Comparative Study on Pollen Viability of Camellia oleifera at Four Ploidy Levels
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pollen Collection
2.2. Pollen Ploidy
2.3. Pollen Size
2.4. Pollen Viability
2.5. Pollen Germination
2.6. Statistical Analysis
3. Results
3.1. Pollen Size Measured after Staining and Scanning Electron Microscopy
3.2. Pollen Viability Assessed by Staining
3.3. Pollen Germination Rate Assessed by In Vitro Germination
4. Discussion
4.1. Pollen Size Related to the Ploidy Level
4.2. Pollen Viability and Pollen Germination at Different Ploidy Levels
4.3. Comparison of Two Vigor Measurement Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.L.; Li, J.A.; Li, Z.; Zhang, F.H.; Tan, X.F. Transcriptomic Analyses of Camellia oleifera ‘Huaxin’ Leaf Reveal Candidate Genes Related to Long-Term Cold Stress. Int. J. Mol. Sci. 2020, 21, 846. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, R.L. Oil-Tea Camellia in China, 2nd ed.; China Forestry Publishing House: Beijing, China, 2011. [Google Scholar]
- Ma, J.; Hang, Y.; Rui, Y.; Chen, G.; Zhang, N. Fatty acid composition of Camellia oleifera oil. J. Für Verbrauch. Und Lebensm. 2011, 6, 9–12. [Google Scholar] [CrossRef]
- Yao, X.H.; Wang, Y.P.; Wang, K.L.; Ren, H.D. Effects of geographic latitude and longitude on fat and its fatty acid composition of oil-tea camellia seeds. China Oils Fats. 2011, 36, 31–34. [Google Scholar]
- He, L.; Zhou, G.Y.; Zhang, H.Y.; Liu, J.A. Research progress on the health function of tea oil. J. Med. Plants Res. 2011, 5, 485–489. [Google Scholar]
- Chen, J.M.; Yang, X.Q.; Huang, X.M.; Duan, S.H.; Long, C.; Chen, J.K.; Rong, J. Leaf transcriptome analysis of a subtropical evergreen broadleaf plant, wild oil-tea camellia (Camellia oleifera), revealing candidate genes for cold acclimation. BMC Genom. 2017, 18, 211. [Google Scholar] [CrossRef]
