1. Introduction
Pollination refers to the process by which plant pollen is transferred from the anthers to the stigma of the pistil to fertilize the plant. Over 85% of flowering plants rely on biological pollination, mainly insect-borne, and the remainder relies on abiotic-borne pollen, mainly wind-borne [
1]. In the production of the world’s leading grains, fruits, vegetables, and seeds, over 75% of crops rely on insect-pollinated pollination, and the annual increase in agricultural yield generated by insect-pollinated pollination exceeds EUR 150 billion, accounting for 9.5% of the worldwide agricultural output value [
2,
3]. As such, pollination is critical to maintaining ecosystem balance and agricultural production and has significant economic significance.
However, due to climate change, land occupation, pests, diseases and overuse of pesticides, the survival of numerous types of pollinators has been seriously threatened, and their numbers have dropped dramatically [
4,
5]. The public, governments, and organizations are increasingly recognizing the detrimental effects of the “pollinating insect crisis”, and research on sustainable pollinators has become a focus [
6,
7]. At present, The domesticated bee pollination, which is easy to feed and manage, and mechanized pollination, which is not restricted by the environment, are considered the primary technical means to alleviate the “pollinating insect crisis” and have attracted the attention of researchers [
8,
9].
This review focuses on the research information of the Science Citation Index, Engineering Village, National Knowledge Infrastructure, and Food and Agriculture Organization of the United Nations database from 2007 to now. Based on an analysis of the differences in pollination methods of different crops, this review summarizes the research progress of efficient crop pollination technology worldwide from the perspectives of bee pollination and mechanized pollination of fields, orchards, and greenhouse crops. In addition, we discuss the prospect of efficient pollination technology for crops to facilitate the modernization and intelligent development of the agricultural pollination industry worldwide. To ensure the integrity of the summary content and logic, a few previous research information was also retrieved.
2. Pollination Methods for Different Crops
2.1. Basic Structural Features of Flowers
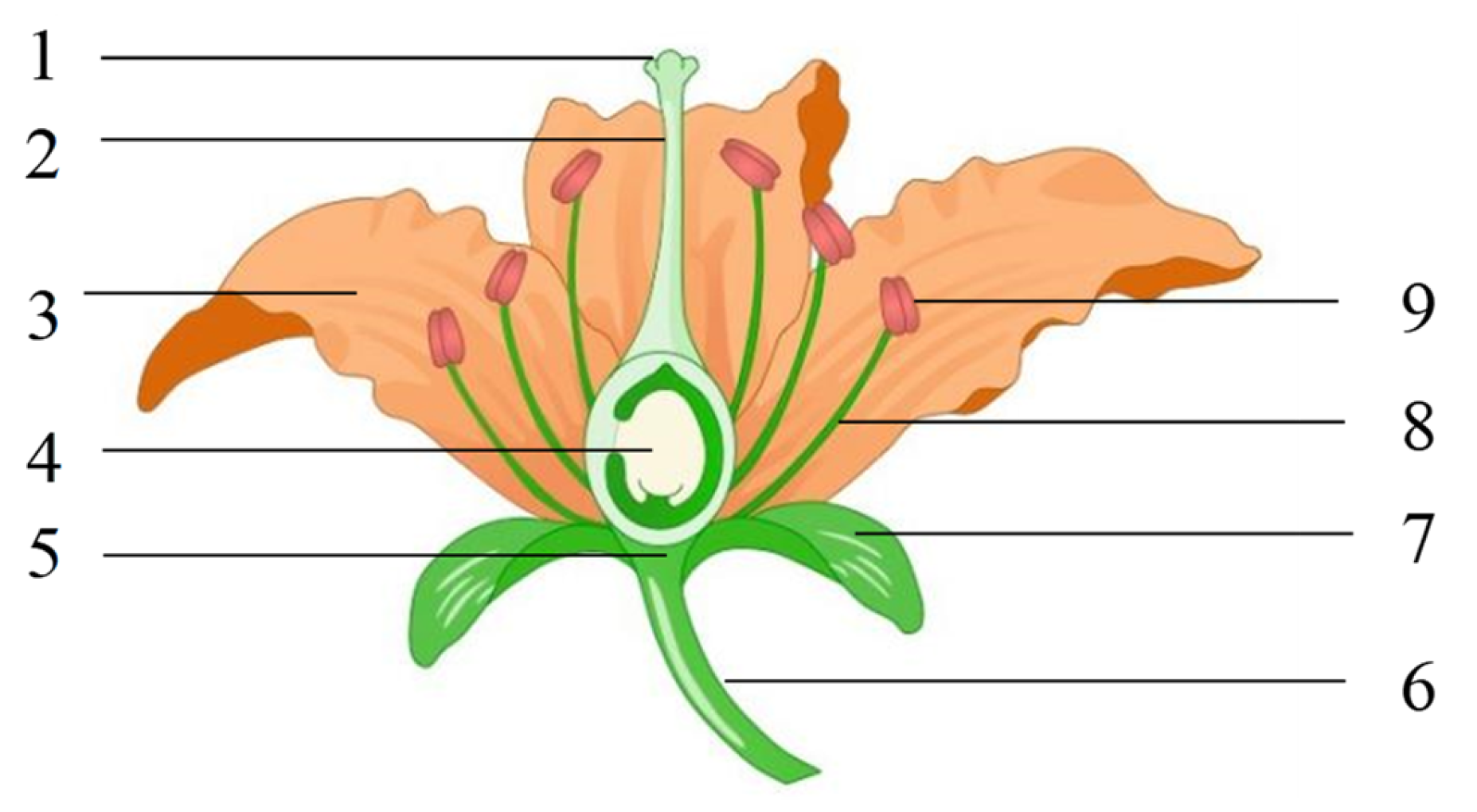
As shown in
Figure 1, a flower comprises six parts: flower stalk, receptacle, calyx, corolla, stamen, and pistil. The stamens comprise filaments and anthers that contain a large amount of pollen; the pistil comprises the stigma, style, and ovary. The stigma is at the top of the pistil and is used to receive pollen grains. A flower with a calyx, corolla, stamen, and pistil is called a complete flower, whereas those lacking one or more parts are called incomplete flowers. Flowers with pistils and stamens are called hermaphroditic flowers. A flower lacking a stamen is called a unisexual flower; a flower with only stamens is a male flower, and a one with only pistils is a female flower. A plant with female and male flowers is called a hermaphrodite, whereas the separation of female and male flowers in different plants is called dioecism [
10].
2.2. Self-Pollination and Cross-Pollination
Self-pollination refers to transferring pollen from one flower to the stigma of the same flower. In contrast, cross-pollination refers to transferring pollen from one flower to the stigma of another flower. The natural outcrossing rate is a common method to distinguish between self- and cross-pollinated crops [
11]. However, it is difficult to find absolute self-pollination and cross-pollination crops under insect- and wind-pollinated conditions.
Compared with self-pollination, cross-pollination is a method of long-term natural selection and crop evolution. This is because the genetic differences between female and male gametes from cross-pollination crops are relatively large. The resulting offspring have strong viability and adaptability, strong plants, numerous flowers, a high fruiting rate, and strong stress resistance [
12,
13]. Furthermore, some crops have also formed physiological characteristics suitable for cross-pollination, mainly including unisexual flowers, pistil heterogeneity, pistil heterosis, ectopic pistils, and self-incompatibility [
14,
15,
16,
17]. Although cross-pollination benefits crop offspring, crop pollination patterns also evolve with changes in natural pollination environmental conditions, varying between self-pollination or cross-pollination, wind- or insect-borne [
18]. Moreover, the quality of the self-pollinated progeny of some crops is not lower than that of cross-pollinated crops [
19,
20].
2.3. Differences in Pollination Methods of Different Crops
As shown in
Table 1, self-pollinated field and orchard crops can be pollinated by natural wind pollination. Cross-pollinated fields, orchards, and greenhouse crops in closed and windless environments require bee pollination. In unfavorable natural pollination environments where the occurrence of natural wind and bee pollination is limited or for crops that cannot attract pollinating insects or have staggered flowering periods of male and female flowers, manual-assisted pollination is needed, usually as a labor-saving and efficient mechanized pollination method. To ensure safe and efficient pollination of crops during a short flowering period, the selection of pollination methods should be based on the physiological characteristics of crops and the actual environmental conditions of natural pollination.
3. Status of Efficient Bee Pollination Technology
3.1. Principle of Efficient Pollination of Bees
Bee pollination is a “bonus” of bees’ out-foraging behavior. In the long-term coevolution of bees and plants, bees have formed numerous characteristics suitable for plant pollination, including being easily attracted by bright colored entomophilous flowers that emit an aroma and secrete nectar; in addition, bees have identifying memory and pollination activity focused on specific species over a long period, high-frequency vibration generated by the wings during foraging that causes pollen to fall off, morphological structures such as villi and pollen baskets that easily adhere to pollen, social group structure, and numerous groups [
21].
The beehive is a place for bees to thrive. Since the American Langstrow proposed the concept of a “bee space” and invented the first live-frame beehive, human bee feeding, and management technology has rapidly developed. At bee same time, the pollination behavior has good trainability, and the nest love of bees makes colonies easy to move, which has extensively promoted the development of the bee pollination industry [
22].
Although most species of bees have apparent advantages in pollination and feeding management compared with ordinary insects, efficient pollination of crops requires bees to actively forage for food. This continuous and active foraging behavior requires a healthy, strong and stable colony state. The colony state is closely related to the bee species and the internal and external environment of the beehive. Therefore, the breeding of high-quality pollinating bee varieties, efficient and continuous monitoring, and control of the internal and external environments of beehives and the status of the bee colony are the mainstream directions of efficient bee pollination technology. As with other pollinator crises, colony collapse syndrome is also an issue; the number of bee colonies raised and managed worldwide is far from sufficient for meeting the needs of agricultural growth based on insect pollination [
23,
24].
3.2. Breeding of High-Quality Pollinating Bee Varieties
As shown in
Figure 2, there are 2
Apis species, and 9 Bombus species, and 8 solidary bee species that provide pollination services for crops. In addition, more bee varieties are waiting to be selected, including 6 bumble bees, 15 stingless bees, and 14 solitary bees. Europe, Asia, North America, and South America have more species that can be more easily used to breed high-quality pollinating bee varieties [
25]. Fertility, population growth rate, bee sorting, collecting ability, pollination habit, disease resistance, and stress resistance are the biological characteristics that need to be closely investigated in pollinating bee breeding. Compared with bees,
Bombus spp. have longer beaks, greater ability for nectar collection, and adaptability to low light density. Because bumblebees lack a developed information exchange system similar to bees, they are more suitable for pollination of crops in greenhouses where they are widely used [
26,
27].
3.3. Climate and Environment Control Technology Outside the Hive
Climate and environmental factors (e.g., temperature, humidity, light, rain and wind) outside the hive directly affect the physiological function of bees, the secretion of nectar, and pollen germination of nectar crops, thus affecting the foraging behavior of bees and their pollination effects.
In a subtropical climate, the initial temperature at which
Apis Spp. commence foraging behavior on apple blossoms is 16 °C. As the temperature increases, the number of bees out-foraging continuously increases and reaches a relatively stable high level when the temperature exceedes 20 °C. Temperatures below 20 °C, wind speeds of over 15 mph, rain, and lower light levels limit bees’ out-foraging behavior [
28]. In the regional climate of Lithuania,
Apis mellifera almost stopped its foraging behavior on rape crops when the temperature reached 43 °C [
29]. A light intensity of 600–1700 lx and solar radiation of 9–20 mW/cm
2 are the minimum climatic and environmental conditions required for the four types of bees (
Apis dorsata F;
A. mellifera L;
A. cerana F; and
A. florea F) to go out for food [
30]. Under high humidity conditions, the nectar secretion rate of crops is higher and the evaporation rate is lower; therefore, the foraging rate of bees is positively correlated with environmental humidity. However, considering both pollen collection and transportation of bees, researchers believe that a lower humidity environment is more conducive to bee foraging [
31]. Clarke et al. [
32] established a least squares generalized linear model using climatic and environmental factors to predict the out-foraging rate of bees; the results showed that 78% of changes in bee foraging activities were due to changes in temperature and solar radiation. Due to differences in bee species, crop varieties, regional climate, and research methods, there are apparent differences in the effective range of the above-mentioned climatic and environmental factors outside the beehive, which impacts the most dynamic range for foraging. However, the trends of change are consistent [
33,
34,
35,
36].
