Unveiling the Impacts of Biochar, Manure and Their Optimal Combinations on Microbiological Soil Health Indicators and Lettuce Biomass
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Manure and Preparation of Amendments
2.2. Pot Experiment and Design
2.3. Soil Analyses
2.4. Statistical Analyses
3. Results
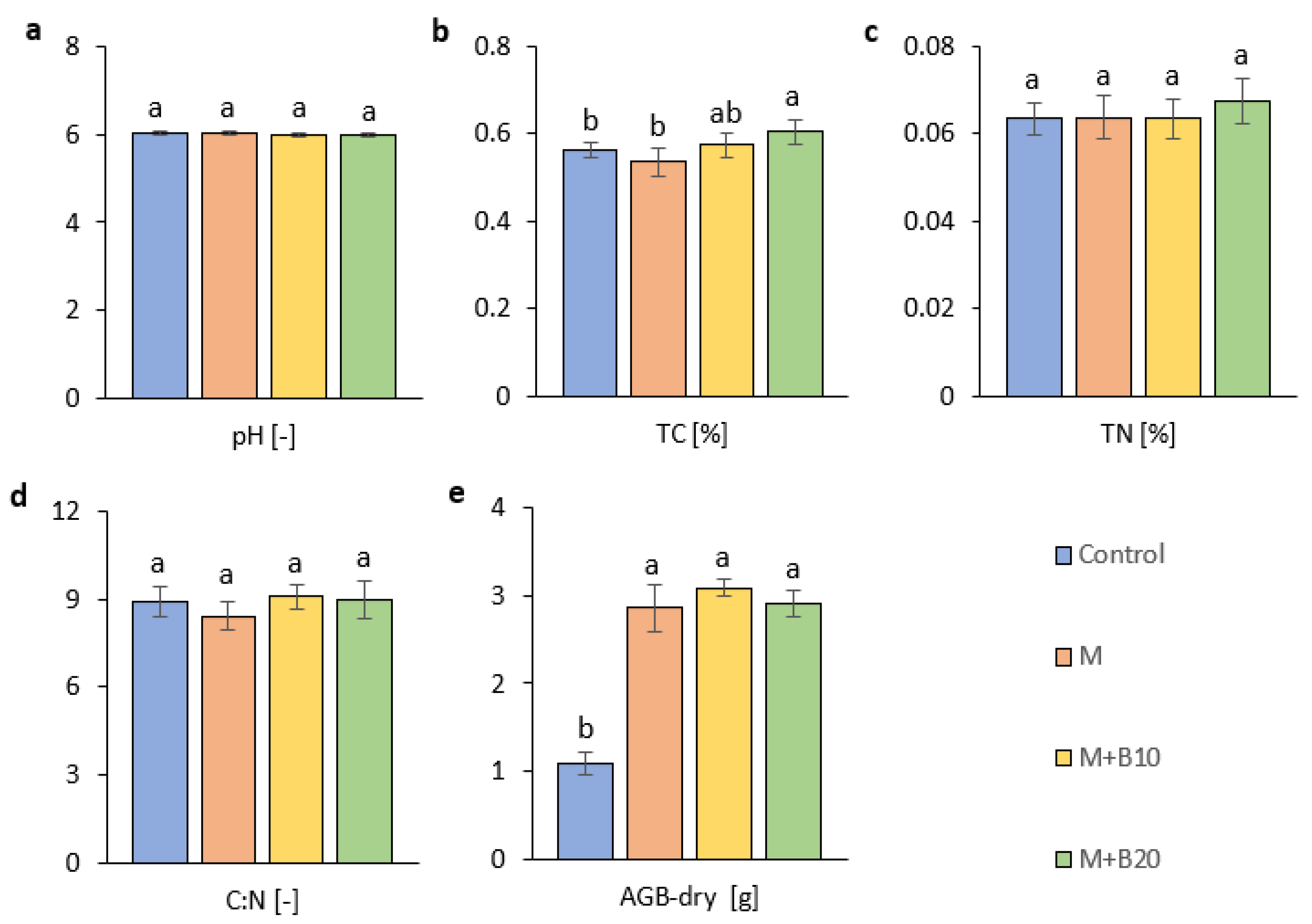
3.1. Effects of Amendments on Soil Properties and Plant Biomass
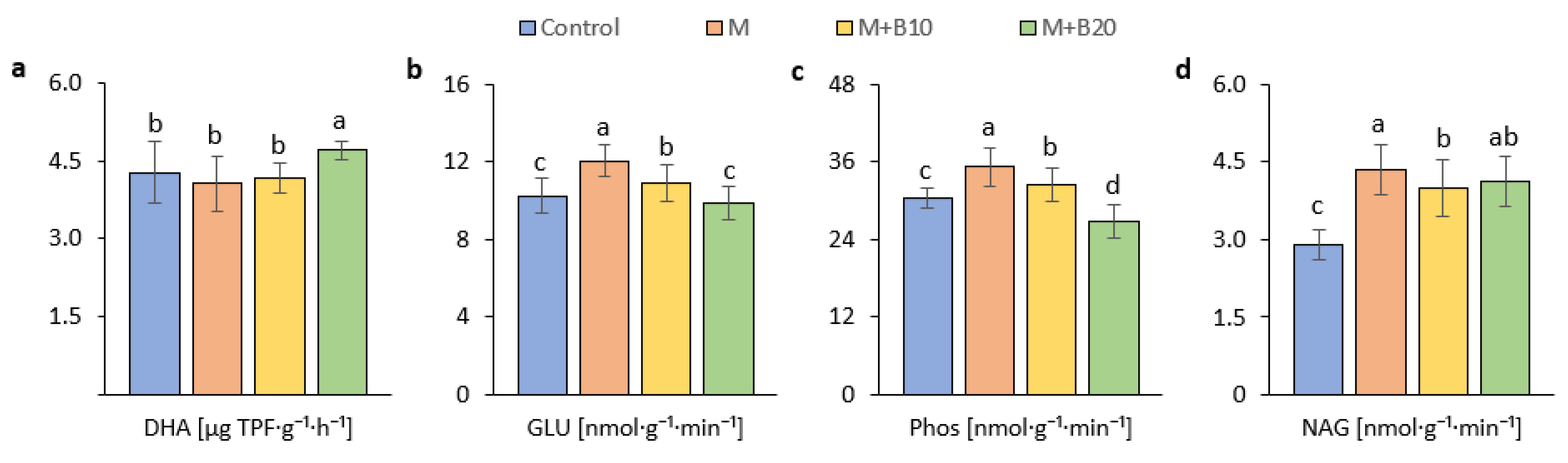
3.2. Effects of Amendments on Soil Microbial Properties
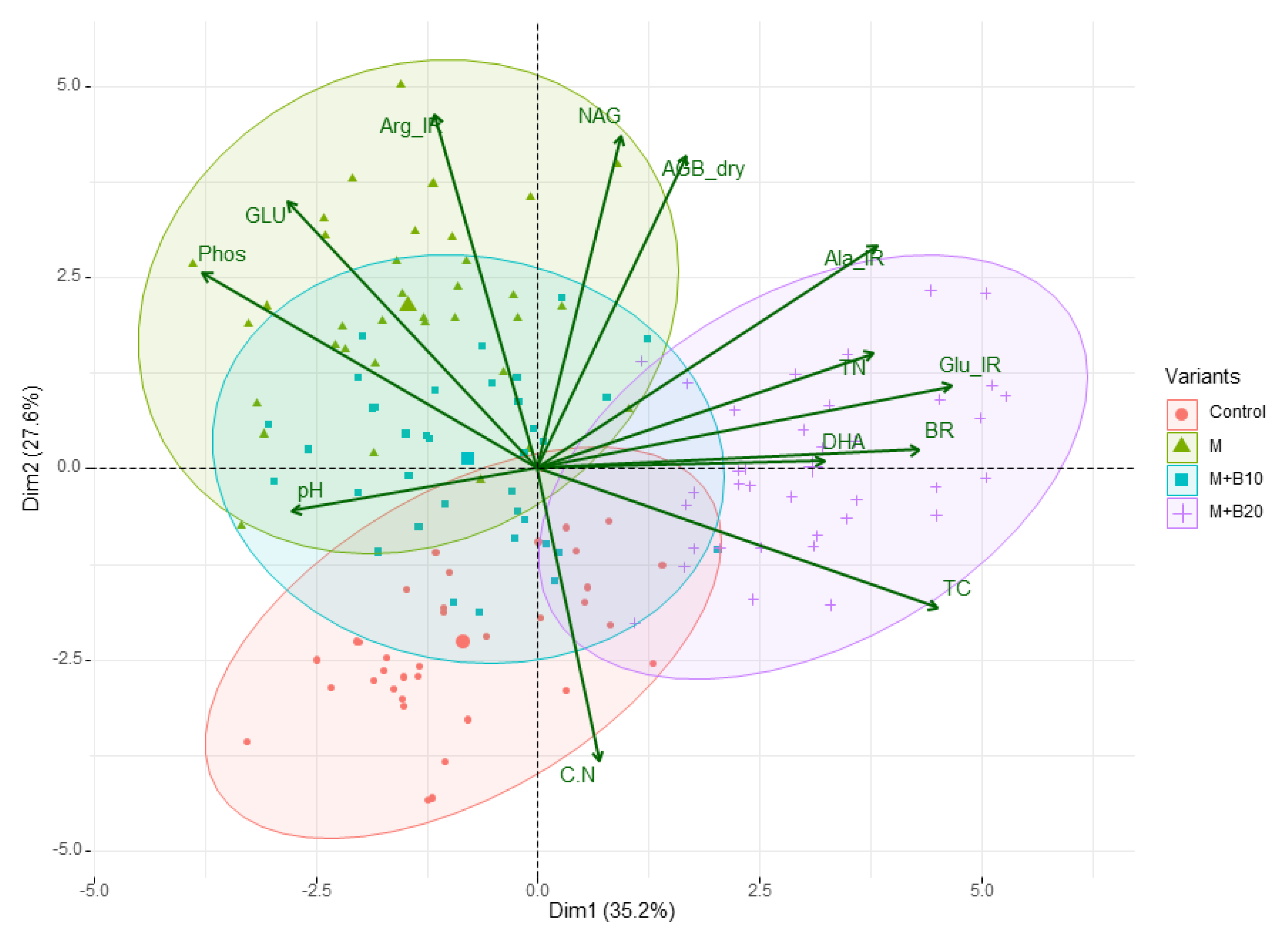
3.3. Results from Principal Component Analysis
4. Discussion
4.1. Effects of Amendments on Soil Physico-Chemical Properties and Plant Biomass
4.2. Effects of Amendments on Microbial Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oldeman, L.R.; Hakkeling, R.T.A.; Sombroek, W.G. World Map of the Status of Human-Induced Soil Degradation: An Explanatory Note; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1990. [Google Scholar]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhou, X.; Luo, Y.; Yang, Y.; Fang, C.; Chen, J.; Li, B. Minor stimulation of soil carbon storage by nitrogen addition: A meta-analysis. Agric. Ecosyst. Environ. 2011, 140, 234–244. [Google Scholar] [CrossRef]
- Wu, J.; He, S.; Liang, Y.; Li, G.; Li, S.; Chen, S.; Nadeem, F.; Hu, J. Effect of phosphate additive on the nitrogen transformation during pig manure composting. Environ. Sci. Pollut. Res. 2017, 24, 17760–17768. