Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Greenhouse
2.2. Crop System
2.3. Plant Diseases Development Quantification
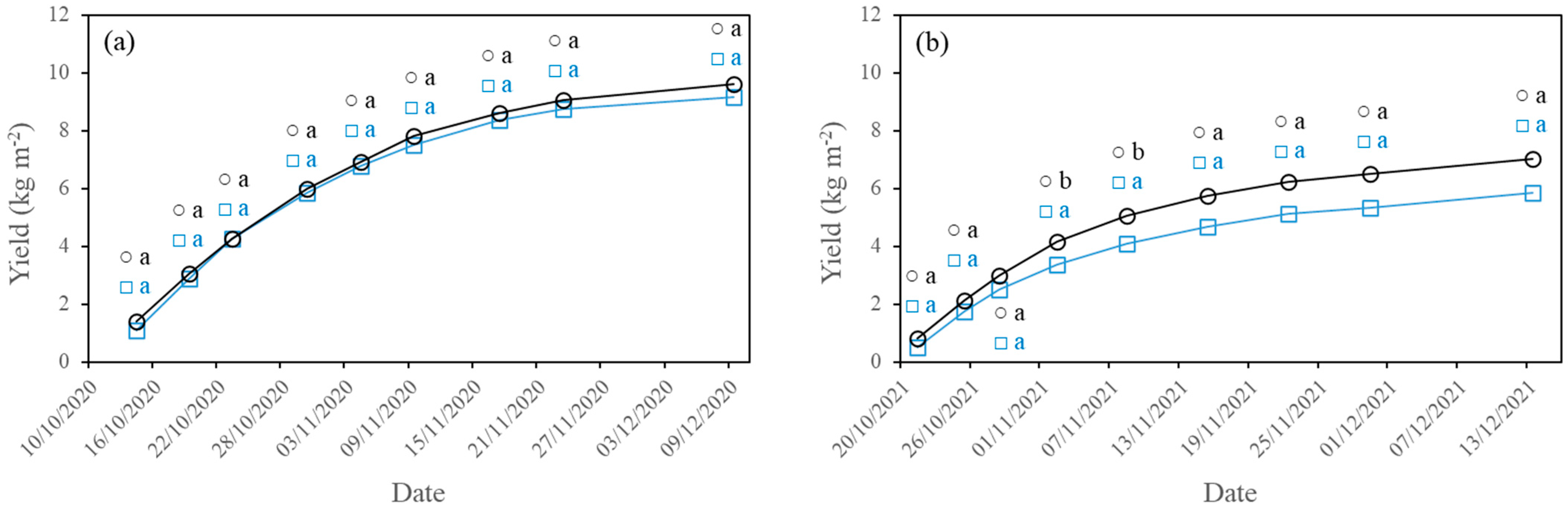
2.4. Yield and Fruit Quality Measurements
2.5. Statistical Analysis
3. Results
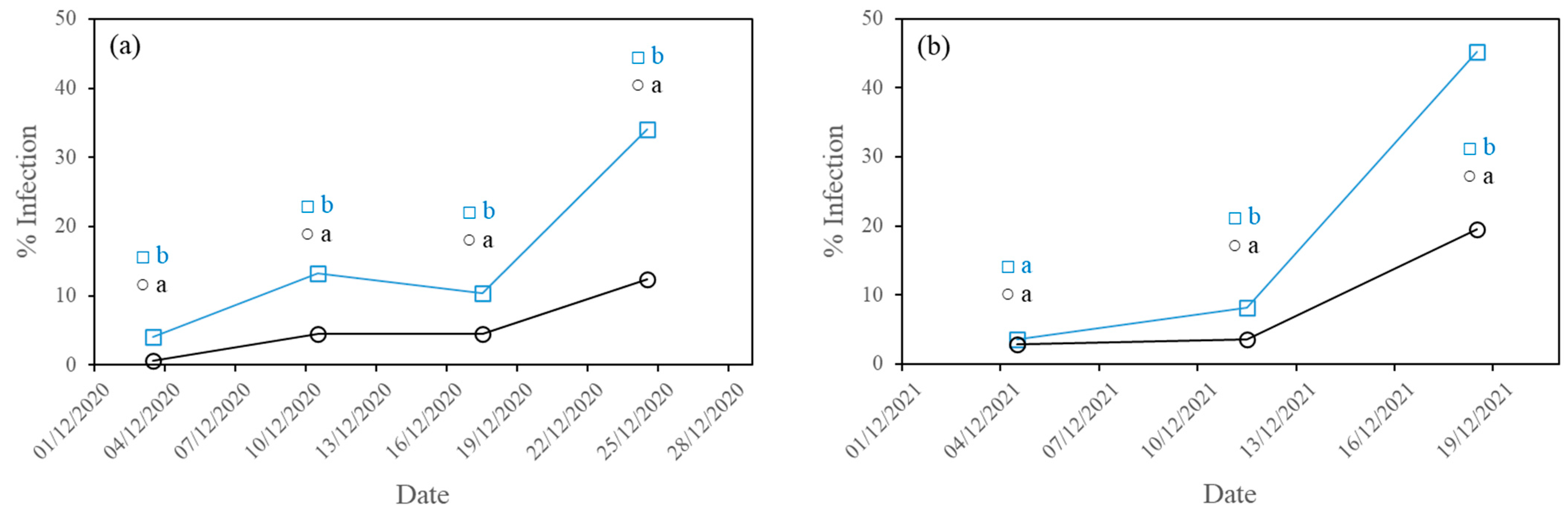
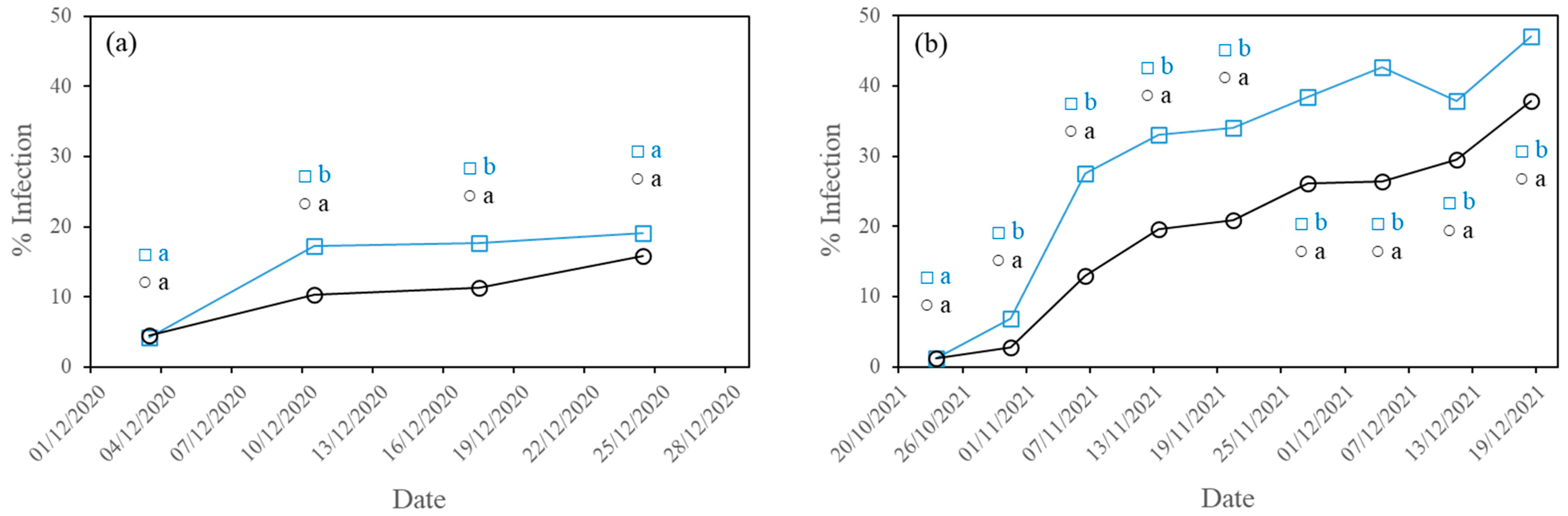
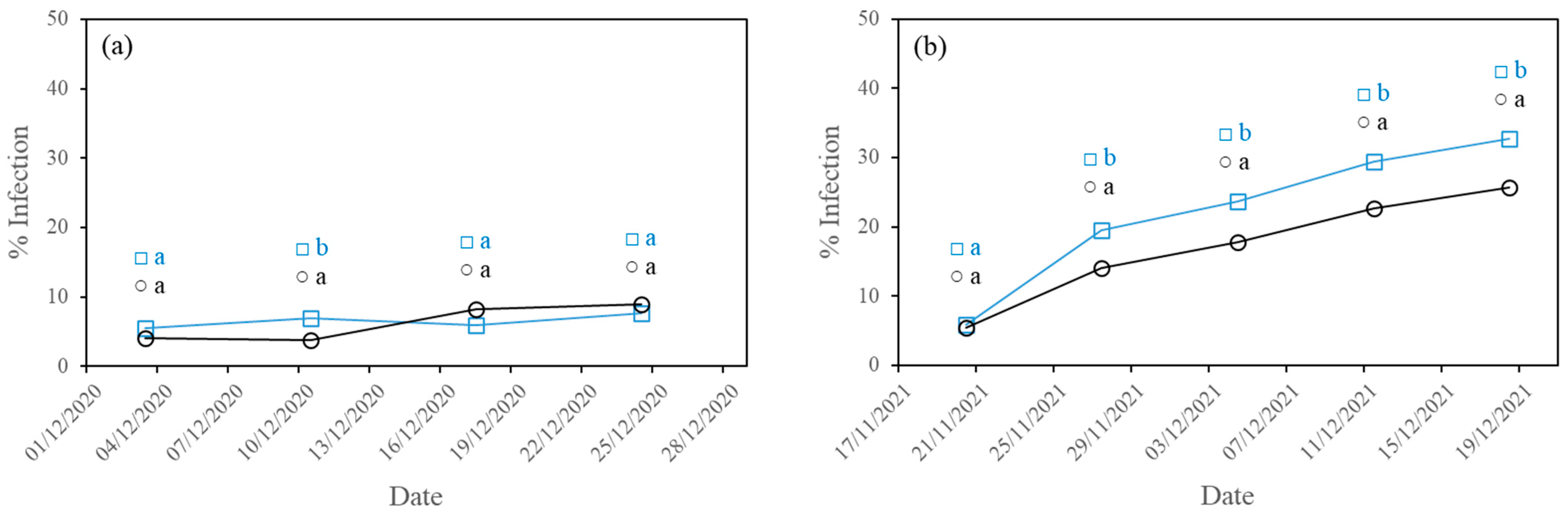
3.1. Development of Diseases
3.1.1. Powdery Mildew
3.1.2. Downy Mildew
3.1.3. Gummy Stem Blight
3.2. Yield and Fruit Quality
4. Discussion
5. Conclusions
- -
- The higher PAR radiation transmissivity of the experimental film reduced the incidence of the three fungal diseases studied (powdery mildew, downy mildew and gummy stem blight) in the second crop cycle and of powdery mildew in the first crop cycle, with statistically significant differences.
- -
- Powdery mildew was the disease most affected by the lower radiation transmissivity of the commercial cover film in both crop cycles. The fast development of powdery mildew caused the end of the crop.
- -
- The marketable yield of the cucumber crop was higher with the experimental film EF (with an increase of 4.9% in the first crop cycle and 14.7% in the second one).
