Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Characterization of Fungal Isolate
2.2. Preparation of C. speciosa Extract
2.3. The Antifungal Activity of C. speciosa Extract against Root Rot Fungus In Vitro
2.4. Greenhouse Experimental Design and Growth Parameters Assessment
2.5. Total Phenolic Content (TPC) in Tomato Plants
2.6. Oxidative Stress Markers
2.6.1. Malondialdehyde Assay (MDA)
2.6.2. Hydrogen Peroxide Assay (H2O2)
2.7. Antioxidant Enzymes
2.7.1. Polyphenol Oxidase (PPO)
2.7.2. Catalase (CAT) Activity
2.7.3. Superoxide Dismutase (SOD) Activity
2.8. Real-Time Quantitative Reverse Transcription PCR Analysis (RT-qPCR) of Defense-Related Genes
2.9. Antioxidant Activity of C. speciosa Extract
2.10. HPLC Analysis of Polyphenolic Components for the Obtained Plant Extract
2.11. Statistical Analysis
3. Results and Discussion
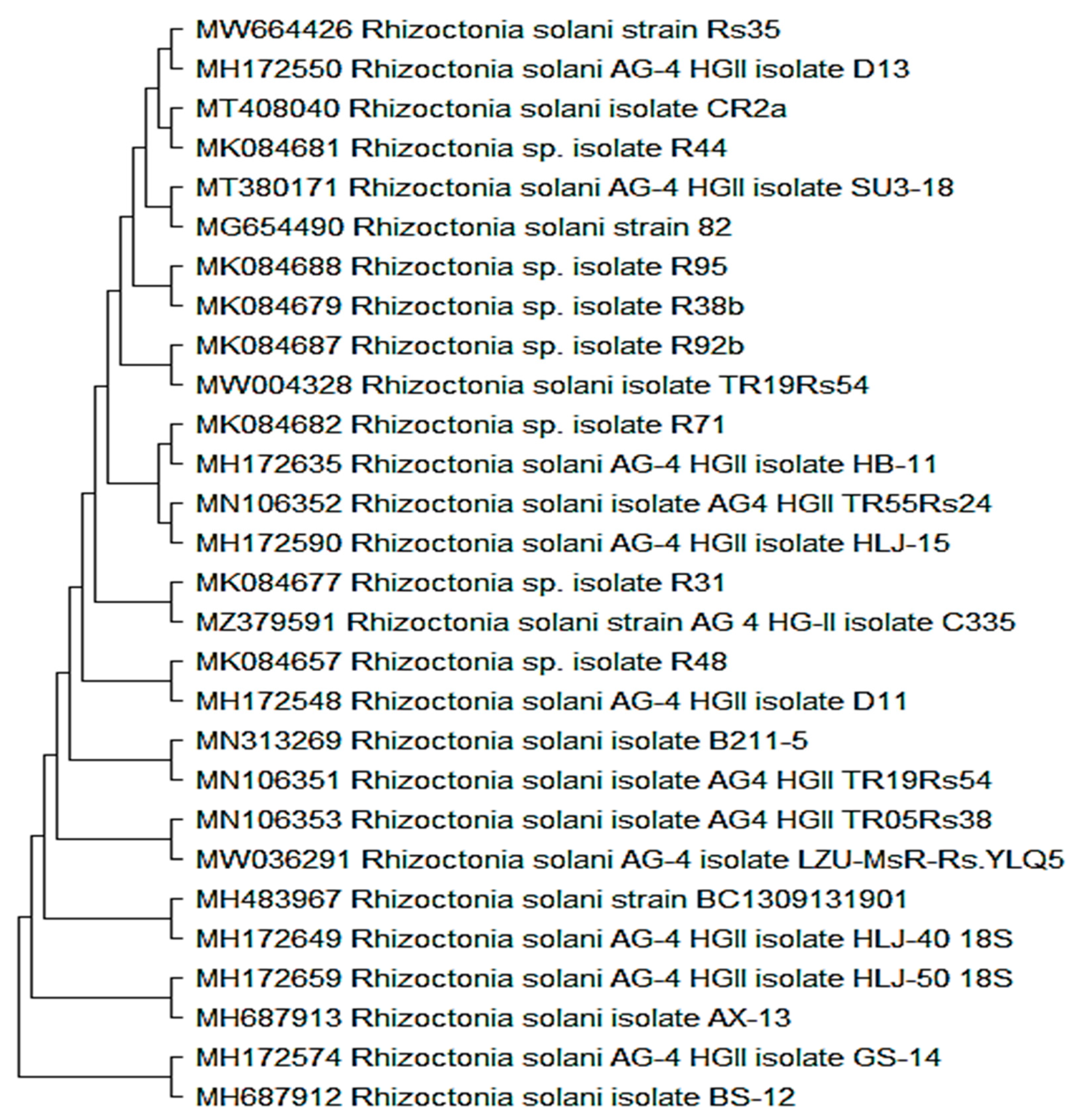
3.1. Rhizoctonia Solani Fungus Identification
3.2. C. speciosa Extract inhibitory Effect In Vitro
3.3. Disease Index of R. solani Pathogen in Response to C. speciosa Extract
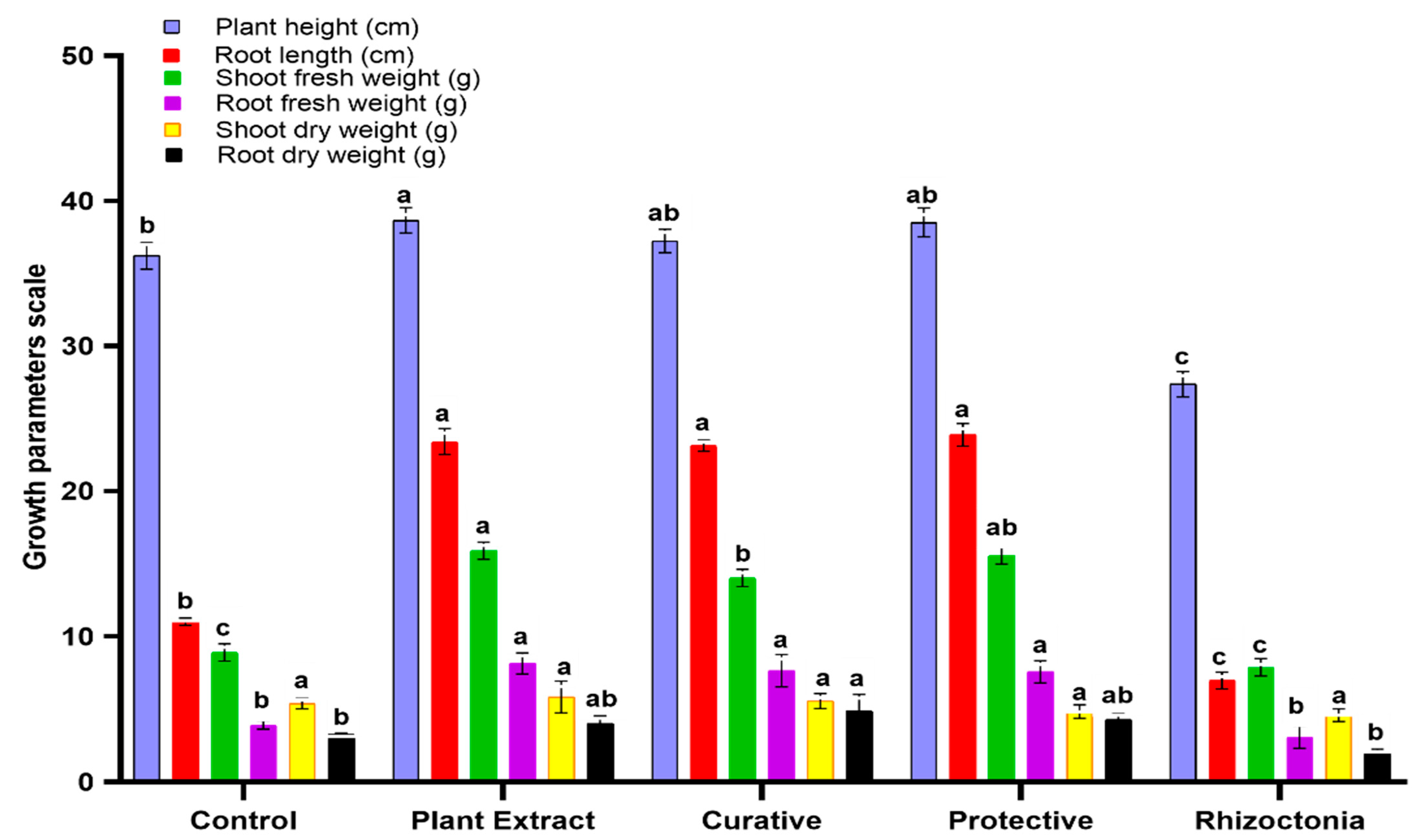
3.4. In Vivo Effect of C. speciosa Extract on Tomato Plants
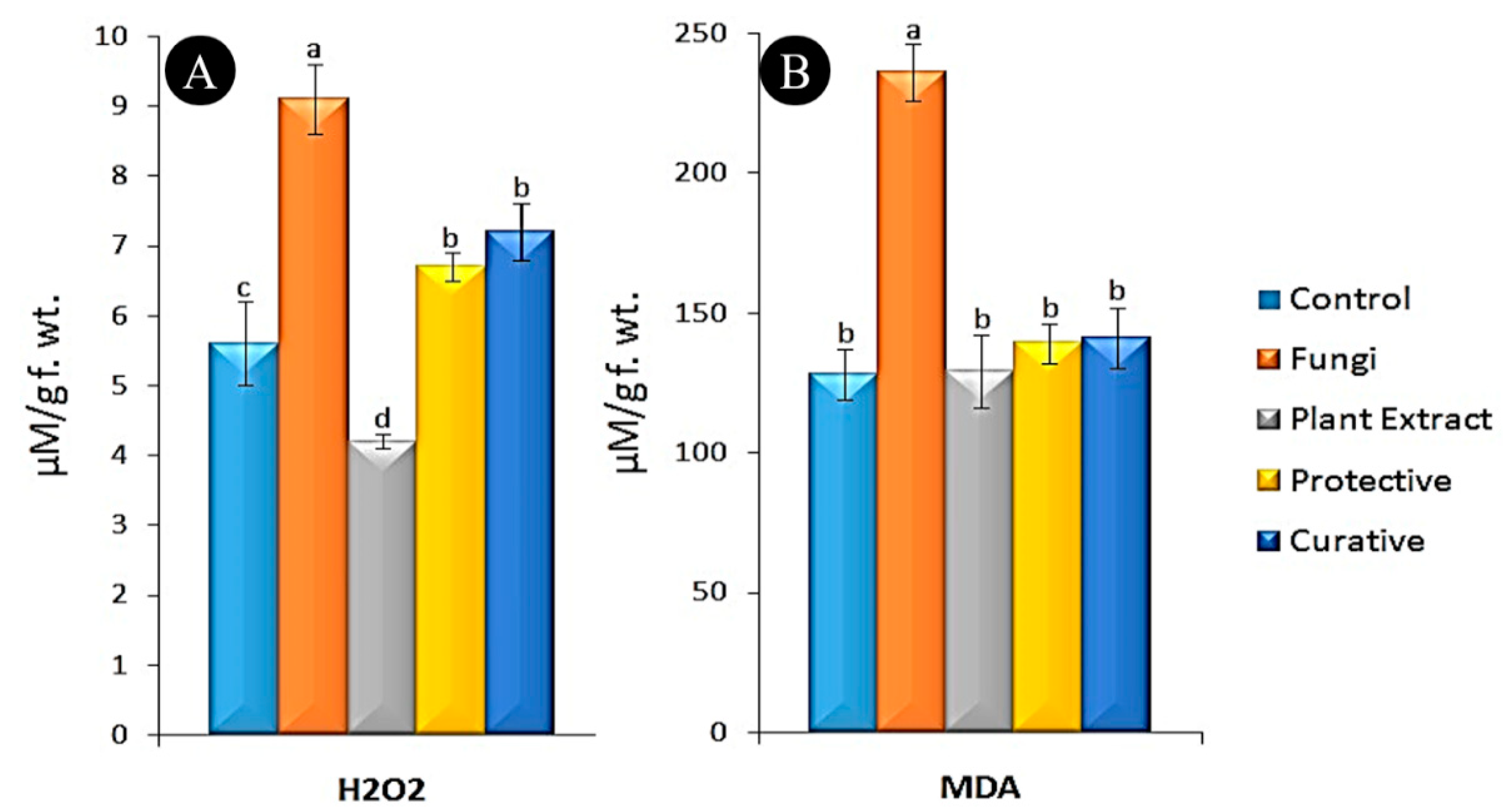
3.5. Oxidative Stress Markers Assay
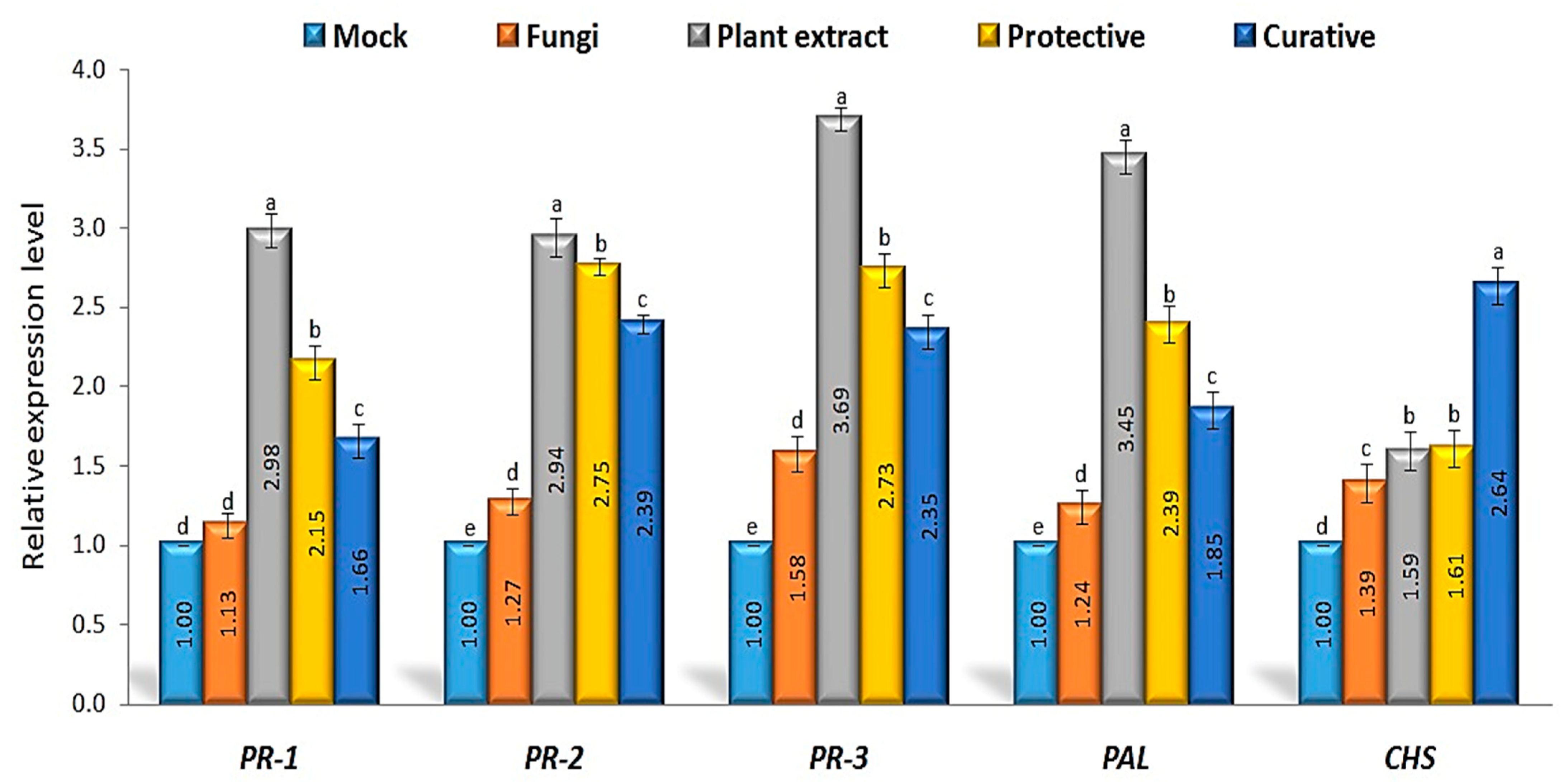
3.6. Defense-Related Genes’ Relative Expression Levels
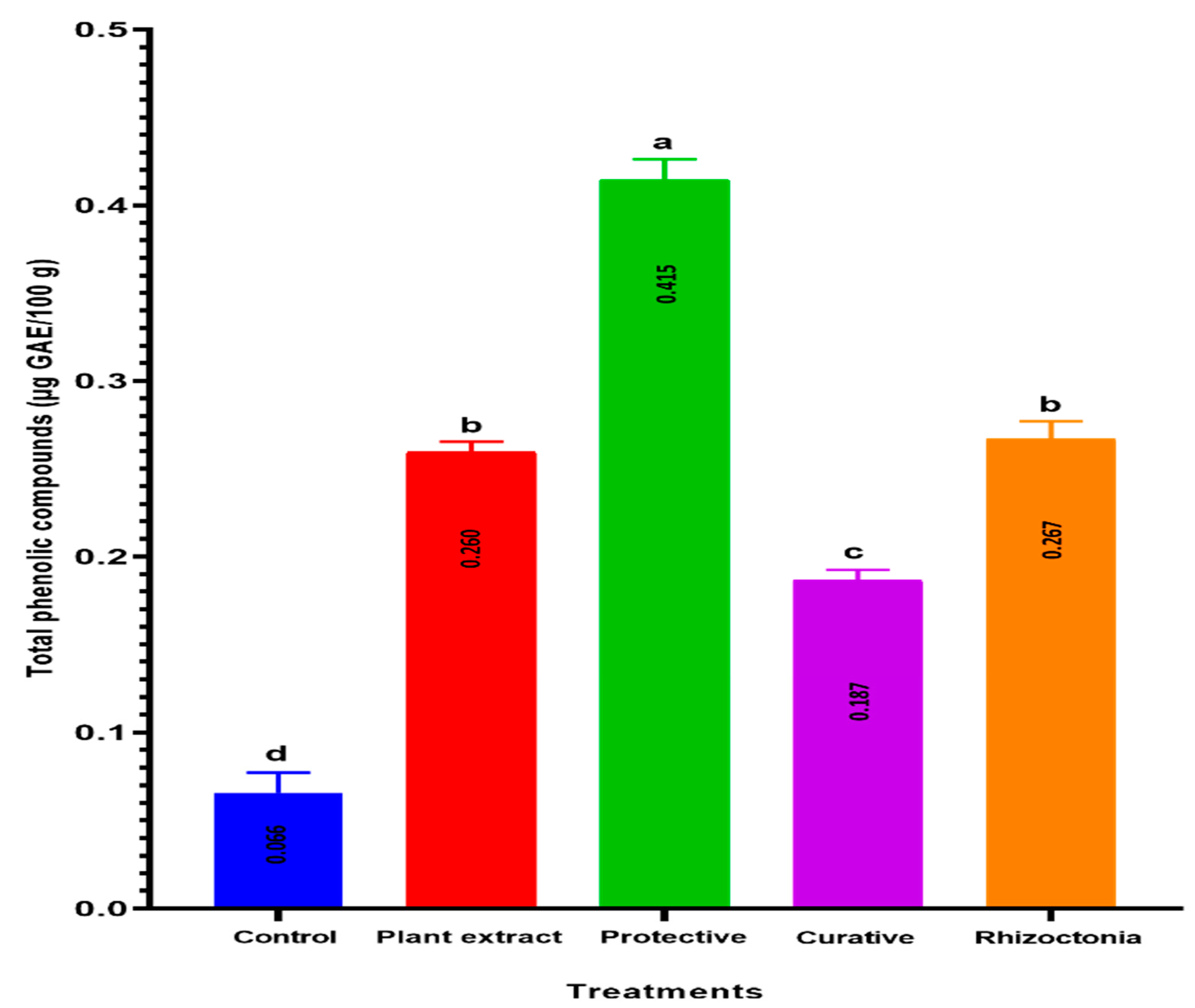
3.7. TPC Accumulation
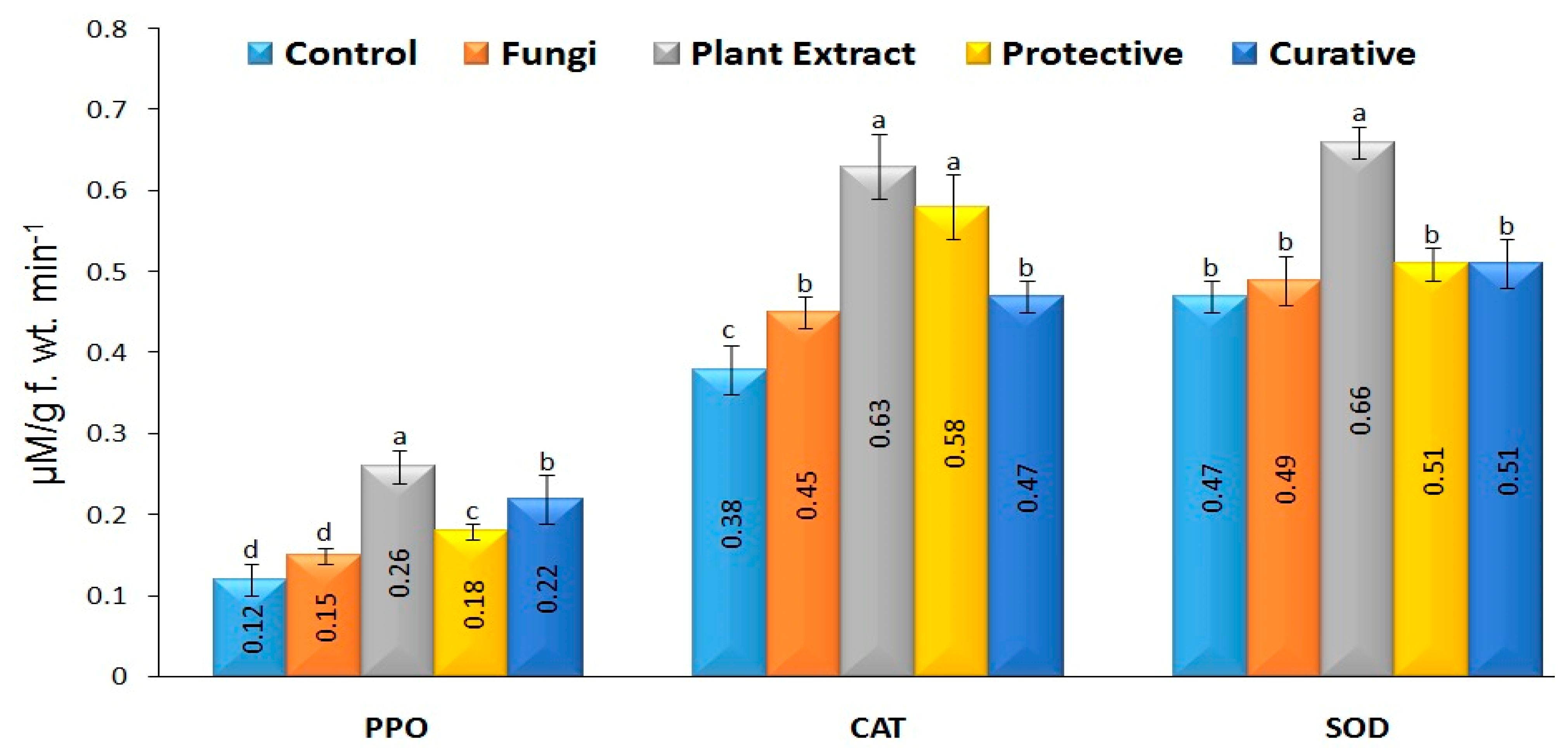
3.8. Antioxidant Enzymes Activity
3.9. Characterization of C. speciosa Extract
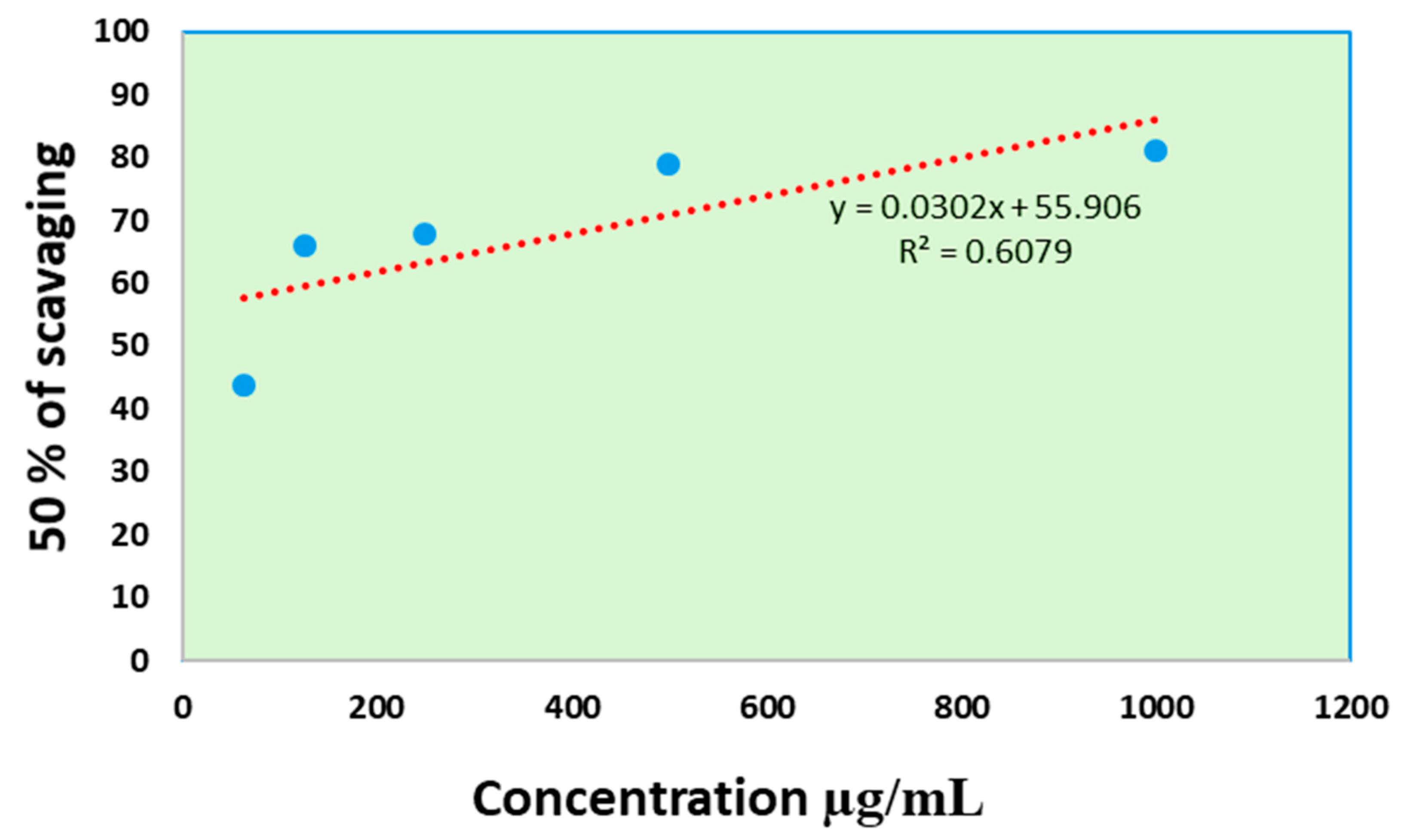
3.9.1. Antioxidant Activity
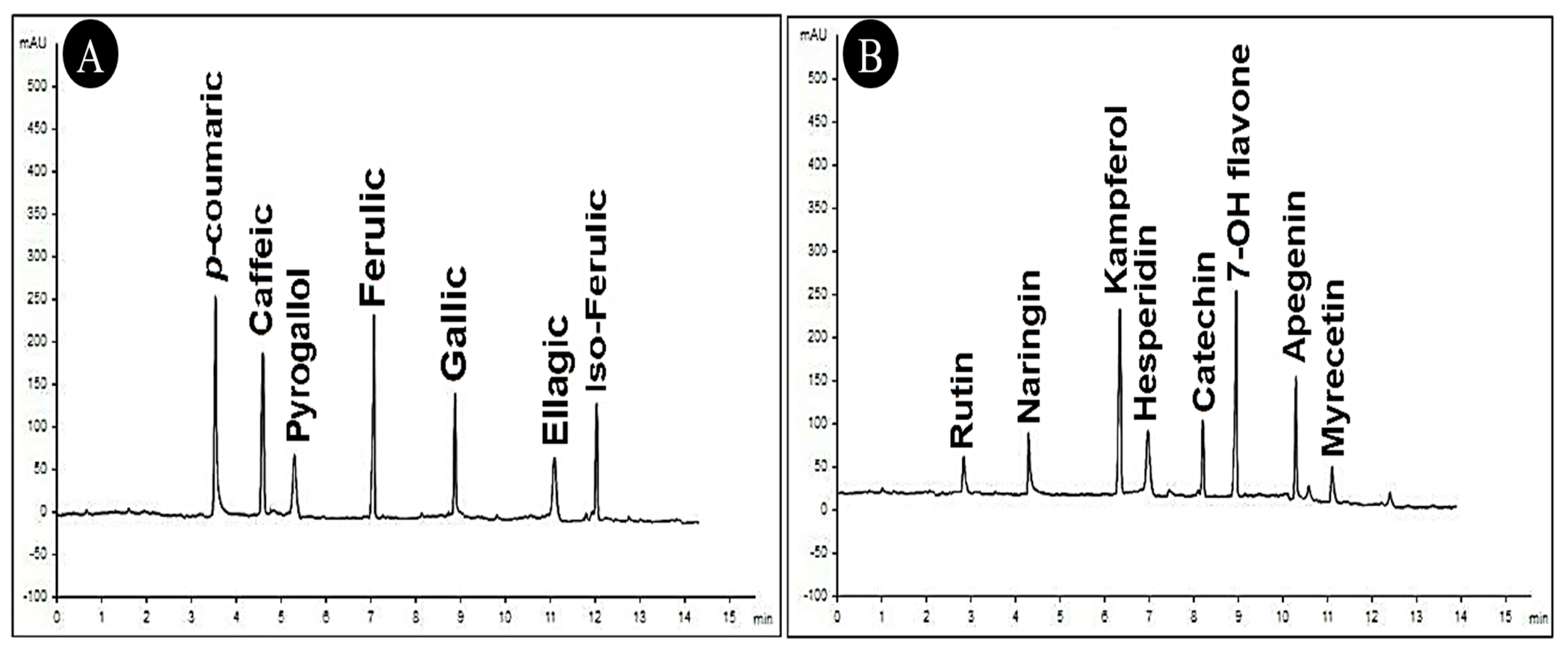
3.9.2. HPLC of C. speciosa Extract
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sabuquillo, P.; De Cal, A.; Melgarejo, P. Biocontrol of tomato wilt by Penicillium oxalicum formulations in different crop conditions. Biol. Control 2006, 37, 256–265. [Google Scholar] [CrossRef]
- Kuprashvili, T.D. The use of phytoncides for seed treatment. Zas. Karantin Rast. 1996, 5, 31. [Google Scholar]
- Gondal, A.S.; Rauf, A.; Naz, F. Anastomosis Groups of Rhizoctonia solani associated with tomato foot rot in Pothohar Region of Pakistan. Sci. Rep. 2019, 9, 3910. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Behiry, S.I.; Al-Askar, A.A. Bacillus velezensis PEA1 Inhibits Fusarium oxysporum Growth and Induces Systemic Resistance to Cucumber Mosaic Virus. Agronomy 2020, 10, 1312. [Google Scholar] [CrossRef]
- Parveen, S.; Wani, A.H.; Bhat, M.Y.; Malik, A.R.; Koka, J.A.; Ashraf, N. Antimycotic potential of some phytoextracts on some pathogenic fungi. J. Biopestic. 2017, 10, 60–65. [Google Scholar]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. African J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Joly, A.B. Botany: An Introduction to Plant Taxonomy; National Publishing Company: São Paulo, Brazil, 1991; Volume 10, p. 462. [Google Scholar]
