A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper- and Multi-Spectral Imagery
Abstract
1. Introduction
2. Methods and Materials
2.1. Field Experiments
2.2. Image Acquisition and Ground Sampling
2.3. Image Processing
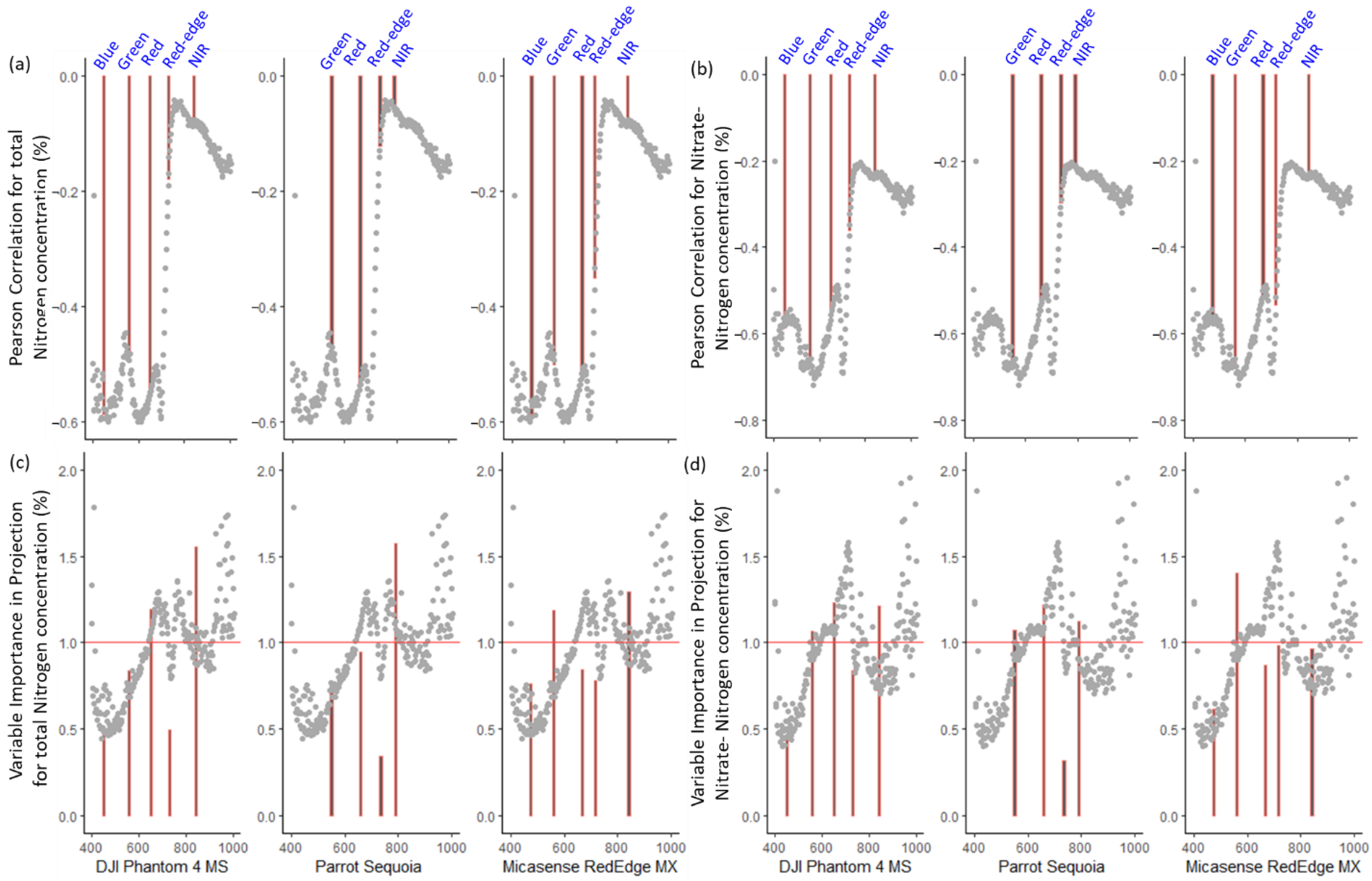
2.4. Synthetization of Multispectral Image Features
2.5. Data Analysis
2.6. Model Development and Evaluation
3. Results and Discussion
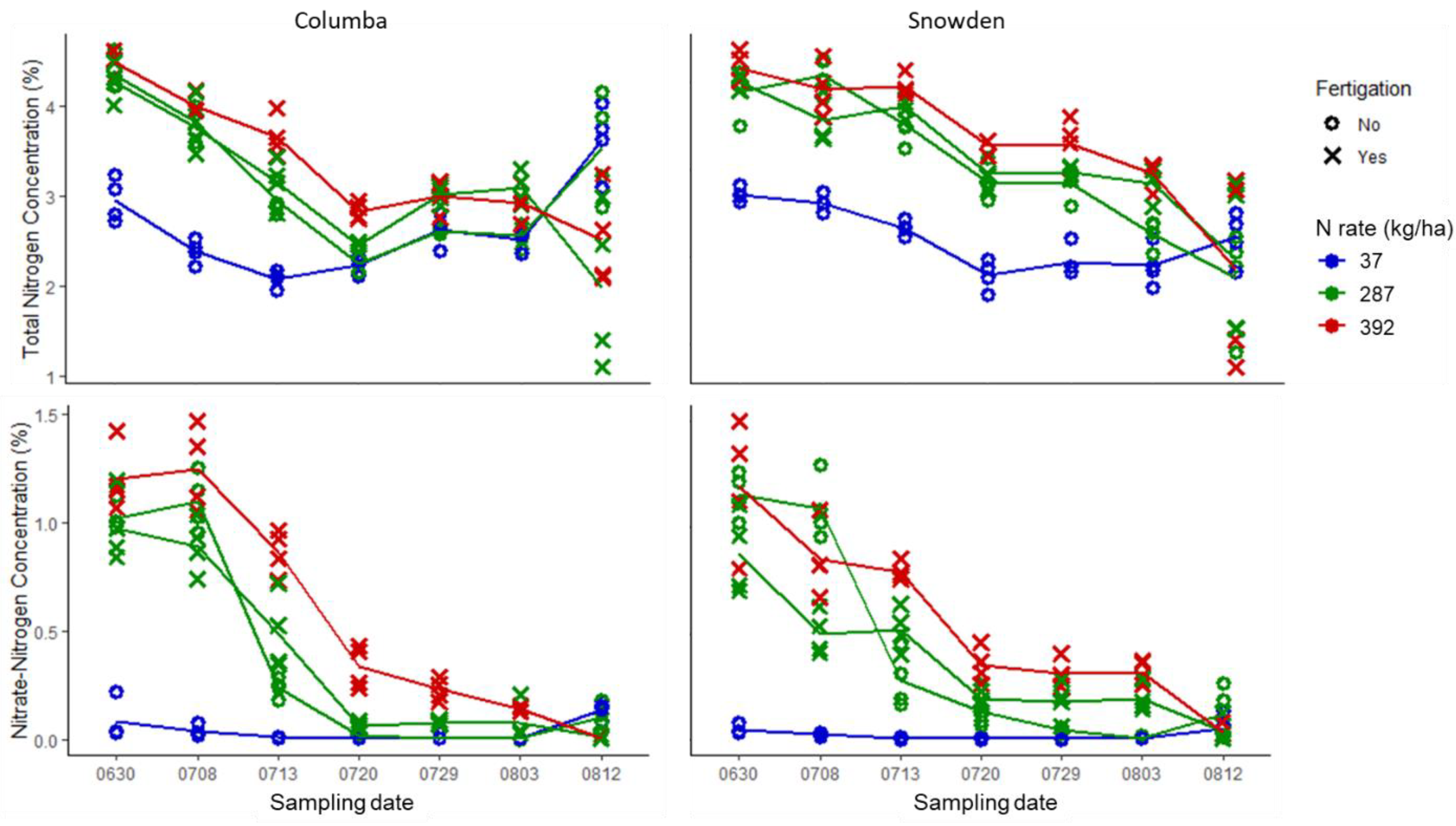
3.1. Potato N Variability by N Treatments
