Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up and Crop Management
2.2. Arbuscular Mycorrhizal Fungal Root Colonization
2.3. Determination of Grain Yield, Yield Components, and Grain Quality
2.4. Analysis of Protein Composition and Quality
2.5. Phytate and Mineral Determination
2.6. Statistical Analysis
3. Results
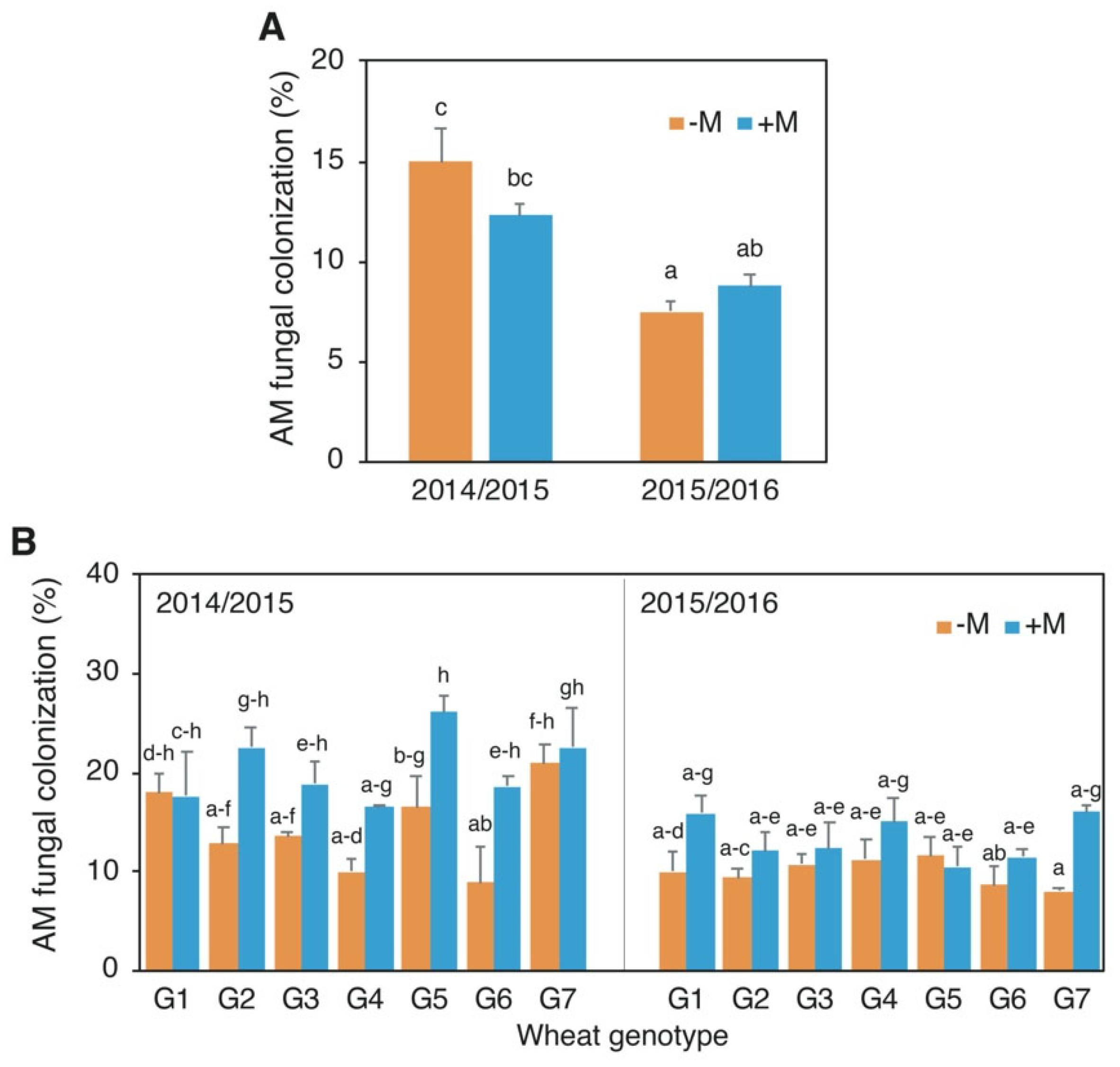
3.1. AM Fungal Root Colonization
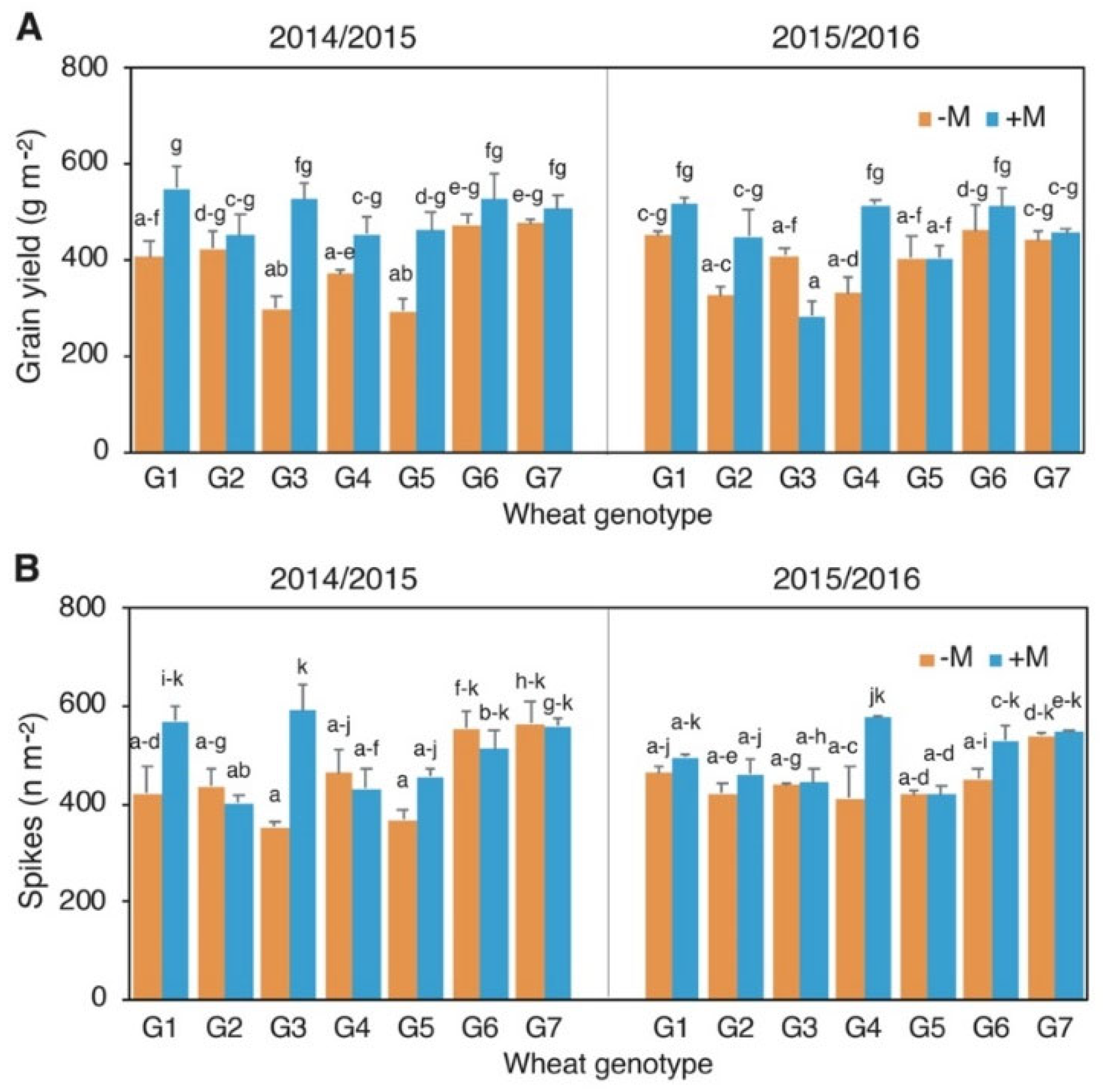
3.2. Grain Yield and Yield Components
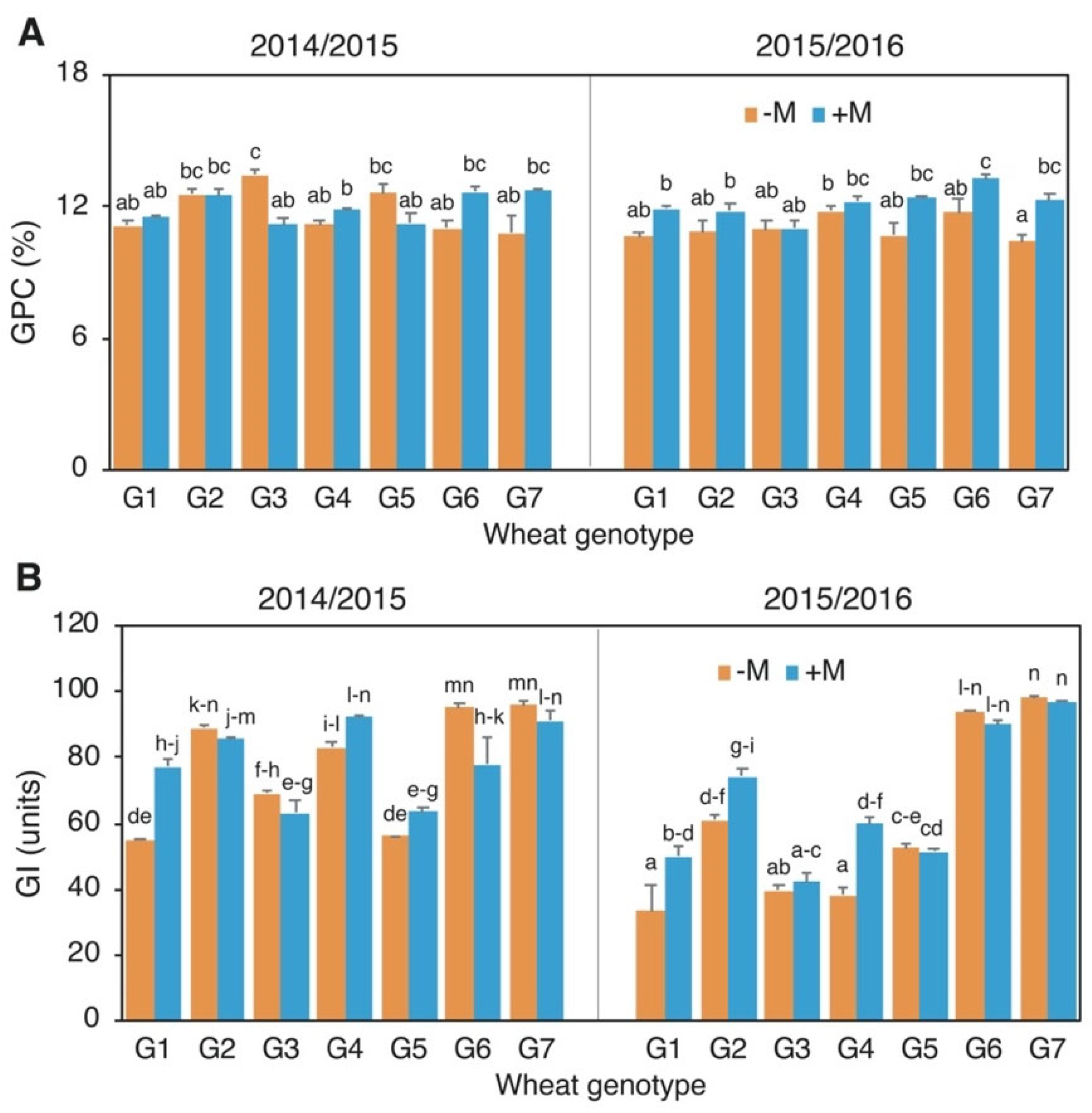
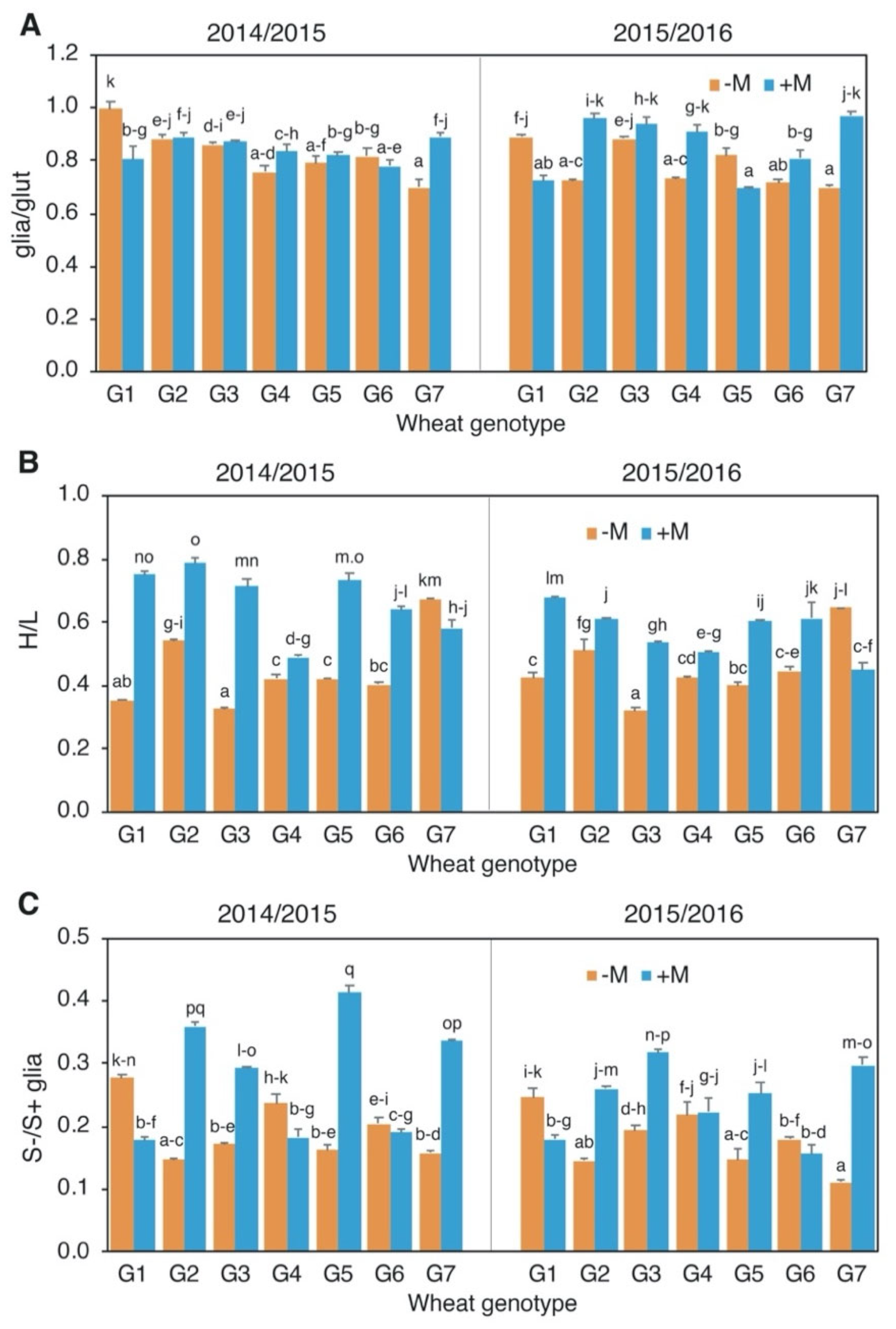
3.3. Protein Content and Composition
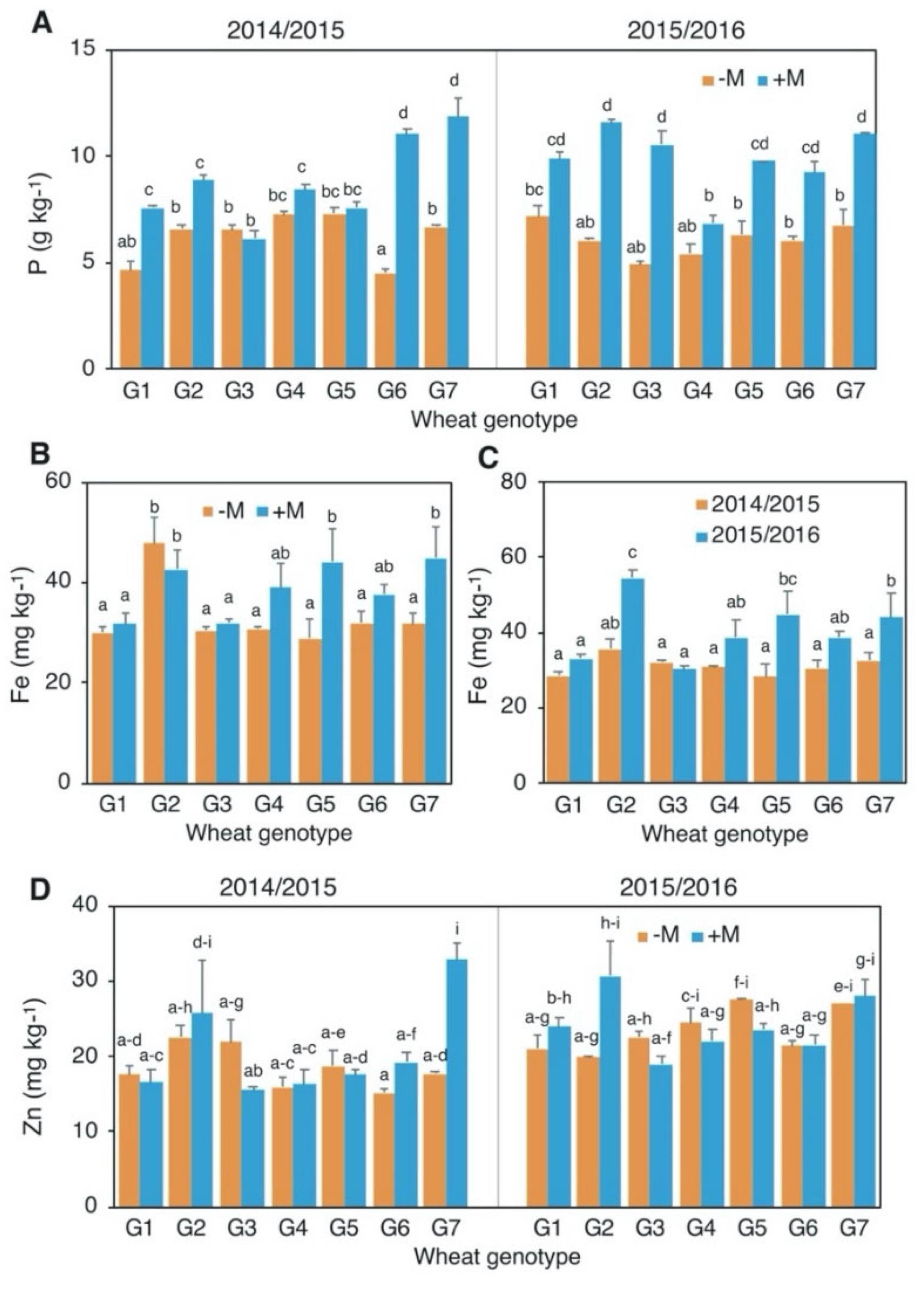
3.4. Grain Mineral Composition
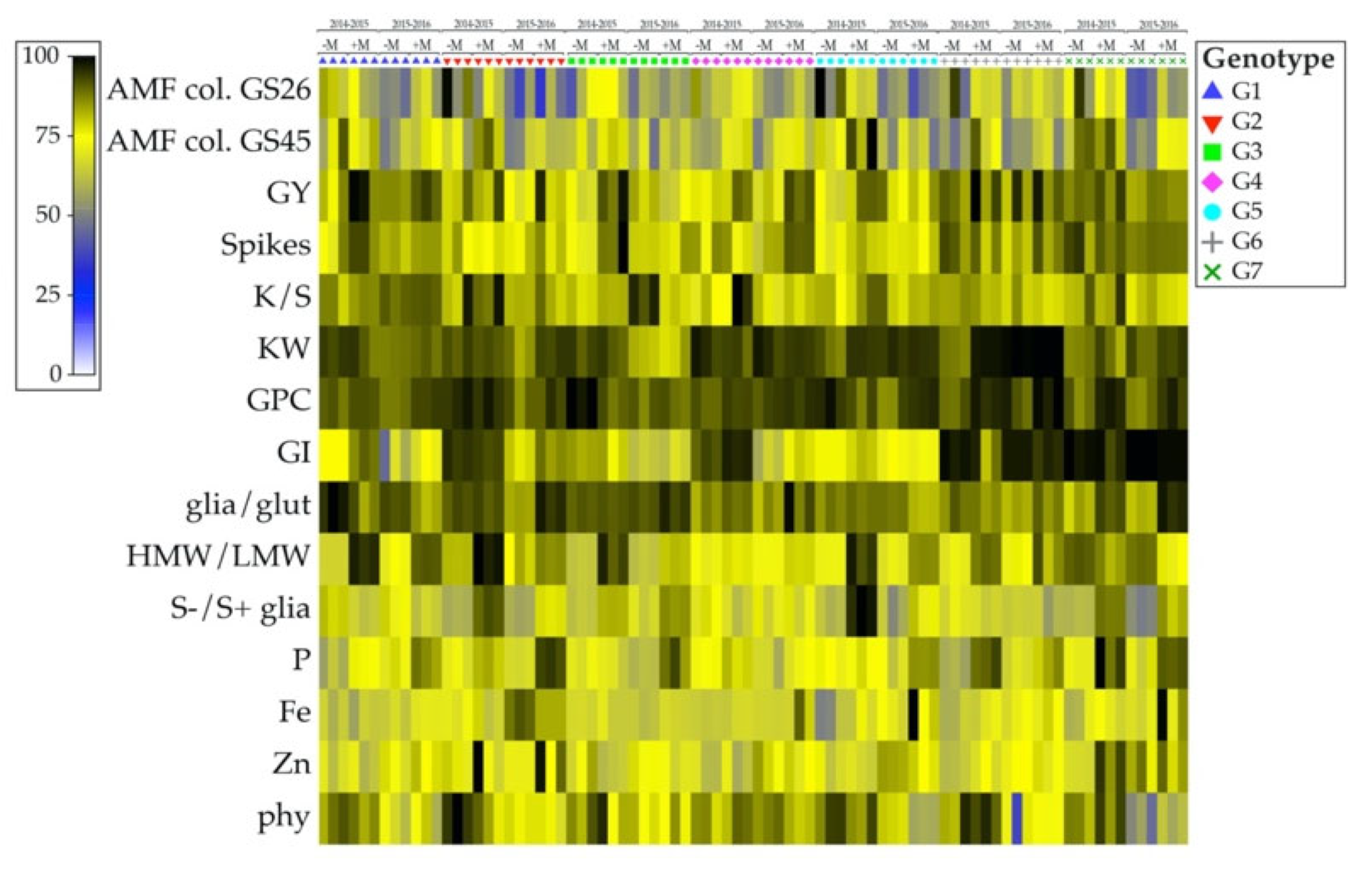
3.5. Relationships among AM Fungal Colonization, Yield and Yield Components, Proteins and Minerals in Grains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Guarda, G.; Padovan, S.; Delogu, G. Grain Yield, Nitrogen-Use Efficiency and Baking Quality of Old and Modern Italian Bread-Wheat Cultivars Grown at Different Nitrogen Levels. Eur. J. Agron. 2004, 21, 181–192. [Google Scholar] [CrossRef]
- Migliorini, P.; Spagnolo, S.; Torri, L.; Arnoulet, M.; Lazzerini, G.; Ceccarelli, S. Agronomic and Quality Characteristics of Old, Modern and Mixture Wheat Varieties and Landraces for Organic Bread Chain in Diverse Environments of Northern Italy. Eur. J. Agron. 2016, 79, 131–141. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in Gluten Protein Composition between Old and Modern Durum Wheat Genotypes in Relation to 20th Century Breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Shewry, P. What Is Gluten—Why Is It Special? Front. Nutr. 2019, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, M.; Álvaro, F.; Peremarti, A.; Martín-Sánchez, J.A.; Royo, C. Changes in Bread-Making Quality Attributes of Bread Wheat Varieties Cultivated in Spain during the 20th Century. Eur. J. Agron. 2015, 63, 79–88. [Google Scholar] [CrossRef]
- De Santis, M.A.; Cunsolo, V.; Giuliani, M.M.; Di Francesco, A.; Saletti, R.; Foti, S.; Flagella, Z. Gluten Proteome Comparison among Durum Wheat Genotypes with Different Release Date. J. Cereal Sci. 2020, 96, 103092. [Google Scholar] [CrossRef]
- Malalgoda, M.; Ohm, J.-B.; Meinhardt, S.; Simsek, S. Association between Gluten Protein Composition and Breadmaking Quality Characteristics in Historical and Modern Spring Wheat. Cereal Chem. 2018, 95, 226–238. [Google Scholar] [CrossRef]