- Adjanohoun, E.J.; et Technique, A.D.C.C.; Ahyi, A.M.R.; Ake Assi, L.; Baniakina, J.; Chibon, P. Médecine Traditionnelle et Pharmacopée: Contribution aux Études Ethnobotaniques et Floristiques en République Populaire du Congo; Agence de Cooperation Culturelle et Technique: Paris, France, 1988. [Google Scholar]
- Khan, A.; Asadsaeed, M.; Chaudhary, M.A.; Ahmad, Q.; Ansari, F. Antimicrobial, anti-inflammatory and antipyretic activity of Chorisia speciosa leaves (bombacaceae). Inter. J. Biol. Pharm. Allied Scien. 2015, 4, 6826–6838. [Google Scholar]
- Refaat, J.; Desoky, S.Y.; Ramadan, M.A.; Kamel, M.S. Bombacaceae: A phytochemical review. Pharm. Biol. 2013, 51, 100–130. [Google Scholar] [CrossRef]
- Hafez, S.S.; Ghani, A.; Afaf, E.; El Shazly, A.M. Pharmacognostical and antibacterial studies of chorisia speciosa st. Hil. flower [Bomhacaceae]. Mansoura J. Pharm. Sci. 2003, 19, 40–59. [Google Scholar]
- Ashmawy, A.M.; Azab, S.S.; Eldahshan, O.A. Effects of Chorisia crispiflora ethyl acetate extract on P21 and NF-κB in breast cancer cells. J. Am. Sci. 2012, 8, 965–972. [Google Scholar]
- Refaat, J.; Yehia Desoukey, S.; Ramadan, M.A.; Kamel, M.S.; Han, J.; Isoda, H. Comparative polyphenol contents, free radical scavenging properties and effects on adipogenesis of Chorisia Chodatii and Chorisia Speciosa. J. Med. Herbs 2015, 5, 193–207. [Google Scholar]
- El-Alfy, T.S.; El-Sawi, S.A.; Sleem, A.; Moawad, D.M. Investigation of flavonoidal content and biological activities of Chorisia insignis Hbk. leaves. Aust. J. Basic Appl. Sci. 2010, 4, 1334–1348. [Google Scholar]
- Youssef, N.H.; Qari, S.H.; Matar, S.; Hamad, N.A.; Dessoky, E.S.; Elshaer, M.M.; Sobhy, S.; Abdelkhalek, A.; Zakaria, H.M.; Heflish, A.A. Licorice, Doum, and Banana Peel Extracts Inhibit Aspergillus flavus Growth and Suppress Metabolic Pathway of Aflatoxin B1 Production. Agronomy 2021, 11, 1587. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Al-Askar, A.A.; Alsubaie, M.M.; Behiry, S.I. First Report of Protective Activity of Paronychia argentea Extract against Tobacco Mosaic Virus Infection. Plants 2021, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. Introductory Mycology; John Wiley and Sons: Hoboken, NJ, USA, 1996; ISBN 0471522295. [Google Scholar]
- Heflish, A.A.; Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Protective and Curative Effects of Trichoderma asperelloides Ta41 on Tomato Root Rot Caused by Rhizoctonia solani Rs33. Agronomy 2021, 11, 1162. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Prasad, C.S.; Dubey, N.K. Assessment of Thymus vulgaris L. essential oil as a safe botanical preservative against post harvest fungal infestation of food commodities. Innov. Food Sci. Emerg. Technol. 2008, 9, 575–580. [Google Scholar] [CrossRef]
