Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301
Abstract
1. Introduction
2. Materials and Methods
2.1. Parental Germplasm
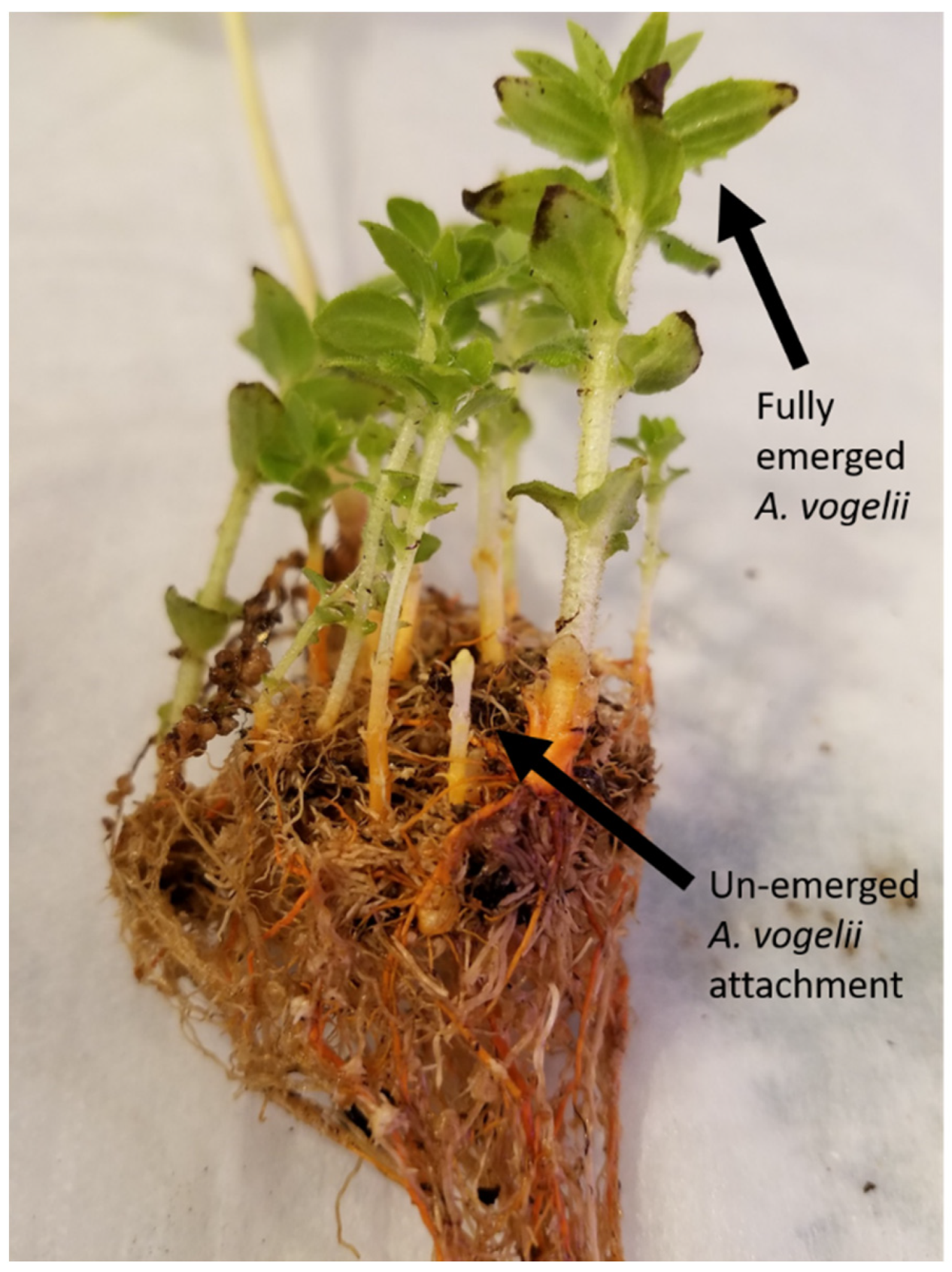
2.2. Analysis of Alectra vogelii Resistance in Cowpea
2.3. DNA Extraction and Genotyping
2.4. Linkage Mapping and QTL Analysis
2.5. Marker Assisted Backcrossing and QTL Validation
3. Results
3.1. Phenotypic Characterization of Alectra Resistance
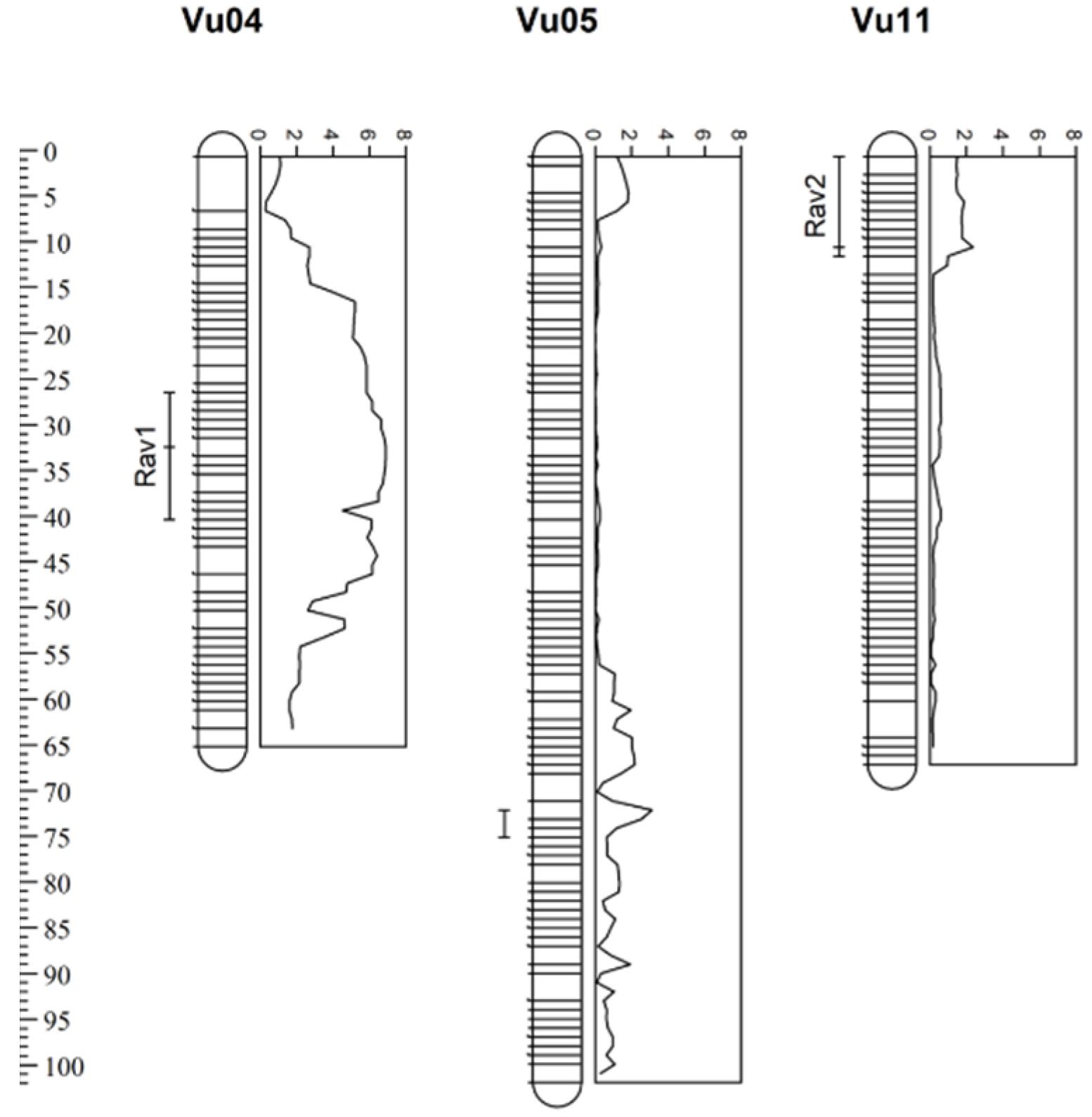
3.2. SNP Genotyping, Linkage Mapping, and QTL Analysis
3.3. Selective Genotyping
3.4. Marker Assisted Backcrossing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, B.B. Advances in Cowpea Research; IITA: Ibadan, Nigeria, 1997. [Google Scholar]
- Singh, B.B. Challenges and Opportunities for Enhancing Sustainable Cowpea Production; Fatokun, C.A., Tarawali, S.A., Singh, B.B., Kormawa, P.M., Tamo, M., Eds.; International Institute of Tropical Agriculture: Ibadan, Nigeria, 2000; pp. 154–163. [Google Scholar]
- Singh, B.B.; Olufajo, O.O.; Ishiyaku, M.E.; Adeleke, R.A.; Ajeigiie, H.A.; Mohammed, S.G. Registration of six improved germplasm lines of cowpea with combined resistance to Striga gesnerioides and Alectra vogelii. Crop Sci. 2006, 46, 2332–2333. [Google Scholar] [CrossRef]
- Atokple, I.D.K.; Singh, B.B.; Emechebe, A.M. Independent inheritance of Striga and Alectra resistance in cowpea genotype B301. Crop Sci. 1993, 33, 714–715. [Google Scholar] [CrossRef]
- Riches, C.R. The Biology and Control of Alectra vogelii Benth.(Scrophylariaceae) in Botswana; University of Reading: Nusajaya, Malaysia, 1988. [Google Scholar]
- Botha, P. The parasitism of Alectra vogelii Benth. with special reference to the germination of its seeds. J. S. Afr. Bot. 1948, 14, 63–80. [Google Scholar]
- Salako, E.A. Observations on the effect of Alectra vogelii infestation on the yield of groundnut. Trop. Pest Manag. 1984, 30, 209–211. [Google Scholar] [CrossRef]
- Visser, J.H.; Dorr, I.; Kollmann, R. Compatibility of Alectra vogelii with different leguminous host species. J. Plant Physiol. 1990, 135, 737–745. [Google Scholar] [CrossRef]
- Riches, C.R.; Hamilton, K.A.; Parker, C. Parasitism of grain legumes by Alectra species (Scrophulariaceae). Ann. Appl. Biol. 1992, 121, 361–370. [Google Scholar] [CrossRef]