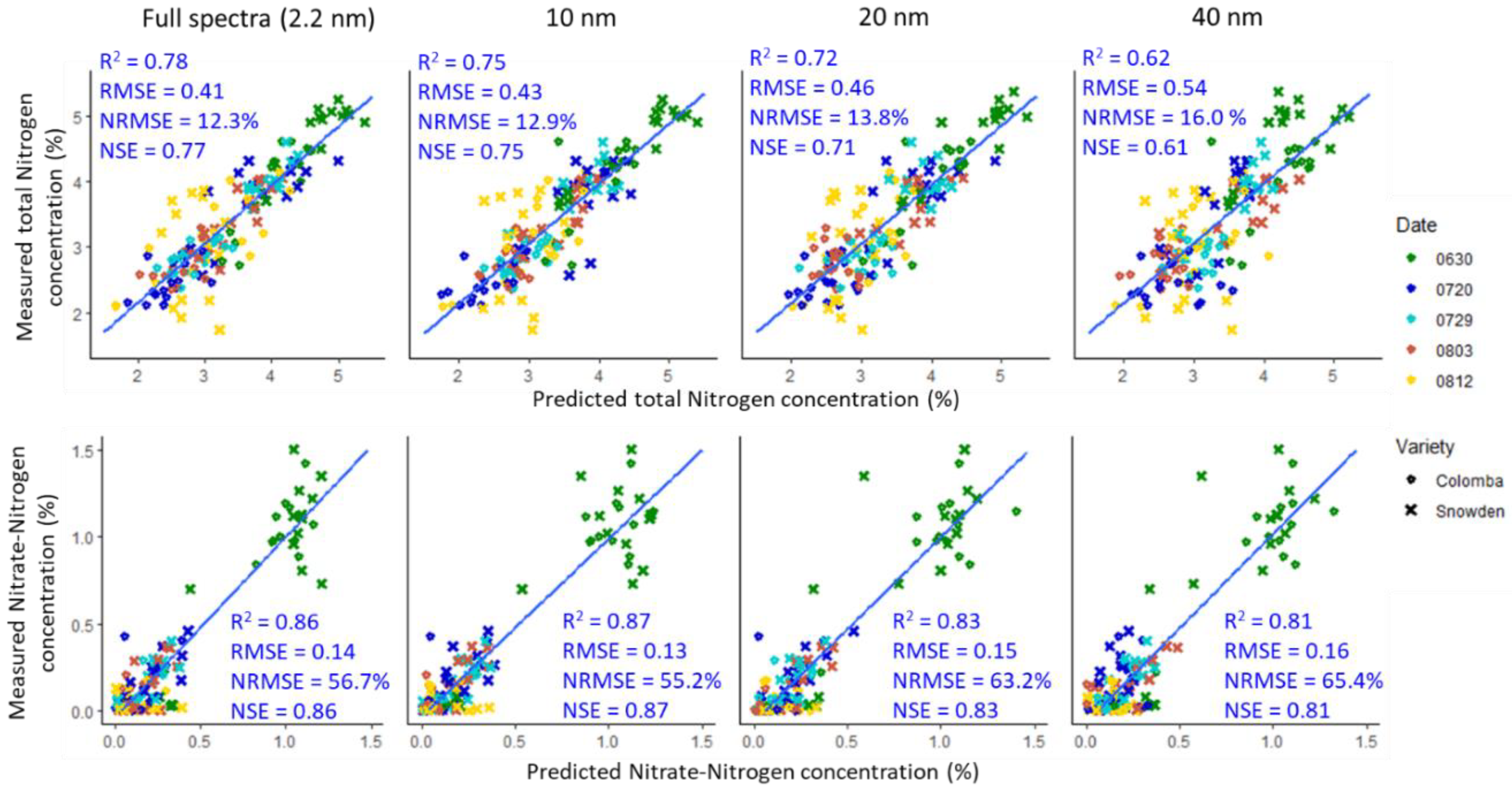
3.2. Model Performance of the Hyperspectral Narrow and Broad Bands
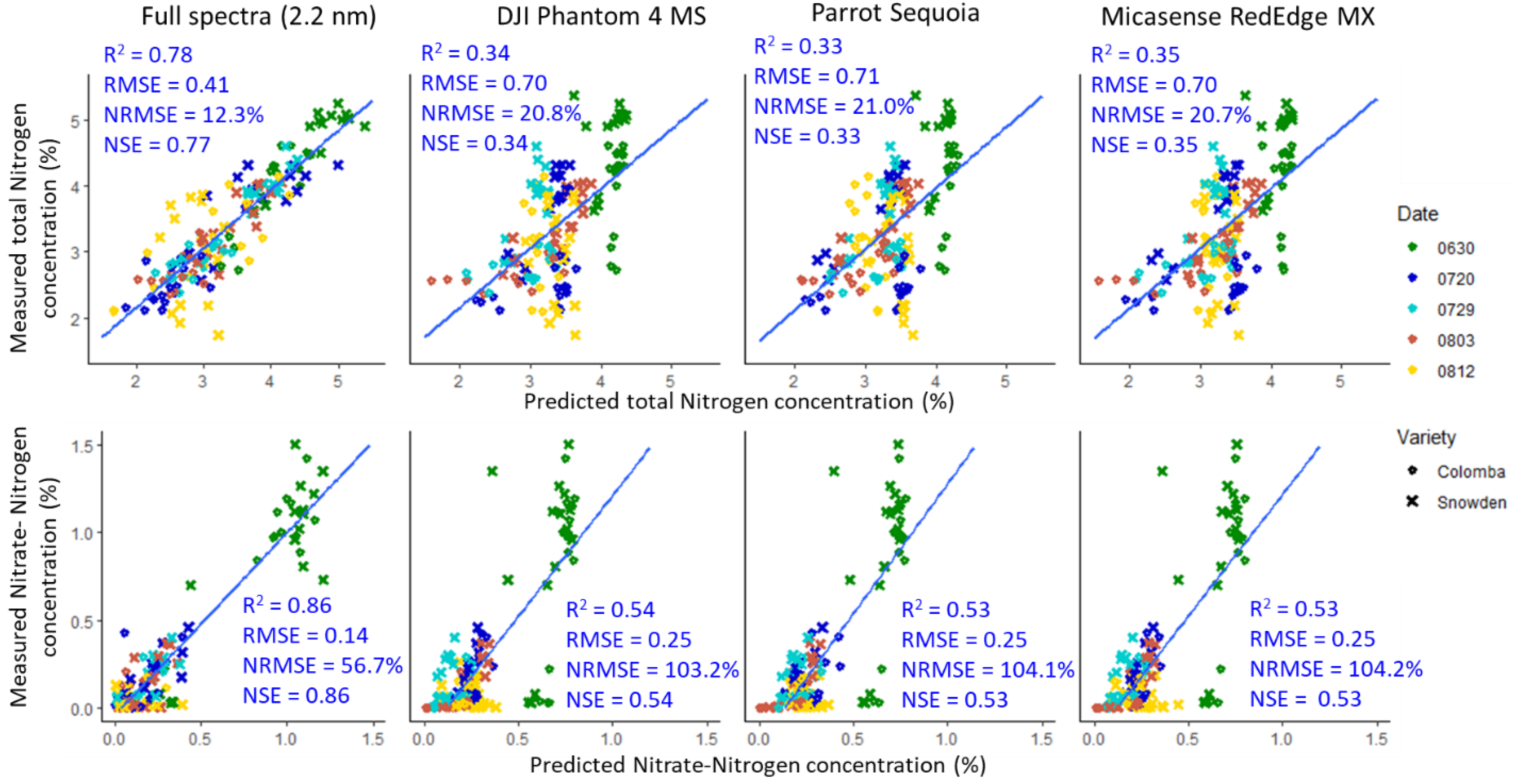
3.3. Model Performance of the Hyperspectral and Synthesized Multispectral Reflectance
3.4. Effects of Potato Variety and Imaging Date on the Model Performance
3.5. Model Performance for the Two N Concentrations
3.6. Evaluation of the Sensor Costs and Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Prosekov, A.Y.; Ivanova, S.A. Food security: The challenge of the present. Geoforum 2018, 91, 73–77. [Google Scholar] [CrossRef]
- Devaux, A.; Goffart, J.-P.; Kromann, P.; Andrade-Piedra, J.; Polar, V.; Hareau, G. The Potato of the Future: Opportunities and Challenges in Sustainable Agri-food Systems. Potato Res. 2021, 64, 681–720. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. International Year of the Potato 2008: New Light on a Hidden Treasure. Available online: https://www.fao.org/potato-2008/en/events/book.html (accessed on 11 February 2022).