Fields and orchards are open-air spaces and bees require favorable natural climate conditions to go out for food. Bees can regulate their body temperature to ensure physiological functions, such as food absorption, respiration, metabolism, and energy supply [
37]. As shown in
Figure 3, foraging bees use solar energy to increase body temperature and save energy in a low-temperature environment. At the same time, they choose between “investment-oriented” and “energy-saving” thermoregulation strategies based on whether increasing body temperature can maximize the efficiency of food absorption [
38]. In a high-temperature environment, foraging bees can dissipate heat and cool through the evaporation of honey sacs and oral water droplets [
39,
40].
Compared with open-air fields and orchards, greenhouses allow for the management and control of the climate environment. Due to the closed and windless environment, greenhouses can significantly increase bee pollination. The climate control of greenhouses is based mainly on the combined work of the heating system, the energy storage system, and the cooling system. Humidity control is achieved through ventilation, heating, and spraying. Light control mainly relies on sunshades and supplementary light systems integrated into heating and cooling systems; furthermore, temperature is coupled to control greenhouse climate [
41,
42,
43]. However, current research on climate and environmental control systems and strategies in greenhouses focuses on high-quality and efficient plant growth and system energy efficiency optimization; few studies have considered the climate and environmental needs of bees for efficient foraging and pollination [
44,
45].
3.4. Temperature and Humidity Control Technology in the Beehive
Healthy breeding of queen bees, drones, and worker bees is the basis for the health, strength, and stability of the colony. The beehive provides a breeding and living place that shields the bee colony from light, rain, and wind. Temperature and humidity in the beehive are the most critical factors that affect colony reproduction and are directly affected by the climate outside the beehive.
3.4.1. Influence of Temperature and Humidity on Beehive Colony Reproduction
The optimum temperature for the development of the fertilized eggs of
Apis mellifera and the queen bee pupae in the capping platform is 35–36 °C; between 32 °C and 36 °C, the fertilized eggs and queen bee pupae develop normally. When the temperature reaches 37 °C, the queen bee pupae cannot develop normally [
46]. The temperate ranges 29–37 °C and 31–37 °C are the development temperatures of
Apis mellifera and
Apis cerana, respectively. With a decrease in temperature, the development period of worker bee capping is significantly prolonged, and this affects developmental and morphological indicators such as birth weight, snout length, and forewing area. Beyond this temperature zone, bees are deformed or die [
47]. The temperature of pupa development can significantly affect the behavioral performance of honeybee adults, thus affecting their foraging tasks. The learning and memory behavior of honeybee adults under a pupa development temperature of 36 °C is better than that under 32 °C and 34.5 °C [
48]. The synaptic organization of the adult honeybee brain is mediated by the temperature experienced during pupal development, which affects its communication and learning behavior. In the olfactory input area of the mushroom body of the brain, the number of microglomers was highest in bees incubated at a temperature of 34.5 °C that is normally maintained by brood cells, and the number of microglomers was significantly reduced in bees incubated at 1 °C below or above this standard [
49]. Pupal developmental temperature affected JH metabolism and octopamine levels in bee brains; bees developing at higher temperatures showed an earlier tendency to go out for food [
50]. The optimum humidity range for normal hatching of bee eggs is 90% to 95%; outside this humidity range, the number of larvae that hatch normally decreases significantly [
51]. In environments with high relative humidity, bees cannot excrete metabolized and food-dissolved water, which shortens their lifespan [
52]. In summary, healthy breeding of bee colonies and their active foraging behavior depend strongly on the temperature and humidity in the beehive.
3.4.2. Colony Self-Regulation Mechanism of Beehive Temperature and Humidity
Bees are typical social insects. Although individual bees have limited ability to regulate temperature and humidity, they can cooperate in groups to maintain temperature and humidity in the beehive within a reasonable range, thus ensuring healthy breeding and colony life of the colony [
20].
Honeybee antennae are multimodal sensory organs that can sense various information, such as temperature, humidity, smell, taste, and mechanical stimuli, among others. Their temperature sensitivity is 0.25 °C. Honeybees receive real-time information on temperature, humidity, and spleen temperature in the hive through their antennae [
53,
54,
55]. When the temperature, humidity, and temperature of the spleen in the hive are beyond the reasonable range for breeding and life, the bee colony takes measures to modify it. When the temperature in the hive or spleen is low, thermogenic bees raise the temperature of their thoracic cavity and attach the breast to the wax cover of the sealing lid, transferring this heat to maintain the temperature in the hive and spleen. Non-thermogenic bees gather and squeeze the cell comb to increase the sealing performance of the hive and reduce heat loss [
56,
57]. When the temperature in the beehive or spleen is high, the bee colony increases airflow between the inside of the beehive and the outside world through ordered directional fans to discharge the overheated air in the beehive; furthermore, the bee colony is also scattered in the beehive, further improving ventilation and heat dissipation [
58]. In addition, according to the temperature in the hive or the temperature of the spleen, the bee colony will adjust its metabolic rate in real-time and control its heat generation rate. The means by which the bee colony regulates humidity in the beehive is like that of temperature, and the two processes are temporally coupled [
50].
The temperature regulation capacity of a bee colony is related to bee species, colony strength, and gene diversity.
Apis cerana is more sensitive to hive temperature than
Apis mellifera and is more motivated to use fan cooling. If the bee colony is strong, the heat generated by it will significantly exceed the heat loss, which is conducive to maintaining a stable temperature in the beehive; feed consumption is also significantly reduced [
20,
32]. The genetic diversity of the honeybee colony results from the queen bee mating with many drones. Different genetic species of bees flap their wings around the hive at different temperatures. When warming a hive, the half-sister worker bees cooperate better, and the temperature fluctuation range is 0.5 °C, whereas in a hive based on artificial insemination, the temperature fluctuation range was 1 °C [
59,
60].
3.4.3. Temperature and Humidity Monitoring Technology in Beehives
The existing temperature and humidity control in the beehive mainly relies on the self-regulating ability of the bee colony. A healthy, strong, and stable bee colony can maintain the temperature and humidity in the beehive within a reasonable range to ensure the breeding and life of the bee colony. A typical winter colony of 17,500 bees can survive for over 300 h even when the temperature outside the hive is as low as −25 °C [
61]. However, when the state of the bee colony changes because of, for example, the occurrence of disease and insect pests, separation of bees, escape, or changes in the temperature and humidity inside the beehive caused by external changes that exceed the ability of the existing bee colony to adjust, the survival of the entire bee colony is seriously threatened. The existing temperature and humidity monitoring technology in the beehive provides an early warning function for the state of the beehive, which is convenient for manual control over time; however, research is needed to reveal the self-regulation mechanisms of temperature and humidity in the beehive.
Kridi et al. [
62] continuously and remotely monitored the climate inside and outside beehives and the behavior of bees under unfavorable temperatures using a single-point temperature sensor and wireless sensor network; using these data, they explored the relationship between colony absconding behavior and temperature and found that, based on a clustering mechanism, early warning of an overheated climate can lead to escape of bee colonies. As shown in
Figure 4, Becher et al. [
63] designed a high-precision comb temperature monitoring system called “the porcupine”, which used 256 negative temperature coefficient thermistor sensors embedded in three adjacent nests in the middle of the spleen hive unit; all the temperature data during the growth process of 768 individuals were recorded simultaneously. Yu et al. [
64] redesigned the array temperature acquisition circuit based on the negative temperature coefficient thermistor sensor to achieve complete coverage monitoring of the temperature of each hive in 90% of the comb; the error between the acquired and actual temperature was less than 0.5 °C.
Due to the spatial structure of a beehive and the self-regulating mechanism of temperature and humidity, there is a gradient in the distribution of temperature and humidity in the beehive. Under harsh external climatic conditions, the temperature and humidity of the beehive outside, inside at the top, and inside at the bottom are significantly different. As such, single-point temperature and humidity sensors cannot accurately reflect the distribution of temperature and humidity in the beehive [
65]. To address this issue, Human et al. [
66] and Evans et al. [
67] designed multi-point humidity monitoring systems for the beehive and revealed the importance and mechanism of worker bees and their fan activity on humidity control in the beehive. As shown in
Figure 5, Li et al. [
68] designed an integrated temperature and humidity monitoring system based on a microsensor array, which can monitor humidity and temperature in real-time at 30 uniformly distributed locations in the beehive, along with the outside ambient temperature and humidity. As shown in
Figure 6, Meitalovs et al. [
69] designed multiple beehive microclimate monitoring systems that integrate video information. When abnormal temperature and humidity are detected at multiple locations in the beehive, the system automatically opens the beehive entrance and external environment cameras; video information can be used to analyze the causes of climate anomalies. Edmund et al. [
70] designed an innovative stingless beehive monitoring system which monitors the temperature, humidity, water level, and geographic location of stingless beehives through multisensors and the internet of things. They built temperature, humidity, and water volume control systems by adding DC fan motors and diaphragm pumps to ensure that the environment in the hives is favorable.
3.5. Bee Colony State Management and Control Technology
Colony state is a broad concept that includes multi-characteristic information about the queen bee, drones, and worker bees (e.g., breeding status, population structure, number, nest, spleen status, degree of diseases, and insect pests). In actual production, beekeepers still need to manually estimate the status of the bee colony regularly before implementation of strategic management; this approach is labor intensive, low efficiency, extremely dependent on professional experience, and interferes with the life of the bee colony and pollination operations. With the development of modern information technologies such as artificial intelligence and the internet of things, researchers have attempted to use non-invasive intelligent equipment to obtain real-time bee colony information using images, sound, vibration, weight, and incoming and outgoing bee activity.
3.5.1. Image Monitoring Technology in Beehives
Image-based information from the beehive is the most intuitive technical means to assess the state of the bee colony. As honey bees have phototaxis and cannot recognize red light, image monitoring technology in beehives often uses red light irradiation or infrared thermal imaging to obtain image information.
As shown in
Figure 7, Knauer et al. [
71] designed an image acquisition test device for the nest spleen in the hive. The adaptive background model method was adopted to improve the detection rate of larvae without the cover of the nest spleen in the image and to help the researchers detect highly resistant bees. As shown in
Figure 8, Chazette et al. [
72] used the deep convolution neural network algorithm to identify mite-infected bees in the beehive, judge the degree of infestation, and give the coordinates of the mites. By using laser elimination, the recognition rate of mites reached 93%.
Kimura et al. [
73] used the vector quantization method to identify and track honeybees to study their individual and social behaviors, with a recognition rate of over 72%, and to determine the active areas of bees in the beehive by tracking their trajectories. Feldman et al. [
74] integrated k-nearest neighbor classification and hidden Markov model technology to realize the identification and extraction of bee activity tracks in video streams. Based on improved particle filter tracking, Maitra et al. [
75] analyzed the relationships between individuals and colonies of bees and studied the interaction behaviors between bees. The root-mean-square error of this method for tracking bees was 8.7 pixels; moreover, compared with particle filter tracking based on Gaussian appearance modeling, the position and angle errors increased by 75% and 58%, respectively. As shown in
Figure 9, to further improve the efficiency of bee identification and tracking, Hendriks et al. [
76] pasted labels on bees to study the position, speed, and interaction of individual bees and their relationship with group activities. Tsai et al. [
77] summarized the different movement modes of bees through label identification and positioning and explored the behavioral characteristics of bees with different ages and roles. As shown in
Figure 10, Wild et al. [
78] proposed an algorithm structure based on two deep convolution neural networks. With the positioning and decoding of self-defined binary marks, the early identification work of bee behavior analysis of bee images in the nest with labels was realized, and the identification accuracy of labels was improved.
3.5.2. Sound and Vibration Monitoring Technology in Beehives
Colony activity in the beehive occurs in the honeycomb, and an image of colony activity at the location of the spleen can directly reflect colony status. However, honeycombs are densely arranged in the beehive based on nest frames, and there are bee spaces between the honeycomb, which are fixed and narrow. Therefore, the existing image detection technology in the hive cannot obtain images of the colony activity of all the honeycombs. Therefore, indirect estimation of bee colony status by analyzing information such as sound, vibration, weight, and incoming and outgoing bee numbers has gradually attracted attention.