- Qin, S.Y.; Rong, J.; Zhang, W.J.; Chen, J.K. Cultivation history of Camellia oleifera and genetic resources in the Yangtze River Basin. Biodivers. Sci. 2018, 26, 384–395. [Google Scholar] [CrossRef]
- Boavida, L.C.; Vieira, A.M.; Becker, J.D.; Feijo, J.A. Gametophyte interaction and sexual reproduction: How plants make a zygote. Int. J. Dev. Biol. 2005, 49, 615–632. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.R.; Dong, X.X.; Li, Y.; Wang, B.S. NaCl treatment markedly enhanced pollen viability and pollen preservation time of euhalophyte Suaeda salsa via up regulation of pollen development-related genes. J. Plant Res. 2020, 133, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Hine, A.; Rojas, A.; Suarez, L.; Murillo, O.; Espinoza, M. Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs. Forests 2019, 10, 908. [Google Scholar] [CrossRef]
- Chagas, E.A.; Pio, R.; Chagas, P.C.; Pasqual, M.; Bettiol, J.E.N. Composição do meio de cultura e condições ambientais para germinação de grãos de pólen de porta-enxertos de pereira. Ciência Rural. 2010, 40, 261–266. [Google Scholar] [CrossRef]
- Nogueira, P.V.; Silva, D.F.; Pio, R.; Silva, P.A.O.; Bisi, R.B.; Balbi, R.V. Germinação de pólen e aplicação de ácido bórico em botões florais de nespereiras. Bragantia 2015, 74, 9–15. [Google Scholar] [CrossRef][Green Version]
- Grant, V. Plant Speciation, 2nd ed.; Columbia Univ. Press: New York, NY, USA, 1981. [Google Scholar]
- Ramsey, J.; Schemske, D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998, 29, 467–501. [Google Scholar] [CrossRef]
- Lim, K.B.; Chung, J.D.; Van Kronenburg, B.C.E.; Ramanna, M.S.; De Jong, J.H.; Van Tuyl, J.M. Introgression of Lilium rubellum Baker chromosomes into L. longiflorum Thunb: A genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromosome Res. 2000, 8, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Alexander, L. Ploidy Level Influences Pollen