- -
- No statistically significant differences were observed in any of the fruit quality parameters (weight, fruit diameter, length and soluble solids content).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castilla, N.; Montero, J.I. Environmental control and crop production in Mediterranean greenhouses. Acta Hortic. 2008, 797, 25–36. [Google Scholar] [CrossRef]
- Papadakis, G.; Briassoulis, D.; Mugnozza, G.S.; Vox, G.; Feuilloley, P.; Stoffers, J.A. Radiometric and thermal properties of, and testing methods for, greenhouse covering materials. J. Agric. Eng. Res. 2000, 77, 7–38. [Google Scholar] [CrossRef]
- Von Elsner, B.; Briassoulis, D.; Waaijenberg, D.; Mistriotis, A.; von Zabeltitz, C.; Gratraud, J.; Russo, G.; Suay-Cortes, R. Review of Structural and Functional Characteristics of Greenhouses in European Union Countries: Part I, Design Requirements. J. Agric. Eng. Res. 2000, 75, 1–16. [Google Scholar] [CrossRef]
- Hemming, S.; Mohammadkhani, V.; Van Ruijven, J. Material technology of diffuse greenhouse covering materials influence on light transmission, light scattering and light spectrum. Acta Hortic. 2014, 1037, 883–895. [Google Scholar] [CrossRef]
- Li, T.; Yang, Q. Advantages of diffuse light for horticultural production and perspectives for further research. Front. Plant Sci. 2015, 6, 704. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zheng, J.M.; Zhang, Y.; Little, C.; Khosla, S. Effects of Diffused Plastic Cover Materials on Greenhouse Microclimate, Plant Growth, Fruit Yield and Quality, and Energy Use in Greenhouse Fruit Vegetable Production. Acta Hortic. 2017, 1182, 73–78. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; Dueck, T.A.; Janse, J.; Gort, G.; Marcelis, L.F.M. Enhancement of crop photosynthesis by diffuse light: Quantifying the contributing factors. Ann. Bot. 2014, 114, 145–156. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C.; Williams, W.E.; Gorton, H.L. A new paradigm in leaf-level photosynthesis: Direct and diffuse lights are not equal. Plant. Cell Environ. 2008, 31, 159–164. [Google Scholar] [CrossRef]
- Brodersen, C.R.; Vogelmann, T.C. Do Epidermal Lens Cells Facilitate the Absorptance of Diffuse Light? Am. J. Bot. 2007, 94, 1061–1066. [Google Scholar] [CrossRef]
- Hemming, S.; Dueck, T.; Janse, J.; Van Noort, F. The effect of diffuse light on crops. Acta Hortic. 2008, 801, 1293–1300. [Google Scholar] [CrossRef]
- Li, T.; Heuvelink, E.; van Noort, F.; Kromdijk, J.; Marcelis, L.F.M. Responses of two Anthurium cultivars to high daily integrals of diffuse light. Sci. Hortic. 2014, 179, 306–313. [Google Scholar] [CrossRef]
- Moreno-Teruel, M.Á.; Molina-Aiz, F.D.; Peña-Fernández, A.; López-Martínez, A.; Valera-Martínez, D.L. The Effect of Diffuse Film Covers on Microclimate and Growth and Production of Tomato (Solanum lycopersicum L.) in a Mediterranean Greenhouse. Agronomy 2021, 11, 860. [Google Scholar] [CrossRef]
- Dueck, T.; Janse, J.; Li, T.; Kempkes, F.; Eveleens, B. Influence of diffuse glass on the growth and production of tomato. Acta Hortic. 2012, 956, 75–82. [Google Scholar] [CrossRef]
- Jarquín-Enríquez, L.; Mercado-Silva, E.M.; Maldonado, J.L.; Lopez-Baltazar, J. Lycopene content and color index of tomatoes are affected by the greenhouse cover. Sci. Hortic. 2013, 155, 43–48. [Google Scholar] [CrossRef]
- Hernández, J.; Bonachela, S.; Granados, M.R.; López, J.C.; Magán, J.J.; Montero, J.I. Microclimate and Agronomical Effects of Internal Impermeable Screens in an Unheated Mediterranean Greenhouse. Biosyst. Eng. 2017, 163, 66–77. [Google Scholar] [CrossRef]