- Dissanayake, M. Inhibitory effect of selected medicinal plant extracts on phytopathogenic fungus Fusarium oxysporum (Nectriaceae) Schlecht. Emend. Snyder and Hansen. Annu. Res. Rev. Biol. 2014, 4, 133–142. [Google Scholar] [CrossRef]
- Montealegre, J.; Valderrama, L.; Sánchez, S.; Herrera, R.; Besoain, X.; Pérez, L.M. Biological control of Rhizoctonia solani in tomatoes with Trichoderma harzianum mutants. Electron. J. Biotechnol. 2010, 13, 1–2. [Google Scholar] [CrossRef][Green Version]
- Abdeljalil, N.O.-B.; Vallance, J.; Gerbore, J.; Bruez, E.; Martins, G.; Rey, P.; Daami-Remadi, M. Biocontrol of Rhizoctonia root rot in tomato and enhancement of plant growth using rhizobacteria naturally associated to tomato. J. Plant Pathol. Microbiol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Velioglu, Y.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- CHO, Y.K.; AHN, H.Y.E.K. Purification and characterization of polyphenol oxidase from potato: II. Inhibition and catalytic mechanism. J. Food Biochem. 1999, 23, 593–605. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Sanan-Mishra, N. Differential expression profiles of tomato miRNAs induced by tobacco mosaic virus. J. Agric. Sci. Technol. 2019, 21, 475–485. [Google Scholar]
- AbdEl-Rahim, W.M.; Khalil, W.K.B.; Eshak, M.G. Evaluation of the gene expression changes in Nile tilapia (Oreochromis niloticus) as affected by the bio-removal of toxic textile dyes from aqueous solution in small-scale bioreactor. Environmentalist 2010, 30, 242–253. [Google Scholar] [CrossRef]
- Abo-Zaid, G.; Abdelkhalek, A.; Matar, S.; Darwish, M.; Abdel-Gayed, M. Application of Bio-Friendly Formulations of Chitinase-Producing Streptomyces cellulosae Actino 48 for Controlling Peanut Soil-Borne Diseases Caused by Sclerotium rolfsii. J. Fungi 2021, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Király, L.; Al-Mansori, A.-N.A.; Younes, H.A.; Zeid, A.; Elsharkawy, M.M.; Behiry, S.I. Defense Responses and Metabolic Changes Involving Phenylpropanoid Pathway and PR Genes in Squash (Cucurbita pepo L.) following Cucumber mosaic virus Infection. Plants 2022, 11, 1908. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef]
- Hof, H. Critical annotations to the use of azole antifungals for plant protection. Antimicrob. Agents Chemother. 2001, 45, 2987–2990. [Google Scholar] [CrossRef]