Tube Growth and Seed Viability in Interploidy Crosses of Hydrangea macrophylla. Front. Plant Sci. 2020, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Hembree, W.G.; Ranney, T.G.; Jackson, B.E.; Weathington, M. Cytogenetics, ploidy, and genome sizes of Camellia and related genera. HortScience 2019, 54, 1124–1142. [Google Scholar] [CrossRef]
- Novara, C.; Ascari, L.; Morgia, V.L.; Reale, L.; Genre, A.; Siniscalco, C. Viability and germinability in long term storage of Corylus avellana pollen. Sci. Hortic. 2017, 214, 295–303. [Google Scholar] [CrossRef]
- Mccallum, B.; Chang, S.M. Pollen competition in style: Effects of pollen size on siring success in the hermaphroditic common morning glory. Ipomoea Purpurea. Am. J. Bot. 2016, 103, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Souza-Pérez, M.; Mourelle, D.; Tr Ujillo, C.; Borges, C.; Speroni, G. Pollen grain performance in Psidium cattleyanum (Myrtaceae): A pseudogamous polyploid species. Flora 2021, 281, 151863. [Google Scholar] [CrossRef]
- Kolarčik, V.; Vašková, D.; Mirková, M.; Mártonf, P. Pollen morphology in natural diploid polyploid hybridogeneous complex of the genus Onosma (Boraginaceae–Lithospermeae). Plant Syst. Evol. 2018, 305, 151–168. [Google Scholar] [CrossRef]
- Han, C.X.; Zhou, T.L.; Li, Y.M.; Ye, T.W.; Hu, X.; Zhao, J.R.; Yuan, D.Y.; Xiao, S.X. Regeneration of different ploidy callus from anther culture of Camellia oleifera. Mol. Plant Breed. 2022, 20, 12. [Google Scholar]
- Li, Y.M.; Ye, T.W.; Han, C.X.; Ye, Z.H.; Zhang, J.; Xiao, S.X.; Yuan, D.Y. Cytogenetic analysis of interspecific hybridization in oil-tea (Camellia oleifera). Euphytica 2021, 217, 28. [Google Scholar] [CrossRef]
- Vrána, J.; Cápal, P.; Bednářová, M.; Doležel, J. Flow cytometry in plant research: A success story. Appl. Plant Cell Biol. 2014, 22, 395–430. [Google Scholar]