- Baptista, F.J.F. Modelling the Climate in Unheated Tomato Greenhouses and Predicting Botrytis Cinerea Infection. Ph.D. Thesis, Universidade de Evora, Evora, Portugal, 2007. [Google Scholar]
- Raviv, M.; Reuveni, R. Fungal photomorphogenesis: A basis for the control of foliar diseases using photoselective covering materials for greenhouses. HortScience 1998, 33, 925–929. [Google Scholar] [CrossRef]
- Xu, L.L.; Li, F.; Xie, H.Y.; Liu, X.Z. A novel method for promoting conidial production by a nematophagous fungus, Pochonia chlamydosporia AS6.8. World J. Micro. Biotechnol. 2009, 25, 1989–1994. [Google Scholar] [CrossRef]
- Suthaparan, A.; Solhaug, K.A.; Bjugstad, N.; Gislerød, H.R.; Gadoury, D.M.; Stensvand, A. Suppression of powdery Mildews by UV-B: Application Frequency and Timing, Dose, Reflectance, and Automation. Plant Dis. 2016, 100, 1643–1650. [Google Scholar] [CrossRef]
- Su, Y.Y.; Qi, Y.L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology 2012, 3, 195–200. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; De Vicente, A.; Pérez-García, A. Field resistance to QoI fungicides in Podosphaera fusca is not supported by typical mutations in the mitochondrial cytochrome b gene. Pest Manag. Sci. 2008, 64, 694–702. [Google Scholar] [CrossRef]
- Pérez-García, A.; Romero, D.; Fernández-Ortuño, D.; López-Ruiz, F.; de Vicente, A.; Torés, J.A. The powdery mildew Fungus Podosphaera Fusca (synonym Podosphaera Xanthii), a Constant Threat to Cucurbits. Mol. Plant Pathol. 2009, 10, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lebeda, A.; Krístková, E.; Sedláková, B.; McCreight, J.D.; Coffey, M.D. Cucurbit powdery mildews: Methodology for objective determination and denomination of races. Eur. J. Plant Pathol. 2016, 144, 399–410. [Google Scholar] [CrossRef]
- Ojiambo, P.S.; Gent, D.H.; Quesada-Ocampo, L.M.; Hausbeck, M.K.; Holmes, G.J. Epidemiology and population biology of Pseudoperonospora cubensis: A model system for management of downy mildews. Annu. Rev. Phytopathol. 2015, 53, 223–246. [Google Scholar] [CrossRef]
- Savory, E.A.; Granke, L.L.; Quesada-Ocampo, L.M.; Varbanova, M.; Hausbeck, M.K.; Day, B. The cucurbit downy mildew pathogen Pseudoperonospora cubensis. Mol. Plant Pathol. 2011, 12, 217–226. [Google Scholar] [CrossRef]
- Keinath, A.P.; Holmes, G.J.; Everts, K.L.; Egel, D.S.; Langston, D.B. Evaluation of combinations of chlorothalonil with azoxystrobin, harpin, and disease forecasting for control of downy mildew and gummy stem blight on melon. Crop Prot. 2007, 26, 83–88. [Google Scholar] [CrossRef]
- Yao, X.; Li, P.; Xu, J.; Zhang, M.; Ren, R.; Liu, G.; Yang, X. Rapid and Sensitive Detection of Didymella bryoniae by Visual Loop-Mediated Isothermal Amplification Assay. Front. Microbiol. 2016, 7, 1372. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, J.; Bruton, B.D.; Biles, C.L. Cell wall-degrading enzymes of Didymella bryoniae in relation to fungal growth and virulence in cantaloupe fruit. Eur. J. Plant Pathol. 2014, 139, 749–761. [Google Scholar] [CrossRef]
- Gusmini, G.; Song, R.; Wehner, T.C. Inheritance of resistance to gummy stem blight in watermelon. HortScience 2017, 52, 1477–1482. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Rahim, M.A.; Natarajan, S.; Robin, A.H.K.; Kim, H.T.; Park, J.I.; Nou, I.S. Gummy stem blight resistance in melon: Inheritance pattern and development of molecular markers. Int. J. Mol. Sci. 2018, 19, 2914. [Google Scholar] [CrossRef]
- UNE-EN 13206: 2017+A1; Plastics. Thermoplastic Covering Films for Use in Agriculture and Horticulture. Asociación Española de Normalización (UNE): Madrid, Spain, 2017; 6p. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?Tipo=N&c=N0064784 (accessed on 16 December 2020).