- Singh, B.B.; Emechebe, A.M.; Atokple, I.D.K. Inheritance of Alectra resistance in cowpea genotype B301. Crop Sci. 1993, 33, 70–72. [Google Scholar] [CrossRef]
- Hussien, T.; Mishra, B.B.; Gebrekidan, H. A new parasitic weed (Alectra vogelii) similar to Striga on groundnut in Ethiopia. Trop. Sci. 2006, 46, 139–140. [Google Scholar] [CrossRef]
- Emechebe, A.; Singh, B.B.; Leleji, O.; Atokple, I.; Adu, J. Cowpea-striga problems and research in Nigeria. In Proceedings of the Combating Striga in Africa: Proceedings of the International Workshop Held in Ibadan, Ibadan, Nigeria, 22–24 August 1988; pp. 18–28. [Google Scholar]
- Parker, C.; Riches, C.R. Parasitic Weeds of the World: Biology and Control; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Berner, D.K.; Cardwell, K.F.; Faturoti, B.O.; Ikie, F.O.; Williams, O.A. Relative roles of wind, crop seeds, and cattle in dispersal of Striga spp. Plant Dis. 1994, 78, 402–406. [Google Scholar] [CrossRef]
- Kroschel, J. Striga—How will it affect African agriculture in the future?—An ecological perspective. Agroecol. Plant Prot. Hum. Environ. Views Concepts 1998, 16, 137–158. [Google Scholar]
- Karanja, J.; Nguluu, S.; Wambua, J.; Gatheru, M. Response of cowpea genotypes to Alectra vogelii parasitism in Kenya. Afr. J. Biotechnol. 2013, 12, 6591–6598. [Google Scholar]
- Polniaszek, T.I.; Parker, C.; Riches, C.R. Variation in the virulence of Alectra vogelii populations on cowpea. Trop. Pest Manag. 1991, 37, 152–154. [Google Scholar] [CrossRef]
- Omoigui, L.; Kamara, A.; Ishiyaku, M.; Boukar, O. Comparative responses of cowpea breeding lines to Striga and Alectra in the dry savanna of northeast Nigeria. Afr. J. Agric. Res. 2012, 7, 747–754. [Google Scholar] [CrossRef]
- Fite, G.L.; Bruce, T.; Foyer, C.; Halford, N.; Keys, A.; Kunert, K.; Lawlor, D.; Parry, M.; Russell, G. Cowpea landraces of Botswana: A potential resistance source for Alectra vogelii. Asp. Appl. Biol. 2010, 96, 111–117. [Google Scholar]
- Kabambe, V.; Mazuma, E.; Bokosi, J.; Kazila, E. Release of cowpea line IT99K-494-6 for yield and resistance to the parasitic weed Alectra vogelii (Benth) in Malawi. Afr. J. Agric. Res. 2014, 8, 196–203. [Google Scholar]