- Johansson, E.; Branlard, G.; Cuniberti, M.; Flagella, Z.; Hüsken, A.; Nurit, E.; Peña, R.J.; Sissons, M.; Vazquez, D. Genotypic and Environmental Effects on Wheat Technological and Nutritional Quality. In Wheat Quality for Improving Processing and Human Health; Springer: Cham, Switzerland, 2020; pp. 171–204. ISBN 978-3-030-34163-3. [Google Scholar] [CrossRef]
- De Santis, M.A.; Soccio, M.; Laus, M.N.; Flagella, Z. Influence of Drought and Salt Stress on Durum Wheat Grain Quality and Composition: A Review. Plants 2021, 10, 2599. [Google Scholar] [CrossRef]
- Schüβler, A.; Schwarzott, D.; Walker, C. A New Fungal Phylum, the Glomeromycota: Phylogeny and Evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Igiehon, N.O.; Babalola, O.O. Biofertilizers and Sustainable Agriculture: Exploring Arbuscular Mycorrhizal Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 4871–4881. [Google Scholar] [CrossRef]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of Wheat to Arbuscular Mycorrhizal Fungi: A Meta-Analysis of Field Studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Coccina, A.; Cavagnaro, T.R.; Pellegrino, E.; Ercoli, L.; McLaughlin, M.J.; Watts-Williams, S.J. The Mycorrhizal Pathway of Zinc Uptake Contributes to Zinc Accumulation in Barley and Wheat Grain. BMC Plant Biol. 2019, 19, 133. [Google Scholar] [CrossRef] [PubMed]
- De Vita, P.; Avio, L.; Sbrana, C.; Laidò, G.; Marone, D.; Mastrangelo, A.M.; Cattivelli, L.; Giovannetti, M. Genetic Markers Associated to Arbuscular Mycorrhizal Colonization in Durum Wheat. Sci. Rep. 2018, 8, 10612. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Soriano, J.M.; Rufo, R.; Guzmán, C. Are the Agronomic Performance and Grain Quality Characteristics of Bread Wheat Mediterranean Landraces Related to the Climate Prevalent in Their Area of Origin? J. Cereal Sci. 2022, 105, 103478. [Google Scholar] [CrossRef]
- Pellegrino, E.; Piazza, G.; Arduini, I.; Ercoli, L. Field Inoculation of Bread Wheat with Rhizophagus irregularis under Organic Farming: Variability in Growth Response and Nutritional Uptake of Eleven Old Genotypes and A Modern Variety. Agronomy 2020, 10, 333. [Google Scholar] [CrossRef]
