Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes
Abstract
1. Introduction
2. Materials and Methods
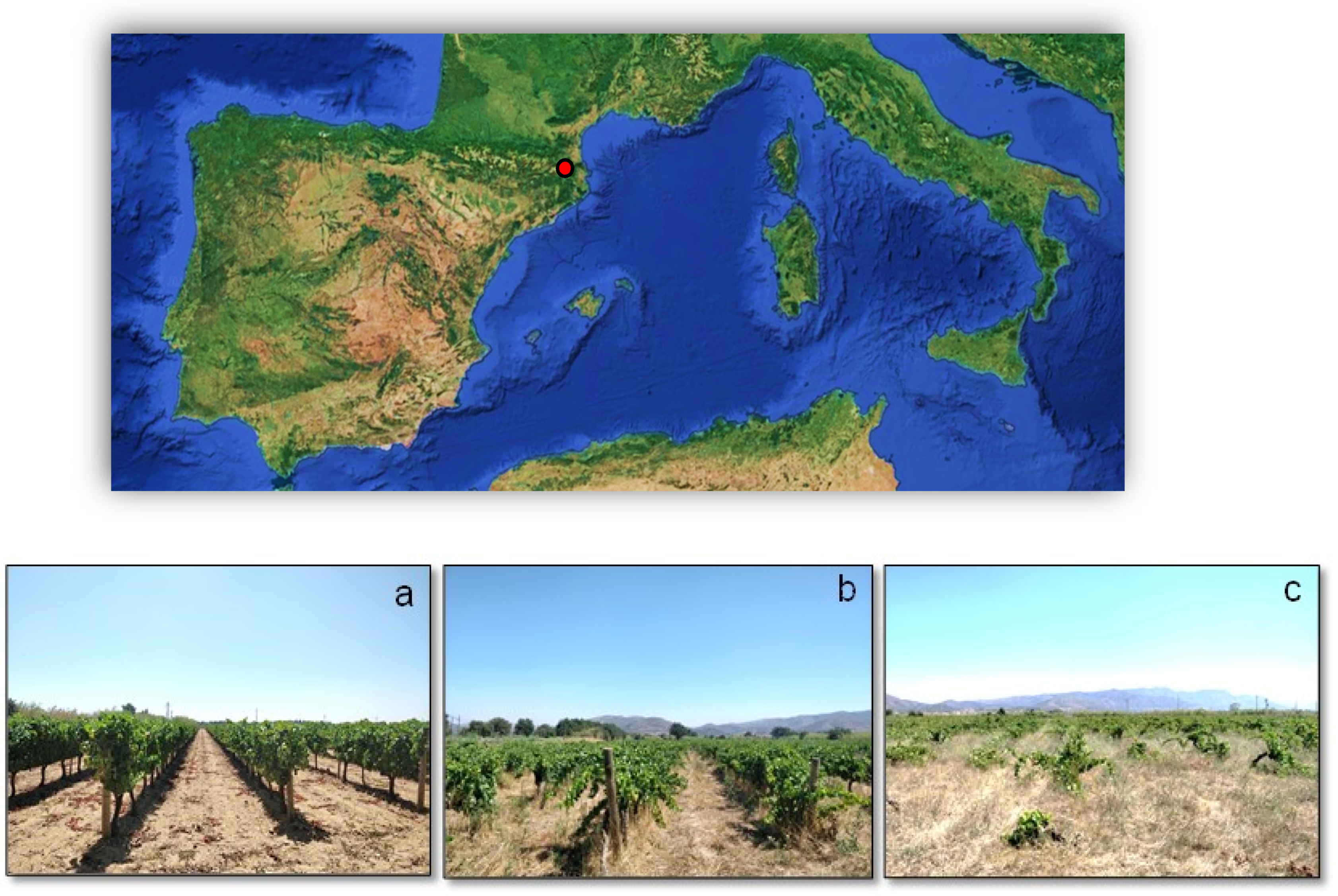
2.1. Study Site
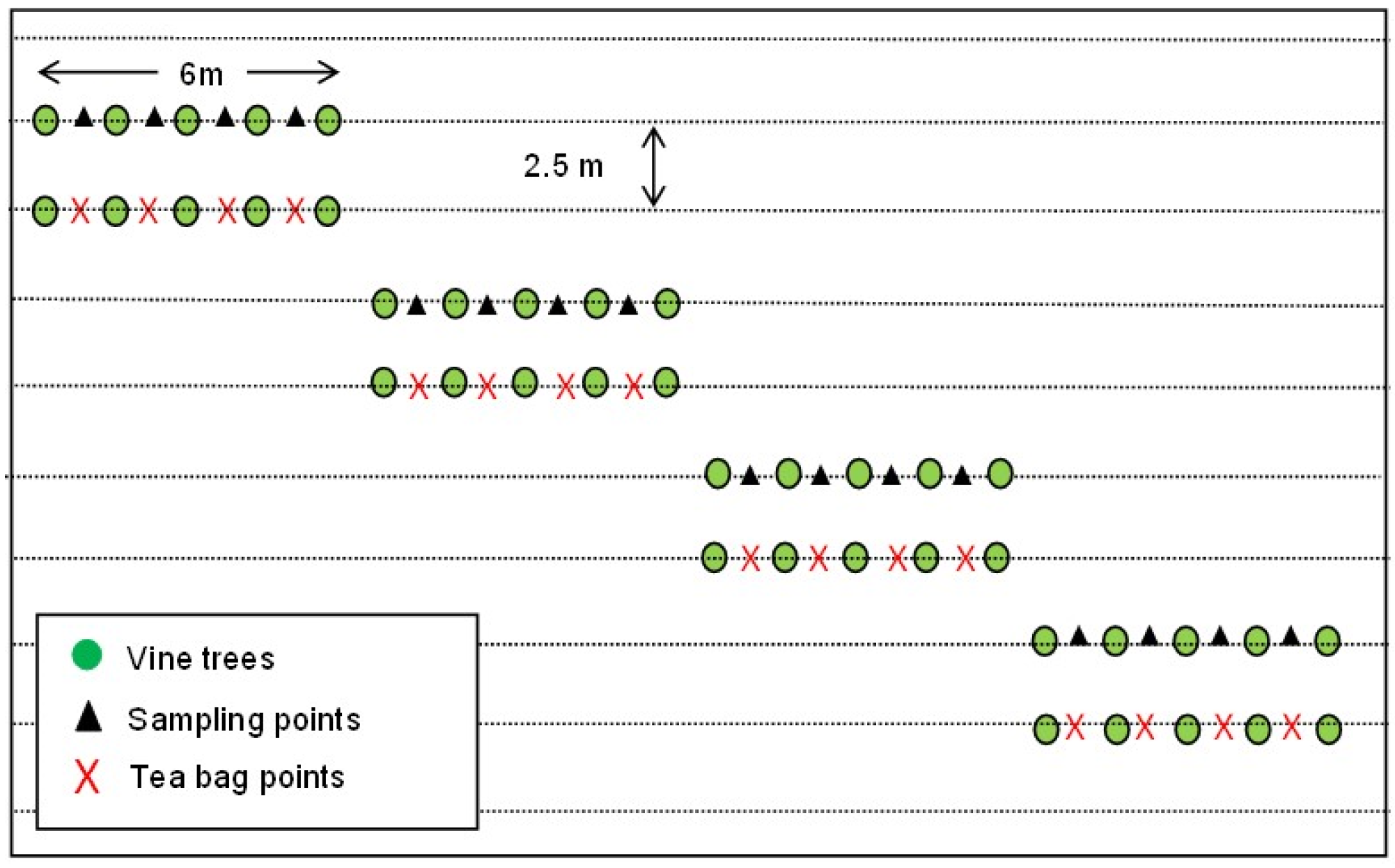
2.2. Sampling Design
2.3. Lab Analyses
2.4. Data Treatment
3. Results
3.1. Physical and Chemical Soil Properties under Contrasting Management
3.2. Abundance of Soil Groups under Diverse Management Strategies
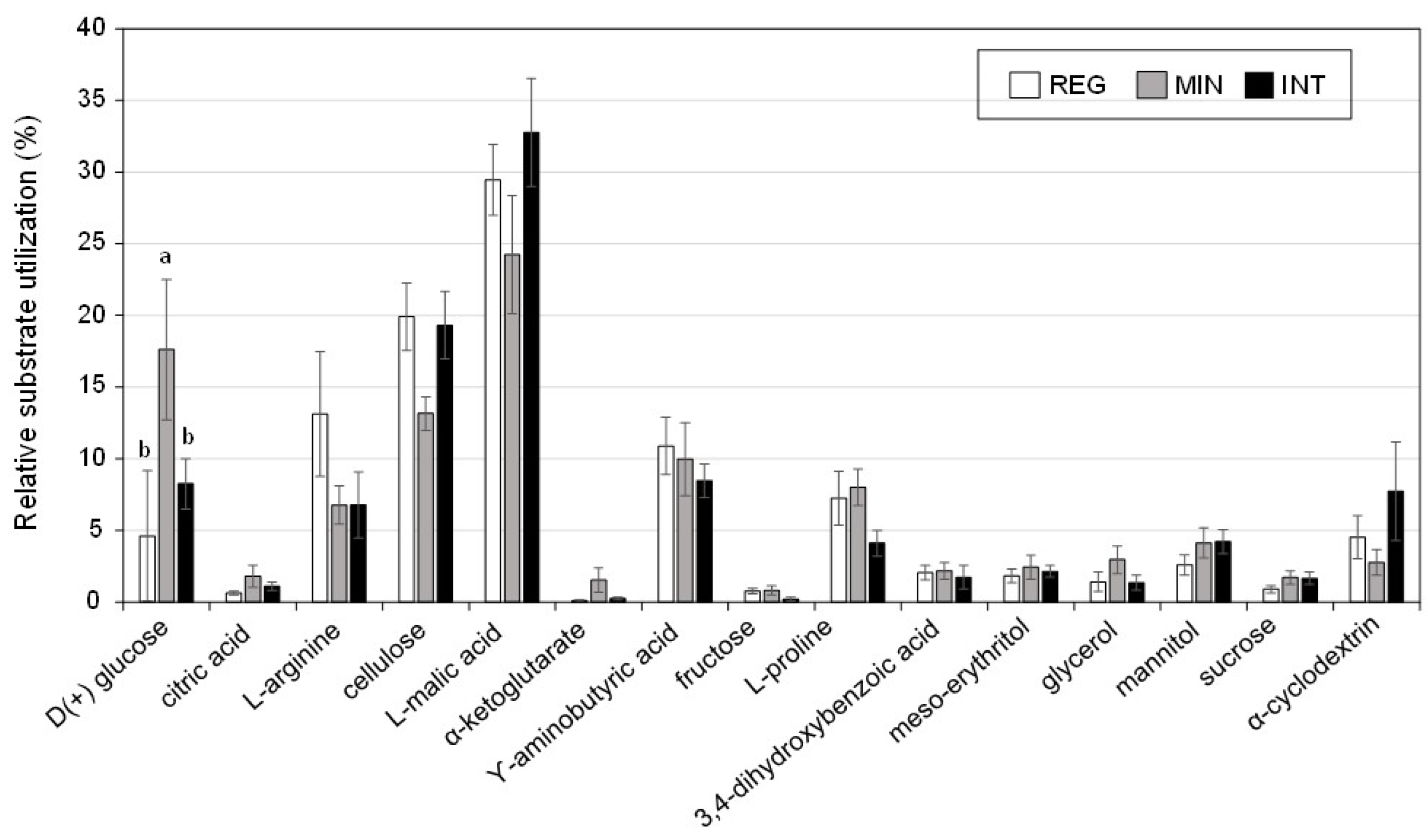
3.3. Soil Microbial Properties under Contrasting Management
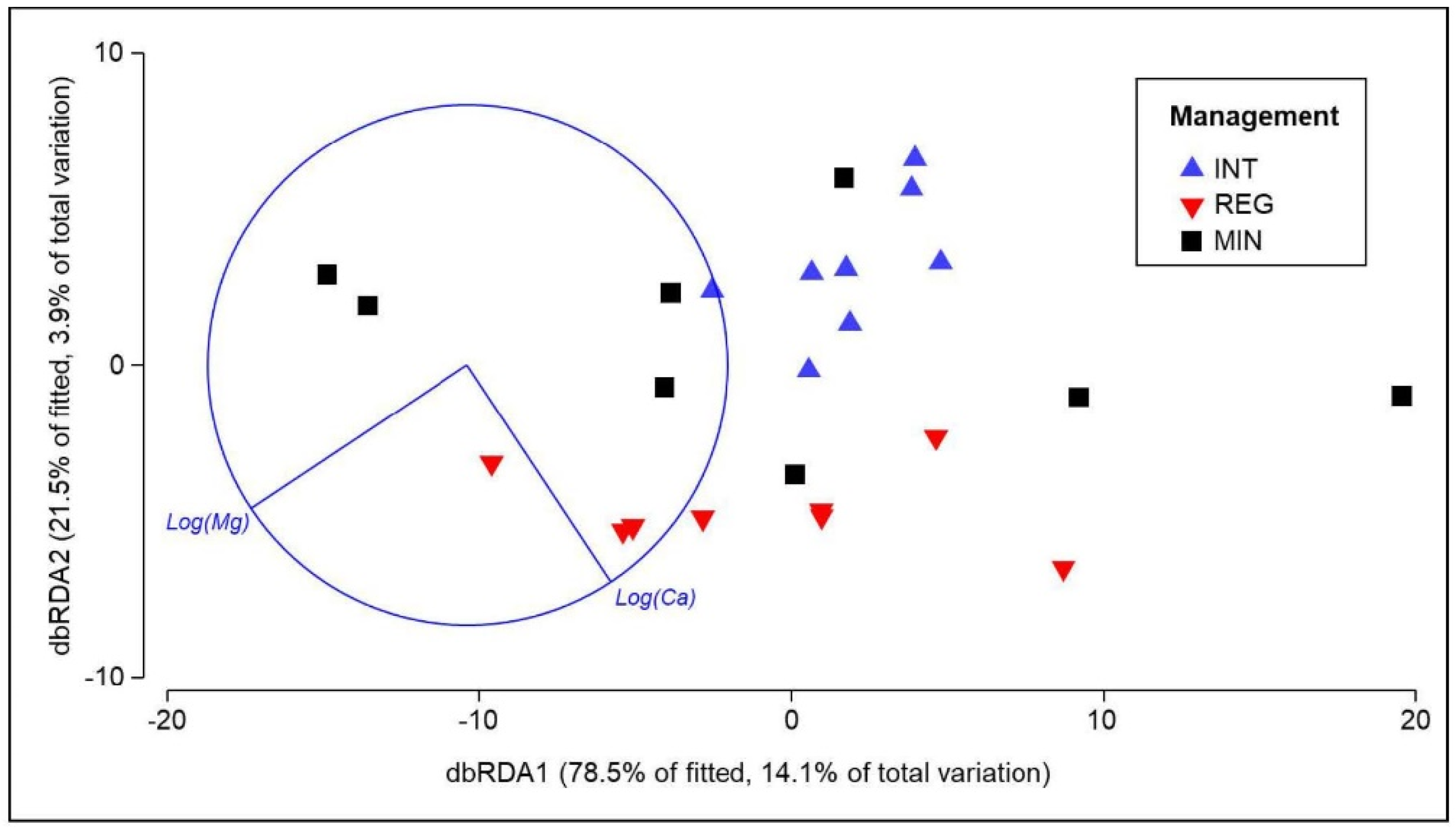
3.4. Response of Soil Properties to Agricultural Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Individual Body Weight (g, d.w.) | |
|---|---|
| Chilopoda (Geophilomorpha) | 2.59 × 10−3 |
| Pseudoescorpionida | 0.00 × 10−5 |
| Predaceous diplurans | 3.40 × 10−5 |
| Predaceous mites | 7.70 × 10−6 |
| Nematophagous mites | 1.00 × 10−6 |
| Predaceous nematodes | 1.04 × 10−6 |
| Omnivorous nematodes | 9.00 × 10−7 |
| Phytophagous nematodes | 6.00 × 10−8 |
| Bacteriophagous nematodes | 7.40 × 10−8 |
| Fungivorous nematodes | 1.10 × 10−7 |
| Ciliates | 1.00 × 10−9 |
| Amoeba | 1.20 × 10−9 |
| Flagellates | 1.90 × 10−11 |
| Fungivorous Cryptostigmata | 2.70 × 10−6 |
| Fungivorous Prostigmata | 1.00 × 10−6 |
| Symphyla | 6.60 × 10−6 |
| Collembolans | 2.70 × 10−6 |
| Bacteria | 6.65 × 10−13 |
| Fungi | 2.30 × 10−6 |
References
- Paustian, K.; Lehmann, J.; Ogle, S.; Reay, D.; Robertson, G.P.; Smith, P. Climate-smart soils. Nature 2016, 532, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Baritz, R.; Amelung, W.; Antoni, V.; Boardman, J.; Horn, R.; Prokop, G.; Römbke, J.; Romkens, P.; Steinhoff-Knopp, B.; Swartjes, F.; et al. Soil Monitoring in Europe. Indicators and Thresholds for Soil Quality Assessments; European Environmental Agency and ETC/ULS, Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- Virto, I.; Imaz, M.J.; Fernández-Ugalde, O.; Urrutia, I.; Enrique, A.; Bescansa, P. Soil quality evaluation following the implementation of permanent cover crops in semi-arid vineyards. Organic matter, physical and biological soil properties. Span. J. Agric. Res. 2012, 4, 1121–1132. [Google Scholar] [CrossRef]
- Tsiafouli, M.A.; Thébault, E.; Sgardelis, S.P.; de Ruiter, P.C.; van der Putten, W.H.; Birkhofer, K.; Hemerik, L.; de Vries, F.T.; Bardgett, R.D.; Brady, M.V.; et al. Intensive agriculture reduces soil biodiversity across Europe. Glob. Chang. Biol. 2015, 21, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, H.; Li, X.; Wang, C.; Zhang, J.; Jiang, R.; Feng, G.; Liu, X.; Zuo, Y.; Yuan, H.; et al. Field management practices drive ecosystem multifunctionality in a smallholder-dominated agricultural system. Agric. Ecosyst. Environ. 2021, 313, 107389. [Google Scholar] [CrossRef]