- Atokple, I.D.K.; Singh, B.B.; Emechebe, A.M. Genetics of resistance to Striga and Alectra in cowpea. J. Hered. 1995, 86, 45–49. [Google Scholar] [CrossRef]
- Omoigui, L.; Ugba, M.; Bello, L.; Gowda, B.; Timlo, M.; Motagi, B. SSR markers linked with Alectra vogelii resistance in cowpea (Vigna unguiculata (L.). In Proceedings of the 5th International conference on Next Generation Genomics and Integrated Breeding for Crop Improvement (NGGIBCI-V), Hyderabad, India, 18–20 February 2015; ICRISAT: Patancheru, India, 2015. [Google Scholar]
- Munoz-Amatriain, M.; Mirebrahim, H.; Xu, P.; Wanamaker, S.I.; Luo, M.; Alhakami, H.; Alpert, M.; Atokple, I.; Batieno, B.J.; Boukar, O.; et al. Genome resources for climate-resilient cowpea, an essential crop for food security. Plant J. 2017, 89, 1042–1054. [Google Scholar] [CrossRef]
- Ohlson, E.W.; Timko, M.P. Race structure of cowpea witchweed (Striga gesnerioides) in West Africa and its implications for Striga resistance breeding of cowpea. Weed Sci. 2020, 68, 125–133. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J.L. Genomic plant DNA preparation from fresh tissue-CTAB method. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Wu, Y.; Bhat, P.; Close, T.J.; Lonardi, S. Efficient and accurate construction of genetic linkage maps from noisy and missing genotyping data. In International Workshop on Algorithms in Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2007; pp. 395–406. [Google Scholar]
- Li, J.; Wang, S.; Zeng, Z.B. Multiple-interval mapping for ordinal traits. Genetics 2006, 173, 1649–1663. [Google Scholar] [CrossRef]
- deVicente, M.C.; Tanksley, S.D. QTL analysis of transgressive segregation in an interspecific tomato cross. Genetics 1993, 134, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Voorrips, R.E. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Lander, E.S.; Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 1989, 121, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Darvasi, A.; Soller, M. Selective genotyping for determination of linkage between a marker locus and a quantitative trait locus. Theor. Appl. Genet. 1992, 85, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, M.; Fara, A.G.; Sabatti, M.; Kuztninsky, E.; Mugnozza, G.S. Single-reaction for SNP genotyping on agarose gel by allele-specific PCR in black poplar (Populus nigra L.). Plant Mol. Biol. Report. 2007, 25, 1–9. [Google Scholar] [CrossRef]