- Hu, Z.; Zhao, Y.; Zhao, C.; Liu, J. Taxonomic importance of pollen morphology in Veratrum L. (Melanthiaceae) using microscopic techniques. Microsc. Res. Tech. 2020, 83, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Coope, R.; Schneider, E.; Osborn, J. Pollen structure and development in Nymphaeales: Insights into character evolution in an ancient angiosperm lineage. Am. J. Bot. 2015, 102, 1685–1702. [Google Scholar] [CrossRef] [PubMed]
- Bispo, R.; Santos, L.; Santos, J.; Karsburg, I. Viability estimation and morphological characterization of pollen Datura suaveolens Willd (Solanaceae). Sci. Electron. Arch. 2018, 11, 61–64. [Google Scholar]
- Ascari, L.; Novara, C.; Dusio, V.; Oddi, L.; Siniscalco, C. Quantitative methods in microscopy to assess pollen viability in different plant taxa. Plant Reprod. 2020, 33, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Rotreklová, O.; Krahulcová, A. Estimating paternal efficiency in an agamic polyploid complex: Pollen stainability and variation in pollen size related to reproduction mode, ploidy level and hybridogenous origin in Pilosella (Asteraceae). Folia Geobot. 2016, 51, 175–186. [Google Scholar] [CrossRef]
- Xiong, H.; Zou, F.; Yuan, D.Y.; Zhang, X.; Tan, X.F. Orthogonal test design for optimizing the culture medium for in vitro pollen germination of feijoa (Acca sellowiana cv. Unique). New Zealand J. Crop Hortic. Sci. 2016, 44, 192–202. [Google Scholar] [CrossRef]
- Yuan, D.Y.; Tan, X.F.; Zou, F.; Cui, M.J.; Liang, Y.Q. Comparative study on pollen viability detection methods of Camellia Oleifera. J. Southwest For. Univ. 2009, 29, 10–12. [Google Scholar]
- Deng, Z.Y.; Xiong, H.; Yuan, D.Y.; Niu, G.H.; Zou, F. Orthogonal Test Design for Optimizing Culture Medium for in vitro Pollen Germination of Chinese Chinquapin (Castanea henryi). Taiwan J. Sci. 2018, 33, 185–195. [Google Scholar]