- de Vries, F.T.; Liiri, M.E.; Bjørnlund, L.; Bowker, M.A.; Christensen, S.; Setälä, H.M.; Bardgett, R.D. Land use alters the resistance and resilience of soil food webs to drought. Nat. Clim. Chang. 2012, 2, 276–280. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; De Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 2010, 91, 460–473. [Google Scholar] [CrossRef]
- Harkes, P.; Suleiman, A.K.A.; van den Elsen, S.J.J.; de Haan, J.J.; Holterman, M.; Kuramae, E.E.; Helder, J. Conventional and organic soil management as divergent drivers of resident and active fractions of major soil food web constituents. Sci. Rep. 2019, 9, 13521. [Google Scholar] [CrossRef]
- Prosdocimi, M.; Cerdà, A.; Tarolli, P. Soil water erosion on Mediterranean vineyards: A review. Catena 2016, 141, 1–21. [Google Scholar] [CrossRef]
- García-Ruiz, J.M. The effects of land uses on soil erosion in Spain: A review. Catena 2010, 81, 1–11. [Google Scholar] [CrossRef]
- Hütsch, B.W. Methane oxidation in non-flooded soils as affected by crop production. Eur. J. Agron. 2001, 14, 237–260. [Google Scholar] [CrossRef]
- Novara, A.; Favara, V.; Novara, A.; Francesca, N.; Santangelo, T.; Columba, P.; Chironi, S.; Ingrassia, M.; Gristina, L. Soil carbon budget account for the sustainability improvement of a Mediterranean vineyard area. Agronomy 2020, 10, 336. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil organic carbon sequestration rates in vineyard agroecosystems under different soil management practices: A meta-analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- FAO; ITPS. Recarbonizing Global Soils: A Technical Manual of Recommended Management Practices. Vol. 3. Cropland, Grassland, Integrated Systems and Farming Approaches—Practices Overview; FAO: Rome, Italy, 2021. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration impacts on global climate change and food security. Science 2004, 304, 1623–1627. [Google Scholar] [CrossRef]