- U.S. Department of Agriculture. North American Potatoes. Available online: https://www.nass.usda.gov/Publications/Todays_Reports/reports/uscp0121.pdf (accessed on 11 February 2022).
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Rud, R. Evaluation of the nitrogen sufficiency index for use with high resolution, broadband aerial imagery in a commercial potato field. Precis. Agric. 2014, 15, 202–226. [Google Scholar] [CrossRef]
- Weyermann, J.; Kneubühler, M.; Schläpfer, D.; Schaepman, M.E. Minimizing Reflectance Anisotropy Effects in Airborne Spectroscopy Data Using Ross–Li Model Inversion With Continuous Field Land Cover Stratification. IEEE Trans. Geosci. Remote Sens. 2015, 53, 5814–5823. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; de Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M. Best Management Practices for Nitrogen Use: Irrigated Potatoes. Available online: https://conservancy.umn.edu/bitstream/handle/11299/198232/n-bmps-irrigated-potatoes-2008.pdf?sequence=1 (accessed on 11 February 2022).
- Liu, N.; Townsend, P.A.; Naber, M.R.; Bethke, P.C.; Hills, W.B.; Wang, Y. Hyperspectral imagery to monitor crop nutrient status within and across growing seasons. Remote Sens. Environ. 2021, 255, 112303. [Google Scholar] [CrossRef]
- Zhang, H.; Smeal, D.; Arnold, R.; Gregory, E. Potato nitrogen management by monitoring petiole nitrate level. J. Plant Nutr. 1996, 19, 1405–1412. [Google Scholar] [CrossRef]
- Wang, Y.; Naber, M.; Crosby, T.; Liang, G. Evaluating Multiple Diagnostic Tools for Monitoring In-season Nitrogen Status of Chipping Potatoes in the Upper Midwest of the USA. Potato Res. 2021, 65, 31–50. [Google Scholar] [CrossRef]
- Bélanger, G.; Walsh, J.; Richards, J.; Milburn, P.; Ziadi, N. Critical petiole nitrate concentration of two processing potato cultivars in eastern Canada. Am. J. Potato Res. 2003, 80, 251–261. [Google Scholar] [CrossRef]
- Kaiser, D.E.; Rosen, C.J. Understanding Plant Analysis for Crops. Available online: https://extension.umn.edu/testing-and-analysis/understanding-plant-analysis-crops (accessed on 11 February 2022).