- van Berloo, R. GGT 2.0: Versatile software for visualization and analysis of genetic data. J. Hered. 2008, 99, 232–236. [Google Scholar] [CrossRef]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, a Biometrical Approach; McGraw-Hill Kogakusha, Ltd.: New York, NY, USA, 1980. [Google Scholar]
- Lebowitz, R.J.; Soller, M.; Beckmann, J.S. Trait-based analyses for the detection of linkage between marker loci and quantitative trait loci in crosses between inbred lines. Theor. Appl. Genet. 1987, 73, 556–562. [Google Scholar] [CrossRef]
- Molinero-Ruiz, M.; García-Ruiz, R.; Melero-Vara, J.M.; Domínguez, J. Orobanche cumana race F: Performance of resistant sunflower hybrids and aggressiveness of populations of the parasitic weed. Weed Res. 2009, 49, 469–478. [Google Scholar] [CrossRef]
- Molinero-Ruiz, M.; Melero-Vara, J.M.; García-Ruiz, R.; Domínguez, J. Pathogenic diversity within field populations of Orobanche cumana and different reactions on sunflower genotypes. Weed Res. 2006, 46, 462–469. [Google Scholar] [CrossRef]
- Molinero-Ruiz, M.; Pérez-Vich, B.; Pineda-Martos, R.; Melero-Vara, J.M. Indigenous highly virulent accessions of the sunflower root parasitic weed Orobanche cumana. Weed Res. 2008, 48, 169–178. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Timko, M.P. Gene-for-gene resistance in Striga-cowpea associations. Science 2009, 325, 1094. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, S.; Li, H.; Deng, Q.; Zheng, A.; Li, S.; Li, P.; Li, Z.; Wang, J. Effects of missing marker and segregation distortion on QTL mapping in F2 populations. Theor. Appl. Genet. 2010, 121, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
| Country | District/State | Village | Year | 524B | B301 | IT81D-994 |
|---|---|---|---|---|---|---|
| Botswana | Marajone | 2008 | S | S | R | |
| Nigeria | Borno | Tila | 2016 | S | R | R |
| Tanzania | Dodoma | Kikombo | 2011 | S | R | S |
| Tanzania | Dodoma | Bihawana | 2011 | S | R | R |
| Tanzania | Iringa | Mkungugu | 2011 | S | R | R |
| Cultivar/ Population | Resistant Plants | Susceptible Plants | Χ2 (15:1) |
|---|---|---|---|