The sound and vibration emitted by bees are an essential part of the communication mechanism of the bee colony. The temperature and humidity regulation of the colony, group activities such as going out for food, separating bees, escaping, losing the king, and disease can all be reflected by sound and vibration information. Kulyyukin et al. [
79,
80,
81] used the Fourier transform to process continuously collected bee colony sound information, converted it into an A440 piano note sequence signal, and proposed an algorithm to estimate the flow level of foragers based on images of import and export bees. To investigate the regularity of group foraging activities and judge the stability of bee colonies, Mezquida et al. [
82] designed a beehive sound monitoring platform, which established a link between the sound intensity of the bee colony and the colony activity, thereby determining the colony potential, and conducted a correlation study between the colony potential and bee colony yield. As shown in
Figure 11, Ferrari et al. [
83] studied the relationship between sound changes in bee colonies in beehives and temperature and humidity. The results showed that when the bee colony was active, the sound increased from 100 to 300 to 500 to 600 Hz; this was closely related to the behavior of wing vibration to adjust temperature and humidity. Murphy et al. [
70,
71] proposed a solution that uses wireless sensor network technology and low-power signal processing to monitor the sound of bee colonies and the temperature and humidity inside the beehive. By filtering the sound of the bee colony and conducting noise analysis, they found that if the noise increases for a long time, it indicates abnormal behavior and an alarm message is sent. The change in different sound information can reflect the agitation degree of the bee colony.
Rangel et al. [
84] used a glass cover to seal the beehive, which facilitated the observation of changes in the density and mobility of bees. Meanwhile, the sound information in the beehive was obtained, and the signal characteristics of large-scale separation and escape behavior of the bee colony were analyzed. The results showed that the generation of piping signals gradually increased during the 60 min before escape and finally reached a peak at the start of the colony. Furthermore, the bee density decreased significantly during colony division, whereas the average bee speed (mm/s) and production of the hum signal increased dramatically. Liu et al. [
85] used extracted sound features to judge whether the bee colony had lost its queen and simultaneously differentiated the old and new situations of the queen bee in the hive. Qandour et al. [
86] used linear discriminant analysis and principal component analysis to reduce the dimensionality of bee colony sounds and using support vector machine, built an acoustic fingerprint of beehives which they then used to detect whether bee colonies were infected or not. Kulyukin [
87] and Amlathe [
88] used convolutional neural networks, logistic regression, K-nearest neighbor, random forest, support vector machine, and other machine learning methods to identify the spectral characteristics of bee colony sounds and constructed several abnormal behavior models to evaluate the health status of a bee colony in the nest.
Ramsey et al. [
89] used an ultrasensitive accelerometer embedded in the honeycomb at the center of the colony for automated, in situ, non-invasive monitoring of short vibration pulses of bees called “stop signals”. Bencsik et al. [
90] proposed using accelerometers to analyze the time course of the normal vibration of beehive walls to predict colony status. By recording data continuously for eight months, they found that the amplitudes of independent signals formed a multidimensional time-varying vector that provided height features specific to the swarming process, based on which swarming phenomena could be predicted in advance.
3.5.3. Bee Colony Weight Monitoring Technology
The colony weight includes the main colony elements such as the adult bee colony, larvae, honey, and pollen, and its changes can accurately reflect the productivity, health, robustness, and stability of the colony in the beehive. To obtain bee colony weight information, professional electronic balances or electronic scales are often used to weigh beehives, comb spleens, adult bees, honey, and pollen.
As shown in
Figure 12, Fitzgerald et al. [
91] designed a low-cost and low-power wireless bee colony weighing platform; for colonies larger than 10 kg, the measurement accuracy was controlled within 100 g. Evans [
67] analyzed the correlation between the daily change in bee colony weight and the foraging activities of bees and showed that when the foraging activities of bees increased, the colony weight increased. Based on this relationship, the change in bee colony weight can be used to evaluate the foraging enthusiasm and collection efficiency of bee colonies. Meikle et al. [
92,
93,
94] constructed a food storage change curve for a colony using the colony weight data and estimated the colony food consumption through the colony weight change. They constructed a multi-period colony, daily activity–colony weight model using the piecewise regression method. They observed the starts and ends of the daily activity cycle and that the increase in colony weight is caused by going out, collecting and returning to the colony. The feasibility of evaluating the productivity, health, robustness, and stability of a colony based on colony weight was also discussed.
3.5.4. Bee Colony Monitoring Technology in the Hive Gate Area
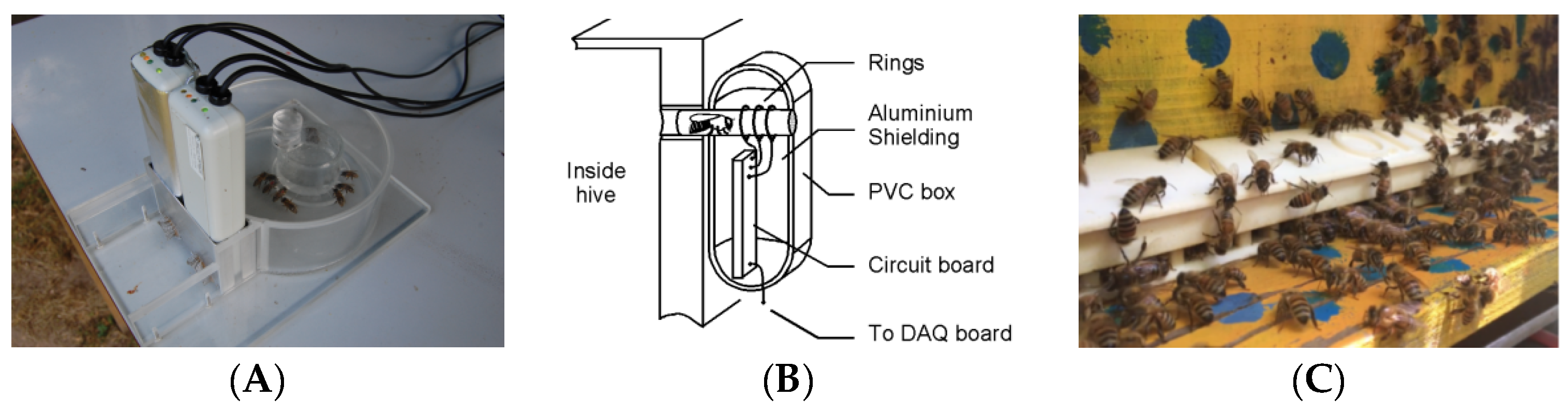
Monitoring bee colony activities in the hive gate area can not only directly provide information on the foraging enthusiasm of bee colonies but also indirectly estimate bee colony status. Research on bee colony monitoring technology outside the hive focuses mainly on the number of bees and the number entering and leaving the hive gate area. As shown in
Figure 13, four main technical solutions are currently used: radio frequency identification tags, capacitive sensors, photoelectric sensors, and machine vision [
95,
96,
97,
98,
99]. However, radio frequency identification tags, capacitive sensors, and photoelectric sensors suffer from regular beehive modifications, high costs, and complex maintenance. The bee colony monitoring system based on machine vision is a non-invasive solution that is easy to install and maintain, low cost, easy to popularize and apply, and has attracted increasing research attention.
Kulyukin et al. [
81] used contour detection and pixel separation methods to conduct a comparative test of bee image recognition detection; the results showed that the pixel separation method was effective and achieved an average accuracy of 73%. On this basis, the image processing space was converted from RGB to HSV, and the environmental contrast of the beehive take-off and landing platform was increased to increase the recognition accuracy to 85.5%. After identifying bees using the background subtraction method, Tashakkori et al. [
100,
101] used the average pixel method and the signal-to-noise ratio to predict the number of bees. By comparing the results, they found that the signal-to-noise ratio method was a good measure of the number of bees when the number was small. Kulyukin [
102] introduced one-dimensional Harr wavelet peaks in bee detection and counted all pixels covered by the upper and lower peaks; the accuracy to within five bees was 63%, and the accuracy to within ten bees was 94%.
As shown in
Figure 14, Campbell et al. [
103] used the background subtraction method to detect bees, set an ellipse matching template for the detected bees, adopted a maximizing bipartite graph-weighted tracking strategy, and used common motion models (wandering, crawling, flying in, fly out) to describe bee activity and count in-and-out. Salas et al. [
104] used SVM as a classifier to detect honeycomb door bees and used a Bayesian tracker to track detected bees and calculate incoming and outgoing numbers. Kale et al. [
100,
101] combined the background subtraction method and blob analysis to extract the bee area and then used the optical flow method and Kalman filter method to track the bees. Kalman tracking was the best approach, and the error range for the in-and-out estimate was 4–20%. As shown in
Figure 15, Tu et al. [
105] monitored bees entering and leaving the hive under constant lighting conditions by limiting the passage of bees to a single layer, segmenting the acquired image to extract the bee area, and using the circular search method between the upper and lower frames to describe the behavior of the bees; they then judged and counted the trajectories. The regression statistic (i.e., R
2) for bee entry and exit, a systematic prediction and comparison of manual counts, was 0.987 for bee counts and 0.953 and 0.888 for measuring intra- and extra-activity, respectively. Chen et al. [
106] designed a single passage for bees and used bee labels. After using the Hough transform method to detect the labels, SVM was used to classify and identify the labels, and counting was realized based on the uniqueness of the labels. The label recognition rate and recognition accuracy of the system were 98% and 86%, respectively. Kulyukin [
107] proposed an omnidirectional honeybee traffic monitoring method that combines motion detection with image classification, where motion detection is responsible for generating a set of regions with possible objects. Each such region is determined by a class-specific classifier (such as a convolutional neural network or SVM) or a set of classifiers (such as a random forest), which enables the automatic monitoring of the number and movement of bees moving in any direction near the landing platform of the hive. Ngo et al. [
108] used the background subtraction method to segment and detect bees and used the integrated Kalman filter and the Hungarian algorithm tracker to track bees. The automatic counting accuracy of the system was 93.9 ± 1.1%.
3.5.5. Bee Colony Monitoring Technology Based on Multi-Feature Information Fusion
The climate outside the hive and the temperature and humidity inside the hive affect bees’ behaviors such as foraging and breeding, thus affecting the colony status. Human activities, pesticides, bee habits, and other factors also significantly affect the state of bee colonies; moreover, these factors are interdependent. Currently, there is no effective model to reasonably estimate the influence of these factors and the self-adjustment mechanism of the colony on the colony state. Furthermore, it is inaccurate to estimate the state of a bee colony only based on single feature such as the climate outside the beehive or the temperature, humidity, sound, or weight inside the beehive.
As shown in
Figure 16, Murphy et al. [
109,
110] designed a bee colony status monitoring system based on wireless sensor network technology using an integrated infrared LED array and an infrared camera to capture images inside the beehive, and the temperature, humidity, sound, and other variables inside and outside the beehive. As shown in
Figure 17, the HiveMind, Arnia, and ToBe companies have all proposed remote beehive monitoring solutions integrating multi-feature data such as sound, vibration, temperature, humidity, and weight. These solutions can effectively reduce the loss of bee colonies and improved honey production [
111,
112,
113]. As shown in
Figure 18, Alibaba Cloud and Tianfu Fenggu Company jointly developed and released a new generation of AI smart beehives. Based on existing remote beehive monitoring technology that integrates multiple data sources such as sound, vibration, temperature, humidity, and weight, a big data platform, and blockchain traceability system increased the overall production efficiency of a single box of honey by 80% [
114]. As shown in
Figure 19, Beewise has developed the world’s first integrated honeycomb, called Beehome, which can accommodate up to 40 bee colonies, obtain or calculate information such as nectar collection, pollen flow, and colony status, and automatically control the climate, pests, and other conditions [
115].
4. Status of Mechanized Pollination Technology
4.1. Principle of Mechanized Pollination
When there is an unfavorable natural pollination environment, or crops cannot attract pollinating insects, or with staggered flowering periods of male and female flowers, natural wind pollination and bee pollination cannot occur, and artificial assisted pollination is required. Therefore, labor-saving and efficient mechanized pollination has attracted increasing attention.