- Yang, H.; Li, F.; Hu, Y.; Yu, K. Hyperspectral indices optimization algorithms for estimating canopy nitrogen concentration in potato (Solanum tuberosum L.). Int. J. Appl. Earth Obs. Geoinf. 2021, 102, 102416. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L. Using Hyperspectral Remote Sensing Data for Retrieving Canopy Chlorophyll and Nitrogen Content. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Morier, T.; Cambouris, A.N.; Chokmani, K. In-Season Nitrogen Status Assessment and Yield Estimation Using Hyperspectral Vegetation Indices in a Potato Crop. Agron. J. 2015, 107, 1295–1309. [Google Scholar] [CrossRef]
- Herrmann, I.; Karnieli, A.; Bonfil, D.J.; Cohen, Y.; Alchanatis, V. SWIR-based spectral indices for assessing nitrogen content in potato fields. Int. J. Remote Sens. 2010, 31, 5127–5143. [Google Scholar] [CrossRef]
- Sun, H.; Li, M.; Zhang, Q.; Alva, A.K.; Zhou, Z. Preliminary study on nitrogen monitoring of potato plants using multispectral image. In Proceedings of the American Society of Agricultural and Biological Engineers Annual International Meeting 2013, ASABE, Kansas City, MO, USA, 21–24 July 2013; pp. 4007–4013. [Google Scholar]
- Zhou, Z.; Jabloun, M.; Plauborg, F.; Andersen, M.N. Using ground-based spectral reflectance sensors and photography to estimate shoot N concentration and dry matter of potato. Comput. Electron. Agric. 2018, 144, 154–163. [Google Scholar] [CrossRef]
- Cohen, Y.; Rud, R.; Rosen, C.; Mulla, D.; Heuer, B.; Dar, Z.; Levi, O.; Cohen, S.; Levi, A.; Brikman, R.; et al. The use of VIS-NIR and thermal ranges for evaluating nitrogen and water status in potato plants. In Proceedings of the Precision Agriculture 2011-Papers Presented at the 8th European Conference on Precision Agriculture 2011, Prague, Czech Republic, 11–14 July 2011; pp. 99–108. [Google Scholar]
- Nigon, T.J.; Mulla, D.J.; Rosen, C.J.; Cohen, Y.; Alchanatis, V.; Knight, J.; Rud, R. Hyperspectral aerial imagery for detecting nitrogen stress in two potato cultivars. Comput. Electron. Agric. 2015, 112, 36–46. [Google Scholar] [CrossRef]
- Hunt, E.R.; Horneck, D.A.; Spinelli, C.B.; Turner, R.W.; Bruce, A.E.; Gadler, D.J.; Brungardt, J.J.; Hamm, P.B. Monitoring nitrogen status of potatoes using small unmanned aerial vehicles. Precis. Agric. 2018, 19, 314–333. [Google Scholar] [CrossRef]
- Yang, C.; Everitt, J.H. Comparison of hyperspectral imagery with aerial photography and multispectral imagery for mapping broom snakeweed. Int. J. Remote Sens. 2010, 31, 5423–5438. [Google Scholar] [CrossRef]
- Sun, J.; Yang, J.; Shi, S.; Chen, B.; Du, L.; Gong, W.; Song, S. Estimating Rice Leaf Nitrogen Concentration: Influence of Regression Algorithms Based on Passive and Active Leaf Reflectance. Remote Sens. 2017, 9, 951. [Google Scholar] [CrossRef]
- Lee, K.-S.; Cohen, W.B.; Kennedy, R.E.; Maiersperger, T.K.; Gower, S.T. Hyperspectral versus multispectral data for estimating leaf area index in four different biomes. Remote Sens. Environ. 2004, 91, 508–520. [Google Scholar] [CrossRef]
- Sluiter, R.; Pebesma, E.J. Comparing techniques for vegetation classification using multi-and hyperspectral images and ancillary environmental data. Int. J. Remote Sens. 2010, 31, 6143–6161. [Google Scholar] [CrossRef]
- Marshall, M.; Thenkabail, P. Advantage of hyperspectral EO-1 Hyperion over multispectral IKONOS, GeoEye-1, WorldView-2, Landsat ETM+, and MODIS vegetation indices in crop biomass estimation. ISPRS J. Photogramm. Remote Sens. 2015, 108, 205–218. [Google Scholar] [CrossRef]
- Tong, A.; He, Y. Estimating and mapping chlorophyll content for a heterogeneous grassland: Comparing prediction power of a suite of vegetation indices across scales between years. ISPRS J. Photogramm. Remote Sens. 2017, 126, 146–167. [Google Scholar] [CrossRef]
- Lu, B.; He, Y.; Dao, P.D. Comparing the Performance of Multispectral and Hyperspectral Images for Estimating Vegetation Properties. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2019, 12, 1784–1797. [Google Scholar] [CrossRef]
- Kniffin, M. Sustaining Central Sands Water Resources. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2014. [Google Scholar]
- University of Wisconsin Soil and Forage Lab. Lab Procedures and Methods. Available online: https://uwlab.webhosting.cals.wisc.edu/wp-content/uploads/sites/17/2015/10/potato_N.pdf (accessed on 11 February 2022).
- Lu, B.; He, Y. Species classification using Unmanned Aerial Vehicle (UAV)-acquired high spatial resolution imagery in a heterogeneous grassland. ISPRS J. Photogramm. Remote Sens. 2017, 128, 73–85. [Google Scholar] [CrossRef]
- Del Pozo, S.; Rodríguez-Gonzálvez, P.; Hernández-López, D.; Felipe-García, B. Vicarious Radiometric Calibration of a Multispectral Camera on Board an Unmanned Aerial System. Remote Sens. 2014, 6, 1918–1937. [Google Scholar] [CrossRef]
- Von Bueren, S.K.; Burkart, A.; Hueni, A.; Rascher, U.; Tuohy, M.P.; Yule, I.J. Deploying four optical UAV-based sensors over grassland: Challenges and limitations. Biogeosciences 2015, 12, 163–175. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Wiklund, S.; Nilsson, D.; Eriksson, L.; Sjöström, M.; Wold, S.; Faber, K. A randomization test for PLS component selection. J. Chemom. A J. Chemom. Soc. 2007, 21, 427–439. [Google Scholar] [CrossRef]
- Mevik, B.-H.; Wehrens, R. Introduction to the pls Package. Available online: https://cran.r-project.org/web/packages/pls/vignettes/pls-manual.pdf (accessed on 11 February 2022).
