Investigations of Multiple Approaches to Reduce Green Spot Incidence in ‘WA 38’ Apple
Abstract
1. Introduction
2. Materials and Methods
2.1. Orchard Location and Design
2.2. Timing of Green Spot (GS) Onset (2020–2021)
2.3. Assessment of Fruit Defects (2020–2021)
2.4. Fruit Bagging (2020–2021)
2.4.1. Tree Selection and Time Points Treatment Imposition
2.4.2. Microclimate Monitoring of Bagged and Control Treatments (2020–2021)
2.5. Mono-Row Drape Netting (2020–2021)
2.6. Application of Parka® Cuticle Supplement (2021)
2.7. Peel Nutrient Analysis (2020)
2.7.1. The Impact of Rootstock Selection and Bagging on ‘WA 38’ Fruit Mineral Content
2.7.2. ‘WA 38’ Fruit Peel Mineral Composition of GS and Relation to Vigor
2.8. Statistical Analysis
3. Results
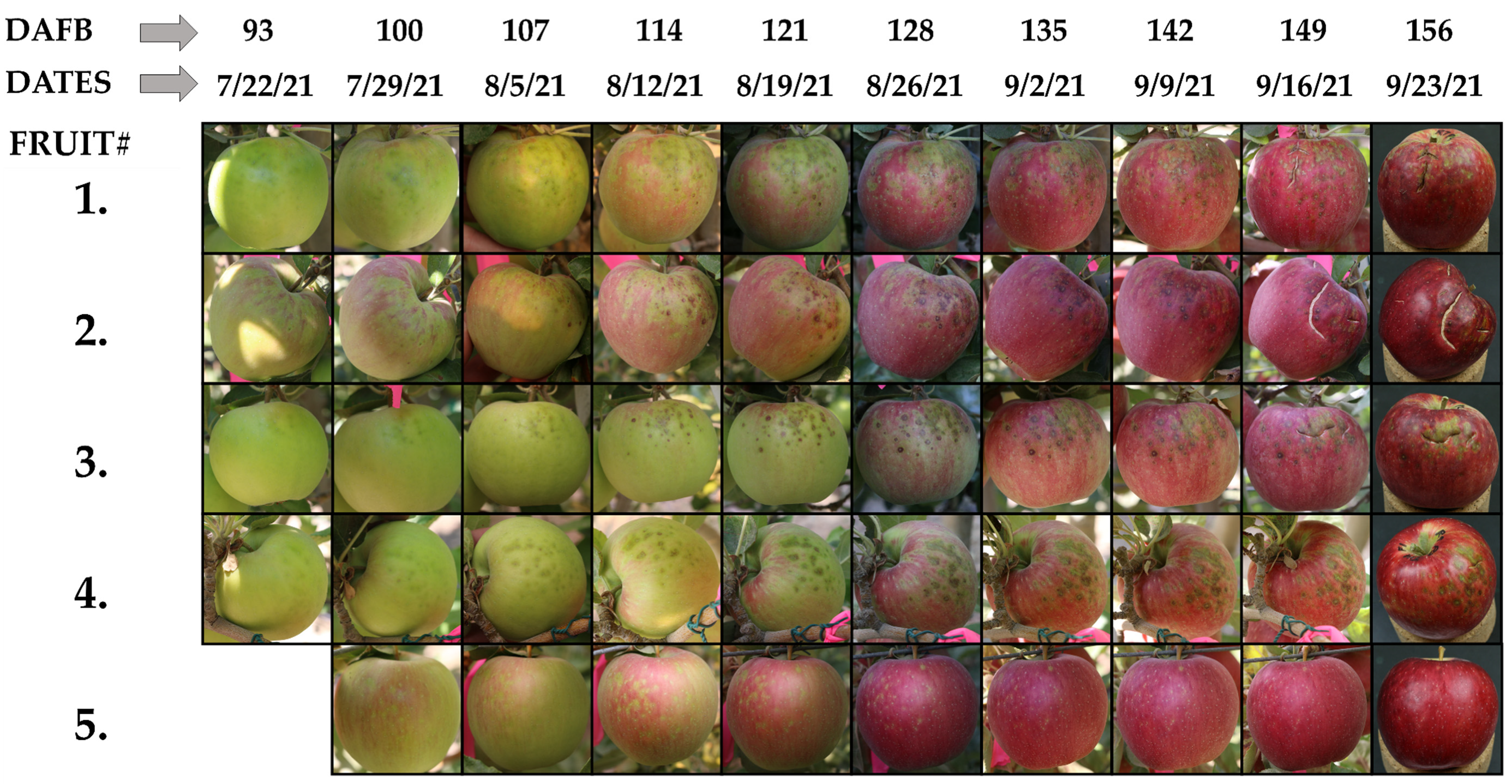
3.1. Onset and Progression of GS Symptoms
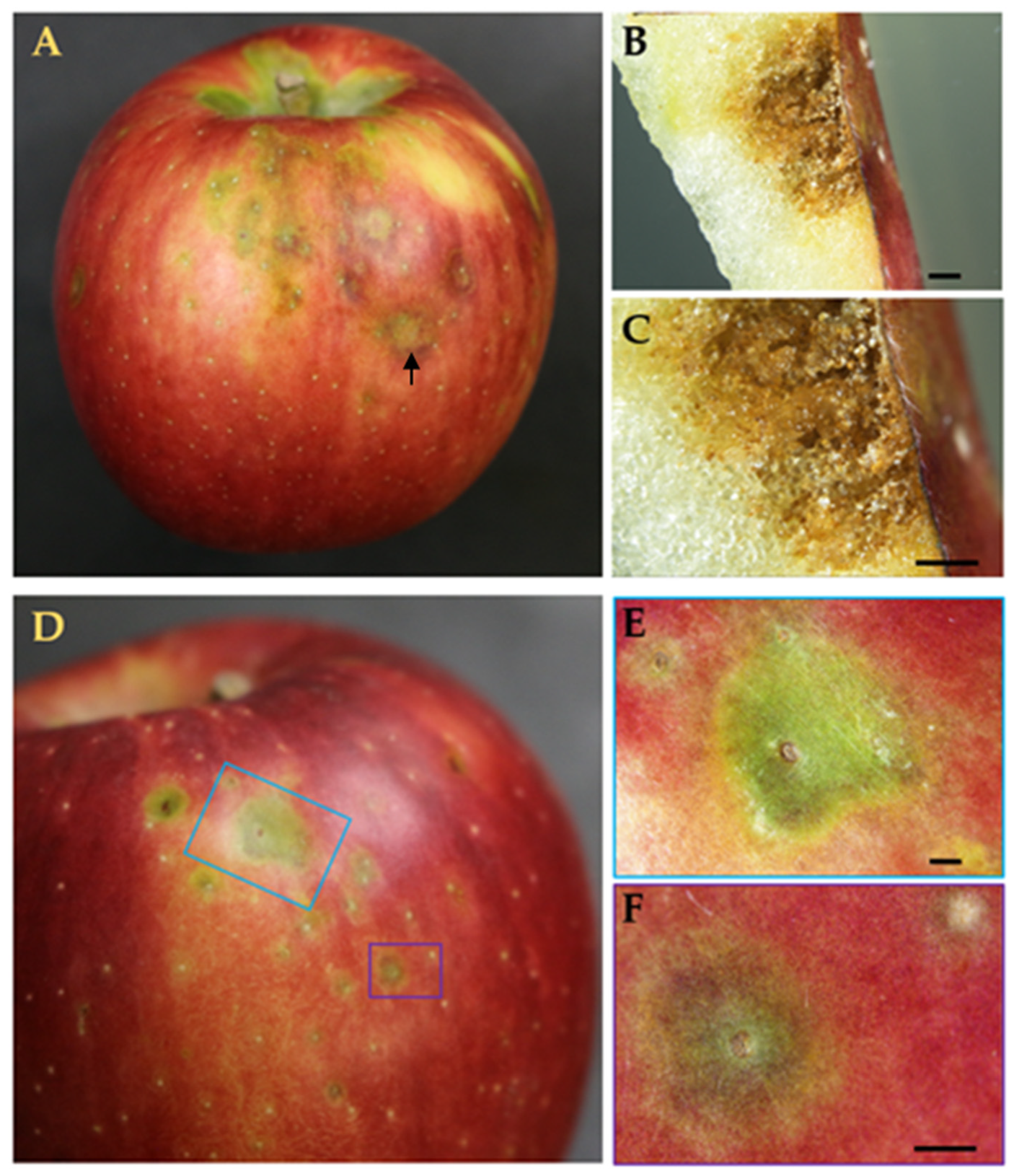
3.2. Visualization of Magnified GS Symptoms
3.3. Bagging
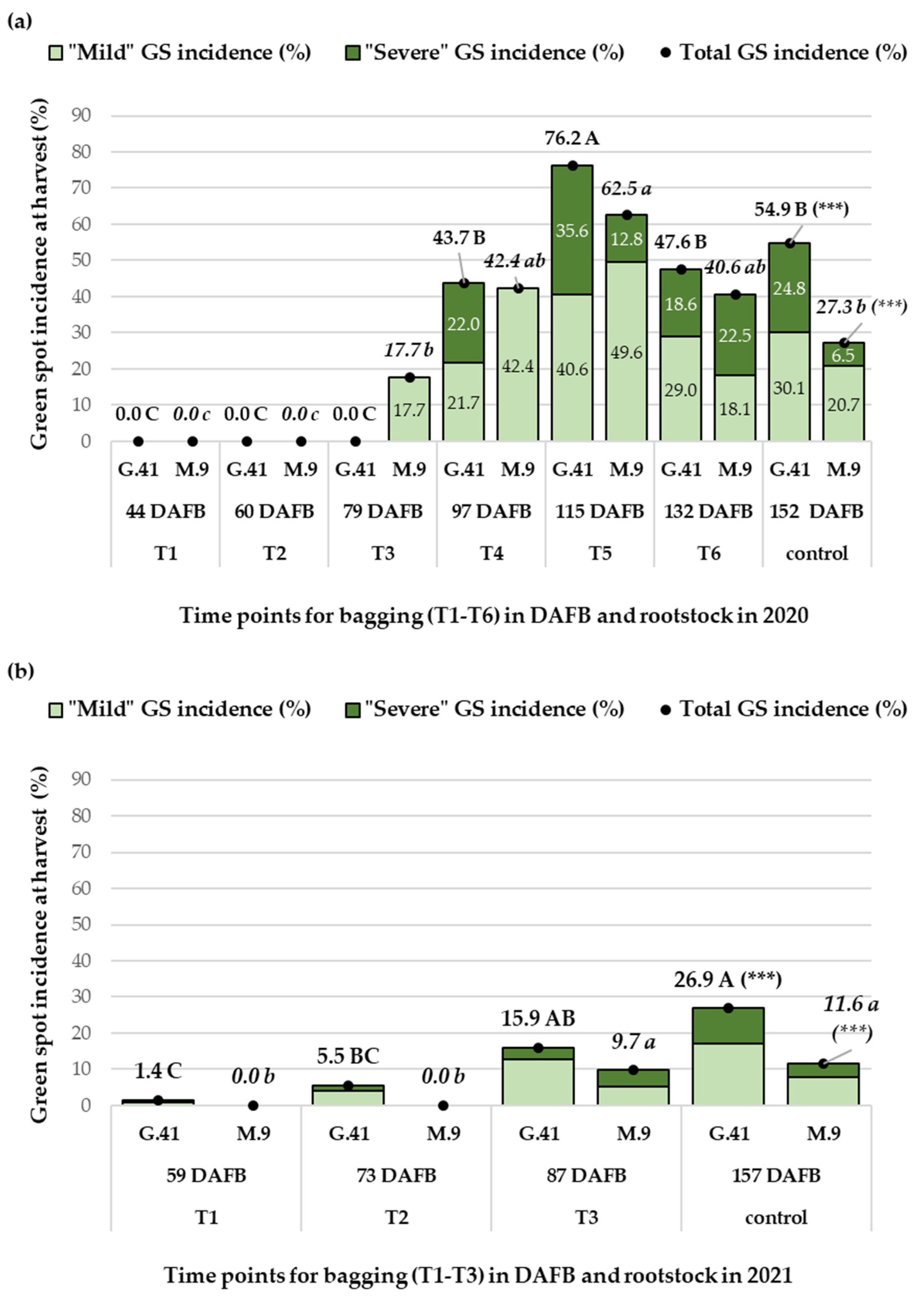
3.3.1. The Influence of Rootstock and Bagging on Total GS Incidence and Severity (2020–2021)
3.3.2. Microclimate Monitoring of Bagged and Control Treatments (2020–2021)
3.4. Mono-Row Drape Netting: Influence of Rootstock and Netting on Total GS Incidence and Severity (2020–2021)
3.5. Application of Parka® Cuticle Supplement: Effect on GS Appearance in ‘WA 38’ (2021)
3.6. Peel Mineral Analysis
3.6.1. The Impact of Rootstock Selection and Bagging on ‘WA 38’ Fruit Mineral Content (2020)
3.6.2. ‘WA 38’ Fruit Peel Mineral Composition of GS and Relation to Vigor (2020)
3.7. Correlative Analysis of Productivity Parameters and GS Incidence
4. Discussion
4.1. The Influence of Bagging, Netting and Rootstock on Total GS Incidence and Severity
4.2. The Effect of Rootstock and the Role of Mineral Nutrition on GS Appearance in ‘WA 38’
4.3. The Potential Role of Lenticels and Cuticle Health
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mix, A.J. Cork, drouth spot, and related diseases of the apple. N. Y. Agric. Exp. Station. Bull. 1916, 426, 473–522. [Google Scholar]
- Tiller, L.W. A superficial spotting disease of the Lord Wolseley apple. N. Z. J. Sci. Technol. 1932, 14, 111–113. [Google Scholar]
- McArthur, M. Histology of some physiological disorders of the apple fruit. Can. J. Res. Sec. C 1940, 18, 26–34. [Google Scholar] [CrossRef]
- Faust, M.; Shear, C.B. Corking disorders of apples: A physiological and biochemical review. Bot. Rev. 1968, 34, 441–469. [Google Scholar] [CrossRef]
- Simons, R.K. The morphological and anatomical comparison of some physiological disorders in apples. J. Am. Soc. Hortic. Sci. 1968, 93, 775–791. [Google Scholar]
- Richmond, A.E.; Dewey, D.H. Distinguishing characteristics of the Jonathan spot and lenticel spot disorders in the ‘Jonathan’ apple fruit. J. Am. Soc. Hortic. Sci. 1969, 94, 245–248. [Google Scholar] [CrossRef]