Mechanized pollination refers to using mechanical equipment to shed crop pollen and transport it to the pistil stigma. For crops with staggered flowering periods of male and female flowers, a common method is to collect the pollen first and then spray or atomize the target pistil. Various methods are available, including mechanical collision, ultrasonic, and pneumatic assistance.
4.2. Mechanized Pollination Technology and Equipment for Field Crops
Mechanized pollination of field crops usually involves pneumatic-assisted pollination to create pollination conditions similar to natural wind pollination. Wang et al. [
116] studied the effect of airflow speed on the pollination of hybrid rice; they suggested that pneumatic-assisted pollination mainly relies on two processes (air flow directly blowing out the pollen and airflow making the plant vibrate) that cause pollen to shed and be transported. Their results showed that airflow speed significantly affects pollen distribution and that horizontal distribution, vertical distribution, and total pollen increase with airflow speed. The horizontal distribution has a unique bimodal image, and the later peak moves away from the pollen source with increasing flow velocity. The vertical distribution moves closer to the airflow center with increasing flow velocity.
Li et al. [
117] studied the influence of airflow position on the pollination effect in
hybrid rice. The results showed that the airflow position significantly affected the horizontal and vertical distribution of pollen but had little effect on the pollen distribution distance. When the air flow center acted on the tassel (100/200 mm below the top of the tassel), the amount of pollen spread was greater, and the horizontal and vertical distributions increased significantly, which is beneficial to the distribution along the airflow direction. Wang et al. [
118] studied the effects of airflow velocity, action position, and action-angle on pollination in hybrid rice. The combination of parameters achieving the best pollination effect was an airflow velocity of 21.98 m/s, an action position of 16.85 cm below spike top, and an action-angle of 0°.
In the United States, small single-rotor helicopters are used to pollinate
hybrid rice. The downwash of a rotorcraft flying above the parent plants blows pollen from the male parents to the stigma of the female parent. Experimental research by RiceTec Co., Ltd. showed that such cross-pollination operations require the male parent pollen to be blown a distance of 6–12 m, which requires the pollination aircraft to maintain a difficult low-altitude flight. Therefore, Floyd Vuncannon Command Company specifically provides a suitable aircraft-based rice pollination service for Rice Tec Company [
119].
As shown in
Figure 20A, Li et al. [
120,
121,
122] measured and analyzed the three-way wind field of the rice canopy during pneumatic-assisted pollination of different types of rotorcrafts and optimized operating parameters such as flight speed and flight height. Li et al. [
123] proposed a combined pollination method using pneumatic-mechanical collision to improve the pollination effect. Compared with a single pollination method, the effective distribution area of pollen was significantly improved, the unevenness of pollen distribution was significantly reduced and its spatial transmission performance was improved. As shown in
Figure 20B, Zhang et al. [
124] showed that the downwash flow field of the quadrotor assisted the pollen dispersal of hybrid rapeseed, confirming the feasibility of using rotorcraft in the pollination operation of hybrid rape.
For field crops such as corn, castration is an integral part of the pollination operation and can ensure yield increases; for other hybrid crops, castration is also a necessary part of field management. In developed countries, such as in Europe and the United States, corn castrating machines are widely used, for example, the Hagie 204 (Hagie Inc., Clarion, PA, USA) castrating machine [
125]. As shown in
Figure 21, China Agricultural University designed a self-adaptive castration operation control system for corn ear height suitable for the current corn castration conditions in China. The castration efficiency is 18 times higher than manual operation, the castration rate is 96.16%, and the castration error is less than 5% [
126].
4.3. Mechanized Pollination Technology and Equipment for Orchards
Compared with field crops, the canopy of orchard crops is large and dense, and the planting row spacing is wide. As such, it is unreasonable to rely on rotorcraft to create wind-pollinated pollination conditions; moreover, different types of fruit trees are planted in the same orchard to ensure the fruit setting rate, but the flowering period of various fruit tree varieties is not synchronized, which increases the difficulty of pollination [
127]. Cross-pollinated orchard crops represented by apples, pears, and kiwi fruit have a large amount of pollen that can be collected and stored in advance, and therefore, air-blown or atomized pollination is now commonly used. This also overcomes the problem that the flowering periods of various fruit tree varieties are not synchronized [
128,
129,
130].
For cross-pollinated orchard crops, the efficient collection of high-quality pollen is the basis of their mechanized pollination. The quality varies with species, climatic conditions during flowering, flower maturity, and agronomic practices [
131,
132,
133,
134]. The pollen germination rate is an important index for measuring the quality of pollen, which determines the amount of pollen sprayed by mechanized pollination [
135]. The germination rate of some commercial kiwi pollen exceeds 80%. Some researchers tried setting a device at the hive entrance to obtain pollen collected by bees, but the quality of the pollen processed and collected by some varieties of bees was not ideal, which affected the success rate of crop pollination [
136]. Mechanized pollen collection is suitable for cross-pollinated crops with a large amount of pollen. A backpack type pollen vacuum cleaner can collect over 500 cc/h [
137]. For crops in which it is difficult to obtain pollen mechanically, manual pollen collection is still the main method [
138]. In 2018, the cost of kiwi pollen in New Zealand was USD 3452/kg, and the recommended application rate is 300–400 g/hm
2. The lower storage temperature and ideal drying conditions affect the pollen life [
139]. Pollen cells with a high water content cannot survive in frozen storage, which may be due to the formation of lethal intracellular ice and subsequent membrane rupture [
140].
Pan et al. [
141,
142] compared the effects of different pollination methods on the pollination of kiwi fruit and verified the feasibility of air-spraying and atomization spraying. As shown in
Figure 22A, Ding et al. [
143,
144] developed a hand-held air-powered duster. The experimental results showed that the vertical distribution of pollen and the amount of dusting were proportional to the outlet wind speed; the peak values of the horizontal distribution and vertical distribution decreased with increasing wind speed, and the peak values of the vertical distribution remained stable within a certain distance from the outlet. With increasing outlet wind speed, the pollen coverage rate on the stigma increased. When the outlet wind speed reached 19.5 m/s, the pollen coverage rate on flower stigmas was 57.66% on average. Michael et al. [
145] used CFD to simulate the air mobility of air-blown powder spraying; the results showed that the pollination efficiency from the front of the flowers was the highest. Vaknin et al. [
146] increased the stigma pollen adhesion of bitter almonds by adding static electricity to pollen; the pollen adhesion and yield increased by 16.2% and 11.3%, respectively.
As shown in
Figure 22B,C, companies in New Zealand have developed a mobile
kiwi fruit air-delivered powder sprayer and an atomizing sprayer, which have been applied for large-scale mechanized pollination; the working efficiency is approximately 0.75 mu/h, and the pollen consumption does not exceed 900 g/hm
2 [
147,
148]. As shown in
Figure 22D, the 2BeTM mobile air-delivered powder sprayer developed by Edete uses light detection and ranging (LiDAR) technology to obtain the outline of the fruit canopy and integrates electrostatic deposition technology to achieve rapid coverage and pollination of the fruit tree canopy flowers [
149]. As shown in
Figure 22E, Dropcopter has developed a pollen spraying device mounted on a rotary-wing UAV, which can perform autonomous and efficient pollination operations according to a planned flight path [
150]. It is still unclear whether the pneumatic powder spraying technology or the atomized powder spraying technology is superior. Because of the poor drift resistance and the high powder consumption of the air-blown powder spraying, atomized powder spraying has higher requirements for the formulation of the stabilizer and the spraying timeliness.
4.4. Mechanized Pollination Technology and Equipment for Greenhouses
Compared with fields and orchards, the space in greenhouses is confined and narrow; as such, pollination equipment requires miniaturization. For greenhouse
strawberries, Hu et al. [
151] proposed first covering strawberry flowers with pollen and then using a rotating brush to gently shake the flowers to form a breeze, causing the flower to flutter in the pollen cover to complete the pollination work. Wu et al. [
152] proposed a method for pollination of greenhouse crops with pneumatic tilting, which involves an automatic cross-pollination mobile operation consisting of the staggered blowing of double-row crops with pneumatic tilting, adaptive humidifying of the first row powder, and then heating and blowing powder. As shown in
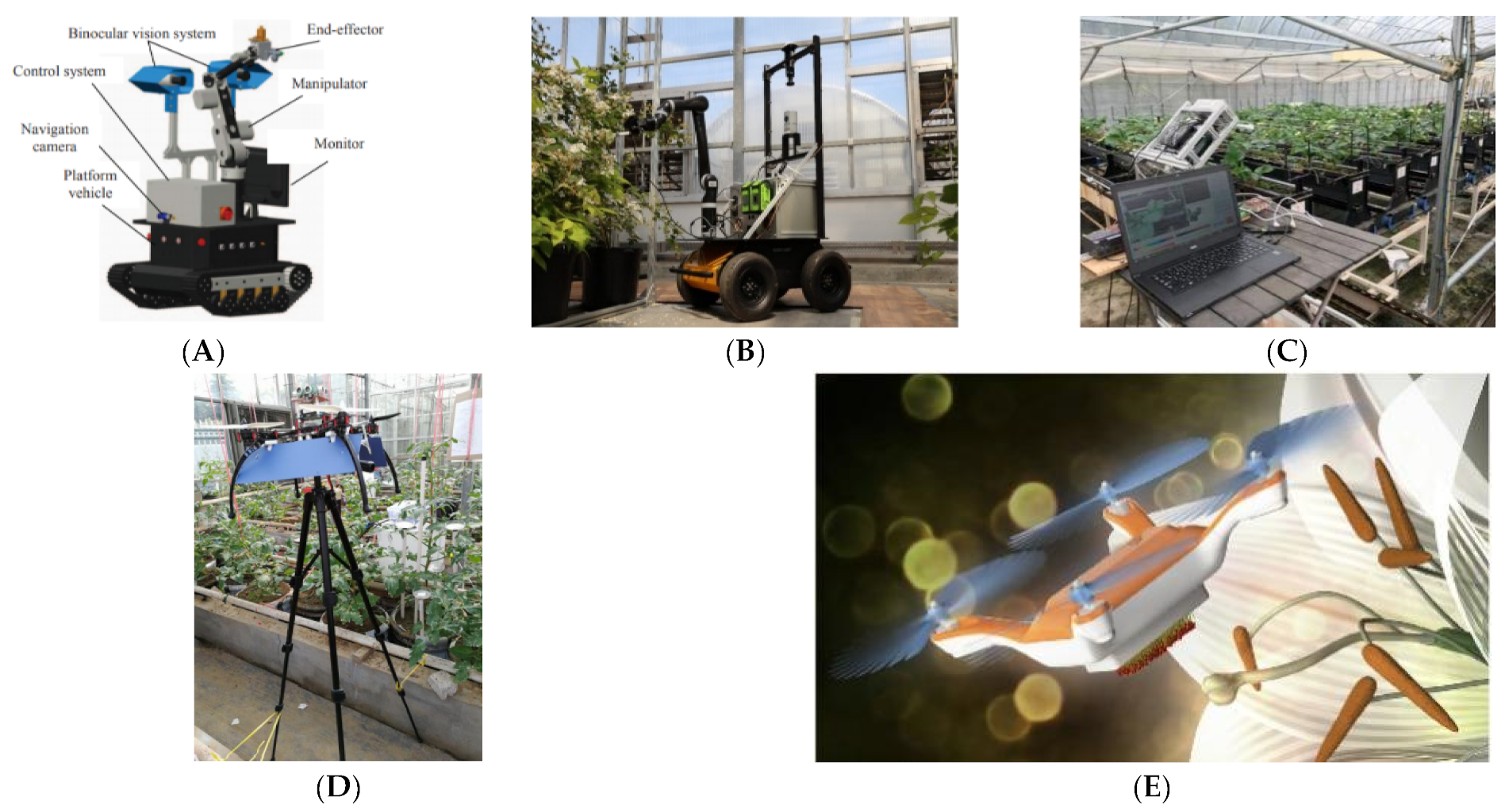
Figure 23A, Ting et al. [
153] developed a target hormone pollination robot based on binocular vision automatic recognition of mature inflorescences for greenhouse tomatoes. The target accuracy is 6.4 mm, the spraying success rate is 69.6%, and the operation time per plant is 15 s. As shown in
Figure 23B, West Virginia University designed an autonomous and precise pollination robot for greenhouse blackberries called the Bramble Bee, which uses RGB-D cameras to obtain information about the greenhouse and the plant flower clusters; it then analyzes the parameters of the pollination operation and pollinates through cotton brushing [
154]. As shown in
Figure 23C, Kyoto University proposed a technical solution for an ultrasonic pressure radiation type pollinator. From the identification and positioning of strawberry flowers based on a 3D camera, the inflorescence was pollinated by ultrasonic vibration, and the experiment preliminarily confirmed its feasibility [
155,
156].