- Ren, R.F.; Li, Z.D.; Zhou, H.; Zhang, L.L.; Jiang, X.R.; Liu, Y. Changes in apoptosis-like programmed cell death and viability during the cryopreservation of pollen from Paeonia suffruticosa. Plant Cell Tissue Organ Cult. (PCTOC) 2020, 140, 357–368. [Google Scholar] [CrossRef]
- Retagnolle, F.; Thompson, J.D.; Lumaret, R. The influence of seed size variation on seed germination and seedling vigour in diploid and tetraploid Dactylis glomerat L. Ann. Bot. 1995, 76, 607–615. [Google Scholar] [CrossRef]
- Qiang, S. Polyploidization, one of the driving forces for weed origin and evolution. J. Nanjing Agric. Univ. 2012, 35, 64–76. [Google Scholar]
- Shi, Q.H.; Liu, P.; Liu, M.J. Advances in Ploidy Breeding of Fruit Trees. Acta Hortic. Sin. 2012, 39, 1639–1654. [Google Scholar]
- Nico, D.S.; Linda, Z.; Martin, M.; Sharbel, T.F.; Danny, G. Volume-based pollen size analysis: An advanced method to assess somatic and gametophytic ploidy in flowering plants. Plant Reprod. 2013, 26, 65–81. [Google Scholar]
- Katsiotis, A.; Forsberg, R.A. Pollen grain size in four ploidy levels of genusAvena. Euphytica 1995, 83, 103–108. [Google Scholar] [CrossRef]
- Den Nijs, J.C.M.; Hooghiemstra, H.; Schalk, P.H. Biosystematic studies of the Rumex acetocella complex (Polygonaceae). IV. Pollen morphology and the possibilities of identification of cytotypes in pollen analysis. Phyton 1980, 20, 307–323. [Google Scholar]
- Jan, F.; Schüler, L.; Behling, H. Trends of pollen grain size variation in C3 and C4 Poaceae species using pollen morphology for future assessment of grassland ecosystem dynamics. Grana Palynol. 2015, 54, 129–145. [Google Scholar] [CrossRef]
- Cui, G.F.; Du, W.W.; Duan, Q.; Jia, W.J.; Wang, X.N. Analysis on Pollen Morphology and Variation of Viability of Lilium Asiatic Hybrids with Different Ploidy. Acta Agric. Boreali-Sin. 2015, 30, 55–60. [Google Scholar]