- ASTM D 1003-13; Standard Test Method for Haze and Luminous Transmittance of Transparent Plastics. American Society for Testing and Materials (ASTM): West Conshohocken, PA, USA, 2021; 64p. Available online: https://www.astm.org/Standards/D1003.htm (accessed on 8 December 2020).
- Valera, D.L.; Belmonte, L.J.; Molina-Aiz, F.D.; López, A. Greenhouse Agriculture in Almería. A Comprehensive Techno-Economic Analysis; Cajamar, Caja Rural: Almería, Spain, 2016; 408p, Available online: https://publicacionescajamar.es/seriestematicas/economia/greenhouse-agriculture-in-almeria-a-comprehensive-techno-economic-analysis (accessed on 11 September 2022).
- Parvatha Reddy, P. Sustainable Crop Protection Under Protected Cultivation, 1st ed.; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Statgraphics® Statgraphics 19. User Manual. Statgraphics Technologies. Available online: Statgraphics_Centurion_19_User_Guide(1).pdf (accessed on 19 September 2022).
- Cohen, Y. The combined effects of temperature, leaf wetness and inoculum concentration on infection of cucumbers with Pseudoperonospora cubensis. Can. J. Bot. 1977, 55, 1478–1487. [Google Scholar] [CrossRef]
- Shetty, N.V.; Wehner, T.C.; Thomas, C.E.; Doruchowski, R.W.; Shetty, V.K.P. Evidence for downy mildew races in cucumber tested in Asia, Europe and North America. Sci. Hortic. 2002, 94, 231–239. [Google Scholar] [CrossRef]
- Pharis, V.L.; Kemp, T.R.; Knavel, D.E. Host plant-emitted volatiles as a factor in susceptibility in vitro of Cucumis and Cucurbita spp. to the fungus Mycosphaerella melonis. Sci. Hortic. 1982, 17, 311–317. [Google Scholar] [CrossRef]
- Schuerger, A.C.; Brown, C.S. Spectral quality affects disease development of three pathogens on hydroponically grown plants. HortScience 1997, 32, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.S. Physiology and Biochemistry of Host-Parasite Interaction; Spencer, D.M., Ed.; The Downy Mildews. Academic Press: London, UK, 1981; pp. 143–163. [Google Scholar]