- Hastie, T.; Tibshirani, R.; Friedman, J.H.; Friedman, J.H. The Elements of Statistical Learning: Data Mining, Inference, and Prediction; Springer: Berlin/Heidelberg, Germany, 2009; Volume 2. [Google Scholar]
- Mukherjee, R.; Sengupta, D.; Sikdar, S.K. Selection of Sustainable Processes Using Sustainability Footprint Method: A Case Study of Methanol Production from Carbon Dioxide. In Computer Aided Chemical Engineering; You, F., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 36, pp. 311–329. [Google Scholar]
- Wang, Z.X.; He, Q.P.; Wang, J. Comparison of variable selection methods for PLS-based soft sensor modeling. J. Process Control. 2015, 26, 56–72. [Google Scholar] [CrossRef]
- Zhou, J.; Yungbluth, D.; Vong, C.N.; Scaboo, A.; Zhou, J. Estimation of the maturity date of soybean breeding lines using UAV-based multispectral imagery. Remote Sens. 2019, 11, 2075. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Enclona, E.A.; Ashton, M.S.; Van Der Meer, B. Accuracy assessments of hyperspectral waveband performance for vegetation analysis applications. Remote Sens. Environ. 2004, 91, 354–376. [Google Scholar] [CrossRef]
- Santos-Rufo, A.; Mesas-Carrascosa, F.-J.; García-Ferrer, A.; Meroño-Larriva, J.E. Wavelength Selection Method Based on Partial Least Square from Hyperspectral Unmanned Aerial Vehicle Orthomosaic of Irrigated Olive Orchards. Remote Sens. 2020, 12, 3426. [Google Scholar] [CrossRef]
- Elvidge, C.D.; Chen, Z. Comparison of broad-band and narrow-band red and near-infrared vegetation indices. Remote Sens. Environ. 1995, 54, 38–48. [Google Scholar] [CrossRef]
- Thenkabail, P.S.; Hall, J.; Lin, T.; Ashton, M.S.; Harris, D.; Enclona, E.A. Detecting floristic structure and pattern across topographic and moisture gradients in a mixed species Central African forest using IKONOS and Landsat-7 ETM+ images. Int. J. Appl. Earth Obs. Geoinf. 2003, 4, 255–270. [Google Scholar] [CrossRef]
- Herrmann, I.; Bdolach, E.; Montekyo, Y.; Rachmilevitch, S.; Townsend, P.A.; Karnieli, A. Assessment of maize yield and phenology by drone-mounted superspectral camera [Article]. Precis. Agric. 2019, 21, 51–76. [Google Scholar] [CrossRef]
- Bernie Zebarth, G.M.; Charles, K. Nitrogen Management for Potatoes: Petiole Nitrate Testing. Available online: https://www2.gnb.ca/content/dam/gnb/Departments/10/pdf/Agriculture/potato_pnit.pdf (accessed on 11 February 2022).
- Stroppiana, D.; Fava, F.; Boschetti, M.; Brivio, P. Estimation of nitrogen content in crops and pastures using hyperspectral vegetation indices. In Hyperspectral Remote Sensing of Vegetation; Thenkabail, P.S., Lyon, J.G., Huete, A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 245–262. [Google Scholar]
- Filella, I.; Serrano, L.; Serra, J.; Penuelas, J. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 1995, 35, 1400–1405. [Google Scholar] [CrossRef]