- Thuzar, M.; Puteh, A.; Abdullah, N.A.P.; Lassim, M.M.; Jusoff, K. The effects of temperature stress on the quality and yield of soya bean (Glycine max L.). J. Agric. Sci. 2010, 2, 172. [Google Scholar]
- Sulusoglu, M.; Cavusoglu, A. In Vitro Pollen Viability and Pollen Germination in Cherry Laurel (Prunus laurocerasus L.). Sci. World J. 2014, 7, 657123. [Google Scholar]
- Ferreira, M.D.S.; Soares, T.L.; Costa, E.M.R.; Silva, R.L.D.; Souza, F.V.D. Optimization of culture medium for the in vitro germination and histochemical analysis of Passiflora spp. pollen grains. Sci. Hortic. 2021, 288, 110298. [Google Scholar] [CrossRef]
- Shivanna, R.K.; Mohan Ram, Y.H. Pollination biology: Contributions to fundamental and applied aspects. Curr. Sci. 1993, 65, 226–233. [Google Scholar]
- Luo, S.; Zhang, K.; Zhong, W.P.; Chen, P.; Fan, X.M.; Yuan, D.Y. Optimization of in vitro pollen germination and pollen viability tests for Castanea mollissima and Castanea henryi. Sci. Hortic. 2020, 271, 109481. [Google Scholar] [CrossRef]
- Atlagić, J.; Terzić, S.; Marjanović-Jeromela, A. Staining and fluorescent microscopy methods for pollen viability determination in sunflower and other plant species. Ind. Crops Prod. 2012, 35, 88–89. [Google Scholar] [CrossRef]
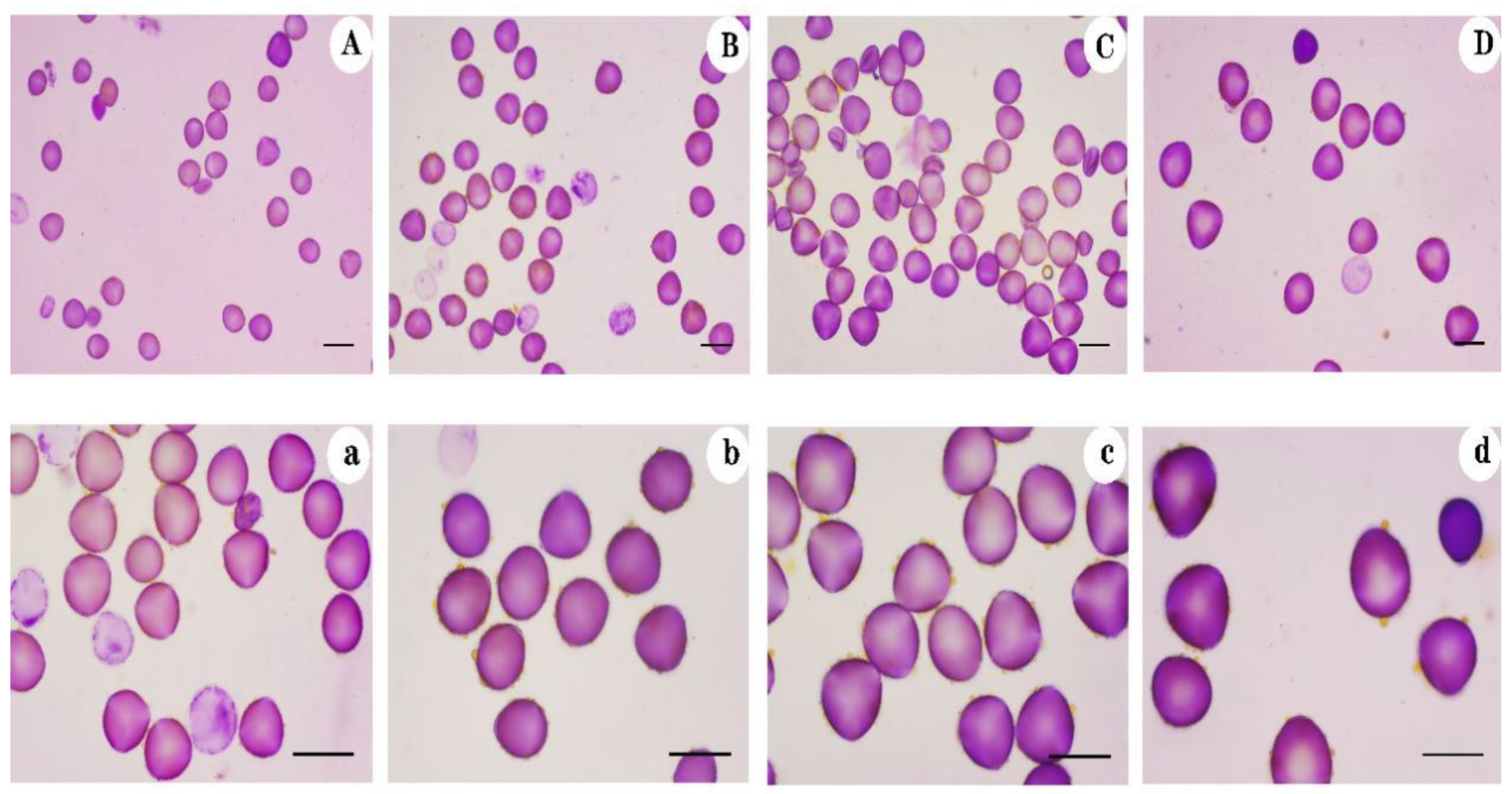
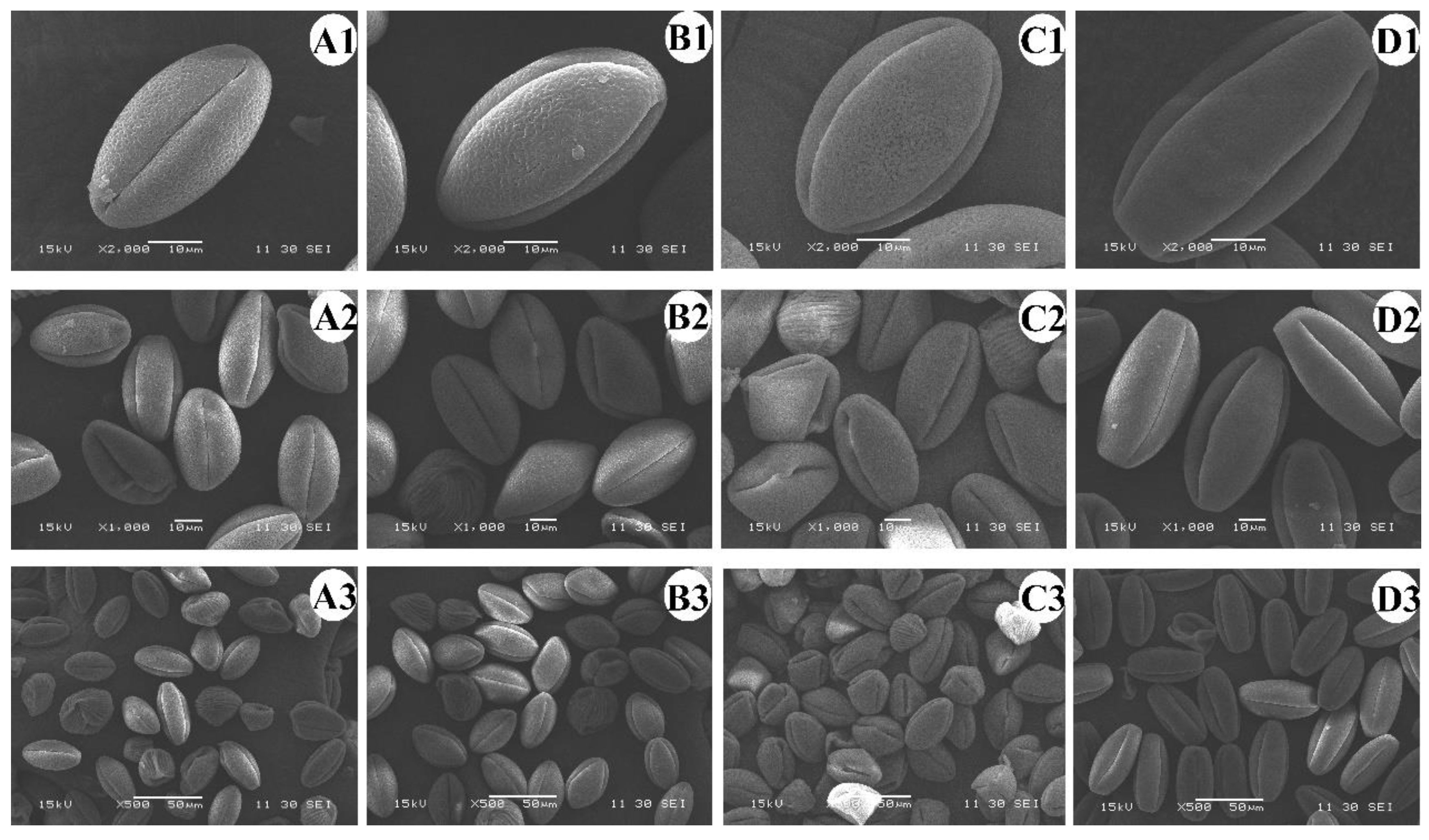
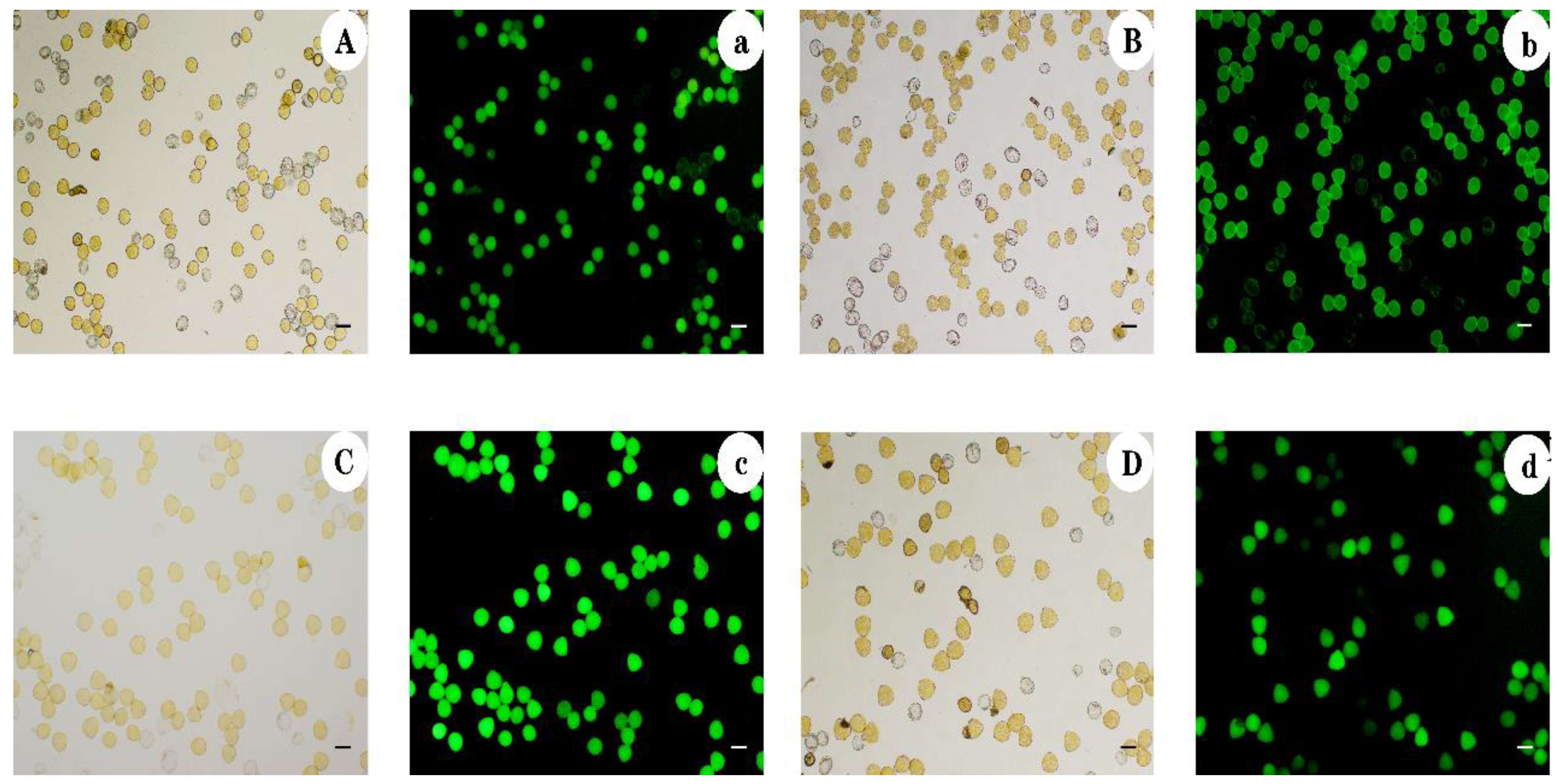
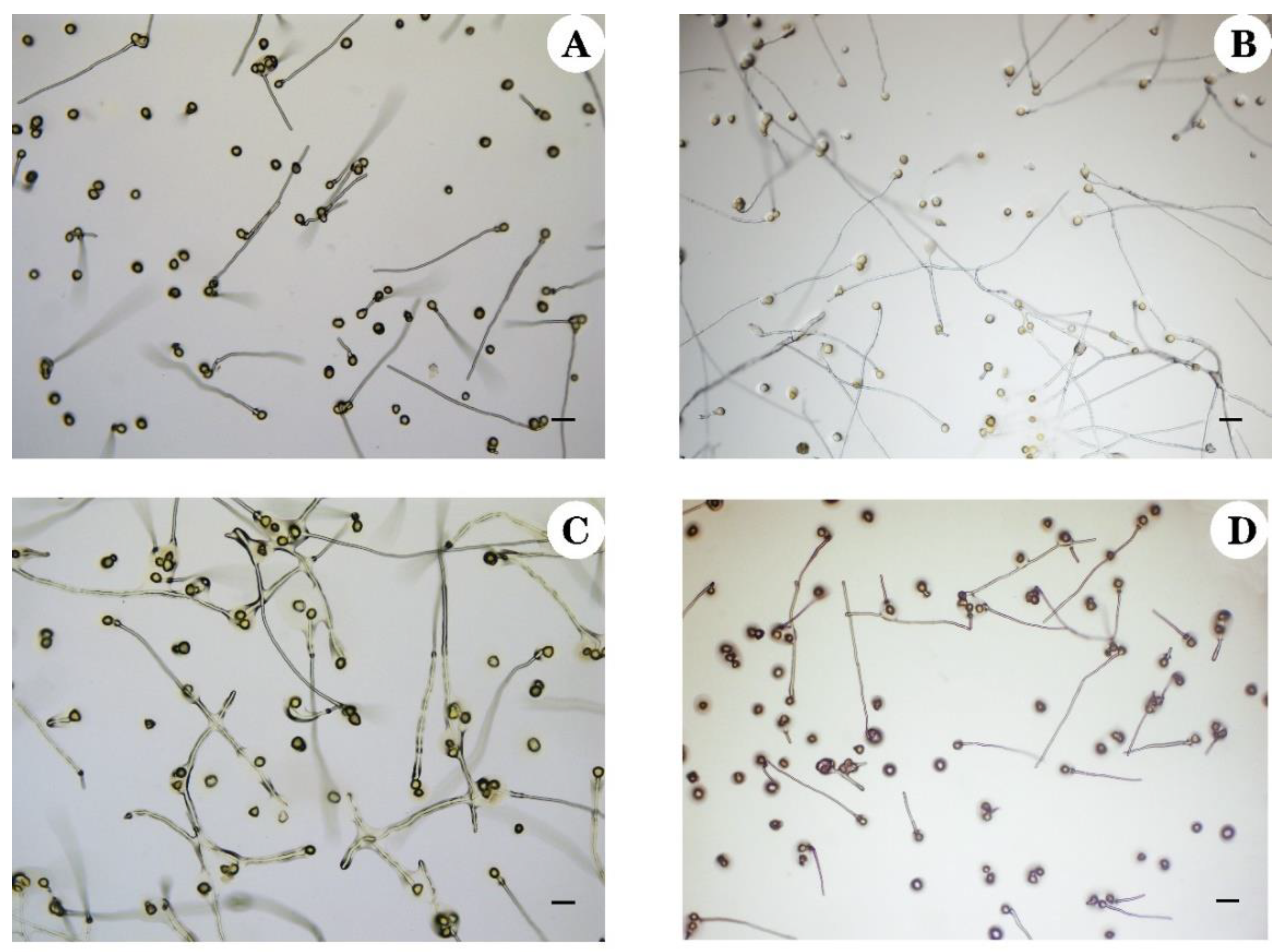
| Ploidy Level | Pollen Type | Pollen Diameter (μm) | Mean Pollen Diameter (μm) | Ploidy Level | Pollen Type | Pollen Diameter | Mean Pollen Diameter (μm) |
|---|---|---|---|---|---|---|---|
| diploid | NR-1 | 38.85 ± 3.11 ab | 37.85 ± 3.00 d | tetraploid | DP43 | 42.81 ± 2.36 ab | 41.92 ± 2.73 c |
| NR-2 | 35.88 ± 3.18 d | DP47 | 40.89 ± 3.67 bc | ||||
| NR-3 | 36.23 ± 3.63 bc | DB(1) | 40.23 ± 2.78 cd | ||||
| NR-4 | 39.30 ± 2.92 a | DF24 | 44.24 ± 2.56 a | ||||
| NR-5 | 39.04 ± 3.41 b | ZX0907 | 40.94 ± 1.87 bc | ||||