- Oerke, E.C.; Steiner, U.; Dehne, H.W.; Lindenthal, M. Thermal imaging of cucumber leaves affected by downy mildew and environmental conditions. J. Exp. Bot. 2006, 57, 2121–2132. [Google Scholar] [CrossRef]
- Virtuoso, M.C.S.; Valente, T.S.; Costa-Silva, E.H.; Trevisan Braz, L.; de Cassia-Panizzi, R.; Forlan-Vargas, P. Implications of the inoculation method and environment in the selection of melon genotypes resistant to Didymella bryoniae. Sci. Hortic. 2022, 300, 111066. [Google Scholar] [CrossRef]
- Bhat, Z.A.; Bhat, M.A.; Ahangar, Z.A.; Badri, G.; Mir, H.; Mohi-u-Din, F. Survival of Didymella bryoniae incitant of ridge gourd blight under temperate conditions. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2632–2638. [Google Scholar] [CrossRef]
- Liu, S.; Shi, Y.; Miao, H.; Wang, M.; Li, B.; Gu, X.; Zhang, S. Genetic analysis and QTL mapping of resistance to gummy stem blight in Cucumis sativus seedling stage. Plant Dis. 2017, 101, 1–8. [Google Scholar] [CrossRef]
- Lebeda, A.; Cohen, Y. Cucurbit downy mildew (Pseudoperonospora cubensis) biology, ecology, epidemiology, host-pathogen interaction and control. Eur. J. Plant. Pathol. 2011, 129, 157–192. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Pandey, S.; Yandell, B.S.; Pathak, M.; Weng, Y. QTL mapping of powdery mildew resistance in WI 2757 cucumber (Cucumis sativus L.). Theor. Appl. Genet. 2013, 126, 2149–2161. [Google Scholar] [CrossRef]
- Olczak-Woltman, H.; Marcinkowska, J.; Niemirowicz-Szczytt, K. The genetic basis of resistance to downy mildew in Cucumis spp. latest developments and prospects. J. Appl. Genet. 2011, 52, 249–255. [Google Scholar] [CrossRef]
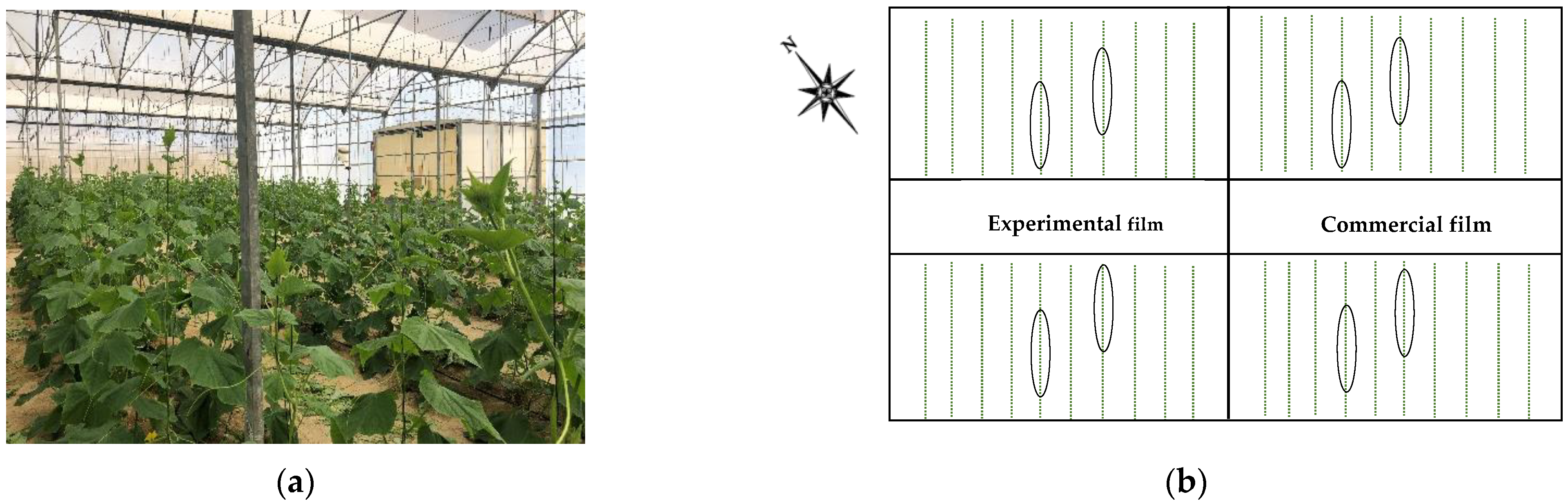

| Sector | Plastic Cover | LG × WG | SC | 20–21 Crop Cycle | 21–22 Crop Cycle | ||
|---|---|---|---|---|---|---|---|
| SV | SV/SC | SV | SV/SC | ||||
| West | EF | 40 × 25 | 1000 | 109.1 | 10.9 | 232.2 | 23.2 |
| East | CF | 40 × 20 | 800 | 84.9 | 10.6 | 193.9 | 24.2 |
| Plastic Cover | TPAR | TUV | D | T |
|---|---|---|---|---|
| Diffuse experimental film | 0.90 | 0.24 | 0.55 | 0.90 |
| Diffuse commercial film | 0.85 | 0.24 | 0.60 | 0.85 |
| Sector | Powdery Mildew S. fuliginea | Downy Mildew P. cubensis | Gummy Stem Blight D. bryoniae |