- Houles, V.; Guerif, M.; Mary, B. Elaboration of a nitrogen nutrition indicator for winter wheat based on leaf area index and chlorophyll content for making nitrogen recommendations. Eur. J. Agron. 2007, 27, 1–11. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of spectral remote sensing for agronomic decisions. Agron. J. 2008, 100, S-117–S-131. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating Corn Leaf Chlorophyll Concentration from Leaf and Canopy Reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and physiological basis for the reflectance of visible and near-infrared radiation from vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
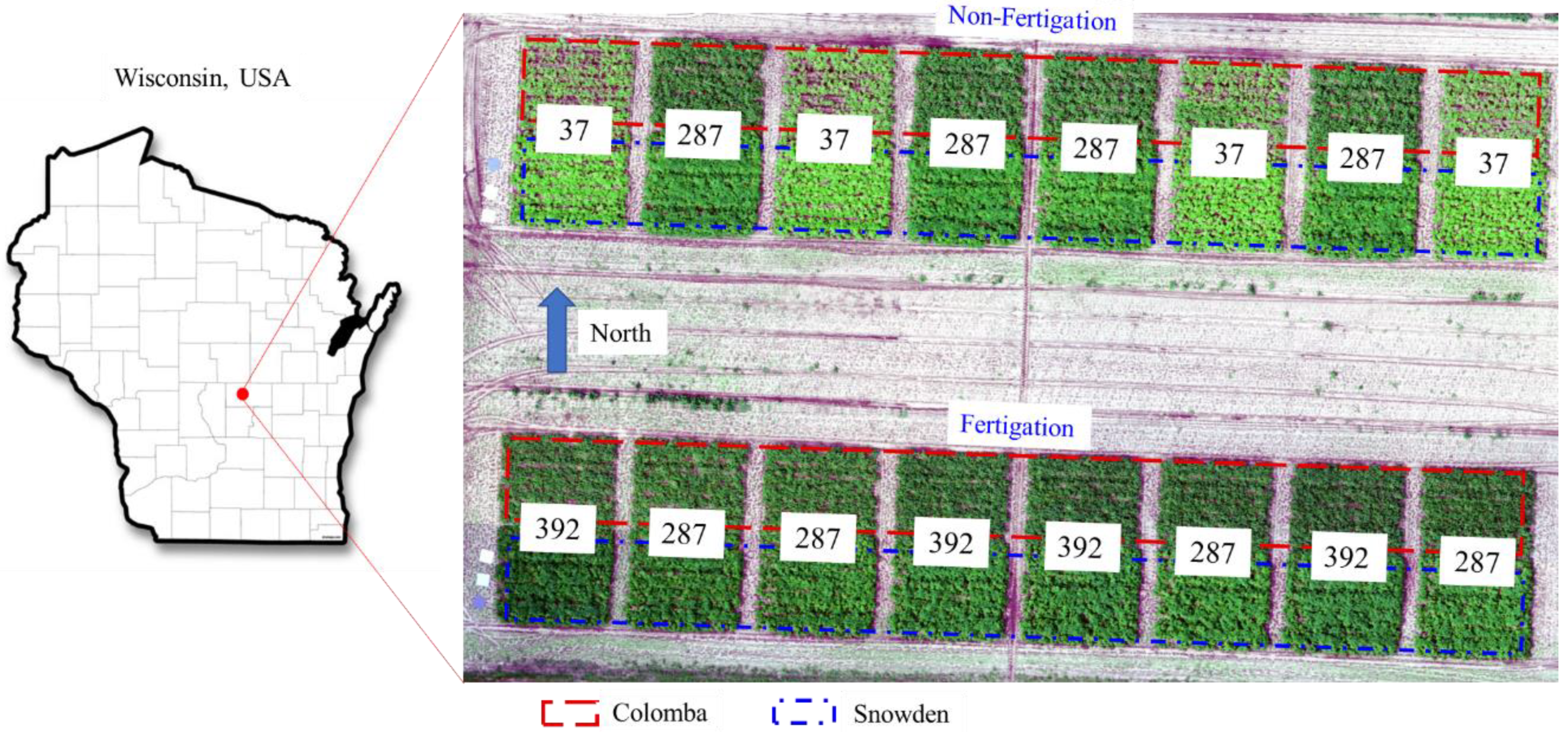
| Fertigation | Seasonal Total N Rate | Planting | Emergence (Hilling) | Tuber Initiation | Fertigation Date | |||
|---|---|---|---|---|---|---|---|---|
| 23 April | 12 May | 2 June | 30 June | 10 July | 20 July | 30 July | ||
| No | 37 | 37 | - | - | - | - | - | - |
| No | 287 | 37 | 85 | 165 | - | - | - | - |
| Yes | 287 | 37 | 85 | 30 | 34 | 34 | 34 | 34 |
| Yes | 392 | 37 | 85 | 134 | 34 | 34 | 34 | 34 |
| Manufacturer | Model | Price | Spectral Bands | Band Center (nm) | Band Center (nm) | Synthesized Band Center (nm) | Synthesized Bandwidth (nm) |
|---|---|---|---|---|---|---|---|
| DJI | P4 Multispectral | $6500 (Including a UAV) | Blue | 450 | 16 | 449.07 | 15.48 |
| Green | 560 | 16 | 559.65 | 15.48 | |||
| Red | 650 | 16 | 650.32 | 15.48 | |||
| Red Edge | 730 | 16 | 729.94 | 15.48 | |||
| NIR * | 840 | 26 | 839.41 | 26.54 | |||
| Parrot | Sequoia+ | $3500 | Green | 550 | 40 | 549.70 | 39.81 |
| Red | 660 | 40 | 660.28 | 39.81 | |||
| Red Edge | 735 | 10 | 736.57 | 11.06 | |||
| NIR * | 790 | 40 | 790.76 | 39.81 | |||
| Micasense | RedEdge MX | $6300 | Blue | 475 | 32 | 475.61 | 33.17 |
| Green | 560 | 27 | 560.75 | 26.54 | |||
| Red | 668 | 14 | 666.91 | 13.27 | |||
| Red Edge | 717 | 12 | 716.67 | 11.06 | |||
| NIR * | 842 | 57 | 841.62 | 57.50 |
| Leaf Total N Concentration (%) | Petiole Nitrate-N Concertation (%) | |||||
|---|---|---|---|---|---|---|
| Coefficients | Sum of Sq † | F-Value | p-Value | Sum of Sq | F-Value | p-Value |
| Sampling Date | 54.394 | 34.256 | <0.001 | 19.326 | 65.192 | <0.001 |
| Fertigation | 0.017 | 0.065 | 1.000 | 0.029 | 0.588 | 0.444 |
| Variety | 0.381 | 1.438 | 1.000 | 0.000 | 0.001 | 0.979 |
| N rate | 5.885 | 22.239 | <0.001 | 2.800 | 56.671 | <0.001 |
| Fertigation: N rate | 0.065 | 0.244 | 0.622 | 0.077 | 1.552 | 0.214 |
| Fertigation: Variety | 0.052 | 0.196 | 1.000 | 0.001 | 0.013 | 0.908 |
| Variety: N rate | 0.036 | 0.137 | 0.712 | 0.002 | 0.037 | 0.849 |
| Variety: Fertigation: N rate | 0.159 | 0.600 | 0.440 | 0.010 | 0.199 | 0.656 |
| Potato Cultivar | Full Spectra (2.2 nm) | 10 nm | 20 nm | 40 nm | DJI Phantom 4 MS | Parrot Sequoia | Micasense RedEdge MX | |