| NR-6 | 38.31 ± 2.38 c | DBH | 42.98 ± 3.70 b | ||||
| NR-9 | 37.60 ± 3.09 bc | MX-5 | 42.31 ± 2.60 c | ||||
| NR-10 | 37.61 ± 2.31 bc | CJ-03 | 40.92 ± 2.28 bc | ||||
| hexaploid | CJ-12 | 48.46 ± 3.71 ab | 49.40 ± 3.42 ab | octaploid | YNYC-1 | 49.47 ± 3.19 e | 50.54 ± 3.63 a |
| ASX0902 | 50.75 ± 2.87 b | YNYC-2 | 53.92 ± 3.39 b | ||||
| TXP-14 | 47.49 ± 2.77 c | YNYC-3 | 58.16 ± 6.19 a | ||||
| CJ-07 | 48.68 ± 5.43 ab | YNYC-4 | 52.40 ± 2.81 c | ||||
| XH097 | 50.06 ± 2.86 ab | YNYC-5 | 50.11 ± 3.35 d | ||||
| LE38 | 50.87 ± 2.73 a | YNYC-7 | 47.64 ± 3.58 de | ||||
| LE3 | 48.80 ± 3.37 ab | YNYC-8 | 46.31 ± 3.74 ef | ||||
| GTDB 02 | 50.06 ± 3.63 ab | YNYC-9 | 46.34 ± 2.79 ef |
| Ploidy Level | Polar Axis Length (μm) | Equatorial Length (μm) |
|---|---|---|
| diploid | 36.68 ± 2.87 d | 22.05 ± 1.13 c |
| tetraploid | 44.27 ± 3.06 c | 27.00 ± 1.42 b |
| hexaploid | 47.93 ± 2.39 b | 29.97 ± 1.71 ab |
| octaploid | 58.51 ± 2.88 a | 30.84 ± 2.08 a |
| Ploidy Level | Pollen Type | Pollen Viability (%) | Pollen Germination Rate (%) | Ploidy Level | Pollen Type | Pollen Viability (%) | Pollen Germination Rate (%) |
|---|---|---|---|---|---|---|---|
| diploid | NR-1 | 45.89 b | 34.52 d | tetraploid | DP43 | 80.25 a | 76.45 a |
| NR-2 | 62.83 ab | 40.17 c | DP47 | 72.76 cd | 72.06 bc | ||
| NR-3 | 74.61 a | 53.71 ab | DB(1) | 75.71 c | 74.05 bc | ||
| NR-4 | 63.56 ab | 50.65 bc | DF24 | 78.24 b | 66.28 bc | ||
| NR-5 | 63.17 ab | 50.75 b | ZX0907 | 56.40 e | 65.56 c | ||
| NR-6 | 63.77 ab | 49.21 bc | DBH | 71.52 cd | 75.47 b | ||
| NR-9 | 72.04 ab | 61.00 a | MX-5 | 67.12 d | 68.15 bc | ||
| NR-10 | 68.91 ab | 55.64 ab | CJ-03 | 65.60 de | 66.22 bc | ||
| hexaploid | CJ-12 | 88.24 a | 84.59 a | octaploid | YNYC-1 | 77.56 a | 69.79 a |
| ASX0902 | 74.28 cd | 77.04 b | YNYC-2 | 65.45 b | 63.25 ab | ||
| TXP-14 | 83.95 ab | 57.03 d | YNYC-3 | 63.57 bc | 25.71 b | ||