|---|---|---|---|
| End of first crop cycle (24 December 2020) | |||
| CF | 34.11 b ± 30.32 | 19.05 a ± 23.37 | 7.69 a ± 19.30 |
| EF | 12.38 a ± 21.18 | 15.79 a ± 23.78 | 8.94 a ± 20.18 |
| End of second crop cycle (18 December 2021) | |||
| CF | 45.22 b ± 34.93 | 47.14 b ± 35.07 | 32.74 b ± 37.58 |
| EF | 19.49 a ± 24.17 | 37.86 a ± 33.27 | 25.71 a ± 33.15 |
| Sector | Weight, [g] | Diameter, [mm] | Length, [cm] | Soluble Solids, [°Brix] |
|---|---|---|---|---|
| 2020–2021 Crop cycle | ||||
| CF | 474.3 a ± 105.4 | 33.8 a ± 4.0 | 44.4 a ± 2.7 | 3.7 a ± 0.8 |
| EF | 463.0 a ± 80.9 | 32.8 a ± 3.1 | 44.7 a ± 4.3 | 3.4 a ± 0.5 |
| 2021–2022 Crop cycle | ||||
| CF | 399.5 a ± 73.9 | 41.5 a ± 3.7 | 33.4 a ± 2.2 | 2.9 a ± 0.4 |
| EF | 427.7 a ± 99.6 | 42.5 a ± 3.8 | 33.9 a ± 2.8 | 2.8 a ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávalos-Sánchez, E.; Moreno-Teruel, M.Á.; Molina-Aiz, F.D.; López-Martínez, A.; Peña-Fernández, A.; Baptista, F.; Valera-Martínez, D.L. Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop. Agronomy 2022, 12, 2743. https://doi.org/10.3390/agronomy12112743
Ávalos-Sánchez E, Moreno-Teruel MÁ, Molina-Aiz FD, López-Martínez A, Peña-Fernández A, Baptista F, Valera-Martínez DL. Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop. Agronomy. 2022; 12(11):2743. https://doi.org/10.3390/agronomy12112743
Chicago/Turabian StyleÁvalos-Sánchez, Eugenio, María Ángeles Moreno-Teruel, Francisco Domingo Molina-Aiz, Alejandro López-Martínez, Araceli Peña-Fernández, Fátima Baptista, and Diego Luis Valera-Martínez. 2022. "Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop" Agronomy 12, no. 11: 2743. https://doi.org/10.3390/agronomy12112743
APA StyleÁvalos-Sánchez, E., Moreno-Teruel, M. Á., Molina-Aiz, F. D., López-Martínez, A., Peña-Fernández, A., Baptista, F., & Valera-Martínez, D. L. (2022). Influence of the Diffusivity and Transmittance of a Plastic Greenhouse Cover on the Development of Fungal Diseases in a Cucumber Crop. Agronomy, 12(11), 2743. https://doi.org/10.3390/agronomy12112743