- Voets, L.; Dupré de Boulois, H.; Renard, L.; Strullu, D.-G.; Declerck, S. Development of an Autotrophic Culture System for the in Vitro Mycorrhization of Potato Plantlets. FEMS Microbiol. Lett. 2005, 248, 111–118. [Google Scholar] [CrossRef]
- Vosátka, M.; Látr, A.; Gianinazzi, S.; Albrechtová, J. Development of Arbuscular Mycorrhizal Biotechnology and Industry: Current Achievements and Bottlenecks. Symbiosis 2012, 58, 29–37. [Google Scholar] [CrossRef]
- De La Providencia, I.E.; De Souza, F.A.; Fernández, F.; Delmas, N.S.; Declerck, S. Arbuscular Mycorrhizal Fungi Reveal Distinct Patterns of Anastomosis Formation and Hyphal Healing Mechanisms between Different Phylogenic Groups. New Phytol. 2005, 165, 261–271. [Google Scholar] [CrossRef]
- Avio, L.; Pellegrino, E.; Bonari, E.; Giovannetti, M. Functional Diversity of Arbuscular Mycorrhizal Fungal Isolates in Relation to Extraradical Mycelial Networks. New Phytol. 2006, 172, 347–357. [Google Scholar] [CrossRef]
- Kokkoris, V.; Li, Y.; Hamel, C.; Hanson, K.; Hart, M. Site Specificity in Establishment of a Commercial Arbuscular Mycorrhizal Fungal Inoculant. Sci. Total Environ. 2019, 660, 1135–1143. [Google Scholar] [CrossRef]
- U. S. Department of Agriculture; Natural Resources Conservation Service; Soil Survey Staff. Keys to Soil Taxonomy, 11th ed.Books Express Publishing: Berkshire, UK, 2010; 346p. [Google Scholar]
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA Soil Test for Zinc, Iron, Manganese, and Copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Zadocks, J.C.; Chang, T.T.; Konzak, C.F. A Decimal Code for the Growth Stages of Cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN18. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An Evaluation of Techniques for Measuring Vesicular Arbuscular Mycorrhizal Infection in Roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Flagella, Z.; Reyneri, A.; Blandino, M. Impact of Nitrogen Fertilisation Strategies on the Protein Content, Gluten Composition and Rheological Properties of Wheat for Biscuit Production. Field Crops Res. 2020, 254, 107829. [Google Scholar] [CrossRef]
- Hussain, S.; Maqsood, M.A.; Miller, L.V. Bioavailable Zinc in Grains of Bread Wheat Varieties of Pakistan. Cereal Res. Commun. 2012, 40, 62–73. [Google Scholar] [CrossRef]
- Isaac, R.A.; Johnson, W.C., Jr. Elemental Determination by Inductively Coupled Plasma Atomic Emission Spectrometry. In Handbook of Reference Methods for Plant Analysis; CRC Press: Boca Raton, FL, USA, 2019; pp. 165–170. [Google Scholar]