| 524B | 0 | 8 | NA |
| B301 | 8 | 0 | NA |
| Population 1 | 88 | 9 | 1.52 |
| Population 2 | 153 | 11 | 0.06 |
| QTL | Chromosome | Genetic Locus | Physical Locus | LOD | aA | dB | d/aC |
|---|---|---|---|---|---|---|---|
| cM | Mbp | ||||||
| Rav1 | Vu04 | 26.2–38.3 | 4.9–40.0 | 6.9 | 4.4 | −6.6 | −1.5 |
| Vu05 D | 71.5–73.3 | 42.5–43.0 | 3.1 | 10.7 | −12.2 | −1.1 | |
| Rav2 | Vu11 | 0–10.7 | 0–6.2 | 2.4 | 7.5 | −1.3 | −0.2 |
| SNP A | Chromosome | Genetic Locus | Physical Locus | pp | pq | qqB | X2 (1:2:1) | pS | pS–pNSC | σp D |
|---|---|---|---|---|---|---|---|---|---|---|
| cM | Mbp | |||||||||
| 2_21345 | 4 | 6.3 | 0.9 | 6 | 5 | 0 | 7.7 * | 0.77 | 0.27 ** | 0.07 |
| 2_07872 | 4 | 30.4 | 8.5 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_23898 | 4 | 37.7 | 33.3 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_05791 | 4 | 38.7 | 34.9 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_22012 | 4 | 38.7 | 36.4 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_04604 | 4 | 40.8 | 37.1 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_04705 | 4 | 46.0 | 38.8 | 11 | 0 | 0 | 22.2 ** | 1.00 | 0.50 ** | 0.00 |
| 2_16297 | 4 | 53.8 | 40.2 | 9 | 2 | 0 | 14.6 ** | 0.91 | 0.41 ** | 0.05 |
| 2_14455 | 11 | 4.6 | 0.8 | 5 | 6 | 0 | 6.6 * | 0.73 | 0.23 ** | 0.07 |
| 2_54689 | 11 | 6.7 | 3.0 | 5 | 6 | 0 | 6.6 * | 0.73 | 0.23 ** | 0.07 |
| 2_41050 | 11 | 7.2 | 3.4 | 5 | 6 | 0 | 6.6 * | 0.73 | 0.23 ** | 0.07 |
| 2_00054 | 11 | 28.4 | 30.4 | 1 | 10 | 0 | 8.2 * | 0.55 | 0.05 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohlson, E.W.; Timko, M.P. Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301. Agronomy 2022, 12, 2654. https://doi.org/10.3390/agronomy12112654
Ohlson EW, Timko MP. Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301. Agronomy. 2022; 12(11):2654. https://doi.org/10.3390/agronomy12112654
Chicago/Turabian StyleOhlson, Erik W., and Michael P. Timko. 2022. "Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301" Agronomy 12, no. 11: 2654. https://doi.org/10.3390/agronomy12112654
APA StyleOhlson, E. W., & Timko, M. P. (2022). Mapping and Validation of Alectra vogelii Resistance in the Cowpea Landrace B301. Agronomy, 12(11), 2654. https://doi.org/10.3390/agronomy12112654