|---|---|---|---|---|---|---|---|---|
| Leaf total N concentration | Colomba | 0.75 (0.39) | 0.71 (0.41) | 0.64 (0.46) | 0.59 (0.52) | 0.35 (0.69) | 0.32 (0.71) | 0.32 (0.72) |
| Snowden | 0.72 (0.44) | 0.70 (0.46) | 0.69 (0.47) | 0.57 (0.56) | 0.37 (0.71) | 0.39 (0.70) | 0.45 (0.68) | |
| Petiole nitrate-N concentration | Colomba | 0.90 (0.12) | 0.90 (0.12) | 0.86 (0.14) | 0.86 (0.14) | 0.59 (0.24) | 0.59 (0.24) | 0.60 (0.23) |
| Snowden | 0.83 (0.16) | 0.84 (0.15) | 0.80 (0.17) | 0.78 (0.18) | 0.49 (0.27) | 0.49 (0.27) | 0.46 (0.27) |
| Imaging Date | Full Spectra (2.2 nm) | 10 nm | 20 nm | 40 nm | DJI Phantom 4 MS | Parrot Sequoia+ | Micasense RedEdge MX | |
|---|---|---|---|---|---|---|---|---|
| Leaf total N concentration | 06/30 | 0.83 (0.30) | 0.76 (0.35) | 0.77 (0.34) | 0.39 (0.57) | 0.01 (0.73) | 0.02 (0.74) | 0.01 (0.74) |
| 07/20 | 0.81 (0.36) | 0.77 (0.40) | 0.76 (0.39) | 0.56 (0.52) | 0.16 (0.73) | 0.12 (0.78) | 0.14 (0.77) | |
| 07/29 | 0.89 (0.21) | 0.81 (0.28) | 0.64 (0.38) | 0.74 (0.33) | 0.20 (0.64) | 0.27 (0.55) | 0.22 (0.60) | |
| 08/03 | 0.79 (0.25) | 0.77 (0.25) | 0.66 (0.37) | 0.72 (0.38) | 0.56 (0.39) | 0.57 (0.35) | 0.52 (0.41) | |
| 08/12 | 0.25 (0.72) | 0.22 (0.71) | 0.19 (0.72) | 0.13 (0.77) | 0.01 (0.90) | 0.07 (0.96) | 0.00 (0.87) | |
| Petiole nitrate-N concentration | 06/30 | 0.81 (0.21) | 0.82 (0.20) | 0.76 (0.23) | 0.76 (0.23) | 0.20 (0.45) | 0.25 (0.45) | 0.16 (0.46) |
| 07/20 | 0.53 (0.11) | 0.46 (0.11) | 0.46 (0.12) | 0.18 (0.14) | 0.36 (0.19) | 0.36 (0.23) | 0.38 (0.19) | |
| 07/29 | 0.70 (0.07) | 0.66 (0.08) | 0.53 (0.09) | 0.55 (0.09) | 0.37 (0.13) | 0.40 (0.12) | 0.38 (0.12) | |
| 08/03 | 0.50 (0.09) | 0.60 (0.08) | 0.49 (0.10) | 0.56 (0.12) | 0.52 (0.13) | 0.55 (0.11) | 0.50 (0.14) | |
| 08/12 | 0.04 (0.16) | 0.04 (0.16) | 0.20 (0.18) | 0.18 (0.17) | 0.06 (0.21) | 0.00 (0.19) | 0.06 (0.21) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, B.; Fan, J.; Ma, Y.; Wang, Y.; Zhang, Z. A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper- and Multi-Spectral Imagery. Agronomy 2022, 12, 2533. https://doi.org/10.3390/agronomy12102533
Zhou J, Wang B, Fan J, Ma Y, Wang Y, Zhang Z. A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper- and Multi-Spectral Imagery. Agronomy. 2022; 12(10):2533. https://doi.org/10.3390/agronomy12102533
Chicago/Turabian StyleZhou, Jing, Biwen Wang, Jiahao Fan, Yuchi Ma, Yi Wang, and Zhou Zhang. 2022. "A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper- and Multi-Spectral Imagery" Agronomy 12, no. 10: 2533. https://doi.org/10.3390/agronomy12102533
APA StyleZhou, J., Wang, B., Fan, J., Ma, Y., Wang, Y., & Zhang, Z. (2022). A Systematic Study of Estimating Potato N Concentrations Using UAV-Based Hyper- and Multi-Spectral Imagery. Agronomy, 12(10), 2533. https://doi.org/10.3390/agronomy12102533