- McTavish, C.K.; Poirier, B.C.; Torres, C.A.; Mattheis, J.P.; Rudell, D.R. A convergence of sunlight and cold chain: The influence of sun exposure on postharvest apple peel metabolism. Postharvest Biol. Technol. 2020, 164, 111164. [Google Scholar] [CrossRef]
- Fan, X.; Mattheis, J.P. Bagging ‘Fuji’ apples during fruit development affects color development and storage quality. HortScience 1998, 33, 1235–1238. [Google Scholar] [CrossRef]
- Curry, E.A.; Torres, C.; Neubauer, L. Preharvest lipophilic coatings reduce lenticel breakdown disorder in ‘Gala’ apples. Horttechnology 2008, 18, 690–696. [Google Scholar] [CrossRef]
- Mupambi, G.; Anthony, B.M.; Layne, D.R.; Musacchi, S.; Serra, S.; Schmidt, T.; Kalcsits, L.A. The influence of protective netting on tree physiology and fruit quality of apple: A review. Sci. Hortic. 2018, 236, 60–72. [Google Scholar] [CrossRef]
- Schrader, L.E.; Felicetti, D.A.; Sun, J.; Xu, J.; Zhang, J.; Kahn, C.B. Effects of high temperature and high solar irradiance on sunburn fruit quality, and skin pigments of apple. Acta Hortic. 2011, 903, 1025–1039. [Google Scholar] [CrossRef]
- Knoche, M.; Khanal, B.P.; Stopar, M. Russeting and microcracking of ‘Golden Delicious’ apple fruit concomitantly decline due to gibberellin A4+7 application. J. Am. Soc. Hortic. Sci. 2011, 136, 159–164. [Google Scholar] [CrossRef]
- Donahue, D.J.; Reig Córdoba, G.; Elone, S.E.; Wallis, A.E.; Basedow, M.R. ‘Honeycrisp’ bitter pit response to rootstock and region under Eastern New York climatic conditions. Plants 2021, 10, 983. [Google Scholar] [CrossRef] [PubMed]
- Khanal, B.P.; Si, Y.; Knoche, M. Lenticels and apple fruit transpiration. Postharvest Biol. Technol. 2020, 167, 111221. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H.; Max, S. Pre-harvest bagging improves packout and fruit quality of pears (Pyrus communis). N. Z. J. Crop. Hortic. Sci. 2002, 30, 93–98. [Google Scholar] [CrossRef]
- Martins, C.R.; Hoffman, A.; Rombaldi, C.V.; Farias, R.D.M.; Teodoro, A.V. Apple biological and physiological disorders in the orchard and postharvest according to production system. Rev. Bras. Frutic. 2013, 35, 1–8. [Google Scholar] [CrossRef]
- Knoche, M.; Grimm, E. Surface moisture induces microcracks in the cuticle of ‘Golden Delicious’ apple. HortScience 2008, 43, 1929–1931. [Google Scholar] [CrossRef]
- Heidenreich, M.C.M.; Corral-Garcia, M.R.; Momol, E.A.; Burr, T.J. Russet of apple fruit caused by Aureobasidium pullulans and Rhodotorula glutinis. Plant Dis. 1997, 81, 337–342. [Google Scholar] [CrossRef]
- Daines, R.; Weber, D.J.; Bunderson, E.D.; Roper, T. Effect of early sprays on control of powdery mildew fruit russet on apples. Plant Dis. 1984, 68, 326–328. [Google Scholar] [CrossRef]