- Ashmawy, N.A.; Salem, M.Z.M.; El Shanhorey, N.; Al-Huqail, A.; Ali, H.M.; Behiry, S.I. Eco-friendly wood-biofungicidal and antibacterial activities of various Coccoloba uvifera L. leaf extracts: HPLC analysis of phenolic and flavonoid compounds. BioResources 2020, 15, 4165–4187. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Behiry, S.I.; EL-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane extracts of three plant species as a wood-treated oil fungicide. J. Appl. Microbiol. 2019, 126, 1683–1699. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.M.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z.M. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef]
- Kausar, F.; Intisar, A.; Din, M.I.; Aamir, A.; Hussain, T.; Aziz, P.; Mutahir, Z.; Fareed, S.; Samreen, B.; Sadaqat, K. Volatile Composition and Antibacterial Activity of Leaves of Chorisia speciosa. J. Mex. Chem. Soc. 2020, 64, 339–348. [Google Scholar] [CrossRef]
- El Sawi, S.A.M.; Hanafy, D.M.M.M.; El Alfy, T.S.M.A. Composition of the non-polar extracts and antimicrobial activity of Chorisia insignis HBK. leaves. Asian Pacific J. Trop. Dis. 2014, 4, 473–479. [Google Scholar] [CrossRef]
- Al-Askar, A.A.; Rashad, Y.M. Efficacy of some plant extracts against Rhizoctonia solani on pea. J. Plant Prot. Res. 2010, 50, 239–243. [Google Scholar] [CrossRef]
- Jat, J.G.; Agalave, H.R. Fungitoxic properties of some leaf extracts against oilseed-borne fungi. Sci. Res. Rep. 2013, 3, 210–215. [Google Scholar]
- Ambikapathy, V.; Gomathi, S.; Panneerselvam, A. Effect of antifungal activity of some medicinal plants against Pythium debaryanum (Hesse). Asian J. Plant Sci. Res. 2011, 1, 131–134. [Google Scholar]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mohebbi, M. Antifungal effect of aqueous and ethanolic mangrove plant extract on pathogenic fungus “in vitro”. Int. J. Agron. Plant Prod. 2013, 4, 1652–1658. [Google Scholar]
- Ashraf, Z.; Muhammad, A.; Imran, M.; Tareq, A.H. In vitro antibacterial and antifungal activity of methanol, chloroform and aqueous extracts of Origanum vulgare and their comparative analysis. Int. J. Org. Chem. 2011, 1, 257–261. [Google Scholar] [CrossRef]
- Moorthy, K.K.; Subramaniam, P.; Senguttuvan, J. In vitro antifungal activity of various extracts of leaf and stem parts of Solena amplexicaulis (Lam.) Gandhi. Int. J. Pharm. Pharm. Sci. 2013, 5, 745–747. [Google Scholar]
- Culver, M.; Fanuel, T.; Chiteka, A.Z. Effect of moringa extract on growth and yield of tomato. Greener J. Agric. Sci. 2012, 2, 207–211. [Google Scholar]
- Mvumi, C.; Tagwira, F.; Chiteka, A.Z. Effect of moringa extract on growth and yield of maize and common beans. Greener J. Agric. Sci. 2013, 3, 55–62. [Google Scholar] [CrossRef]
- Abdalla, M.M. Boosting the growth of rocket plants in response to the application of Moringa oleifera extracts as a biostimulant. Life Sci. J. 2014, 11, 1113–1121. [Google Scholar]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef]
- Biradar, S.R.; Rachetti, B.D. Extraction of some secondary metabolites & thin layer chromatography from different parts of Centella asiatica L.(URB). Am. J. Life Sci. 2013, 1, 243–247. [Google Scholar]