- Six, J.; Paustian, K. Aggregate-associated soil organic matter as an ecosystem property and a measurement tool. Soil Biol. Biochem. 2014, 68, A4–A9. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation: A mechanism for C sequestration under no-tillage agriculture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Jat, M.L.; Gerard, B.G.; Palm, C.A.; Sanchez, P.A.; Cassman, K.G. Limited potential of no-till agriculture for climate change mitigation. Nat. Clim. Chang. 2014, 4, 678–683. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, E.; Sun, O.J. Can no-tillage stimulate carbon sequestration in agricultural soils? A meta-analysis of paired experiments. Agric. Ecosyst. Environ. 2010, 139, 224–231. [Google Scholar] [CrossRef]
- Palm, C.; Blanco-Canqui, H.; DeClerck, F.; Gatere, L.; Grace, P. Conservation agriculture and ecosystem services: An overview. Agric. Ecosyst. Environ. 2014, 187, 87–105. [Google Scholar] [CrossRef]
- Poulton, P.; Johnston, J.; Macdonald, A.; White, R.; Powlson, D. Major limitations to achieving “4 per 1000” increases in soil organic carbon stock in temperate regions: Evidence from long-term experiments at Rothamsted Research, United Kingdom. Glob. Chang. Biol. 2018, 24, 2563–2584. [Google Scholar] [CrossRef] [PubMed]
- Morlat, R.; Jacquet, A. Grapevine root system and soil characteristics in a vineyard maintained long-term with or without interrow sward. Am. J. Enol. Vitic. 2003, 54, 1–7. [Google Scholar]
- Guzmán, G.; Cabezas, J.M.; Sánchez-Cuesta, R.; Lora, A.; Bauer, T.; Strauss, P.; Winter, S.; Zallere, J.G.; Gómeza, J.A. A field evaluation of the impact of temporary cover crops on soil properties and vegetation communities in southern Spain vineyards. Agric. Ecosyst. Environ. 2019, 272, 135–145. [Google Scholar] [CrossRef]
- Messiga, A.J.; Sharifi, M.; Hammermeister, A.; Gallant, K.; Fuller, K.; Tango, M. Soil quality response to cover crops and amendments in a vineyard in Nova Scotia, Canada. Sci. Hortic. 2015, 188, 6–14. [Google Scholar] [CrossRef]
- Vicente-Vicente, J.L.; García-Ruiz, R.; Francaviglia, R.; Aguilera, E.; Smith, P. Soil carbon sequestration rates under Mediterranean woody crops using recommended management practices: A meta-analysis. Agric. Ecosyst. Environ. 2016, 235, 204–214. [Google Scholar] [CrossRef]
- Morugán-Coronado, A.; Linares, C.; Gómez-López, M.D.; Faz, Á.; Zornoza, R. The impact of intercropping, tillage and fertilizer type on soil and crop yield in fruit orchards under Mediterranean conditions: A meta-analysis of field studies. Agric. Syst. 2020, 178, 102736. [Google Scholar] [CrossRef]
- Aguilera, E.; Lassaletta, L.; Gattinger, A.; Gimeno, B.S. Managing soil carbon for climate change mitigation and adaptation in Mediterranean cropping systems: A meta-analysis. Agric. Ecosyst. Environ. 2013, 168, 25–36. [Google Scholar] [CrossRef]