- Easterbrook, M.A.; Fuller, M.M. Russeting of apples caused by apple rust mite Aculus schlechtendali (Acarina: Eriophyidae). Ann. Appl. Biol. 1986, 109, 1–9. [Google Scholar] [CrossRef]
- Singh, V.; Gamrasni, D.; Ben Arie, R.; Naschitz, S.; Friedman, H. Identification of open lenticels in apples after harvest in relation to lenticel breakdown development during storage. Postharvest Biol. Technol. 2016, 121, 165–170. [Google Scholar] [CrossRef]
- Turketti, S.S.; Curry, E.; Lötze, E. Role of lenticel morphology, frequency, and density on incidence of lenticel breakdown in ‘Gala’ apples. Sci. Hortic. 2012, 138, 90–95. [Google Scholar] [CrossRef]
- McLarty, H.R. Tree injections with boron and other materials as a control for drought spot and corky core of apple. Sci. Agric. 1936, 16, 625–633. [Google Scholar]
- Dozier, W.A.; Odom, J.W.; Carlton, C.C.; Short, K.C.; Knowles, J.W. Foliar spraying with boron and calcium reduces cork spot of apples. Highlights Agric. Res. Ala. Agric. Exp. Station. 1982, 29, 5. [Google Scholar]
- Hewett, E.W.; Watkins, C.B. Bitter pit control by sprays and vacuum infiltration of calcium in ‘Cox’s Orange Pippin’ apples. Hortscience 1991, 26, 284–286. [Google Scholar] [CrossRef]
- Rosenberger, D.A.; Schupp, J.R.; Hoying, S.A.; Cheng, L.; Watkins, C.B. Controlling bitter pit in ‘Honeycrisp’ apples. Horttechnology 2004, 14, 342–349. [Google Scholar] [CrossRef]
- Perring, M.A. The effects of environment and cultural practices on calcium concentrations in the apple fruit. Commun. Soil Sci. Plant Anal. 1979, 10, 279–293. [Google Scholar] [CrossRef]
- Serra, S.; Leisso, R.; Giordani, L.; Kalcsits, L.; Musacchi, S. Crop load influences fruit quality, nutritional balance, and return bloom in ‘Honeycrisp’ apple. Hortscience. 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Anthony, B.M.; Serra, S.; Musacchi, M. Optimizing crop load for new apple cultivar: “WA 38”. Agronomy 2019, 9, 107. [Google Scholar] [CrossRef]
- Uselis, N.; Viškelis, J.; Lanauskas, J.; Liaudanskas, M.; Janulis, V.; Kviklys, D. Effects of growth control on yield and fruit quality of the apple cultivar ‘Rubin’. Agric. Food Sci. 2020, 29, 245–252. [Google Scholar]
- Amarante, C.V.T.; Silveira, J.P.G.; Steffens, C.A.; Paes, F.N.; Argenta, L.C. Tissue sampling method and mineral attributes to predict bitter pit occurrence in apple fruit: A multivariate approach. Acta Hortic. 2013, 1012, 1133–1140. [Google Scholar] [CrossRef]
- Khanal, B.P.; Shrestha, R.; Hückstädt, L.; Knoche, M. Russeting in apple seems unrelated to the mechanical properties of the cuticle at maturity. HortScience 2013, 48, 1135–1138. [Google Scholar] [CrossRef]