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Naveed, M.; Saeed, Q.; Ashraf, M.N.; Hussain, A.; Abbas, T.; Kamran, M.; Minggang, X. Application potentials of plant growth promoting rhizobacteria and fungi as an alternative to conventional weed control methods. In Sustainable Crop Production; IntechOpen: London, UK, 2019. [Google Scholar]
- Khan, A.; Lal, R. Potential for Carbon Sequestration in the Soils of Afghanistan and Pakistan. In Climate Change and Terrestrial Carbon Sequestration in Central Asia; CRC Press: Boca Raton, FL, USA, 2007; pp. 235–250. [Google Scholar]
- Sadaf, J.; Shah, G.A.; Shahzad, K.; Ali, N.; Shahid, M.; Ali, S.; Hussain, R.A.; Ahmed, Z.I.; Traore, B.; Ismail, I.M.; et al. Improvements in wheat productivity and soil quality can accomplish by co-application of biochars and chemical fertilizers. Sci. Total Environ. 2017, 607, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An introduction. In Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Lehmann, J.; Joseph, S. (Eds.) Biochar for Environmental Management: Science, Technology and Implementation; Routledge: Abingdon, UK, 2015. [Google Scholar]
- Bass, A.M.; Bird, M.I.; Kay, G.; Muirhead, B. Soil properties, greenhouse gas emissions and crop yield under compost, biochar and co-composted biochar in two tropical agronomic systems. Sci. Total Environ. 