As shown in
Figure 22D, in their preliminary study using drones for greenhouse pollination, Shi et al. [
157] installed a deflector on a small multi-rotor UAV to guide the down-rotating airflow of the UAV to the front and test the pneumatic-assisted pollination of tomatoes. As shown in
Figure 23E, Chechetka et al. [
158] imitated the pollination principle of bees and designed and completed an indoor experiment on lilies by combining a micro drone and new composite stucco material.
5. Conclusions
To ensure safe and efficient pollination of crops, bee pollination and mechanized pollination are critical reserve technologies. The selection of pollination method should be based on the physiological characteristics and actual natural pollination environmental conditions of crops. For field and orchard crops that require self-pollination, the natural wind medium can meet pollination needs; for field and orchard crops that require cross-pollination, and for crops in closed and windless greenhouses, bees are suitable for pollination. When the climatic environment restricts the use of natural wind and bee pollination, it needs to be supplemented by mechanized pollination. Even if there is an excellent natural pollination environment, bee pollination and mechanized pollination can still be reasonably utilized for improved pollination, with economic benefits being the leading indicators that need to be considered.
The pollination ability of bees is closely related to the status of bees. Maintaining the pollination ability of bees in a reasonable range is the goal of bee pollination services. Currently, there is no effective model that can reasonably incorporate the influence of various factors (including climate outside the hive, temperature, and humidity inside the hive, and the self-regulation mechanism of the hive) to accurately estimate the state of the hive. As such, colony state control technology needs to develop in two directions. First, there is a need to develop colony state monitoring technology based on multi-feature information fusion and to explore the self-regulation mechanisms of the colony in response to various factors. Second, based on the self-regulation mechanisms of bee colonies in response to various factors, there is a need to develop low-cost and non-invasive bee colony state and pollination capacity estimation models, monitoring technology, and equipment based on single feature information.
The goals of mechanized pollination are “efficiency” and “precision”. Different operation scenarios and crops rely on different mobile carriers, execution devices, and technologies for mechanized pollination. Mechanized pollination technology must be developed in two directions. First, the mechanisms of abscission, transport, and sedimentation of pollen should be explored in different crops and mechanized pollination conditions. Second, the research and development of efficient and accurate pollination equipment and technology based on the integration of multiple technologies such as pneumatic assistance, auxiliaries, static electricity, target, variables, and navigation are needed.
Efficient pollination of agricultural crops should not be regarded as an isolated subject that requires substantial investment. Instead, pollination is an essential link in crop production and management, and it should be included in the concept and application systems of common “smart agriculture” methods. Efficient crop pollination requires the acquisition of information from the massive data generated by “smart agriculture” to guide efficient and accurate operation. Moreover, understanding the feedback of the pollination effect will provide important dimensional information for “smart agriculture”.
Author Contributions
S.W.; writing original draft preparation, J.L. (Jizhan Liu); funding acquisition, X.L.; language proofreading, S.Z., J.L. (Jiajun Lu), Y.J., B.X. and M.W.; reference collection; All authors contributed to the article. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Jiangsu Provincial Key Research and Development Program (BE2020383) and the Changzhou Science and Technology Bureau (CE20202014).
Acknowledgments
The authors thank the editor and reviewers of the journal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ollerton, J.; Winfree, R.; Tarrant, S. How many flowering plants are pollinated by animals? Oikos 2011, 120, 321–326. [Google Scholar] [CrossRef]
- Klein, A.-M.; Vaissiere, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B-Biol. Sci. 2007, 274, 303–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissiere, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Kjøhl, M.; Nielsen, A.; Stenseth, N.C. Potential Effects of Climate Change on Crop Pollination; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Connolly, C. The risk of insecticides to pollinating insects. Commun. Integr. Biol. 2013, 6, e25074. [Google Scholar] [CrossRef] [PubMed]
- Maes, J.; Hauck, J.; Paracchini, M.L.; Ratamaki, O.; Hutchins, M.; Termansen, M.; Furman, E.; Perez-Soba, M.; Braat, L.; Bidoglio, G. Mainstreaming ecosystem services into EU policy. Curr. Opin. Environ. Sustain. 2013, 5, 128–134. [Google Scholar] [CrossRef]
- FitzPatrick, Ú.; Stout, J.; Bertrand, C.; Bradley, K.; Clabby, G.; Keena, C.; Walsh, J. All-Ireland Pollinator Plan 2015–2020; National Biodiversity Data Centre: Waterford, Ireland, 2015. [Google Scholar]
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B-Biol. Sci. 2018, 285, 20172140. [Google Scholar] [CrossRef] [Green Version]
- Binns, C. Robotic Insects Could Pollinate Flowers and Find Disaster Victims Popular Science USA. 2009. Available online: https://www.popsci.com/technology/article/2009-12/flight-robobee/ (accessed on 10 November 2022).
- Leins, P.; Erbar, C. Flower and fruit. Hoppea 2010, 71, 354–355. [Google Scholar]
- Brown, A.H.D.; Zohary, D.; Nevo, E. Outcrossing rates and heterozygosity in natural populations of Hordeum spontaneum Koch in Israel. Heredity 1978, 41, 49–62. [Google Scholar] [CrossRef] [Green Version]
- Vuletin Selak, G.; Perica, S.; Ban Goreta, S.; Radunic, M.; Poljak, M. Reproductive Success after Self-pollination and Cross-pollination of Olive Cultivars in Croatia. HortScience 2011, 46, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Chavez, J.D.; Lyrene, M.P. Effects of Self-pollination and Cross-pollination of Vaccinium darrowii (Ericaceae) and Other Low-chill Blueberries. HortScience 2009, 44, 1538–1541. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, D.G.; Webb, C.J. The avoidance of interference between the presentation of pollen and stigmas in angiosperms I. Dichogamy. N. Z. J. Bot. 2011, 24, 135–162. [Google Scholar] [CrossRef]
- Webb, C.J.; Lloyd, D.G. The avoidance of interference between the presentation of pollen and stigmas in angiosperms II. Herkogamy. N. Z. J. Bot. 1986, 24, 163–178. [Google Scholar] [CrossRef]
- Trelease, W. The Heterogony of Oxalis violacea. Am. Nat. 1882, 16, 13–19. [Google Scholar] [CrossRef]
- East, E.M. The distribution of self-sterility in the flowering plants. Proc. Am. Philos. Soc. 1940, 82, 449–518. [Google Scholar]
- Lord, E.M. Cleistogamy: A Tool for the Study of Floral Morphogenesis, Function and Evolution. Bot. Rev. 1981, 47, 421–449. [Google Scholar] [CrossRef]
- Kumar, S.; Rao, M.; Gupta, N.C. Breeding Strategies of Self Pollinated Crop with Special Emphasis on Hybrid Rice: Present and Future Perspectives. Res. Rev. J. Agric. Sci. Technol. 2014, 3, 2349–3682. [Google Scholar]
- Dicenta, F.; Ortega, E.; Canovas, J.A.; Egea, J. Self-pollination vs. cross-pollination in almond: Pollen tube growth, fruit set and fruit characteristics. Plant Breed. 2002, 121, 163–167. [Google Scholar] [CrossRef]
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Johansson, K.T.S.; Johansson, M.P. Langstroth and the Bee Space. Bee World 1967, 48, 133–143. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Aizen, M.A.; Harder, L.D. The Global Stock of Domesticated Honey Bees Is Growing Slower Than Agricultural Demand for Pollination. Curr. Biol. 2009, 19, 915–918. [Google Scholar] [CrossRef] [Green Version]
- Osterman, J.; Aizen, M.A.; Biesmeijer, J.C.; Bosch, J.; Howlett, B.G.; Inouye, D.W.; Jung, C.; Martins, D.J.; Medel, R.; Pauw, A. Global trends in the number and diversity of managed pollinator species. Agric. Ecosyst. Environ. 2021, 322, 107653. [Google Scholar] [CrossRef]
- Zhigang, L.; Hongsong, R.; Guangyu, F.; Aihemaiti, M.; Hu Xidan, M.; Kurban, A.; Ruihua, W.; Hongmei, G.; Jian, W.; Haifeng, L. Effect of Bumblebee Pollination on Yield and Quality of Greenhouse Tomato; Jiangsu Academy of Agricultural Sciences: Nanjing, China, 2021. [Google Scholar]
- Hongdong, W.; Shuang, H.; Bing, H.; Mingfei, W.; Guanxiong, Z.; Donggang, L. Research progress of bumblebee pollination technology in protected agriculture. Yangtze River Veg. 2022, 8, 34–37. [Google Scholar]
- Joshi, N.C.; Joshi, P.C. Foraging Behaviour of Apis spp. on Apple Flowers in a Subtropical Environment. N. Y. Sci. J. 2010, 3, 71–76. [Google Scholar]
- Blazyte-Cereskiene, L.; Vaitkeviciene, G.; Venskutonyte, S.; Buda, V. Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirb. Agric. 2010, 97, 61–70. [Google Scholar]
- Abrol, D.P. Diversity of pollinating insects visiting litchi flowers (Litchi chinensis Sonn.) and path analysis of environmental factors influencing foraging behaviour of four honeybee species. J. Apic. Res. 2006, 45, 180–187. [Google Scholar] [CrossRef]