- James, J.; Dubery, I. Identification and quantification of triterpenoid centelloids in Centella asiatica (L.) Urban by densitometric TLC. JPC-J. Planar Chromatogr. TLC 2011, 24, 82–87. [Google Scholar] [CrossRef]
- Singh, D.; Singh, P.; Gupta, A.; Solanki, S.; Sharma, E.; Nema, R. Qualitative estimation of the presence of bioactive compound in Centella asiatica: An important medicinal plant. Int. J. Life Sci. Med. Sci. 2012, 2, 4–7. [Google Scholar]
- Sobhy, S.E.; Abo-Kassem, E.-E.M.; Sewelam, N.A.; Hafez, E.E.; Aseel, D.G.; Saad-Allah, K.M. Pre-soaking in Weed Extracts is a Reasonable Approach to Mitigate Fusarium graminearum Infection in Wheat. J. Plant Growth Regul. 2021, 41, 2261–2278. [Google Scholar] [CrossRef]
- Anthony, K.K.; George, D.S.; Baldev Singh, H.K.; Fung, S.M.; Santhirasegaram, V.; Razali, Z.; Somasundram, C. Reactive oxygen species activity and antioxidant properties of Fusarium infected bananas. J. Phytopathol. 2017, 165, 213–222. [Google Scholar] [CrossRef]
- Mondal, S.; Phadke, R.R.; Badigannavar, A.M. Genetic variability for total phenolics, flavonoids and antioxidant activity of testaless seeds of a peanut recombinant inbred line population and identification of their controlling QTLs. Euphytica 2015, 204, 311–321. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Kuźniak, E.; Urbanek, H. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol. Plant 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Grene, R. Oxidative stress and acclimation mechanisms in plants. In The Arabidopsis Book; American Society of Plant Biologists: Rockville, MD, USA, 2002; Volume 1. [Google Scholar]
- Taheri, P.; Kakooee, T. Reactive oxygen species accumulation and homeostasis are involved in plant immunity to an opportunistic fungal pathogen. J. Plant Physiol. 2017, 216, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Hoegen, E.; Strömberg, A.; Pihlgren, U.; Kombrink, E. Primary structure and tissue-specific expression of the pathogenesis-related protein PR-1b in potato. Mol. Plant Pathol. 2002, 3, 329–345. [Google Scholar] [CrossRef] [PubMed]
- D’Maris Amick Dempsey, A.C.; Vlot, M.C.W.; Daniel, F.K.; Dempsey, D.A.; Vlot, A.C.; Wildermuth, M.C.; Klessig, D.F.; D’Maris Amick Dempsey, A.C.; Vlot, M.C.W.; Daniel, F.K.; et al. Salicylic acid biosynthesis and metabolism. Arab. Book/Am. Soc. Plant Biol. 2011, 9, e0156. [Google Scholar] [CrossRef]
- Su, Z.-Z.; Mao, L.-J.; Li, N.; Feng, X.-X.; Yuan, Z.-L.; Wang, L.-W.; Lin, F.-C.; Zhang, C.-L. Evidence for biotrophic lifestyle and biocontrol potential of dark septate endophyte Harpophora oryzae to rice blast disease. PLoS ONE 2013, 8, e61332. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Chun, S.C. Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 2016, 80, 2277–2283. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Di Pietro, A. The CAP protein superfamily: Function in sterol export and fungal virulence. Biomol. Concepts 2013, 4, 519–525. [Google Scholar] [CrossRef]
