.21 | 0.43 | 0.63 | |||||||||||
| TH | 0.37 | −0.66 | 0.62 | 0.68 * | 0.6 | 0.42 | 0.57 | 0.04 | ||||||||||
| LN | 0.15 | −0.03 | 0.31 | 0.27 | 0.27 | −0.25 | 0.04 | −0.1 | 0.16 | |||||||||
| LL | 0.16 | −0.38 | 0.86 ** | 0.79 * | 0.79 * | 0.38 | 0.33 | −0.3 | 0.68 * | 0.33 | ||||||||
| LW | 0.03 | −0.39 | 0.59 | 0.58 | 0.76 * | 0.54 | 0.36 | −0.1 | 0.74 * | 0 | 0.76 * | |||||||
| QEP | 0 | 0.12 | −0.81 ** | −0.55 | −0.21 | −0.23 | −0.4 | 0.14 | −0.3 | −0.4 | −0.57 | −0.1 | ||||||
| FMAP | 0.17 | −0.46 | 0.87 ** | 0.79 * | 0.79 * | 0.47 | 0.47 | −0.1 | 0.78 * | 0.38 | 0.97 *** | 0.83 ** | −0.54 | |||||
| DWAP | 0.26 | −0.44 | 0.67 * | 0.70 * | 0.84 ** | 0.29 | 0.27 | −0.3 | 0.64 | 0.54 | 0.89 ** | 0.77 * | −0.37 | 0.93 *** | ||||
| SH | 0.6 | −0.67* | 0.06 | 0.28 | 0.08 | 0.31 | 0.28 | −0.1 | 0.3 | −0.5 | 0.01 | 0.1 | −0.1 | 0.02 | −0.12 | |||
| CLD | 0.14 | −0.15 | 0.52 | 0.46 | 0.48 | 0.14 | 0.16 | 0 | 0.15 | 0.57 | 0.69 * | 0.24 | −0.35 | 0.61 | 0.69 * | −0.4 | ||
| FLD | −0.01 | 0.21 | −0.84 ** | −0.46 | −0.23 | −0.21 | −0.3 | 0.25 | −0.32 | −0.5 | −0.70 * | −0.16 | 0.89 ** | −0.64 | −0.53 | 0.07 | −0.64 | |
| NBI | 0.08 | −0.19 | 0.72 * | 0.5 | 0.4 | 0.19 | 0.25 | −0.1 | 0.26 | 0.6 | 0.76 * | 0.23 | −0.65 | 0.68 * | 0.67 * | −0.3 | 0.93 *** | −0.88 ** |
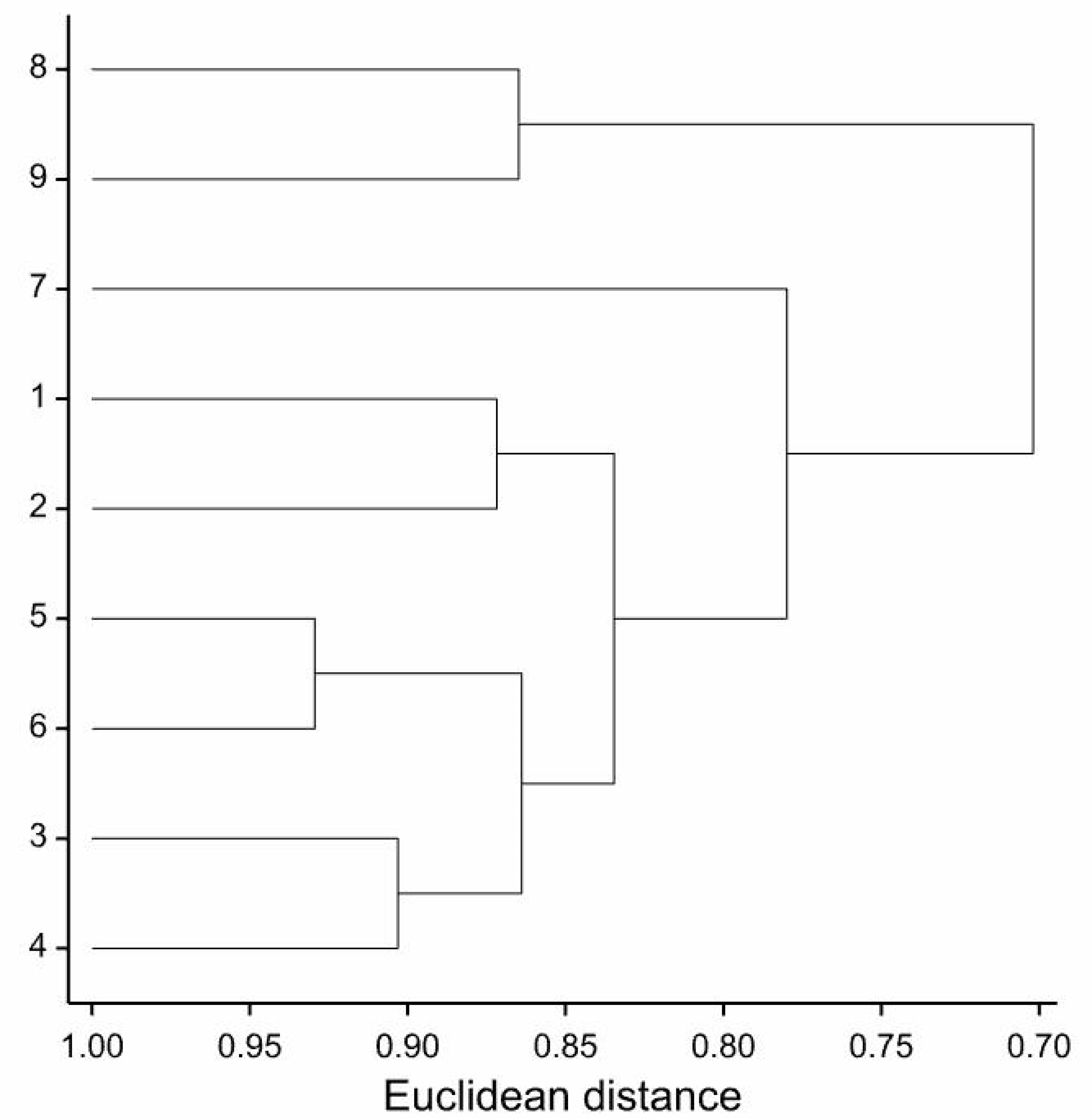
| Trait | PC1 | PC2 |
|---|---|---|
| EN | 0.151 | 0.132 |
| PW | −0.322 | −0.582 |
| FD | 0.786 * | 0.427 |
| IH | 0.629 | 0.351 |
| IW | 0.572 | 0.255 |
| FN | 0.342 | 0.737 * |
| FMF | 0.375 | 0.725 * |
| DMF | −0.075 | 0.246 |
| TH | 0.439 | 0.740 * |
| LN | 0.542 | −0.32 |
| LL | 0.873 ** | 0.281 |
| LW | 0.454 | 0.638 |