- Peat, J.; Goulson, D. Effects of experience and weather on foraging rate and pollen versus nectar collection in the bumblebee, Bombus terrestris. Behav. Ecol. Sociobiol. 2005, 58, 152–156. [Google Scholar] [CrossRef]
- Clarke, D.; Robert, D. Predictive modelling of honey bee foraging activity using local weather conditions. Apidologie 2018, 49, 386–396. [Google Scholar] [CrossRef] [Green Version]
- Tan, K.; Yang, S.; Wang, Z.-W.; Radloff, S.E.; Oldroyd, B.P. Differences in foraging and broodnest temperature in the honey bees Apis cerana and A-mellifera. Apidologie 2012, 43, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Woyke, J.; Wilde, J.; Wilde, M. Flight activity reaction to temperature changes in Apis dorsata, Apis laboriosa and Apis mellifera. J. Apic. Sci. 2003, 47, 73–80. [Google Scholar]
- Begna, T.; Ulziibayar, D.; Noor-ul-Ane, M.; Shin, J.H.; Jung, C. Offering Pollen as Reward Enhances Foraging Activity of Honey Bee, Apis mellifera on Strawberry Greenhouse during Winter Season. J. Apic. 2020, 35, 111–118. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lim, J.H.; Ko, H.; Ko, H.-J. Effect of Climatic Conditions on Pollination Behavior of Honeybees (Apis mellifera L.) in the Greenhouse Cultivation of Watermelon (Citrullus lanatus L.). J. Apic. 2018, 33, 239–250. [Google Scholar] [CrossRef]
- Kovac, H.; Stabentheiner, A. Thermoregulation of foraging honeybees on flowering plants: Seasonal variability and influence of radiative heat gain. Ecol. Entomol. 2011, 36, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Stabentheiner, A.; Kovac, H. Honeybee economics: Optimisation of foraging in a variable world. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, B. Mechanisms of body temperature regulation in honeybees, Apis mellifera. I. Regulation of Head Temperature. J. Exp. Biol. 1980, 85, 61–72. [Google Scholar] [CrossRef]
- Heinrich, B. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. II. Regulation of thoracic temperature at high air temperatures. J. Exp. Biol. 1980, 85, 73–87. [Google Scholar] [CrossRef]
- Attar, I.; Farhat, A. Efficiency evaluation of a solar water heating system applied to the greenhouse climate. Sol. Energy 2015, 119, 212–224. [Google Scholar] [CrossRef]
- Vadiee, A.; Martin, V. Thermal energy storage strategies for effective closed greenhouse design. Appl. Energy 2013, 109, 337–343. [Google Scholar] [CrossRef]
- Ghoulem, M.; El Moueddeb, K.; Nehdi, E.; Boukhanouf, R.; Calautit, J.K. Greenhouse design and cooling technologies for sustainable food cultivation in hot climates: Review of current practice and future status. Biosyst. Eng. 2019, 183, 121–150. [Google Scholar] [CrossRef]
- Golzar, F.; Heeren, N.; Hellweg, S.; Roshandel, R. A novel integrated framework to evaluate greenhouse energy demand and crop yield production. Renew. Sustain. Energy Rev. 2018, 96, 487–501. [Google Scholar] [CrossRef]
- Iddio, E.; Wang, L.; Thomas, Y.; McMorrow, G.; Denzer, A. Energy efficient operation and modeling for greenhouses: A literature review. Renew. Sustain. Energy Rev. 2020, 117, 109480. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, S.; Jing, S.U.; Xue, F.; Jiang, T. Effects of temperature on the developments of honeybee oosperms and queen pupae. J. Fujian Agric. Univ. 2002, 31, 511–513. [Google Scholar]
- Zhu, X.; Zhou, B.; Luo, Q.; Luo, F.; Zhong, X.; Shi, M. The effect of temperature on the development of worker honey bee during sealed brood period. New Agric. Technol. 2006, 7, 57–60. [Google Scholar]
- Tautz, J.; Maier, S.; Groh, C.; Rossler, W.; Brockmann, A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc. Natl. Acad. Sci. USA 2003, 100, 7343–7347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groh, C.; Tautz, J.; Rossler, W. Synaptic organization in the adult honey bee brain is influenced by brood-temperature control during pupal development. Proc. Natl. Acad. Sci. USA 2004, 101, 4268–4273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, M.A.; Scharpenberg, H.; Moritz, R.F.A. Pupal developmental temperature and behavioral specialization of honeybee workers (Apis mellifera L.). J. Comp. Physiol. A-Neuroethol. Sens. Neural Behav. Physiol. 2009, 195, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Doull, K.M. The effects of different humidities on the hatching of the eggs of honeybees. Apidologie 1976, 7, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Woodrow, W.A. Some Effects of Relative Humidity on the Length of Life and Food Consumption of Honeybees. J. Econ. Entomol. 1935, 28, 565–568. [Google Scholar] [CrossRef]
- Jain, R.; Brockmann, A. Sex-specific molecular specialization and activity rhythm-dependent gene expression in honey bee antennae. J. Exp. Biol. 2020, 223, jeb217406. [Google Scholar] [CrossRef]
- Lacher, V. Elektrophysiologische Untersuchungen an einzelnen Rezeptoren für Geruch, Kohlendioxyd, Luftfeuchtigkeit und Tempratur auf den Antennen der Arbeitsbiene und der Drohne (Apis mellifica L.). Z. Vgl. Physiol. 1964, 48, 587–623. [Google Scholar] [CrossRef]
- Koeniger, N.; Veith, H.J. Glyceryl-1,2-dioleate-3-palmitate, a brood pheromone of the honey bee (Apis mellifera L.). Experientia 1983, 39, 1051–1052. [Google Scholar] [CrossRef]
- Bujok, B.; Kleinhenz, M.; Fuchs, S.; Tautz, J. Hot spots in the bee hive. Naturwissenschaften 2002, 89, 299–301. [Google Scholar] [CrossRef]
- Kleinhenz, M.; Bujok, B.; Fuchs, S.; Tautz, H. Hot bees in empty broodnest cells: Heating from within. J. Exp. Biol. 2003, 206, 4217–4231. [Google Scholar] [CrossRef] [Green Version]
- Kronenberg, F.; Heller, H.C. Colonial thermoregulation in honey bees (Apis mellifera). J. Comp. Physiol. B 1982, 148, 65–76. [Google Scholar] [CrossRef]
- Gloria, D.-H.; Marla, S.; Martin, J.H. Role of Thermoregulation by Nestmates on the Development Time of Honey Bee (Hymenoptera: Apidae) Queens. Ann. Entomol. Soc. Am. 1993, 86, 165–172. [Google Scholar] [CrossRef]
- Michel, C.; Fuchs, S.; Heldmaier, G. Regulation of temperature in worker bees of 4 lines from different races. Apidologie 1995. [Google Scholar]
- Southwick, E.E. HOGG. Temperature Control in Honey Bee Colonies. BioScience 1987, 37, 395–399. [Google Scholar] [CrossRef]
- Kridi, D.S.; de Carvalho, C.G.N.; Gomes, D.G. Application of wireless sensor networks for beehive monitoring and in-hive thermal patterns detection. Comput. Electron. Agric. 2016, 127, 221–235. [Google Scholar] [CrossRef]
- Becher, M.A.; Moritz, R.F.A. A new device for continuous temperature measurement in brood cells of honeybees (Apis mellifera). Apidologie 2009, 40, 577–584. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Pan, W.; Jiang, Z.H.; Yu, L.S. Design and experiment of the temperature acquisition system for the full beehive. J. Hunan Agric. Univ. (Nat. Sci.) 2016, 42, 460–464. [Google Scholar] [CrossRef]
- Szabo, T.I. Thermology of wintering honey-bee colonies in 4-colony packs. 3. Cluster positions. Am. Bee J. 1989, 3, 552–553. [Google Scholar]
- Hannelie, H.; W, N.S.; Vincent, D. Do honeybees, Apis mellifera scutellata, regulate humidity in their nest? Naturwissenschaften 2006, 93, 397–401. [Google Scholar] [CrossRef]
- Evans, S.K. Electronic beehive monitoring—Applications to research. In Hazards of Pesticides to Bees, Proceedings of the 12th International Symposium of the ICP-PR Bee Protection Group, Ghent, Belgium, 15–17 September 2014; Ghent University, Faculty of Bioscience Engineering: Ghent, Belgium, 2014; pp. 121–129. [Google Scholar]
- Xiang, L.; Zhao-Hui, J.; Yuan-Zhou, L.; Wei, P.; Lin-Sheng, Y. Temperature monitoring and analysis system ofbeehive based on micro-sensor array. Transducer Microsyst. Technol. 2015, 34, 63–65+68. [Google Scholar] [CrossRef]
- Meitalovs, J.; Histjajevs, A.; Stalidzans, E. Automatic microclimate controlled beehive observation system. In Proceedings of the 8th International Scientific Conference on Engineering for Rural Development, Jelgava, Latvia, 28–29 May 2009; pp. 265–271. [Google Scholar]
- Edmund, C.; Rahman, M.A. Smart Stingless Beehive Monitoring System. In Computational Science and Technology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 537–549. [Google Scholar]
- Knauer, U.; Himmelsbach, M.; Winkler, F.; Zautke, F.; Meffert, B. Application of an Adaptive Background Model for Monitoring Honeybees. 2005. Available online: https://d1wqtxts1xzle7.cloudfront.net/47142109/knauer05a-libre.pdf?1468265240=&response-content-disposition=inline%3B+filename%3DApplication_of_an_Adaptive_Background_Mo.pdf&Expires=1668173789&Signature=Rs1U1PET2bx~-DQ1KJQFrEGe~lpTfDKYwSlzWBkidI~sArkWjrxet7CLzaqPazp-p2Z~3v60bAYLkx3NMzupoklUHdvM5VZu-KU7q74Jrf0xalznSmp7-gtzKbxoE7iVBbwiBdCgIJiAahlqUHc~zHSTVrCacnHKpJOfVnjjROxqBqN3DptnC2cLtqVF4S1rM6LFLZ~lQ0lj4Aoo2NL0pCl2d~Gbw9gVdSTZcM36ZFYqQYMfTqBKoxaGVR8E8B8su2lg7CCwt-7P7KPolpvLf6APOt-lfFj7RawkAhys0Jm0yM4KM~jUs3hLXp72B1Bxap~dUvqUlYCAl~oQMAOvpv1Q__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 10 November 2022).
- Chazette, L.; Becker, M.; Szczerbicka, H.; IEEE. Basic Algorithms for Bee Hive Monitoring and Laser-based Mite Control. In Proceedings of the IEEE Symposium Series on Computational Intelligence (IEEE SSCI), Athens, Greece, 6–9 December 2016.
- Kimura, T.; Ohashi, M.; Okada, R.; Ikeno, H. A new approach for the simultaneous tracking of multiple honeybees for analysis of hive behavior. Apidologie 2011, 42, 607–617. [Google Scholar] [CrossRef] [Green Version]
- Feldman, A.; Balch, T. Automatic Identification of Bee Movement Using Human Trainable Models of Behavior. 2003. Available online: https://sites.cc.gatech.edu/~borg/biotracking/pubs/FeldmanMASI2003.pdf (accessed on 10 November 2022).