2016, 550, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Marks, E.A.; Mattana, S.; Alcañiz, J.M.; Pérez-Herrero, E.; Domene, X. Gasifier biochar effects on nutrient availability, organic matter mineralization, and soil fauna activity in a multi-year Mediterranean trial. Agric. Ecosyst. Environ. 2016, 215, 30–39. [Google Scholar] [CrossRef]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, M.F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Holatko, J.; Bielska, L.; Hammerschmiedt, T.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Baltazar, T.; Latal, O.; Brtnicky, M. Cattle Manure Fermented with Biochar and Humic Substances Improve the Crop Biomass, Microbiological Properties and Nutrient Status of Soil. Agronomy 2022, 12, 368. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Influence of biochar on potential enzyme activities in two calcareous soils of contrasting texture. Geoderma 2017, 308, 149–158. [Google Scholar] [CrossRef]
- Zheng, X.; Song, W.; Guan, E.; Wang, Y.; Hu, X.; Liang, H.; Dong, J. Response in Physicochemical Properties of Tobacco-Growing Soils and N/P/K Accumulation in Tobacco Plant to Tobacco Straw Biochar. J. Soil Sci. Plant Nutr. 2020, 20, 293–305. [Google Scholar] [CrossRef]
- Du, Z.J.; Xiao, Y.T.; Qi, X.B.; Liu, Y.A.; Fan, X.Y.; Li, Z.Y. Peanutshell biochar and biogas slurry improve soil properties in the North China Plain: A four-year feld study. Sci. Rep. 2018, 14, 1032. [Google Scholar] [CrossRef]