- Abdelkhalek, A. Expression of tomato pathogenesis related genes in response to Tobacco mosaic virus. JAPS J. Anim. Plant Sci. 2019, 29, 1596–1602. [Google Scholar]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Roylawar, P.; Panda, S.; Kamble, A. Comparative analysis of BABA and Piriformospora indica mediated priming of defence-related genes in tomato against early blight. Physiol. Mol. Plant Pathol. 2015, 91, 88–95. [Google Scholar] [CrossRef]
- Abdelkhalek, A.; Dessoky, E.S.; Hafez, E. Polyphenolic genes expression pattern and their role in viral resistance in tomato plant infected with Tobacco mosaic virus. Biosci. Res. 2018, 15, 3349–3356. [Google Scholar]
- Abdelkhalek, A.; Salem, M.Z.M.; Hafez, E.; Behiry, S.I.; Qari, S.H. The Phytochemical, Antifungal, and First Report of the Antiviral Properties of Egyptian Haplophyllum tuberculatum Extract. Biology 2020, 9, 248. [Google Scholar] [CrossRef] [PubMed]
- Marais, J.P.J.; Deavours, B.; Dixon, R.A.; Ferreira, D. The stereochemistry of flavonoids. In The Science of Flavonoids; Springer: Amsterdam, The Netherlands, 2006; pp. 1–46. [Google Scholar]
- Abdelkhalek, A.; Al-Askar, A.A.; Behiry, S.I. Bacillus licheniformis strain POT1 mediated polyphenol biosynthetic pathways genes activation and systemic resistance in potato plants against Alfalfa mosaic virus. Sci. Rep. 2020, 10, 16120. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.; Regente, M.; Jacobi, S.; Del Rio, M.; Pinedo, M.; de la Canal, L. Chlorogenic acid is a fungicide active against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017, 140, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Ahn, I.-P.; Lee, S.-W.; Suh, S.-C. Rhizobacteria-induced priming in Arabidopsis is dependent on ethylene, jasmonic acid, and NPR1. Mol. Plant-Microbe Interact. 2007, 20, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.-D.; Liu, H.-X.; Jiang, C.-H.; Wang, Y.-P.; Wang, Q.-Y.; Jin, H.-L.; Guo, J.-H. The plant growth–promoting rhizobacterium Bacillus cereus AR156 induces systemic resistance in Arabidopsis thaliana by simultaneously activating salicylate-and jasmonate/ethylene-dependent signaling pathways. Mol. Plant-Microbe Interact. 2011, 24, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.; Vesentini, D.; Rego, C.; Monteiro, S.; Oliveira, H.; Ferreira, R.B. Phaeomoniella chlamydospora infection induces changes in phenolic compounds content in Vitis vinifera. Phytopathol. Mediterr. 2009, 48, 101–116. [Google Scholar]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. Phenolic compounds in apple leaves after infection with apple scab. Biol. Plant 2011, 55, 725–730. [Google Scholar] [CrossRef]