- De Freitas, S.T.; do Amarante, C.V.T.; Mitcham, E.J. Mechanisms regulating apple cultivar susceptibility to bitter pit. Sci. Hortic. 2015, 186, 54–60. [Google Scholar] [CrossRef]
- Gomez, R.; Kalcsits, L. Physiological factors affecting nutrient uptake and distribution and fruit quality in ‘Honeycrisp’ and ‘WA 38’ apple (Malus × domestica Borkh.). Hortscience 2020, 55, 1327–1336. [Google Scholar] [CrossRef]
- Bentley, W.J.; Viveros, M. Brown-bagging Granny Smith apples on trees stops codling moth damage. Calif. Agric. 1992, 46, 30–32. [Google Scholar] [CrossRef]
- Kitagawa, H.; Manabe, K.; Esguerra, E.B. Bagging of fruit on the tree to control disease. Acta Hortic. 1992, 321, 871–875. [Google Scholar] [CrossRef]
- Frank, D.L. Evaluation of fruit bagging as a pest management option for direct pests of apple. Insects 2018, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Racsko, J.; Schrader, L.E. Sunburn of Apple Fruit: Historical Background, Recent Advances and Future Perspectives. Crit. Rev. Plant Sci. 2012, 31, 455–504. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zhang, D.; Wang, Y.-Q.; Li, P.-M.; Ma, F.-W. Effects of fruit bagging on the contents of phenolic compounds in the peel and flesh of ‘Golden Delicious’, ‘Red Delicious’, and ‘Royal Gala’ apples. Sci. Hortic. 2012, 142, 68–73. [Google Scholar] [CrossRef]
- Feng, F.; Li, M.; Ma, F.; Cheng, L. The effects of bagging and debagging on external fruit quality, metabolites, and the expression of anthocyanin biosynthetic genes in ‘Jonagold’ apple (Malus domestica Borkh.). Sci. Hortic. 2014, 165, 123–131. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kobayashi, T.; Kougo, T.; Fujita, T.; Sato, S.; Moriguchi, T. Prevention of new cork spot-like physiological disorder in ‘Kurenainayume’ apples by pre-harvest fruit bagging. Hortic. J. 2018, 87, 174–183. [Google Scholar] [CrossRef]
- Kalcsits, L.; Asteggiano, L.; Schmidt, T.; Musacchi, S.; Serra, S.; Layne, D.R.; Mupambi, G. Shade netting reduces sunburn damage and soil moisture depletion in ‘Granny Smith’ apples. Acta Hortic. 2018, 1228, 85–90. [Google Scholar] [CrossRef]
- Serra, S.; Borghi, S.; Mupambi, G.; Camargo-Alvarez, H.; Layne, D.; Schmidt, T.; Kalcsits, L.; Musacchi, S. Photoselective Protective Netting Improves “Honeycrisp” Fruit Quality. Plants 2020, 9, 1708. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.S.; Barritt, B.H.; Konishi, B.S.; Brutcher, L.J.; Ross, C.F. ‘WA 38’ apple. Hortscience 2012, 47, 1177–1179. [Google Scholar] [CrossRef]
- Musacchi, S.; Whiting, M.; Lewis, L.; Gallardo, K.; Auvil, T. WA 38 Rootstocks and Training Systems. Final Project Report. 2017. Available online: https://treefruitresearch.org/report/wa-38-rootstocks-and-training-systems/ (accessed on 27 September 2022).