- Maitra, P.; Schneider, S.; Shin, M.C. Robust bee tracking with adaptive appearance template and geometry-constrained resampling. In Proceedings of the 2009 Workshop on Applications of Computer Vision (WACV), Snowbird, UT, USA, 7–8 December 2009; pp. 1–6. [Google Scholar] [CrossRef]
- Luengo Hendriks, C.; Yu, Z.; Lecocq, A.; Bakker, T.; Locke, B.; Terenius, O. Identifying all individuals in a honeybee hive: Progress towards mapping all social interactions. In Proceedings of the 21st International Conference on Pattern Recognition, Tsukuba, Japan, 11–15 November 2012; pp. 5–8. [Google Scholar]
- Tsai, C.; Ngo, T.; Yang, E.; Lin, T. Image processing algorithms of tracking and movement pattern analysis for honeybees in a beehive. In Proceedings of the CIGR-AgEng Conference, Aarhus, Denmark, 26–29 June 2016; Abstracts and Full Papers. pp. 1–8. [Google Scholar]
- Wild, B.; Sixt, L.; Landgraf, T. Automatic localization and decoding of honeybee markers using deep convolutional neural networks. arXiv 2018, arXiv:1802.04557. [Google Scholar]
- Kulyukin, V.A.; Putnam, M.; Reka, S.K. Digitizing buzzing signals into A440 piano note sequences and estimating forager traffic levels from images in solar-powered, electronic beehive monitoring. In Proceedings of the International MultiConference of Engineers and Computer Scientists, Hong Kong, China, 16–18 March 2016; Volume 1, pp. 82–87. [Google Scholar]
- Kulyukin, V.A.; Reka, S.K. Toward Sustainable Electronic Beehive Monitoring: Algorithms for Omnidirectional Bee Counting from Images and Harmonic Analysis of Buzzing Signals. Eng. Lett. 2016, 24, 72–82. [Google Scholar]
- Kulyukin, V.; Reka, S.K. A Computer Vision Algorithm for Omnidirectional Bee Counting at Langstroth Beehive Entrances. In Proceedings of the International Conference on Image Processing, Computer Vision, & Pattern Recognition, Las Vegas, NV, USA, 27–30 June 2016; pp. 229–234. [Google Scholar]
- Mezquida, D.A.; Martínez, J.L. Platform for bee-hives monitoring based on sound analysis. A perpetual warehouse for swarm apos; s daily activity. Span. J. Agric. Res. 2009, 7, 824–828. [Google Scholar] [CrossRef]
- Ferrari, S.; Silva, M.; Guarino, M.; Berckmans, D. Monitoring of swarming sounds in bee hives for early detection of the swarming period. Comput. Electron. Agric. 2008, 64, 72–77. [Google Scholar] [CrossRef]
- Rangel, J.; Seeley, T.D. The signals initiating the mass exodus of a honeybee swarm from its nest. Anim. Behav. 2008, 76, 1943–1952. [Google Scholar] [CrossRef]
- Yibo, L.; Zhijiang, Z. Automatic detection of queen bees in colonies using sound analysis technology. J. Bee 2018, 38, 3. [Google Scholar]
- Qandour, A.; Ahmad, I.; Habibi, D.; Leppard, M. Remote Beehive Monitoring Using Acoustic Signals. Acoust. Aust. 2014, 42, 204–209. [Google Scholar]
- Kulyukin, V.; Mukherjee, S.; Amlathe, P. Toward Audio Beehive Monitoring: Deep Learning vs. Standard Machine Learning in Classifying Beehive Audio Samples. Appl. Sci. 2018, 8, 1573. [Google Scholar] [CrossRef] [Green Version]
- Amlathe, P. Standard Machine Learning Techniques in Audio Beehive Monitoring: Classification of Audio Samples with Logistic Regression, K-Nearest Neighbor, Random Forest and Support Vector Machine. Master’s Thesis, Utah State University, Logan, UT, USA, 2018. [Google Scholar]
- Michael, R.; Martin, B.; Newton, M.I.; Wulfila, G. Long-term trends in the honeybee ‘whooping signal’ revealed by automated detection. PLoS ONE 2017, 12, e0171162. [Google Scholar] [CrossRef] [Green Version]
- Bencsik, M.; Bencsik, J.; Baxter, M.; Lucian, A.; Romieu, J.; Millet, M. Identification of the honey bee swarming process by analysing the time course of hive vibrations. Comput. Electron. Agric. 2011, 76, 44–50. [Google Scholar] [CrossRef]
- Fitzgerald, D.W.; Murphy, F.E.; Wright, W.M.D.; Whelan, P.M.; Popovici, E.M.; IEEE. Design and Development of a Smart Weighing Scale for Beehive Monitoring. In Proceedings of the 26th Irish Signals and Systems Conference (ISSC), Inst Technol Carlow, Carlow, Ireland, 24–25 June 2015; pp. 1–6. [Google Scholar]
- Meikle, W.G.; Rector, B.G.; Mercadier, G.; Holst, N. Within-day variation in continuous hive weight data as a measure of honey bee colony activity. Apidologie 2008, 39, 694–707. [Google Scholar] [CrossRef]
- Meikle, W.G.; Weiss, M.; Stilwell, A.R. Monitoring colony phenology using within-day variability in continuous weight and temperature of honey bee hives. Apidologie 2016, 47, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Meikle, W.G.; Holst, N.; Colin, T.; Weiss, M.; Carroll, M.J.; McFrederick, Q.S.; Barron, A.B. Using within-day hive weight changes to measure environmental effects on honey bee colonies. PLoS ONE 2018, 13, e0197589. [Google Scholar] [CrossRef]
- Schneider, C.W.; Tautz, J.; Gruenewald, B.; Fuchs, S. RFID Tracking of Sublethal Effects of Two Neonicotinoid Insecticides on the Foraging Behavior of Apis mellifera. PLoS ONE 2012, 7, e30023. [Google Scholar] [CrossRef] [Green Version]
- Henry, M.; Beguin, M.; Requier, F.; Rollin, O.; Odoux, J.-F.; Aupinel, P.; Aptel, J.; Tchamitchian, S.; Decourtye, A. A Common Pesticide Decreases Foraging Success and Survival in Honey Bees. Science 2012, 336, 348–350. [Google Scholar] [CrossRef]
- Campbell, J.M.; Dahn, D.C.; Ryan, D.A.J. Capacitance-based sensor for monitoring bees passing through a tunnel. Meas. Sci. Technol. 2005, 16, 2503–2510. [Google Scholar] [CrossRef]
- Jiang, J.-A.; Wang, C.-H.; Chen, C.-H.; Liao, M.-S.; Su, Y.-L.; Chen, W.-S.; Huang, C.-P.; Yang, E.-C.; Chuang, C.-L. A WSN-based automatic monitoring system for the foraging behavior of honey bees and environmental factors of beehives. Comput. Electron. Agric. 2016, 123, 304–318. [Google Scholar] [CrossRef]
- BeeHero. Available online: https://www.beehero.io/ (accessed on 10 August 2022).
- Tashakkori, R.; Kae, D.; Parry, R.M. Automated beehive surveillance using computer vision. In Proceedings of the IEEE Southeast Conference, Fort Lauderdale, FL, USA, 9–12 April 2015. [Google Scholar]
- Ghadiri, A. Implementation of an Automated Image Processing System for Observing the Activities of Honey Bees; Appalachian State University: Boone, NC, USA, 2013. [Google Scholar]
- Kulyukin, V.A. In situ omnidirectional vision-based bee counting using 1d haar wavelet spikes. In Proceedings of the International Multiconference of Engineers and Computer Scientists, Hong Kong, China, 15–17 March 2017; pp. 182–187. [Google Scholar]
- Campbell, J.; Mummert, L.; Sukthankar, R. Video monitoring of honey bee colonies at the hive entrance. Vis. Obs. Anal. Anim. Insect Behav. ICPR 2008, 8, 1–4. [Google Scholar]
- Salas, J.; Vera, P. Counting the Bumblebees Entering and Leaving a Beehive. In Proceedings of the Visual Observation and Analysis of Animal and Insect Behaviour, Tsukuba, Japan, 11 November 2012. [Google Scholar]
- Tu, G.J.; Hansen, M.K.; Kryger, P.; Ahrendt, P. Automatic behaviour analysis system for honeybees using computer vision. Comput. Electron. Agric. 2016, 122, 10–18. [Google Scholar] [CrossRef]
- Chen, C.; Yang, E.-C.; Jiang, J.-A.; Lin, T.-T. An imaging system for monitoring the in-and-out activity of honey bees. Comput. Electron. Agric. 2012, 89, 100–109. [Google Scholar] [CrossRef]
- Kulyukin, V.; Mukherjee, S. On Video Analysis of Omnidirectional Bee Traffic: Counting Bee Motions with Motion Detection and Image Classification. Appl. Sci. 2019, 9, 3743. [Google Scholar] [CrossRef] [Green Version]
- Thi Nha, N.; Wu, K.-C.; Yang, E.-C.; Lin, T.-T. A real-time imaging system for multiple honey bee tracking and activity monitoring. Comput. Electron. Agric. 2019, 163, 104841. [Google Scholar] [CrossRef]
- Murphy, F.E.; Magno, M.; O’Leary, L.; Troy, K.; Whelan, P.; Popovici, E.M.; IEEE. Big Brother for Bees (3B)—Energy Neutral Platform for Remote Monitoring of Beehive Imagery and Sound. In Proceedings of the 6th IEEE International Workshop on Advances in Sensors and Interfaces (IWASI), Gallipoli, Italy, 18–19 June 2015; pp. 106–111.
- Murphy, F.E.; Magno, M.; Whelan, P.; Popovici, E.; IEEE. B plus WSN: Smart Beehive for Agriculture, Environmental, and Honey Bee Health Monitoring—Preliminary Results and Analysis. In Proceedings of the IEEE Sensors Applications Symposium (SAS), Zadar, Croatia, 13–15 April 2015; pp. 125–130. [Google Scholar]
- HiveMind. Available online: http://hivemind.co.nz/ (accessed on 25 June 2022).
- Arnia. Available online: http://www.arnia.co.uk/ (accessed on 25 June 2022).
- ToBe. Available online: https://www.beewise.ag/ (accessed on 19 August 2022).
- Available online: https://developer.aliyun.com/article/808252 (accessed on 19 August 2022).
- Beewise. Available online: https://www.beewise.ag/#start-1/ (accessed on 19 August 2022).
- Huimin, W.; Chuzhou, T.; Ming, L.; Zhongqiu, L.; Zhen, H.; Mingliang, W.; Juying, H.; Haiqing, Z. Effect of airflow speed on pollens distribution for hybrid rice breeding pollination. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2012, 28, 63–69. [Google Scholar]
- Zhongqiu, L.; Chuzhou, T.; Huimin, W.; Ming, L.; Zhen, H.; Shilun, F. Effects of location of airflow on pollen distribution for pneumatic pollination in hybrid rice breeding. Editor. Off. Trans. Chin. Soc. Agric. Eng. 2012, 28, 107–113. [Google Scholar] [CrossRef]
- Huimin, W.; Chuzhou, T.; Zhongqiu, L.; Ming, L.; Zhen, H.; Shiluen, F. Experiment and optimization of parameters for pneumatic pollination in hybrid rice breeding. Editor. Off. Trans. Chin. Soc. Agric. Eng. 2012, 28, 101–106. [Google Scholar] [CrossRef]
- Chuzhou, T.; Huimin, W.; Ming, L.; Zhongqiu, L.; Zhen, H.; Haifeng, L.; Min, J.; Haiqing, Z. Study status and developmental strategies of mechanical pollination for hybrid rice breeding. Trans. CSAE 2012, 28, 1–7. [Google Scholar] [CrossRef]
- Jiyu, L.; Zhiyan, Z.; Lian, H.; Ying, Z.; Sai, X.; Aimin, L.; Xiwen, L.; Tiemin, Z. Optimization of operation parameters for supplementary pollination in hybrid rice breeding using round multi-axis multi-rotor electric unmanned helicopter. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2014, 11, 1–9. [Google Scholar] [CrossRef]
- Jiyu, L.; Zhiyan, Z.; Lian, H.; Ying, Z.; Menglu, Y.; Aimin, L.; Xiwen, L.; Tiemin, Z. Optimization of operation parameters for supplementary pollination in hybrid rice breeding using uniaxial single-rotor electric unmanned helicopter. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2014, 10, 10–17. [Google Scholar] [CrossRef]
- Li, J.; Lan, Y.; Wang, J.; Chen, S.; Huang, C.; Liu, Q.; Liang, Q. Distribution law of rice pollen in the wind field of small UAV. Int. J. Agric. Biol. Eng. 2017, 10, 32–40. [Google Scholar] [CrossRef]
- Zhongqiu, L.; Chuzhou, T.; Ming, L.; Haifeng, L. Pollen distribution of pneumatic and collision combined pollination forhybrid rice breeding. J. Hunan Agric. Univ. (Nat. Sci.) 2015, 41, 325–331. [Google Scholar] [CrossRef]
- Zhang, S.; Cai, C.; Li, J.; Sun, T.; Liu, X.; Tian, Y.; Xue, X. The Airflow Field Characteristics of the Unmanned Agricultural Aerial System on Oilseed Rape (Brassica napus) Canopy for Supplementary Pollination. Agronomy 2021, 11, 2035. [Google Scholar] [CrossRef]
- Yang, L.; Yaxiong, L.; Yongtao, L.; Bin, L.; Tao, W. Several foreign seed corn emasculation machines. Xinjiang Agric. Mech. 2010, 14. [Google Scholar] [CrossRef]
- Tao, X.; Bin, X.; Enrong, M.; Yuefeng, D. Design and experiment of emasculation control system of corn detasseling machine. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2015, 31, 49–54. [Google Scholar] [CrossRef]
- Sedgley, M.; Griffin, A.R. Sexual Reproduction of Tree Crops; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Pinillos, V.; Cuevas, J. Artificial pollination in tree crop production. Hortic. Rev. 2008, 34, 239–276. [Google Scholar]
- Shu, Q.; Liu, Z.; Zhang, J.; Yun, J. Effects of different pollinators on the pollination effect of kiwifruit. J. Zhejiang Agric. Sci. 2015, 56, 1416–1417. [Google Scholar] [CrossRef]
- Jianye, C.; Zhanhong, L.; Yuxia, N. Preparation method and biological effects of pollen suspension liquid forspraying pollination of Actinidia chinensis. J. Fruit Sci. 2014, 31, 1105–1109. [Google Scholar] [CrossRef]