- Al-Karaki, G.N.; Al-Raddad, A. Effects of Arbuscular Mycorrhizal Fungi and Drought Stress on Growth and Nutrient Uptake of Two Wheat Genotypes Differing in Drought Resistance. Mycorrhiza 1997, 7, 83–88. [Google Scholar] [CrossRef]
- Mardukhi, B.; Rejali, F.; Daei, G.; Ardakani, M.R.; Malakouti, M.J.; Miransari, M. Arbuscular Mycorrhizas Enhance Nutrient Uptake in Different Wheat Genotypes at High Salinity Levels under Field and Greenhouse Conditions. Comptes Rendus Biol. 2011, 334, 564–571. [Google Scholar] [CrossRef]
- Singh, A.K.; Hamel, C.; DePauw, R.M.; Knox, R.E. Genetic Variability in Arbuscular Mycorrhizal Fungi Compatibility Supports the Selection of Durum Wheat Genotypes for Enhancing Soil Ecological Services and Cropping Systems in Canada. Can. J. Microbiol. 2012, 58, 293–302. [Google Scholar] [CrossRef]
- Yao, Q.; Li, X.; Christie, P. Factors Affecting Arbuscular Mycorrhizal Dependency of Wheat Genotypes with Different Phosphorus Efficiencies. J. Plant Nutr. 2001, 24, 1409–1419. [Google Scholar] [CrossRef]
- de Souza Campos, P.M.; Borie, F.; Cornejo, P.; Meier, S.; López-Ráez, J.A.; López-Garcia, Á.; Seguel, A. Wheat Root Trait Plasticity, Nutrient Acquisition and Growth Responses Are Dependent on Specific Arbuscular Mycorrhizal Fungus and Plant Genotype Interactions. J. Plant Physiol. 2021, 256, 153297. [Google Scholar] [CrossRef] [PubMed]
- Stefani, F.; Dupont, S.; Laterrière, M.; Knox, R.; Ruan, Y.; Hamel, C.; Hijri, M. Similar Arbuscular Mycorrhizal Fungal Communities in 31 Durum Wheat Cultivars (Triticum turgidum L. var. durum) Under Field Conditions in Eastern Canada. Front. Plant Sci. 2020, 11, 1206. [Google Scholar] [CrossRef] [PubMed]
- Suri, V.K.; Choudhary, A.K.; Chander, G.; Verma, T.S. Influence of Vesicular Arbuscular Mycorrhizal Fungi and Applied Phosphorus on Root Colonization in Wheat and Plant Nutrient Dynamics in a Phosphorus-Deficient Acid Alfisol of Western Himalayas. Commun. Soil Sci. Plant Anal. 2011, 42, 1177–1186. [Google Scholar] [CrossRef]
- Renaut, S.; Daoud, R.; Masse, J.; Vialle, A.; Hijri, M. Inoculation with Rhizophagus irregularis Does Not Alter Arbuscular Mycorrhizal Fungal Community Structure within the Roots of Corn, Wheat, and Soybean Crops. Microorganisms 2020, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.; Ash, J. Colonisation of Wheat in Southern New South Wales by Vesicular-Arbuscular Mycorrhizal Fungi Is Significantly Reduced by Drought. Aust. J. Exp. Agric. 1996, 36, 563–569. [Google Scholar] [CrossRef]