| CJ-07 | 74.70 cd | 63.58 bc | YNYC-4 | 74.76 ab | 65.10 ab | ||
| XH097 | 85.38 ab | 83.98 ab | YNYC-5 | 75.11 ab | 68.87 ab | ||
| LE38 | 76.34 bc | 64.70 c | YNYC-7 | 24.06 cd | 14.86 bc | ||
| LE3 | 79.33 b | 63.11 bc | YNYC-8 | 27.87 c | 19.68 c | ||
| GTDB 02 | 75.29 c | 80.12 ab | YNYC-9 | 12.10 d | 8.89 cd |
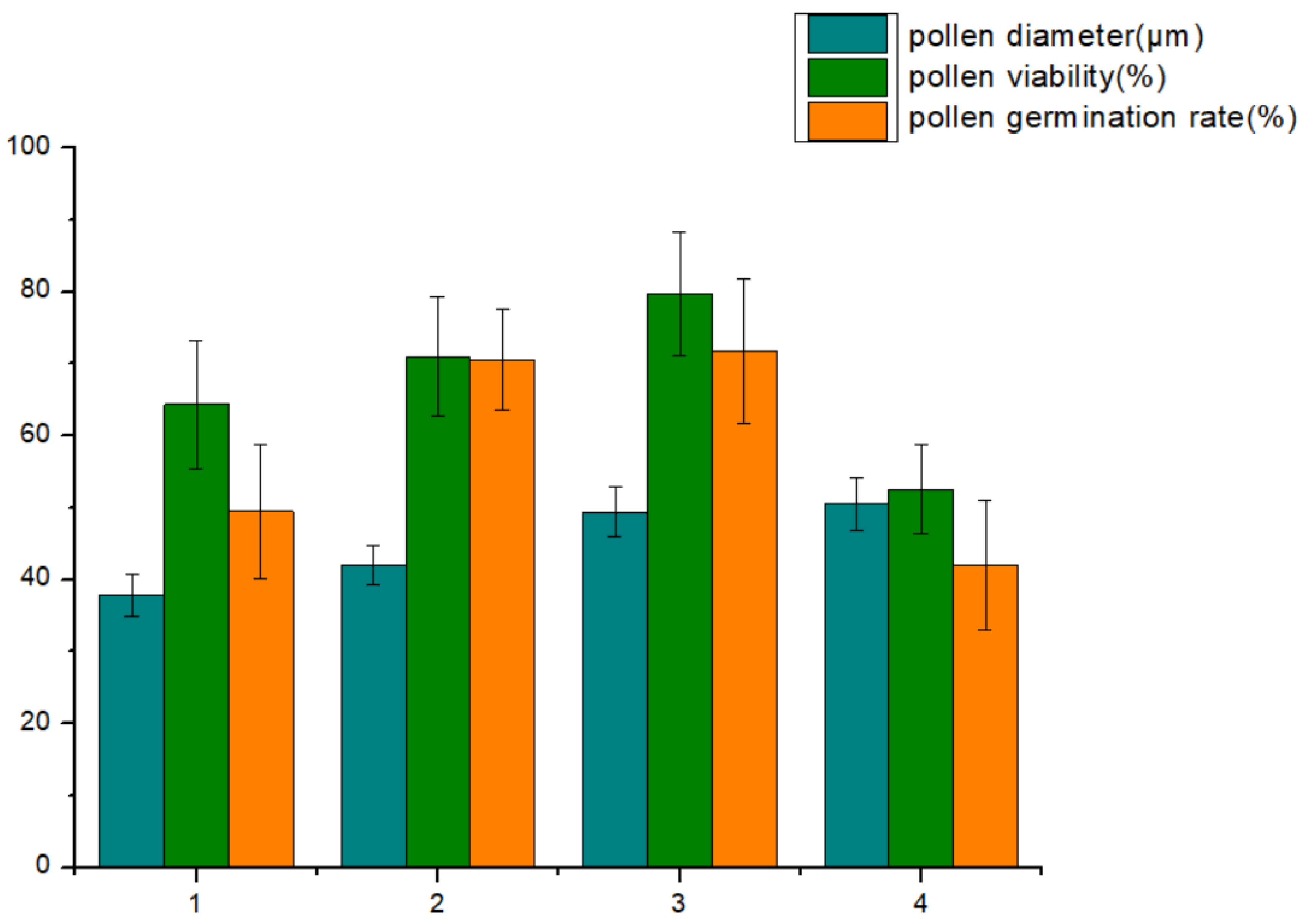
| Ploidy Level | Mean Pollen Diameter (μm) | Mean Pollen Viability (%) | Mean Pollen Germination Rate (%) |
|---|---|---|---|
| diploid | 37.85 ± 3.00 d | 64.35 ± 8.90 b | 49.46 ± 9.30 b |
| tetraploid | 41.92 ± 2.73 c | 70.95 ± 8.33 ab | 70.53 ± 7.00 ab |
| hexaploid | 49.40 ± 3.42 ab | 79.69 ± 8.58 a | 71.78 ± 10.00 a |
| octaploid | 50.54 ± 3.63 a | 52.56 ± 6.14 c | 42.02 ± 9.00 c |
| Factor | SS | DF | F | P | Significance |
|---|---|---|---|---|---|
| pollen diameter | 885.142 | 3 | 53.936 | 0.000 | * |
| pollen viability | 3136.838 | 3 | 4.774 | 0.008 | * |
| pollen germination rate | 5396.710 | 3 | 7.704 | 0.001 | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Xu, L.; Xu, X.; Li, Y.; Xiao, S.; Yuan, D. Comparative Study on Pollen Viability of Camellia oleifera at Four Ploidy Levels. Agronomy 2022, 12, 2592. https://doi.org/10.3390/agronomy12112592
Zhao R, Xu L, Xu X, Li Y, Xiao S, Yuan D. Comparative Study on Pollen Viability of Camellia oleifera at Four Ploidy Levels. Agronomy. 2022; 12(11):2592. https://doi.org/10.3390/agronomy12112592
Chicago/Turabian StyleZhao, Rui, Linjie Xu, Xiangshuai Xu, Yanmin Li, Shixin Xiao, and Deyi Yuan. 2022. "Comparative Study on Pollen Viability of Camellia oleifera at Four Ploidy Levels" Agronomy 12, no. 11: 2592. https://doi.org/10.3390/agronomy12112592
APA StyleZhao, R., Xu, L., Xu, X., Li, Y., Xiao, S., & Yuan, D. (2022). Comparative Study on Pollen Viability of Camellia oleifera at Four Ploidy Levels. Agronomy, 12(11), 2592. https://doi.org/10.3390/agronomy12112592