- Soto, R.L.; Martínez-Mena, M.; Cuéllar Padilla, M.; de Vente, J. Restoring soil quality of woody agroecosystems in Mediterranean drylands through regenerative agriculture. Agric. Ecosyst. Environ. 2021, 306, 107191. [Google Scholar] [CrossRef]
- Costantini, E.; Agnelli, A.E.; Fabiani, A.; Gagnarli, E.; Mocali, S.; Priori, S.; Simoni, S.; Valboa, G. Short-term recovery of soil physical, chemical, micro- and mesobiological functions in a new vineyard under organic farming. Soil 2015, 1, 443–457. [Google Scholar] [CrossRef]
- Nielsen, U.N.; Wall, D.H.; Six, J. Soil Biodiversity and the Environment. Annu. Rev. Environ. Resour. 2015, 40, 63–90. [Google Scholar] [CrossRef]
- El Titi, A. Effects of tillage on invertebrates in soil ecosystems. In Soil Tillage in Agroecosystems; El Titi, A., Ed.; CRC Press: Boca Raton, FL, USA, 2003; pp. 261–296. [Google Scholar]
- Roger-Estrade, J.; Anger, C.; Bertrand, M.; Richard, G. Tillage and soil ecology: Partners for sustainable agriculture. Soil Tillage Res. 2010, 111, 33–40. [Google Scholar] [CrossRef]
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Bardgett, R.D.; Caruso, T. Soil microbial community responses to climate extremes: Resistance, resilience and transitions to alternative states. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190112. [Google Scholar] [CrossRef] [PubMed]
- Andrés, P.; Doblas-Miranda, E.; Rovira, P.; Bonmatí, A.; Ribas, À.; Mattana, S.; Romanyà, J. Research for AGRI Committee—Agricultural Potential in Carbon Sequestration-Humus Content of Land Used for Agriculture and CO2 Storage; European Parliament, Policy Department for Structural and Cohesion Policies: Brussels, Belgium, 2022. [Google Scholar]
- LaCanne, C.E.; Lundgren, J.G. Regenerative agriculture: Merging farming and natural resource conservation profitably. PeerJ 2018, 6, e4428. [Google Scholar] [CrossRef] [PubMed]
- Elevitch, C.R.; Mazaroli, D.N.; Ragone, D. Agroforestry standards for regenerative agriculture. Sustainability 2018, 10, 3337. [Google Scholar] [CrossRef]
- Ranganathan, J.; Waite, R.; Searchinger, T.; Zionts, J. Regenerative Agriculture: Good for Soil Health, but Limited Potential to Mitigate Climate Change; World Resources Institute: Washington, DC, USA, 2020; Available online: https://www.wri.org/insights/regenerative-agriculture-good-soil-health-limited-potential-mitigate-climate-change (accessed on 15 May 2022).