- Mozafar, A.; Anken, T.; Ruh, R.; Frossard, E. Tillage Intensity, Mycorrhizal and Nonmycorrhizal Fungi, and Nutrient Concentrations in Maize, Wheat, and Canola. Agron. J. 2000, 92, 1117–1124. [Google Scholar] [CrossRef]
- Ercoli, L.; Schüßler, A.; Arduini, I.; Pellegrino, E. Strong Increase of Durum Wheat Iron and Zinc Content by Field-Inoculation with Arbuscular Mycorrhizal Fungi at Different Soil Nitrogen Availabilities. Plant Soil 2017, 419, 153–167. [Google Scholar] [CrossRef]
- Pellegrino, E.; Nuti, M.; Ercoli, L. Multiple Arbuscular Mycorrhizal Fungal Consortia Enhance Yield and Fatty Acids of Medicago Sativa: A Two-Year Field Study on Agronomic Traits and Tracing of Fungal Persistence. Front. Plant Sci. 2022, 13, 814401. [Google Scholar] [CrossRef]
- Lafiandra, D.; Shewry, P.R. Wheat Glutenin Polymers 2. The Role of Wheat Glutenin Subunits in Polymer Formation and Dough Quality. J. Cereal Sci. 2022, 106, 103487. [Google Scholar] [CrossRef]
- Ormoli, L.; Costa, C.; Negri, S.; Perenzin, M.; Vaccino, P. Diversity Trends in Bread Wheat in Italy during the 20th Century Assessed by Traditional and Multivariate Approaches. Sci. Rep. 2015, 5, 8574. [Google Scholar] [CrossRef]
- Igrejas, G.; Ikeda, T.M.; Guzmán, C. (Eds.) Wheat Quality for Improving Processing and Human Health; Springer: Cham, Switzerland, 2020; ISBN 978-3-030-34162-6. [Google Scholar] [CrossRef]
- Ghiselli, L.; Rossi, E.; Whittaker, A.; Dinelli, G.; Baglio, A.P.; Andrenelli, L.; Benedettelli, S. Nutritional Characteristics of Ancient Tuscan Varieties of Triticum aestivum L. Ital. J. Agron. 2016, 11, 237–245. [Google Scholar] [CrossRef]
- Dal Cortivo, C.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of Seed-Applied Biofertilizers on Rhizosphere Biodiversity and Growth of Common Wheat (Triticum aestivum L.) in the Field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Pereira, N.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Bending, G.D. Growth and Nutritional Responses to Arbuscular Mycorrhizal Fungi Are Dependent on Onion Genotype and Fungal Species. Biol. Fertil. Soils 2015, 51, 801–813. [Google Scholar] [CrossRef]