- Sallato, B.; Whiting, M.; Munguia, J. Rootstock and nutrient imbalance leads to “green spot” development in ‘WA 38’. HortScience. 2021, 56, 1542–1548. [Google Scholar] [CrossRef]
- Elsysy, M.; Serra, S.; Schwallier, P.; Musacchi, S.; Einhorn, T. Net enclosure of ‘Honeycrisp’ and ‘Gala’ apple trees at different bloom stages affects fruit set and alters seed production. Agronomy 2019, 9, 478. [Google Scholar] [CrossRef]
- Parka Cuticle Supplement. Available online: https://www.cultiva.com/wp-content/uploads/2021/11/Cultiva-Parka-Tech-Sheet-WEB.pdf (accessed on 25 August 2022).
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; JohnWiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- Fallahi, E.; Colt, M.W.; Baird, C.R.; Fallahi, B.; Chun, I.-J. Influence of nitrogen and bagging on fruit quality and mineral concentrations of ‘BC-2 Fuji’ apple. Horttechnology 2001, 11, 462–466. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Jhalegar, M.J. Pre-harvest fruit bagging: A useful approach for plant protection and improved post-harvest fruit quality—A review. J. Hortic. Sci. Biotechnol. 2014, 89, 101–113. [Google Scholar] [CrossRef]
- Proctor, J.T.A.; Lougheed, E.C. The effect of covering apples during development. Hortscience 1976, 11, 108–109. [Google Scholar] [CrossRef]
- Ali, M.M.; Anwar, R.; Yousef, A.F.; Li, B.; Luvisi, A.; De Bellis, L.; Aprile, A.; Chen, F. Influence of bagging on the development and quality of fruits. Plants 2021, 10, 358. [Google Scholar] [CrossRef] [PubMed]
- Boyaci, S. Effects of “bagging” treatment on some pomological and quality features of Red Chief species. Fresenius Environ. Bull. 2019, 28, 150–155. [Google Scholar]
- Xu, G.; Nie, J.; Wu, Y.; Yan, Z.; Ye, M. The effects of fruit bagging on residue behavior and dietary risk for four pesticides in apple. Sci. Rep. 2018, 8, 14348. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, K.; Harada, Y.; Kikuchi, T.; Ogawa, J.M. The apple industry in Japan: A historical sketch and diseases specific to the region. Plant Dis. 1993, 77, 546–552. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.-F.; Zhang, P.-F.; Bian, Y.-H.; Liu, Z.-Y.; Zhang, C.; Liu, X.; Wang, C.-L. An integrated metabolomic and transcriptomic analysis reveals the mechanism through which fruit bagging alleviates exocarp semi-russeting in pear fruit. Tree Physiol. 2021, 41, 1306–1318. [Google Scholar] [CrossRef]
- Shi, C.-H.; Wang, X.-Q.; Zhang, X.-Y.; Shen, L.-Y.; Luo, J.; Zhang, Y.-X. Response of fruit bagging to lignin biosynthesis and expression of related genes in fruit peel of sand pear (Pyrus pyrifolia Nakai) cv. Cuiguan. Hortscience 2019, 54, 1989–1997. [Google Scholar] [CrossRef]
- Shahak, Y.; Ratner, K.; Giller, Y.E.; Zur, N.; Or, E.; Gussakovsky, E.E.; Stern, R.; Sarig, P.; Raban, E.; Harcavi, E.; et al. Improving solar energy utilization, productivity and fruit quality in orchards and vineyards by photoselective netting. Acta Hortic. 2008, 772, 65–72. [Google Scholar] [CrossRef]
- Arthurs, S.P.; Stamps, R.H.; Giglia, F.F. Environmental modification inside photoselective shadehouses. Hortscience 2013, 48, 975–979. [Google Scholar] [CrossRef]
- Urban, J.; Ingwers, M.W.; McGuire, M.A.; Teskey, R.O. Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides × nigra. J. Exp. Bot. 2017, 68, 1757–1767. [Google Scholar] [CrossRef] [PubMed]
- Cline, J.A.; Hanson, E.J. Relative humidity around apple fruit influences its accumulation of calcium. J. Am. Soc. Hortic. 1992, 117, 542–546. [Google Scholar] [CrossRef]
- Witney, G.W.; Kushad, M.M.; Barden, J.A. Induction of bitter pit in apple. Sci. Hortic. 1991, 47, 173–176. [Google Scholar] [CrossRef]
- Fallahi, E.; Westwood, M.N.; Chaplin, M.H.; Richardson, D.G. Influence of apple rootstocks and K and N fertilizers on leaf mineral composition and yield in high density orchard. J. Plant Nutr. 1984, 7, 1161–1177. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, D.; Grusak, M.A.; Robinson, T. Phenotypic diversity and QTL mapping of absorption and translocation of nutrients by apple rootstocks. Asp. Appl. Biol. 2013, 119, 37–50. [Google Scholar]
- Baugher, T.A.; Marini, R.; Schupp, J.R.; Watkins, C.B. Prediction of bitter pit in ‘Honeycrisp’ apples and best management implications. HortScience 2017, 52, 1368–1374. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, J.; Francescatto, P.; Cheng, L.; Wallis, A.; Grusak, M.A.; Robinson, T.L. ‘Honeycrisp’ apple fruit nutrient concentration affected by apple rootstocks. Acta Hortic. 2018, 1128, 223–228. [Google Scholar] [CrossRef]