- Jordon, M.W.; Smith, P.; Long, P.R.; Bürkner, P.C.; Petrokofsky, G.; Willis, K.J. Can regenerative agriculture increase national soil carbon stocks? Simulated country-scale adoption of reduced tillage, cover cropping, and ley-arable integration using RothC. Sci. Total Environ. 2022, 825, 153955. [Google Scholar] [CrossRef]
- Arias, M.E.; González-Pérez, J.A.; González-Vila, F.J.; Ball, A.S. Soil health: A new challenge for microbiologists and chemists. Int. Microbiol. 2005, 8, 13–21. [Google Scholar] [CrossRef]
- Köninger, J.; Panagos, P.; Jones, A.; Briones, M.J.I.; Orgiazzi, A. In defence of soil biodiversity: Towards an inclusive protection in the European Union. Biol. Conserv. 2022, 268, 109475. [Google Scholar] [CrossRef]
- Zeiss, R.; Eisenhauer, N.; Orgiazzi, A.; Rillig, M.; Buscot, F.; Jones, A.; Lehmann, A.; Reitz, T.; Smith, L.; Guerra, C.A. Challenges of and opportunities for protecting European soil biodiversity. Conserv. Biol. 2022, 36, e13930. [Google Scholar] [CrossRef]
- FAO. Keep Soil Alive, Protect Soil Biodiversity. Global Symposium on Soil Biodiversity; Outcome document; Agriculture Organization of the United Nations: Rome, Italy, 22 April 2021. [Google Scholar]
- Luján, R.; Cuéllar Padilla, M.; de Vente, J. Participatory selection of soil quality indicators for monitoring the impacts of regenerative agriculture on ecosystem services. Ecosyst. Serv. 2020, 45, 101157. [Google Scholar] [CrossRef]
- Cameron, E.K.; Martins, I.S.; Lavelle, P.; Mathieu, J.; Tedersoo, L.; Gottschall, F.; Guerra, C.A.; Hines, J.; Patoine, G.; Siebert, J.; et al. Global gaps in soil biodiversity data. Nat. Ecol. Evol. 2018, 2, 1042–1043. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.A.; Martin, S. Identifying critical limits for soil quality indicators in agro-ecosystems. Agric. Ecosyst. Environ. 2002, 88, 153–160. [Google Scholar] [CrossRef]
- Cho, Y. JADAM Organic Farming: ULTRA Powerful Pest and Disease Control Solution, Make all-Natural Pesticide. The Way to Ultra-Low-Cost Agriculture; JADAM: Daejeon, Korea, 2016. [Google Scholar]
- Bardgett, R.D.; Jones, A.C.; Jones, D.L.; Kemmitt, S.J.; Cook, R.; Hobbs, P.J. Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol. Biochem. 2001, 33, 1653–1664. [Google Scholar] [CrossRef]
- de Fátima Tavares, L.; de Carvalho, A.M.X.; Machado, L.G. An evaluation of the Use of statistical procedures in soil science. Rev. Bras. Cienc. Solo 2016, 40, 1–17. [Google Scholar] [CrossRef]
- Kemper, W.D. Aggregate stability. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 511–519. [Google Scholar]
- Kemper, W.D.; Chepil, W.S. Size distribution of aggregates. In Methods of Soil Analysis; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 499–510. [Google Scholar]
- Campbell, C.D.; Grayston, S.J.; Hirst, D.J. Use of rhizosphere carbon sources in sole carbon source tests to discriminate soil microbial communities. J. Microbiol. Methods. 1997, 30, 33–41. [Google Scholar] [CrossRef]
- Hunt, H.W.; Coleman, D.C.; Ingham, E.R.; Ingham, R.E.; Elliott, E.T.; Moore, J.C.; Rose, S.L.; Reid, C.P.P.; Morley, C.R. The detrital food web in a shortgrass prairie. Biol. Fertil. Soils 1987, 3, 57–68. [Google Scholar] [CrossRef]
- Darbyshire, J.F.; Wheatley, R.E.; Greaves, M.P.; Inkson, R.H.E. A rapid micromethod for estimating bacterial and protozoan populations. Soil. Rev. Ecol. Biol. Sol. 1974, 11, 465–475. [Google Scholar]
- Keuskamp, J.A.; Dingemans, B.J.J.; Lehtinen, T.; Sarneel, J.M.; Hefting, M.M. Tea Bag Index: A novel approach to collect uniform decomposition data across ecosystems. Methods Ecol. Evol. 2013, 4, 1070–1075. [Google Scholar] [CrossRef]
- Petraglia, A.; Cacciatori, C.; Chelli, S.; Fenu, G.; Calderisi, G.; Gargano, D.; Abeli, T.; Orsenigo, S.; Carbognan, M. Litter decomposition: Effects of temperature driven by soil moisture and vegetation type. Plant Soil. 2019, 435, 187–200. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Legendre, P.; Andersson, M.J. Distance-based redundancy analysis: Testing multispecies responses in multifactorial ecological experiments. Ecol. Monogr. 1999, 69, 1–24. [Google Scholar] [CrossRef]
- Wardle, D.A.; Parkinson, D. Analysis of co-occurrence in a fungal community. Mycol. Res. 1991, 95, 504–507. [Google Scholar] [CrossRef]
- Starr, G.C.; Lal, R.; Malone, R.; Hothem, D.; Owens, L.; Kimble, J. Modeling soil carbon transported by water erosion processes. Land Degrad. Dev. 2000, 11, 83–91. [Google Scholar] [CrossRef]