- Wieser, H.; Gutser, R.; von Tucher, S. Influence of Sulphur Fertilisation on Quantities and Proportions of Gluten Protein Types in Wheat Flour. J. Cereal Sci. 2004, 40, 239–244. [Google Scholar] [CrossRef]
- Lehmann, A.; Veresoglou, S.D.; Leifheit, E.F.; Rillig, M.C. Arbuscular Mycorrhizal Influence on Zinc Nutrition in Crop Plants—A Meta-Analysis. Soil Biol. Biochem. 2014, 69, 123–131. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular Mycorrhizal Contribution to Copper, Manganese and Iron Nutrient Concentrations in Crops—A Meta-Analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Rengel, Z.; Graham, R.D. Wheat Genotypes Differ in Zn Efficiency When Grown in Chelate-Buffered Nutrient Solution. Plant Soil 1995, 176, 307–316. [Google Scholar] [CrossRef]
- Magallanes-López, A.M.; Hernandez-Espinosa, N.; Velu, G.; Posadas-Romano, G.; Ordoñez-Villegas, V.M.G.; Crossa, J.; Ammar, K.; Guzmán, C. Variability in Iron, Zinc and Phytic Acid Content in a Worldwide Collection of Commercial Durum Wheat Cultivars and the Effect of Reduced Irrigation on These Traits. Food Chem. 2017, 237, 499–505. [Google Scholar] [CrossRef]
- Ciccolini, V.; Pellegrino, E.; Coccina, A.; Fiaschi, A.I.; Cerretani, D.; Sgherri, C.; Quartacci, M.F.; Ercoli, L. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017, 65, 5443–5452. [Google Scholar] [CrossRef]
- Ma, X.; Luo, W.; Li, J.; Wu, F. Arbuscular Mycorrhizal Fungi Increase Both Concentrations and Bioavilability of Zn in Wheat (Triticum aestivum L.) Grain on Zn-Spiked Soils. Appl. Soil Ecol. 2019, 135, 91–97. [Google Scholar] [CrossRef]


| Group | Genotype | Code | YR 1 | Pedigree | Glu-A1 | Glu-B1 | Glu-D1 | Score |
|---|---|---|---|---|---|---|---|---|
| old | Gentil Rosso | G1 | - | landrace | 1 | 13 + 19 | 2 + 12 | 7 |
| Risciola | G2 | - | landrace | 1 | 7 + 8 | 2 + 12 | 8 | |
| Frassineto | G3 | 1922 | selection from Gentil Rosso | 1 | 7 | 2 + 12 | 7 | |
| Autonomia B | G4 | 1938 | Frassineto 405/Mentana | 1 | 7 + 8 | 2 + 12 | 9 | |
| Verna | G5 | 1953 | Est Mottin 72/Mont Calme 245 | - | 6 | 5 + 10 | 6 | |
| modern | Blasco | G6 | 2002 | Oderzo/Barra | 2 | 7 + 8 | 5 + 10 | 15 |
| Bologna | G7 | 2002 | H89092/H89136/Soissons | 2 | 7 + 8 | 5 + 10 | 15 |
| Traits | Parameter | G | AMF | Y | G × AMF | G × Y | Y × AMF | G × AMF × Y |
|---|---|---|---|---|---|---|---|---|