- Reig, G.; Lordan, J.; Fazio, G.; Grusak, M.A.; Hoying, S.; Cheng, L.; Francescatto, P.; Robinson, T. Horticultural performance and elemental nutrient concentrations on ‘Fuji’ grafted on apple rootstocks under New York State climatic conditions. Sci. Hortic. 2018, 227, 22–37. [Google Scholar] [CrossRef]
- Ferguson, I.B.; Watkins, C.B. Crop load affects mineral concentrations and incidence of bitter pit in ‘Cox’s Orange Pippin’ apple fruit. J. Am. Soc. Hort. Sci. 1992, 117, 373–376. [Google Scholar] [CrossRef]
- Fazio, G.; Lordan, J.; Grusak, M.A.; Francescatto, P.; Robinson, T.L.I. Mineral nutrient profiles and relationships of ‘Honeycrisp’ grown on a genetically diverse set of rootstocks under Western New York climatic conditions. Sci. Hortic. 2020, 266, 108477. [Google Scholar] [CrossRef]
- Gomez, R.; Kalcsits, L. Multi-elemental isotope and analog tracer application to measure nutrient uptake and distribution in Malus × dometstica Borkh. Acta Hortic. 2022, 1333, 393–403. [Google Scholar] [CrossRef]
- Fazio, G.; Kviklys, D.; Grusak, M.A.; Robinson, T.L. Soil pH, soil type and replant disease affect growth and nutrient absorption in apple rootstocks. N. Y. Fruit Q. 2012, 20, 22–28. [Google Scholar]
- Perring, M.A. Incidence of bitter pit in relation to the calcium content of apples: Problems and paradoxes, a review. J. Sci. Food Agric. 1986, 37, 591–606. [Google Scholar] [CrossRef]
- Saure, M.C. Reassessment of the role of calcium in development of bitter pit in apple. Aust. J. Plant Physiol. 1996, 23, 237–243. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Marini, R.P.; Lavely, E.K.; Baugher, T.A.; Crassweller, W.; Schupp, J.R. Using logistic regression to predict the probability that individual ‘Honeycrisp’ apples will develop bitter pit. HortScience 2022, 57, 391–399. [Google Scholar] [CrossRef]
- Brooks, C. The fruit spot of apples. Bul. Torrey. Bot. Club 1908, 35, 423–456. [Google Scholar] [CrossRef]
- Amarante, C.V.T.; Miqueloto, A.; Steffens, C.A.; Maciel, T.M.; Denardi, V.; Argenta, L.C.; de Freitas, S.T. Optimization of fruit tissue sampling method to quantify calcium, magnesium and potassium contents to predict bitter pit in apples. Acta Hortic. 2018, 1194, 487–492. [Google Scholar] [CrossRef]
- Drazeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Causes and effects of changes in xylem functionality in apple fruit. Ann. Bot. 2004, 93, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Clements, H.F. Morphology and physiology of the pome lenticels of Pyrus malus. Bot. Gazette. 1935, 97, 101–117. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Appezzato-da-Glória, B.; Antoniolli, L.R. Influence of growing sites and physiochemical features on the incidence of lenticel breakdown in ‘Gala’ and ‘Galaxy’ apples. Sci. Hortic. 2016, 205, 119–126. [Google Scholar] [CrossRef]
- Brown, K.; Considine, J. Physical aspects of fruit growth. Plant Physiol. 1982, 69, 585–590. [Google Scholar] [CrossRef]
- Dai, P.; Liang, X.; Wang, Y.; Gleason, M.L.; Zhang, R.; Sun, G. High humidity and age-dependent fruit susceptibility promote development of Trichothecium black spot on apple. Plant Dis. 2019, 103, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Conway, W.S.; Watada, A.E.; Sams, C.E.; Erbe, E.F.; Wergin, W.P. Changes in the ultrastructure of the epicuticular wax and postharvest calcium uptake in apples. Hortscience 1999, 34, 121–124. [Google Scholar] [CrossRef]
- Wenneker, M.; Pham, K.T.K.; Lemmers, M.E.C.; de Boer, F.A.; van Leeuwen, P.J.; Hollinger, T.C.; van de Geijn, F.G.; Thomma, B.P.H.J. Fibulorhizoctonia psychrophilia is the causal agent of lenticel spot on apple and pear fruit in the Netherlands. Eur. J. Plant. Pathol. 2017, 148, 213–217. [Google Scholar] [CrossRef]
- Đurić, G.; Mićić, N.; Pašalić, B. Lenticels as pomological characteristic of apple and pear fruits. Acta Hortic. 2015, 1099, 771–776. [Google Scholar] [CrossRef]
- Talbott, L.D.; Zeiger, E. Central roles for potassium and sucrose in guard-cell osmoregulation. Plant Physiol. 1996, 111, 1051–1057. [Google Scholar] [CrossRef]
- Schroeder, J.I. Knockout of the guard cell K+ out channel and stomatal movements. Proc. Natl. Acad. Sci. USA 2003, 100, 4976–4977. [Google Scholar] [CrossRef]
- Cochrane, T.T.; Cochrane, T.A. Differences in the way potassium chloride and sucrose solutions effect osmotic potential of significance to stomata aperture modulation. Plant. Physiol. Biochem. 2009, 47, 205–209. [Google Scholar] [CrossRef]
| 2020 | Temperature (°C) | Relative Humidity (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Month | Treatment | N = | Average | Minimum | Maximum | Average | Minimum | Maximum |