- Jilling, A.; Kane, D.; Williams, A.; Yannarell, A.C.; Davis, A.; Jordan, N.R.; Koide, R.T.; Mortensen, D.A.; Smith, R.G.; Snapp, S.S.; et al. Rapid and distinct responses of particulate and mineral-associated organic nitrogen to conservation tillage and cover crops. Geoderma 2020, 359, 114001. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Lal, R. Mechanisms of carbon sequestration in soil aggregates. Crit. Rev. Plant Sci. 2004, 23, 481–504. [Google Scholar] [CrossRef]
- Haruna, S.I.; Anderson, S.H.; Udawatta, R.P.; Gantzer, C.J.; Phillips, N.C.; Cui, S.; Gao, Y. Improving soil physical properties through the use of cover crops: A review. Agrosyst. Geosci. Environ. 2020, 3, e20105. [Google Scholar] [CrossRef]
- USDA. Soil Quality Test Kit Guide; Soil Quality Institute, National Resources Conservation Service, US Department of Agriculture: Auburn, AL, USA, 1999. Available online: https://efotg.sc.egov.usda.gov/references/public/WI/Soil_Quality_Test_Kit_Guide.pdf (accessed on 8 May 2022).
- Gómez, J.A.; Álvarez, S.; Soriano, M.A. Development of a soil degradation assessment tool for organic olive groves in southern Spain. Catena 2009, 79, 9–17. [Google Scholar] [CrossRef]
- López-Vicente, M.; Gómez, J.A.; Guzmán, G.; Calero, J.; García-Ruiz, R. The role of cover crops in the loss of protected and non-protected soil organic carbon fractions due to water erosion in a Mediterranean olive grove. Soil Tillage Res. 2021, 213, 105119. [Google Scholar] [CrossRef]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Wang, J.; Wang, D.; She, L.; Wang, J.; Cai, C. Effects of soil physicochemical properties on aggregate stability along a weathering gradient. Catena 2017, 156, 205–215. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W. A Review of the Use of the Basic Cation Saturation Ratio and the “Ideal” Soil. Soil Sci. Soc. Am. J. 2007, 71, 259–265. [Google Scholar] [CrossRef]
- West, T.O.; Post, W.M. Soil organic carbon sequestration rates by tillage and crop rotation. Soil Sci. Soc. Am. J. 2002, 66, 1930–1946. [Google Scholar] [CrossRef]
- Baker, J.M.; Ochsner, T.E.; Venterea, R.T.; Griffis, T.J. Tillage and soil carbon sequestration-What do we really know? Agric. Ecosyst. Environ. 2007, 118, 1–5. [Google Scholar] [CrossRef]
- Costantini, E.; Priori, S.; Akca, E.; Castaldini, M.; D’Avino, L.; Fulchin, E.; Gagnarli, E.; Giffard, B.; Erdem Kiraz, M.; Lagomarsino, A.; et al. Effects of reduced soil functionality in European vineyards. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 23–28 April 2017; p. 2296. [Google Scholar]
- Pingel, M.; Reineke, A.; Leyer, I. A 30-years vineyard trial: Plant communities, soil microbial communities and litter decomposition respond more to soil treatment than to N fertilization. Agric. Ecosyst. Environ. 2019, 272, 114–125. [Google Scholar] [CrossRef]
- Zaller, J.G.; Cantelmo, C.; Santos, G.D.; Muther, S.; Gruber, E.; Pallua, P.; Mandl, K.; Friedrich, B.; Hofstetter, I.; Schmuckenschlager, B.; et al. Herbicides in vineyards reduce grapevine root mycorrhization and alter soil microorganisms and the nutrient composition in grapevine roots, leaves, xylem sap and grape juice. Environ. Sci. Pollut. Res. 2018, 25, 23215–23226. [Google Scholar] [CrossRef]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBryun, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilization effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Yu, J.; Unc, A.; Zhang, X.; Steinberger, Y. Responses of the soil microbial catabolic profile and diversity to vegetation rehabilitation in degraded semiarid grassland. Appl. Soil Ecol. 2016, 101, 124–131. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Brussaard, L.; Mäder, P.; Oguejiofor, C.U.; de Goede, R.G.M. Soil management intensity shifts microbial catabolic profiles across a range of European long-term field experiments. Appl. Soil Ecol. 2020, 154, 103596. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Shine, A. Linkages between plant litter diversity, soil microbial biomass and ecosystem function in temperate grasslands. Soil Biol. Biochem. 1999, 31, 317–321. [Google Scholar] [CrossRef]
- Coll, P.; Le Cadre, E.; Blanchart, E.; Hinsinger, P.; Villenave, C. Organic viticulture and soil quality: A long-term study in Southern France. Appl. Soil Ecol. 2011, 50, 37–44. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Hobbs, P.J.; Frostegård, Å. Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fertil. Soils 1996, 22, 261–264. [Google Scholar] [CrossRef]