| QEP | −0.577 | −0.106 |
| FMAP | 0.829 ** | 0.439 |
| DWAP | 0.810 ** | 0.24 |
| SH | −0.18 | 0.567 |
| CLD | 0.927 *** | −0.305 |
| FLD | −0.805 ** | 0.04 |
| NBI | 0.963 *** | −0.205 |
| Percentage of explained multivariate variability | 65.14 | 19.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojdyła, A.T.; Nowak, J.S.; Bocianowski, J.; Wiśniewski, J.; Waszkiewicz, E. Effect of Hyacinth Treatment by Hydrogen Peroxide Stabilized with Silver and Some Fungicides on the Fungal Infection of Substrate and Bulbs and on Plant Growth and Development. Agronomy 2022, 12, 2894. https://doi.org/10.3390/agronomy12112894
Wojdyła AT, Nowak JS, Bocianowski J, Wiśniewski J, Waszkiewicz E. Effect of Hyacinth Treatment by Hydrogen Peroxide Stabilized with Silver and Some Fungicides on the Fungal Infection of Substrate and Bulbs and on Plant Growth and Development. Agronomy. 2022; 12(11):2894. https://doi.org/10.3390/agronomy12112894
Chicago/Turabian StyleWojdyła, Adam T., Jacek S. Nowak, Jan Bocianowski, Jacek Wiśniewski, and Emilia Waszkiewicz. 2022. "Effect of Hyacinth Treatment by Hydrogen Peroxide Stabilized with Silver and Some Fungicides on the Fungal Infection of Substrate and Bulbs and on Plant Growth and Development" Agronomy 12, no. 11: 2894. https://doi.org/10.3390/agronomy12112894
APA StyleWojdyła, A. T., Nowak, J. S., Bocianowski, J., Wiśniewski, J., & Waszkiewicz, E. (2022). Effect of Hyacinth Treatment by Hydrogen Peroxide Stabilized with Silver and Some Fungicides on the Fungal Infection of Substrate and Bulbs and on Plant Growth and Development. Agronomy, 12(11), 2894. https://doi.org/10.3390/agronomy12112894