- Cerović, R.; Fotirić Akšić, M.; Meland, M. Success Rate of Individual Pollinizers for the Pear Cultivars “Ingeborg” and “Celina” in a Nordic Climate. Agronomy 2020, 10, 970. [Google Scholar] [CrossRef]
- Santiago, J.P.; Sharkey, T.D. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant Cell Environ. 2019, 42, 2759–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, W.; Law, S.E.; McCoy, D.; Wetzstein, H.Y. Stigma development and receptivity in almond (Prunus dulcis). Ann. Bot. 2006, 97, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Mahamad, H.K.; Ali, I. Effects of fungicides on pollen germination peach and nectarine in vitro. Afr. J. Plant Sci. 2011, 5, 643–647. [Google Scholar]
- Ascari, L.; Guastella, D.; Sigwebela, M.; Engelbrecht, G.; Stubbs, O.; Hills, D.; De Gregorio, T.; Siniscalco, C. Artificial pollination on hazelnut in South Africa: Preliminary data and perspectives. In Proceedings of the IX International Congress on Hazelnut 1226, Samsun, Turkey, 15–18 August 2017; pp. 141–148. [Google Scholar]
- Parker, A.J.; Tran, J.L.; Ison, J.L.; Bai, J.D.K.; Weis, A.E.; Thomson, J.D. Pollen packing affects the function of pollen on corbiculate bees but not non-corbiculate bees. Arthropod-Plant Interact. 2015, 9, 197–203. [Google Scholar] [CrossRef]
- Wizenberg, S.B.; Weis, A.E.; Campbell, L.G. Comparing methods for controlled capture and quantification of pollen in Cannabis sativa. Appl. Plant Sci. 2020, 8, e11389. [Google Scholar] [CrossRef] [PubMed]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Effects of desiccation and dilution on germinability of almond pollen. J. Hortic. Sci. Biotechnol. 1999, 74, 321–327. [Google Scholar] [CrossRef]
- Buitink, J.; Claessens, M.M.; Hemminga, M.A.; Hoekstra, F.A. Influence of water content and temperature on molecular mobility and intracellular glasses in seeds and pollen. Plant Physiol. 1998, 118, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Payne, W.W. Structure and function in angiosperm pollen wall evolution. Rev. Palaeobot. Palynol. 1981, 35, 39–59. [Google Scholar] [CrossRef]
- Delin, P.; Jiyu, Z.; Jinghui, C.; Shengnan, H.; Gang, W.; Min, Z.; Zhongren, G. Effects of different pollination methods on the fruit setting rate of kiwifruit. South China Fruits 2017, 46, 120–122. [Google Scholar] [CrossRef]
- Razeto, B.; Reginato, G.; Larrain, A. Hand and machine pollination of kiwifruit. Int. J. Fruit Sci. 2005, 5, 37–44. [Google Scholar] [CrossRef]
- Ding, S.; Xue, X.; Cai, C.; Qin, W.; Fang, J.; Zhu, S. Design and experiment on handheld air-assisted pollination device. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2014, 30, 20–27. [Google Scholar] [CrossRef]
- Suming, D.; Xinyu, X.; Jinbao, F.; Zhu, S.; Chen, C.; Liangfu, Z.; Weicai, Q. Parameter optimization and experiment of air-assisted pollination device. Trans. Chin. Soc. Agric. Eng. (Trans. CSAE) 2015, 31, 68–75. [Google Scholar]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Improving pollination of almond (Amygdalus communis L., Rosaceae) using electrostatic techniques. J. Hortic. Sci. Biotechnol. 2001, 76, 208–212. [Google Scholar] [CrossRef]
- Vaknin, Y.; Gan-Mor, S.; Bechar, A.; Ronen, B.; Eisikowitch, D. Electrostatic pollination of pistachio (Pistacia vera L.): A novel technique of pollen supplementation in agriculture. Cah. Options Mediterr. 2001, 56, 53–57. [Google Scholar]
- Kempe, K.; Gils, M. Pollination control technologies for hybrid breeding. Mol. Breed. 2011, 27, 417–437. [Google Scholar] [CrossRef]
- Tacconi, G.; Michelotti, V.; Cacioppo, O.; Vittone, G. Kiwifruit pollination: The interaction between pollen quality, pollination systems and flowering stage. J. Berry Res. 2016, 6, 417–426. [Google Scholar] [CrossRef] [Green Version]
- edete. Available online: https://www.edetepta.com/ (accessed on 2 September 2022).
- dropcopter. Available online: https://www.dropcopter.com/ (accessed on 2 September 2022).
- Jiayu, H.; Yigang, Z.; Yuanping, C.; Chunqing, W.; Youjin, L. A Strawberry Artificial Pollinator. No. ZL201920082178.3, 24 September 2019. [Google Scholar]
- Shuo, W.; Jizhan, L.; Jiangshan, W.; Meng, H. A Kind of Pneumatic Strawberry Pollinating Robot in Slightly Raised Greenhouse and Its Realization Method. No. CN109588305A, 9 April 2019. [Google Scholar]
- Yuan, T.; Zhang, S.; Sheng, X.; Wang, D.; Gong, Y.; Li, W. An autonomous pollination robot for hormone treatment of tomato flower in greenhouse. In Proceedings of the 2016 3rd International Conference on Systems and Informatics (ICSAI), Shanghai, China, 19–21 November 2016; pp. 108–113. [Google Scholar]
- Ohi, N.; Lassak, K.; Watson, R.; Strader, J.; Du, Y.; Yang, C.; Hedrick, G.; Nguyen, J.; Harper, S.; Reynolds, D.; et al. Design of an Autonomous Precision Pollination Robot. In Proceedings of the 25th IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 7711–7718. [Google Scholar]
- Shimizu, H.; Sato, T. Development of strawberry pollination system using ultrasonic radiation pressure. In Proceedings of the 6th International-Federation-of-Automatic-Control (IFAC) Conference on Bio-Robotics (BIOROBOTICS), Beijing, China, 13–15 July 2018; pp. 57–60. [Google Scholar]
- Shimizu, H.; Hoshi, T.; Nakamura, K.; Park, J.-E. Development of a Non-contact Ultrasonic Pollination Device. Environ. Control Biol. 2015, 53, 85–88. [Google Scholar] [CrossRef] [Green Version]
- Shi, Q.; Liu, D.; Mao, H.; Shen, B.; Ou, M. Study on Assistant Pollination of Facility Tomato by UAV. In Proceedings of the 2019 SABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019. [Google Scholar]
- Chechetka, S.A.; Yu, Y.; Tange, M.; Miyako, E. Materially Engineered Artificial Pollinators. Chem 2017, 2, 224–239. [Google Scholar] [CrossRef]
Figure 1.
Basic structure of flowers: 1. stigma; 2. style; 3. corolla; 4. ovary; 5. receptacle; 6. flower stalk; 7. calyx; 8. filament; and 9. anther.
Figure 1.
Basic structure of flowers: 1. stigma; 2. style; 3. corolla; 4. ovary; 5. receptacle; 6. flower stalk; 7. calyx; 8. filament; and 9. anther.
Figure 4.
Temperature monitoring system for the comb of the beehive: (A) negative temperature coefficient thermistor sensors, and (B) sensor distribution characteristics.
Figure 4.
Temperature monitoring system for the comb of the beehive: (A) negative temperature coefficient thermistor sensors, and (B) sensor distribution characteristics.
Figure 5.
Integrated temperature and humidity monitoring system based on a microsensor array.
Figure 5.
Integrated temperature and humidity monitoring system based on a microsensor array.
Figure 6.
Schematic of multiple beehive microclimate monitoring systems combined with video information.
Figure 6.
Schematic of multiple beehive microclimate monitoring systems combined with video information.
Figure 7.
Image acquisition test device for a honeycomb spleen in a beehive.
Figure 7.
Image acquisition test device for a honeycomb spleen in a beehive.
Figure 8.
Mite recognition based on deep convolution neural network algorithm: (A) original image, and (B) modified image.
Figure 8.
Mite recognition based on deep convolution neural network algorithm: (A) original image, and (B) modified image.
Figure 9.
Label-based identification and localization of bees. The number in the figure represents the bee number.
Figure 9.
Label-based identification and localization of bees. The number in the figure represents the bee number.
Figure 10.
Localization and decoding of custom binary markers for bee labels: (A) image acquisition experimental device, and (B) visual recognition effect. The color in the figure is the label corresponding to each bee.
Figure 10.
Localization and decoding of custom binary markers for bee labels: (A) image acquisition experimental device, and (B) visual recognition effect. The color in the figure is the label corresponding to each bee.
Figure 11.
Sound monitoring system in a beehive.
Figure 11.
Sound monitoring system in a beehive.
Figure 12.
Wireless swarm weighing platform.
Figure 12.
Wireless swarm weighing platform.
Figure 13.
Technology for monitoring a bee colony outside the hive: (A) RFID tags, (B) capacitive sensor, and (C) photoelectric sensor.
Figure 13.
Technology for monitoring a bee colony outside the hive: (A) RFID tags, (B) capacitive sensor, and (C) photoelectric sensor.
Figure 14.
Modeling bee tracking and movement. (A) Image acquisition device for honeycomb inlet and outlet. (B) Real-time image. (C) Visual identification and tracking effect. The arrows indicate tracking of bees with flight behavior.
Figure 14.
Modeling bee tracking and movement. (A) Image acquisition device for honeycomb inlet and outlet. (B) Real-time image. (C) Visual identification and tracking effect. The arrows indicate tracking of bees with flight behavior.
Figure 15.
Measurement of bee entry and exit statistics based on the circular template matching method. (A) Image acquisition device for honeycomb inlet and outlet. (B) Real-time image. (C) Visual identification and tracking effect. The explanation for variables in the subfigure (C) is visual identification and tracking effect.
Figure 15.
Measurement of bee entry and exit statistics based on the circular template matching method. (A) Image acquisition device for honeycomb inlet and outlet. (B) Real-time image. (C) Visual identification and tracking effect. The explanation for variables in the subfigure (C) is visual identification and tracking effect.
Figure 16.
Beehive multi-feature information monitoring system based on a wireless sensor network.
Figure 16.
Beehive multi-feature information monitoring system based on a wireless sensor network.
Figure 17.
Remote beehive monitoring solutions based on multi-feature information fusion. (A) HiveMind, (B) Arnia, and (C) ToBe.
Figure 17.
Remote beehive monitoring solutions based on multi-feature information fusion. (A) HiveMind, (B) Arnia, and (C) ToBe.
Figure 18.
Artificial intelligence beehive.
Figure 18.
Artificial intelligence beehive.
Figure 19.
Integrated Honeycomb (Beehome).
Figure 19.
Integrated Honeycomb (Beehome).
Figure 20.
Application of the rotorcraft in field crop pollination. (A) Hybrid rice, (B) hybrid rape.
Figure 20.
Application of the rotorcraft in field crop pollination. (A) Hybrid rice, (B) hybrid rape.
Figure 21.
Corn castration machine made by China Agricultural University.
Figure 21.
Corn castration machine made by China Agricultural University.
Figure 22.
Mechanized orchard pollination equipment. (A) Hand-held air-delivered powder sprayer, (B) kiwi air-delivered powder sprayer, (C) kiwi atomizing sprayer, (D) 2BeTM air-delivered powder sprayer, and (E) Dropcopter drone pollination.
Figure 22.
Mechanized orchard pollination equipment. (A) Hand-held air-delivered powder sprayer, (B) kiwi air-delivered powder sprayer, (C) kiwi atomizing sprayer, (D) 2BeTM air-delivered powder sprayer, and (E) Dropcopter drone pollination.
Figure 23.
Mechanized greenhouse pollination equipment. (A) Tomato hormone pollination robot, (B) blackberry pollination robot, (C) ultrasonic pollination robot, (D) greenhouse tomato drone pollination, and (E) greenhouse lily micro-drone pollination.
Figure 23.
Mechanized greenhouse pollination equipment. (A) Tomato hormone pollination robot, (B) blackberry pollination robot, (C) ultrasonic pollination robot, (D) greenhouse tomato drone pollination, and (E) greenhouse lily micro-drone pollination.
Table 1.
Pollination methods of main crops.
Table 1.
Pollination methods of main crops.
| Physiological Characteristics | Crop Name | Pollination Method |
|---|
| Unisexual flower, cross-pollination | Watermelon, Melon, Kiwifruit, Cucumber | Bee pollination, artificially assisted pollination |
| Bisexual flower, self-pollination | Grape, Eggplant, Pepper, Rice, Wheat, Cotton | Bee pollination, natural wind pollination, artificially assisted pollination |
| Bisexual flower, cross-pollination | Apple, Pear, Peach, Kiwifruit, Cabbage, Tomato, Corn, Rapeseed | Bee pollination, artificially assisted pollination |
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).