- Bailey, V.L.; Smith, J.L.; Bolton, H., Jr. Fungal-to-bacterial ratios in soils investigated for enhanced C sequestration. Soil Biol. Biochem. 2002, 34, 997–1007. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Cluzeau, D.; Guernion, M.; Chaussod, R.; Martin-Laurent, F.; Villenave, C.; Cortet, J.; Ruiz-Camacho, N.; Pernin, C.; Mateille, T.; Philippot, L.; et al. Integration of biodiversity in soil quality monitoring: Baselines for microbial and soil fauna parameters for different land-use types. Eur. J. Soil Biol. 2012, 49, 63–72. [Google Scholar] [CrossRef]
- Finney, D.M.; Buyer, J.S.; Kaye, J.P. Living cover crops have immediate impacts on soil microbial community structure and function. J. Soil Water Conserv. 2017, 72, 361–373. [Google Scholar] [CrossRef]
- Gagnarli, E.; Goggioli, D.; Tarchi, F.; Guidi, S.; Nannelli, R.; Vignozzi, N. Case study of microarthropod communities to assess soil quality in different managed vineyards. Soil 2015, 1, 527–536. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Simonetto, A.; Orlando, F.; Donna, P.; Tonni, M.; Valenti, L.; Gilioli, G. Response of the arthropod community to soil characteristics and management in the Franciacorta viticultural area (Lombardy, Italy). Agronomy 2020, 10, 740. [Google Scholar] [CrossRef]
- Bedano, J.C.; Domínguez, A.; Arolfo, R.A.; Wall, L.G. Effect of Good Agricultural Practices under no-till on litter and soil invertebrates in areas with different soil types. Soil Tillage Res. 2016, 158, 100–109. [Google Scholar] [CrossRef]
- Coulibaly, S.F.M.A.; Coudrain, V.A.; Hedde, M.; Brunet, N.; Mary, B.; Recous, S.; Chauvat, M. Effect of different crop management practices on soil Collembola assemblages: A 4-year follow-up. Appl. Soil Ecol. 2017, 119, 354–366. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Simonetto, A.; Donna, P.; Tonni, M.; Valenti, L.; Bedussi, F.; Gilioli, G. Soil biological quality assessment to improve decision support in the wine sector. Agronomy 2019, 9, 593. [Google Scholar] [CrossRef]
- Mantoni, C.; Pellegrini, M.; Dapporto, L.; Del Gallo, M.M.; Pace, L.; Silveri, D.; Fattorini, S. Comparison of soil biology quality in organically and conventionally managed agro-ecosystems using microarthropods. Agriculture 2021, 11, 1022. [Google Scholar] [CrossRef]
- Ott, D.; Digel, C.; Klarner, B.; Maraun, M.; Pollierer, M.; Rall, B.C.; Scheu, S.; Seelig, G.; Brose, U. Litter elemental stoichiometry and biomass densities of forest soil invertebrates. Oikos 2014, 123, 1212–1223. [Google Scholar] [CrossRef]
- Bonkowski, M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2004, 162, 617–631. [Google Scholar] [CrossRef]
- Fox, O.; Vetter, S.; Ekschmitt, K.; Wolters, V. Soil fauna modifies the recalcitrance-persistence relationship of soil carbon pools. Soil Biol. Biochem. 2006, 38, 1353–1363. [Google Scholar] [CrossRef]
- Doran, J.W.; Parkin, T.B. Defining and assessing soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; SSSA Special Publications: Madison, WI, USA, 1994; Volume 35, pp. 1–21. [Google Scholar]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Fauci, M.F.; Dick, R.P. Microbial biomass as an indicator of soil quality: Effects of long-term management and recent soil amendments. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; SSSA Special Publications: Madison, WI, USA, 1994; Volume 35, pp. 229–234. [Google Scholar]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161–168. [Google Scholar]
- Yeates, G.W. Nematodes as soil indicators: Functional and biodiversity aspects. Biol. Fertil. Soils 2003, 37, 199–210. [Google Scholar] [CrossRef]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
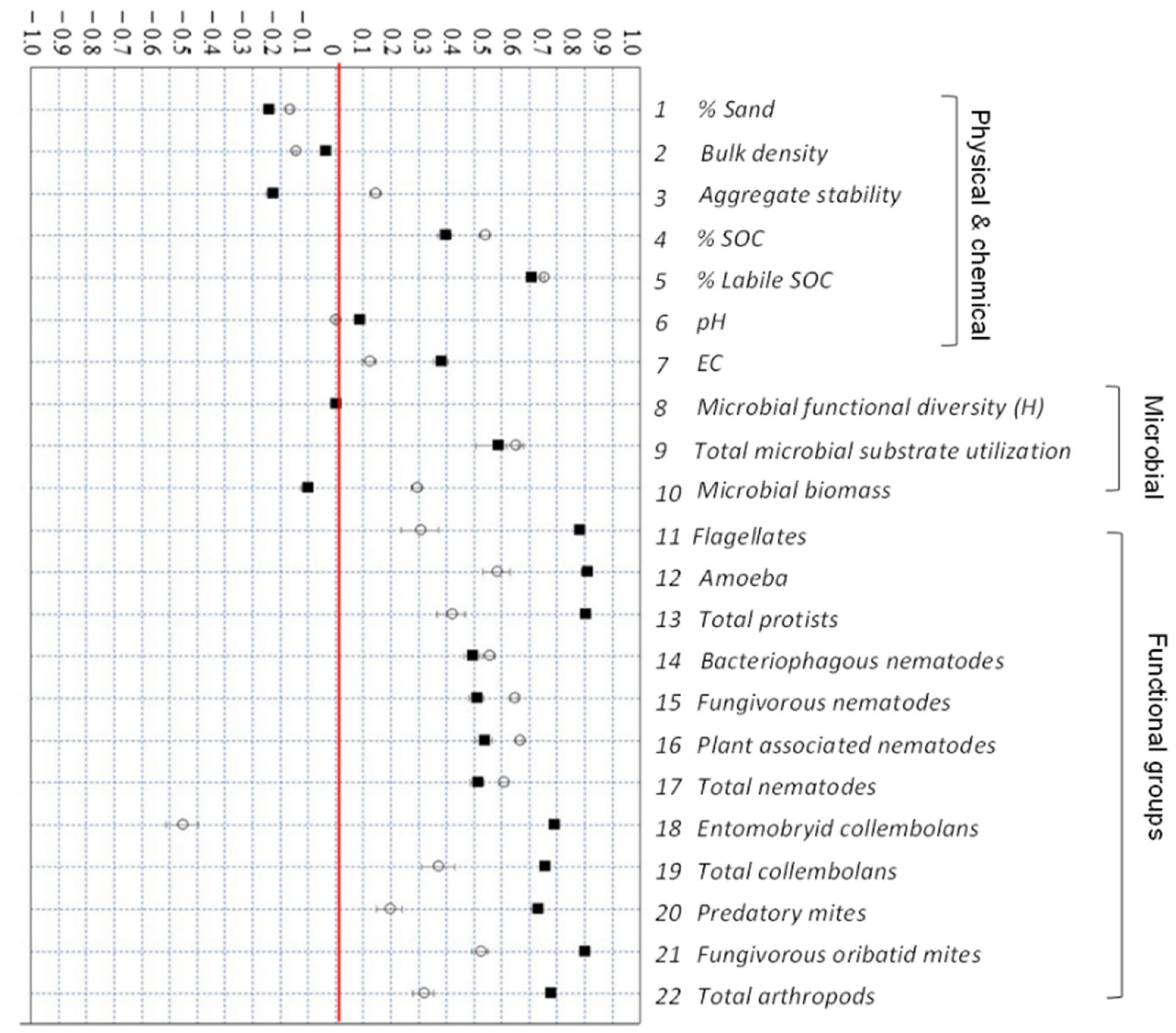
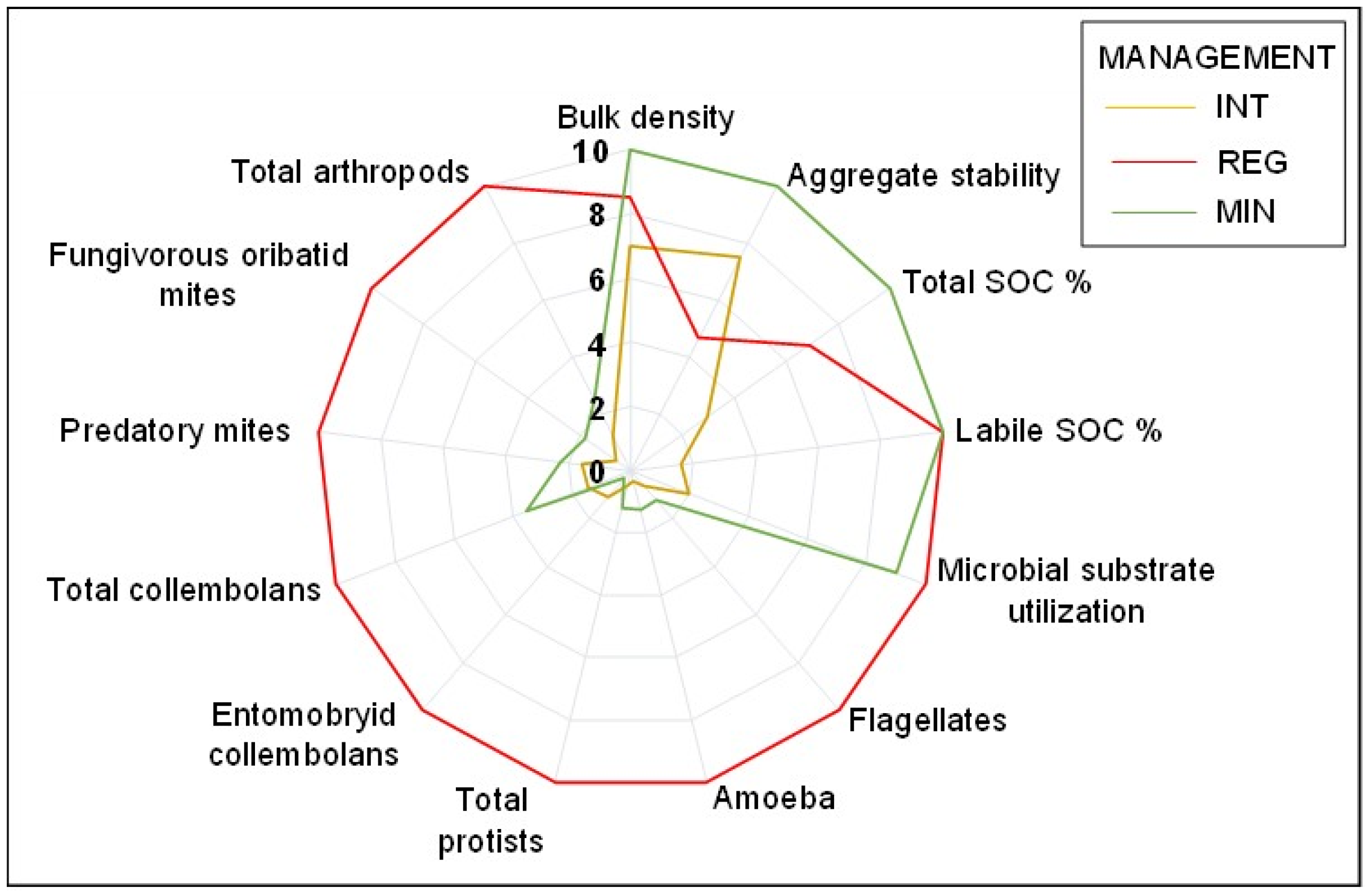
| Unit | Intensive Management (INT) | Regenerative Management (REG) | Minimal Management (MIN) | p | |
|---|---|---|---|---|---|
| Physical and chemical properties | |||||
| Silt + clay | % | 24.3 ± 2.3 b | 53.7 ± 6.7 a | 45.6 ± 4.9 a | 0.002 |
| Bulk density | g cm−3 | 1.64 ± 0.05 a | 1.36 ± 0.10 b | 1.15 ± 0.02 b | <0.0001 |
| Aggregate stability (MWD) | mm | 0.87 ± 0.14 ab | 0.54 ± 0.06 b | 1.16 ± 0.13 a | 0.005 |
| pH | 5.97 ± 0.25 b | 7.1 ± 0.09 a | 5.97 ± 0.08 b | 0.002 | |
| EC | dS m−1 | 0.06 ± 0.01 b | 0.14 ± 0.05 a | 0.08 ± 0.01 ab | 0.014 |
| Total SOC | % | 0.54 ± 0.08 b | 1.27 ± 0.20 a | 1.84 ± 0.32 a | 0.002 |
| Labile SOC | % | 0.007 ± 0.001 b | 0.043 ± 0.007 a | 0.052 ± 0.052 a | <0.0001 |
| Nitrogen | % | 0.056 ± 0.006 b | 0.121 ± 0.017 a | 0.121 ± 0.009 a | 0.004 |
| Available P | mg kg−1 | 7.27 ± 0.75 b | 39.07 ± 11.19 a | 19.85 ± 3.81 ab | 0.003 |