| AMF root colonization | AMF col. GS26 1 | 0.977 | 0.487 | <0.001 | 0.514 | 0.400 | 0.039 | 0.606 |
| AMF col. GS45 | 0.023 | <0.001 | <0.001 | 0.882 | 0.019 | 0.115 | 0.038 | |
| Yield and their components | GY | <0.001 | <0.001 | 0.147 | 0.313 | 0.367 | 0.019 | <0.001 |
| Spikes | <0.001 | <0.001 | 0.697 | 0.056 | 0.399 | 0.811 | <0.001 | |
| K/S | 0.015 | 0.289 | 0.225 | 0.019 | 0.005 | 0.006 | 0.26 | |
| KW | <0.001 | 0.002 | 0.733 | <0.001 | <0.001 | 0.716 | <0.001 | |
| Grain proteins | GPC | <0.001 | <0.001 | 0.913 | <0.001 | <0.001 | 0.327 | <0.001 |
| GI | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.008 | 0.011 | |
| glia/glut | <0.001 | <0.001 | 0.087 | <0.001 | 0.001 | 0.001 | 0.001 | |
| H/L | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | |
| S-/S+ glia | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.012 | <0.001 | |
| Grain minerals | P | 0.326 | 0.008 | 0.612 | 0.203 | 0.117 | 0.539 | 0.027 |
| Fe | <0.001 | <0.001 | <0.001 | 0.004 | 0.005 | 0.128 | 0.398 | |
| Zn | <0.001 | 0.104 | <0.001 | <0.001 | 0.252 | 0.420 | 0.025 | |
| phy | 0.001 | 0.398 | <0.001 | 0.002 | <0.001 | 0.854 | 0.165 | |
| phy/Fe | <0.001 | 0.275 | <0.001 | 0.024 | 0.024 | 0.561 | 0.207 | |
| phy/Zn | 0.001 | <0.001 | <0.001 | 0.001 | <0.001 | 0.547 | 0.231 |
| AMF col. | GY | Spikes | K/S | KW | GPC | GI | Glia/Glut | H/L | S−/S+ Glia | P | Fe | Zn | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GS26 1 | GS45 | |||||||||||||
| GS45 | 0.305 | |||||||||||||
| GY | 0.098 | 0.244 | ||||||||||||
| Spikes | 0.045 | 0.185 | 0.703 | |||||||||||
| K/S | 0.008 | 0.113 | 0.417 | −0.145 | ||||||||||
| KW | 0.122 | 0.011 | 0.277 | −0.013 | −0.311 | |||||||||
| GPC | 0.161 | 0.177 | −0.003 | −0.151 | −0.098 | 0.357 | ||||||||
| GI | 0.211 | 0.148 | 0.366 | 0.351 | −0.091 | 0.297 | 0.215 | |||||||
| glia/glut | 0.068 | 0.244 | 0.048 | 0.034 | 0.131 | −0.147 | 0.101 | −0.109 | ||||||
| H/L | 0.046 | 0.419 | 0.464 | 0.385 | 0.123 | 0.100 | 0.049 | 0.291 | −0.181 | |||||
| S-/S+ glia | 0.059 | 0.437 | 0.091 | 0.037 | 0.108 | −0.032 | 0.042 | −0.156 | 0.505 | 0.217 | ||||
| P | −0.055 | 0.244 | 0.180 | 0.126 | 0.009 | 0.106 | 0.380 | 0.187 | 0.137 | 0.459 | 0.325 | |||
| Fe | −0.367 | −0.140 | 0.054 | 0.034 | −0.105 | 0.183 | 0.052 | 0.088 | −0.023 | 0.152 | 0.065 | 0.344 | ||
| Zn | −0.258 | −0.077 | 0.020 | 0.010 | −0.042 | 0.080 | 0.118 | −0.008 | 0.166 | 0.048 | 0.070 | 0.359 | 0.383 | |