| June | bag | 180 | 20.7 | 14.5 | 28.4 | 54.0 a | 38.3 a | 71.3 |
| June | control | 180 | 20.9 | 14.4 | 29.0 | 47.6 b | 28.6 b | 69.1 |
| Significance | ns | ns | ns | *** | *** | ns | ||
| July | bag | 186 | 24.6 | 17.4 | 32.8 b | 45.1 a | 29.7 a | 62.9 |
| July | control | 186 | 24.8 | 17.1 | 33.7 a | 41.7 b | 24.0 b | 62.4 |
| Significance | ns | ns | * | *** | *** | ns | ||
| August | bag | 186 | 24.3 | 17.3 | 32.2 b | 45.2 a | 30.3 a | 62.5 |
| August | control | 186 | 24.5 | 16.8 | 33.5 a | 41.7 b | 23.6 b | 62.8 |
| Significance | ns | ns | *** | *** | *** | ns | ||
| September | bag | 132 | 20.1 | 13.9 | 26.9 | 58.8 | 42.3 a | 75.5 b |
| September | control | 132 | 20.2 | 13.5 | 28.1 | 57.8 | 37.4 b | 78.5 a |
| Significance | ns | ns | ns (p = 0.059) | ns | ** | * | ||
| 2021 | Temperature (°C) | Relative Humidity (%) | ||||||
| Month | Treatment | N = | Average | Minimum | Maximum | Average | Minimum | Maximum |
| June | bag | 78 | 30.4 | 22.2 | 39.5 | 43.4 a | 31.5 a | 57.9 a |
| June | control | 78 | 29.9 | 21.8 | 39.2 | 35.6 b | 19.9 b | 53.5 b |
| Significance | ns | ns | ns | *** | *** | ** | ||
| July | bag | 186 | 28.3 a | 20.8 a | 37.1 a | 41.7 a | 28.7 a | 56.4 a |
| July | control | 186 | 27.7 b | 20.3 b | 36.2 b | 37.4 b | 21.9 b | 54.4 b |
| Significance | ** | * | *** | *** | *** | ** | ||
| August | bag | 186 | 24.5 | 18.5 | 32.2 | 48.9 a | 34.0 a | 63.7 |
| August | control | 186 | 24.0 | 17.7 | 31.8 | 46.6 b | 28.5 b | 65.4 |
| Significance | ns | ns | ns | ** | *** | ns | ||
| September | bag | 144 | 18.7 | 12.6 a | 26.3 | 59.4 | 41.0 a | 77.6 |
| September | control | 144 | 18.2 | 11.9 b | 25.6 | 57.6 | 35.8 b | 79.9 |
| Significance | ns | * | ns | ns | *** | ns | ||
| 2020 | |||||||
|---|---|---|---|---|---|---|---|
| GS Incidence (%) | |||||||
| Treatment | N Trees | “Mild” 1 | “Severe” 2 | Total 3 | |||
| ‘WA 38’/‘G.41’ | Control | 3 | 30.1 | 24.8 | ab | 54.9 | ab |
| ‘WA 38’/‘G.41’ | Net | 3 | 23.4 | 41.5 | a | 64.9 | a |
| ‘WA 38’/‘M.9’ | Control | 3 | 20.7 | 6.5 | b | 27.3 | b |
| ‘WA 38’/‘M.9’ | Net | 3 | 15.8 | 10.9 | ab | 26.7 | b |
| Significance | ns | ns (p = 0.0503) | * | ||||
| 2021 | |||||||
| GS Incidence (%) | |||||||
| Treatment | N Trees | “Mild” 1 | “Severe” 2 | Total 3 | |||
| ‘WA 38’/‘G.41’ | Control | 9 | 17.1 | 9.8 | a | 26.9 | a |
| ‘WA 38’/‘G.41’ | Net | 9 | 9.9 | 6.9 | ab | 16.8 | b |
| ‘WA 38’/‘M.9’ | Control | 9 | 7.7 | 3.9 | b | 11.6 | b |
| ‘WA 38’/‘M.9’ | Net | 9 | 7.9 | 1.8 | b | 9.7 | b |
| Significance | *** | * | *** | ||||
| 2021 | ||||||||
|---|---|---|---|---|---|---|---|---|
| GS Incidence (%) | ||||||||
| Treatment | N Trees | “Mild” 1 | “Severe” 2 | Total 3 | ||||
| ‘WA 38’/‘G.41’ | control | 9 | 17.1 | a | 9.8 | ab | 26.9 | a |
| ‘WA 38’/‘G.41’ | PARKA® | 3 | 16.9 | a | 17.3 | a | 34.2 | a |
| ‘WA 38’/‘M.9’ | control | 9 | 7.7 | b | 3.9 | b | 11.6 | b |
| ‘WA 38’/‘M.9’ | PARKA® | 3 | 6.2 | b | 1.5 | b | 7.7 | b |
| Significance | *** | ** | *** | |||||
| Macro-Nutrients | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Total N (%) | P (%) | K (%) | S (%) | Ca (%) | Mg (%) | ||||||
| ‘WA 38’/‘G.41’ | bag | 0.392 | 0.077 | 0.784 | 0.040 | 0.039 | B | 0.094 | ||||
| control (GS-) | 0.383 | a | 0.061 | 0.693 | a | 0.036 | a | 0.049 | A | 0.089 | ||
| Significance | ns | ns | ns | ns | * | ns | ||||||
| ‘WA 38’/‘M.9’ | bag | 0.346 | 0.079 | A | 0.801 | A | 0.032 | 0.045 | 0.088 | |||
| control (GS-) | 0.320 | b | 0.048 | B | 0.598 | Bb | 0.028 | b | 0.045 | 0.086 | ||
| Significance | ns | ** | ** | ns | ns | ns | ||||||
| Micro-Nutrients | ||||||||||||
| Treatment | Zn (ppm) | Mn (ppm) | Cu (ppm) | Fe (ppm) | B (ppm) | |||||||
| ‘WA 38’/‘G.41’ | bag | 2.92 | 2.76 | 2.19 | 9.38 | 63.92 | A | |||||
| control (GS-) | 1.91 | 3.82 | 2.01 | 11.97 | 47.79 | Ba | ||||||
| Significance | ns | ns | ns | ns | * | |||||||
| ‘WA 38’/‘M.9’ | bag | 0.93 | B | 2.46 | 2.51 | 8.43 | 34.09 | |||||
| control (GS-) | 3.03 | A | 3.49 | 1.94 | 9.87 | 28.36 | b | |||||
| Significance | * | ns | ns | ns | ns | |||||||
| Ratios | ||||||||||||
| Treatment | N:Ca | K:Ca | Mg:Ca | (K+Mg):Ca | (N+Mg+K):Ca | |||||||
| ‘WA 38’/‘G.41’ | bag | 10.2 | 20.3 | A | 2.4 | A | 22.7 | A | 32.9 | A | ||
| control (GS-) | 7.9 | 14.3 | B | 1.8 | B | 16.2 | B | 24.1 | B | |||
| Significance | ns | ** | ** | ** | * | |||||||
| ‘WA 38’/‘M.9’ | bag | 7.7 | 17.8 | A | 2.0 | 19.8 | A | 27.5 | ||||
| control (GS-) | 7.2 | 13.4 | B | 1.9 | 15.4 | B | 22.6 | |||||
| Significance | ns | * | ns | * | ns | |||||||
| Macro-Nutrients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Total N (%) | P (%) | K (%) | S (%) | Ca (%) | Mg (%) | |||||||