| Ca | mg kg−1 | 739.5 ± 86.4 b | 1498.5 ± 174.9 a | 904.7 ± 162.1 ab | 0.016 |
| K | mg kg−1 | 69.5 ± 4.4 b | 251.2 ± 27.6 a | 202.7 ± 18.7 a | <0.0001 |
| Mg | mg kg−1 | 130.5 ± 14.9 | 228.2 ± 30.8 | 167.5 ± 24.4 | ns |
| Na | mg kg−1 | 30.5 ± 1.9 b | 42.5 ± 2.5 a | 39.0 ± 2.1 a | 0.008 |
| Microbial properties | |||||
| Microbial substrate utilization | µg CO2-C g−1 h−1 | 0.382 ± 0.067 b | 2.032 ± 0.718 a | 1.82 ± 0.406 a | 0.001 |
| Microbial functional diversity (H′) | 2.772 ± 0.0002 a | 2.764 ± 0.002 b | 2.766 ± 0.0024 ab | 0.019 | |
| Microbial functional evenness (E′) | 0.999 ± 0.0002 a | 0.991 ± 0.002 b | 0.994 ± 0.0024 b | 0.014 | |
| Decomposition rate (Kr) | −year | 0.010 ± 0.001 | 0.011 ± 0.002 | 0.014 ± 0.003 | ns |
| Soil functional groups’ properties | |||||
| Bacterial biomass C | g C m−2 | 7.31 ± 2.68 | 12.78 ± 3.98 | 12.73 ± 2.63 | ns |
| Fungal biomass C | g C m−2 | 8.33 ± 1.07 | 13.90 ± 2.42 | 16.65 ± 2.61 | ns |
| Total microbial biomass C | g C m−2 | 15.64 ± 4.16 b | 26.68 ± 5.68 ab | 29.38 ± 7.59 a | 0.01 |
| Fungal-to-bacterial biomass C | unitless | 0.595 ± 0.057 | 0.593 ± 0.064 | 0.564 ± 0.056 | ns |
| Flagellates | g C m−2 | 0.0006 ± 0.0002 b | 0.009 ± 0.002 a | 0.001 ± 0.0004 b | <0.0001 |
| Amoeba | g C m−2 | 0.016 ± 0.007 b | 0.423 ± 0.193 a | 0.057 ± 0.027 b | 0.004 |
| Ciliates | g C m−2 | 0.0006 ± 0.0002 | 0.013 ± 0.009 | 0.0003 ± 0.0001 | ns |
| All protists | g C m−2 | 0.017 ± 0.007 b | 0.445 ± 0.034 a | 0.059 ± 0.027 b | <0.0001 |
| Bacteriophagous nematodes | g C m−2 | 2.703 ± 0.699 b | 8.243 ± 2.028 a | 9.439 ± 2.632 a | 0.037 |
| Fungivorous nematodes | g C m−2 | 0.82 ± 0.14 b | 2.70 ± 0.83 b | 3.87 ± 0.98 a | 0.004 |
| Plant associated nematodes | g C m−2 | 0.90 ± 0.23 b | 3.24 ± 0.24 a | 4.48 ± 1.18 a | 0.012 |
| Omnivorous nematodes | g C m−2 | 1.148 ± 0.483 | 3.102 ± 1.052 | 7.945 ± 2.10 | ns |
| All nematodes | g C m−2 | 5.57 ± 1.32 b | 17.29 ± 4.03 a | 26.22 ± 5.95 a | 0.011 |
| Poduromorpha (collembola) | g C m−2 | 2.19 ± 1.13 | 11.62 ± 3.53 | 7.37 ± 2.12 | ns |
| Entomobryomorpha (collembola) | g C m−2 | 0.0007 ± 0.0002 b | 0.006 ± 0.001 a | 0.0002 ± 0.0001 b | <0.0001 |
| All collembola | g C m−2 | 0.002 ± 0.001 b | 0.015 ± 0.002 a | 0.005 ± 0.001 b | <0.0001 |
| Predatory mites | g C m−2 | 0.0001 ± 0.0001 b | 0.0006 ± 0.0004 a | 0.0004 ± 0.545 b | <0.0001 |
| Fungivorous oribatid mites | g C m−2 | 0.001 ± 0.0003 c | 0.02 ± 0.003 a | 0.003 ± 0.001 b | <0.0001 |
| All microarthropods | g C m−2 | 0.023 ± 0.005 b | 0.676 ± 0.183 a | 0.122 ± 0.082 b | <0.0001 |
| REG-INT % Δ | MIN-INT % Δ | MIN-REG % Δ | |
|---|---|---|---|
| % Sand | −63.5 | −39.2 | 14.9 |
| Bulk density | −20.6 | −42.6 | −18.3 |
| Aggregate stability | −61.1 | 25.0 | 53.4 |
| % SOC | 57.5 | 70.7 | 31.0 |
| % Labile SOC | 83.7 | 86.5 | 17.3 |
| pH | 15.9 | 0.0 | −18.9 |
| EC | 57.1 | 25.0 | −75.0 |
| Microbial functional diversity (H’) | −0.3 | −0.2 | 0.1 |
| Total microbial substrate utilization | 81.2 | 79.0 | −11.6 |
| Microbial biomass | 41.4 | 46.8 | 9.2 |
| Flagellates | 93.7 | 47.7 | −728.2 |
| Amoeba | 96.7 | 73.1 | −703.1 |
| Total protists | 95.0 | 58.6 | −727.4 |
| Bacteriophagous nematodes | 67.2 | 71.4 | 12.7 |
| Fungivorous nematodes | 69.7 | 78.8 | 30.2 |
| Plant associated nematodes | 72.1 | 79.9 | 27.8 |
| Total nematodes | 68.8 | 75.6 | 21.6 |
| Entomobryid collembolans | 89.0 | −222.6 | −2822.6 |
| Total collembolans | 85.7 | 59.5 | −183.1 |
| Predatory mites | 84.6 | 31.9 | −341.9 |
| Fungivorous oribatid mites | 94.6 | 69.6 | −465.3 |
| Total arthropods | 87.4 | 49.7 | −297.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, P.; Doblas-Miranda, E.; Silva-Sánchez, A.; Mattana, S.; Font, F. Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes. Agronomy 2022, 12, 2643. https://doi.org/10.3390/agronomy12112643
Andrés P, Doblas-Miranda E, Silva-Sánchez A, Mattana S, Font F. Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes. Agronomy. 2022; 12(11):2643. https://doi.org/10.3390/agronomy12112643
Chicago/Turabian StyleAndrés, Pilar, Enrique Doblas-Miranda, Alex Silva-Sánchez, Stefania Mattana, and Francesc Font. 2022. "Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes" Agronomy 12, no. 11: 2643. https://doi.org/10.3390/agronomy12112643
APA StyleAndrés, P., Doblas-Miranda, E., Silva-Sánchez, A., Mattana, S., & Font, F. (2022). Physical, Chemical, and Biological Indicators of Soil Quality in Mediterranean Vineyards under Contrasting Farming Schemes. Agronomy, 12(11), 2643. https://doi.org/10.3390/agronomy12112643