| phy | 0.435 | 0.388 | −0.087 | −0.121 | 0.063 | −0.062 | 0.200 | 0.015 | 0.210 | −0.029 | 0.108 | −0.152 | −0.354 | −0.250 |
| Parameter | Unit | Old | +M vs. −M | t-Test | Modern | +M vs. −M | t-Test |
|---|---|---|---|---|---|---|---|
| AMF col. GS26 1 | % | 10.7 ± 5.1 | −11% | ns | 11.3 ± 5.0 | 6% | ns |
| AMF col. GS45 | % | 14.6 ± 5.3 | 35% | * | 14.4 ± 6.5 | 48% | * |
| GY | mg/m2 | 416 ± 91 | 24% | * | 482 ± 56 | 8% | ns |
| Spike | heads/m2 | 452 ± 77 | 16% | * | 532 ± 55 | 2% | ns |
| K/S | n/m2 | 23 ± 3.8 | 5% | ns | 21.8 ± 2.4 | −2% | ns |
| KW | mg | 40.3 ± 4.0 | 1% | ns | 42.1 ± 5.0 | 8% | ns |
| PC | % | 11.7 ± 0.9 | 1% | ns | 11.9 ± 1.1 | 16% | * |
| GI | units | 61.8 ± 17.5 | 14% | ns | 92.4 ± 7.7 | −7% | ns |
| glia/glut | - | 0.84 ± 0.09 | 1% | ns | 0.80 ± 0.10 | 18% | * |
| H/L | - | 0.53 ± 0.14 | 54% | * | 0.56 ± 0.11 | 5% | ns |
| S−/S+ glia | - | 0.23 ± 0.07 | 37% | * | 0.20 ± 0.07 | 50% | * |
| P | g/kg | 7.5 ± 1.9 | 7% | * | 8.4 ± 2.7 | 44% | * |
| Fe | mg/kg | 35.7 ± 10.5 | 13% | ns | 36.5 ± 10.0 | 30% | * |
| Zn | mg/kg | 21.1 ± 5.3 | −1% | ns | 22.9 ± 6.1 | 25% | * |
| phy | mg/kg | 13.0 ± 3.0 | −10% | ns | 11.5 ± 4.6 | 20% | ns |
| phy/Fe | mg/kg | 65.2 ± 23.7 | −3% | ns | 54.9 ± 28.6 | −10% | ns |
| phy/Zn | mg/kg | 33.7 ± 13.0 | −19% | ns | 29.1 ± 14.6 | −5% | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, M.A.; Giuliani, M.M.; Flagella, Z.; Pellegrino, E.; Ercoli, L. Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes. Agronomy 2022, 12, 2418. https://doi.org/10.3390/agronomy12102418
De Santis MA, Giuliani MM, Flagella Z, Pellegrino E, Ercoli L. Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes. Agronomy. 2022; 12(10):2418. https://doi.org/10.3390/agronomy12102418
Chicago/Turabian StyleDe Santis, Michele Andrea, Marcella Michela Giuliani, Zina Flagella, Elisa Pellegrino, and Laura Ercoli. 2022. "Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes" Agronomy 12, no. 10: 2418. https://doi.org/10.3390/agronomy12102418
APA StyleDe Santis, M. A., Giuliani, M. M., Flagella, Z., Pellegrino, E., & Ercoli, L. (2022). Effect of Arbuscular Mycorrhizal Fungal Seed Coating on Grain Protein and Mineral Composition of Old and Modern Bread Wheat Genotypes. Agronomy, 12(10), 2418. https://doi.org/10.3390/agronomy12102418