- Rusjan, D.; Veberič, R.; Mikulič-Petkovšek, M. The response of phenolic compounds in grapes of the variety ‘Chardonnay’(Vitis vinifera L.) to the infection by phytoplasma Bois noir. Eur. J. Plant Pathol. 2012, 133, 965–974. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, A.; Singh, S.P.; Singh, H.B. Trichoderma harzianum-mediated reprogramming of oxidative stress response in root apoplast of sunflower enhances defence against Rhizoctonia solani. Eur. J. Plant Pathol. 2011, 131, 121–134. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Changes in phenolic content induced by infection with Didymella applanata and Leptosphaeria coniothyrium, the causal agents of raspberry spur and cane blight. Plant Pathol. 2014, 63, 185–192. [Google Scholar] [CrossRef]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with Fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, M.P.; Zhang, X.; Huo, Y.; Ma, H.X. Change of defensive-related enzyme in wheat crown rot seedlings infected by Fusarium graminearum. Cereal Res. Commun. 2013, 41, 431–439. [Google Scholar] [CrossRef]
- Blackman, L.M.; Hardham, A.R. Regulation of catalase activity and gene expression during Phytophthora nicotianae development and infection of tobacco. Mol. Plant Pathol. 2008, 9, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Nasr, E.M.; Assaf, M.H.; Darwish, F.M.; Ramadan, M.A. Phytochemical and biological study of Chorisia speciosa A. St. Hil. cultivated in Egypt. J. Pharmacogn. Pharmacol 2018, 7, 649–656. [Google Scholar]
- Mani, J.S.; Johnson, J.B.; Steel, J.C.; Broszczak, D.A.; Neilsen, P.M.; Walsh, K.B.; Naiker, M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020, 284, 197989. [Google Scholar] [CrossRef] [PubMed]
- Shaygannia, E.; Bahmani, M.; Zamanzad, B.; Rafieian-Kopaei, M. A review study on Punica granatum L. J. Evid. Based Complement. Altern. Med. 2016, 21, 221–227. [Google Scholar] [CrossRef]
- El Sawi, S.; Moawad, D.; El Alfy, S. Activity of Chorisia insignis HBK. against larynx carcinoma and chemical investigation of its polar extracts. J. Appl. Sci. Res. 2012, 8, 5564–5571. [Google Scholar]
- Youssef, N.H.; Qari, S.H.; Behiry, S.I.; Dessoky, E.S.; El-Hallous, E.I.; Elshaer, M.M.; Kordy, A.; Maresca, V.; Abdelkhalek, A.; Heflish, A.A. Antimycotoxigenic Activity of Beetroot Extracts against Altenaria alternata Mycotoxins on Potato Crop. Appl. Sci. 2021, 11, 4239. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Behiry, S.I.; Ali, H.M.; EL-Hefny, M.; Salem, M.Z.M.; Ashmawy, N.A. Phytochemical compounds of branches from P. halepensis oily liquid extract and S. terebinthifolius essential oil and their potential antifungal activity. Processes 2020, 8, 330. [Google Scholar] [CrossRef]