| ‘WA 38’/‘G.41’ | GS- | 0.342 | b | 0.059 | b | 0.727 | b | 0.034 | b | 0.112 | a | 0.120 | b |
| GS+ | 0.484 | a | 0.076 | a | 0.872 | a | 0.046 | a | 0.065 | b | 0.141 | a | |
| Significance | *** | ** | ** | ** | ** | ** | |||||||
| ‘WA 38’/‘G.41’ | V+ GS- | 0.379 | 0.057 | 0.721 | 0.037 | ab | 0.072 | 0.128 | |||||
| V+ GS+ | 0.416 | 0.064 | 0.811 | 0.041 | a | 0.075 | 0.132 | ||||||
| V- GS- | 0.342 | 0.050 | 0.693 | 0.031 | b | 0.060 | 0.108 | ||||||
| Significance | ns | ns | ns | * (0.0430) | ns | ns | |||||||
| Micro-Nutrients | |||||||||||||
| Treatment | Zn (ppm) | Mn (ppm) | Cu (ppm) | Fe (ppm) | B (ppm) | ||||||||
| ‘WA 38’/‘G.41’ | GS- | 2.01 | 4.56 | 2.88 | 14.56 | 55.27 | |||||||
| GS+ | 2.00 | 6.16 | 2.16 | 13.68 | 58.70 | ||||||||
| Significance | ns | ns | ns | ns | ns | ||||||||
| ‘WA 38’/‘G.41’ | V+ GS- | 2.04 | 5.13 | 1.50 | 14.79 | 44.42 | |||||||
| V+ GS+ | 2.04 | 6.32 | 2.95 | 15.58 | 49.23 | ||||||||
| V- GS- | 1.91 | 3.92 | 1.69 | 10.36 | 46.39 | ||||||||
| Significance | ns | ns | ns | ns | ns | ||||||||
| Ratios | |||||||||||||
| Treatment | N:Ca | K:Ca | Mg:Ca | (K+Mg):Ca | (N+Mg+K):Ca | ||||||||
| ‘WA 38’/‘G.41’ | GS- | 3.1 | b | 6.5 | b | 1.1 | b | 7.6 | b | 10.6 | b | ||
| GS+ | 7.7 | a | 14.0 | a | 2.3 | a | 16.2 | a | 24.0 | a | |||
| Significance | * | * | * | * | * | ||||||||
| ‘WA 38’/‘G.41’ | V+ GS- | 5.4 | 10.3 | 1.8 | 12.1 | 17.4 | |||||||
| V+ GS+ | 5.7 | 11.1 | 1.8 | 12.9 | 18.6 | ||||||||
| V- GS- | 5.7 | 11.5 | 1.8 | 13.3 | 19.0 | ||||||||
| Significance | ns | ns | ns | ns | ns | ||||||||
| GS Incidence (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| (a) 2020 | Number of Fruits Per Tree | Yield (kg Per Tree) | Avg. Fruit Weight | TCSA | Crop Load at Harvest (Fruit per cm2 TCSA) | “Mild” | “Severe” | Total | |
| Number of fruits per tree | 1 | 0.90509 | −0.48653 | −0.31343 | 0.75001 | −0.53144 | −0.75764 | −0.83332 | |
| 0.0131 | 0.3278 | 0.5452 | 0.0859 | 0.2779 | 0.081 | 0.0394 | |||
| Yield (kg per tree) | 1 | −0.07064 | −0.54724 | 0.86191 | −0.82239 | −0.66836 | −0.8756 | ||
| 0.8942 | 0.2611 | 0.0273 | 0.0445 | 0.1467 | 0.0223 | ||||
| Avg. fruit weight | 1 | −0.37263 | −0.00356 | −0.44573 | 0.45013 | 0.19255 | |||
| 0.4669 | 0.9947 | 0.3757 | 0.3704 | 0.7147 | |||||
| TCSA | 1 | −0.84674 | 0.54966 | 0.54816 | 0.66853 | ||||
| 0.0334 | 0.2585 | 0.2601 | 0.1466 | ||||||
| Crop load at harvest (fruit per cm2 TCSA) | 1 | −0.63558 | −0.80897 | −0.91685 | |||||
| 0.175 | 0.0513 | 0.0101 | |||||||
| GS incidence (%) | “Mild” | 1 | 0.25713 | 0.60846 | |||||
| 0.6228 | 0.1999 | ||||||||
| “Severe” | 1 | 0.92335 | |||||||
| 0.0086 | |||||||||
| Total | 1 | ||||||||
| GS incidence (%) | |||||||||
| (b) 2021 | Number of Fruits Per Tree | Yield (kg Per Tree) | Avg. Fruit Weight | TCSA | Crop Load at Harvest (Fruit Per cm2 TCSA) | “Mild” | “Severe” | Total | |
| Number of fruits per tree | 1 | 0.92947 | −0.46376 | 0.63642 | 0.66778 | 0.55941 | 0.32726 | 0.51971 | |
| <0.0001 | 0.0608 | 0.006 | 0.0034 | 0.0196 | 0.1998 | 0.0325 | |||
| Yield (kg per tree) | 1 | −0.11501 | 0.61314 | 0.56785 | 0.49007 | 0.25809 | 0.44217 | ||
| 0.6603 | 0.0089 | 0.0174 | 0.0458 | 0.3172 | 0.0755 | ||||
| Avg. fruit weight | 1 | −0.28121 | −0.39342 | −0.36469 | −0.28623 | −0.37226 | |||
| 0.2742 | 0.1182 | 0.1501 | 0.2654 | 0.1412 | |||||
| TCSA | 1 | −0.12725 | 0.77687 | 0.34271 | 0.67045 | ||||
| 0.6265 | 0.0002 | 0.1781 | 0.0032 | ||||||
| Crop load at harvest (fruit per cm2 TCSA) | 1 | 0.00192 | 0.16588 | 0.07739 | |||||
| 0.9942 | 0.5246 | 0.7678 | |||||||
| GS incidence (%) | “Mild” | 1 | 0.58234 | 0.92781 | |||||
| 0.0142 | <0.0001 | ||||||||
| “Severe” | 1 | 0.84357 | |||||||
| <0.0001 | |||||||||
| Total | 1 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheick, R.; Serra, S.; Rudell, D.; Musacchi, S. Investigations of Multiple Approaches to Reduce Green Spot Incidence in ‘WA 38’ Apple. Agronomy 2022, 12, 2822. https://doi.org/10.3390/agronomy12112822
Sheick R, Serra S, Rudell D, Musacchi S. Investigations of Multiple Approaches to Reduce Green Spot Incidence in ‘WA 38’ Apple. Agronomy. 2022; 12(11):2822. https://doi.org/10.3390/agronomy12112822
Chicago/Turabian StyleSheick, Ryan, Sara Serra, David Rudell, and Stefano Musacchi. 2022. "Investigations of Multiple Approaches to Reduce Green Spot Incidence in ‘WA 38’ Apple" Agronomy 12, no. 11: 2822. https://doi.org/10.3390/agronomy12112822
APA StyleSheick, R., Serra, S., Rudell, D., & Musacchi, S. (2022). Investigations of Multiple Approaches to Reduce Green Spot Incidence in ‘WA 38’ Apple. Agronomy, 12(11), 2822. https://doi.org/10.3390/agronomy12112822