| Gene | Abbreviation | Nucleotide Sequences |
|---|---|---|
| Internal Transcribed Spacer | ITS | ITS1-TCCGTAGGTGAACCTGCGG |
| ITS4-TCCTCCGCTTATTGATATGC | ||
| Pathogenesis-related protein-1 | PR-1 | For-GTTCCTCCTTGCCACCTTC |
| Rev-TATGCACCCCCAGCATAGTT | ||
| Endoglucanase | PR-2 | For-TATAGCCGTTGGAAACGAAG |
| Rev-CAACTTGCCATCACATTCTG | ||
| Chitinase | PR-3 | For-ATGGAGCATTGTGCCCTAAC |
| Rev-TCCTACCAACATCACCACCA | ||
| Phenylalanine ammonia-lyase | PAL | For-GTTATGCTCTTAGAACGTCGCCC |
| Rev-CCGTGTAATGCCTTGTTTCTTGA | ||
| Chalcone Synthase | CHS | For-CACCGTGGAGGAGTATCGTAAGGC |
| Rev-TGATCAACACAGTTGGAAGGCG | ||
| β-actin | β-actin | For-TGGCATACAAAGACAGGACAGCCT |
| Rev-ACTCAATCCCAAGGCCAACAGAGA |
| Treatment (µg/mL) | Growth Inhibition % |
|---|---|
| Negative control | 00.00 ± 0.00 e |
| 1 | 00.00 ± 0.00 e |
| 2 | 40.37 ± 0.29 d |
| 4 | 89.77 ± 0.12 c |
| 8 | 92.43 ± 0.15 b |
| 10 | 100.00 ± 0.00 a |
| Fungicide (Rizolex, 2 µg/mL) | 100.00 ± 0.00 a |
| Compounds | Retention Time (min.) | Amount (µg/mL) |
|---|---|---|
| p-Coumaric acid | 3.5 | 8.65 |
| Caffeic acid | 4.8 | 7.59 |
| Pyrogallol | 5.2 | 1.22 |
| Ferulic acid | 7.0 | 8.14 |
| Gallic acid | 9.0 | 6.33 |
| Ellagic acid | 11.0 | 1.18 |
| Iso-Ferulic | 12.0 | 5.71 |
| Rutin | 3.0 | 0.88 |
| Naringenin | 4.1 | 3.14 |
| Kaempferol | 6.1 | 9.23 |
| Hesperidin | 7.0 | 2.89 |
| Catechin | 8.1 | 3.51 |
| 7-OH flavone | 9.0 | 10.36 |
| Apigenin | 10.1 | 5.47 |
| Myricetin | 11.0 | 0.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behiry, S.I.; Soliman, S.A.; Al-Mansori, A.-N.A.; Al-Askar, A.A.; Arishi, A.A.; Elsharkawy, M.M.; Abdelkhalek, A.; Heflish, A.A. Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani. Agronomy 2022, 12, 2309. https://doi.org/10.3390/agronomy12102309
Behiry SI, Soliman SA, Al-Mansori A-NA, Al-Askar AA, Arishi AA, Elsharkawy MM, Abdelkhalek A, Heflish AA. Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani. Agronomy. 2022; 12(10):2309. https://doi.org/10.3390/agronomy12102309
Chicago/Turabian StyleBehiry, Said I., Seham A. Soliman, Al-Naji A. Al-Mansori, Abdulaziz A. Al-Askar, Amr A. Arishi, Mohsen Mohamed Elsharkawy, Ahmed Abdelkhalek, and Ahmed A. Heflish. 2022. "Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani" Agronomy 12, no. 10: 2309. https://doi.org/10.3390/agronomy12102309
APA StyleBehiry, S. I., Soliman, S. A., Al-Mansori, A.-N. A., Al-Askar, A. A., Arishi, A. A., Elsharkawy, M. M., Abdelkhalek, A., & Heflish, A. A. (2022). Chorisia speciosa Extract Induces Systemic Resistance against Tomato Root Rot Disease Caused by Rhizoctonia solani. Agronomy, 12(10), 2309. https://doi.org/10.3390/agronomy12102309