- Wang, D.; Felice, M.L.; Scow, K.M. Impacts and interactions of biochar and biosolids on agricultural soil microbial communities during dry and wet-dry cycles. Appl. Soil Ecol. 2020, 152, 103570. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, Y.; Zhao, H.; Li, Q. A comparative study on behavior of heavy metals in pyrochar and hydrochar from sewage sludge. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 565–571. [Google Scholar] [CrossRef]
- Visioli, G.; Conti, F.D.; Menta, C.; Bandiera, M.; Malcevschi, A.; Jones, D.L.; Vamerali, T. Assessing biochar ecotoxicology for soil amendment by root phytotoxicity bioassays. Environ. Monit. Assess. 2016, 188, 166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, R.; Si, X.; Duan, X.; Quan, X. The adverse effect of biochar to aquatic algae- the role of free radicals. Environ. Pollut. 2019, 248, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Liu, S.; Ye, S.; Yang, H.; Song, B.; Qin, F.; Shen, M.; Tan, C.; Zeng, G.; Tan, X. Potential hazards of biochar: The negative environmental impacts of biochar applications. J. Hazard. Mater. 2021, 420, 126611. [Google Scholar] [CrossRef]
- Garrido, M.S.; Menezes, R.S.C.; Sampaio, E.V.S.B.; Marques, T.R.R.; Olszevski, N. Accumulation and apparent recovery of N, P and K after the incorporation of gliricidia and manure in intercropping during the cultivation of corn–cowpea–cotton. Nutr. Cycl. Agroecosystems 2017, 2, 187–196. [Google Scholar] [CrossRef]
- Bohara, H.; Dodla, S.; Wang, J.J.; Darapuneni, M.; Acharya, B.S.; Magdi, S.; Pavuluri, K. Influence of poultry litter and biochar on soil water dynamics and nutrient leaching from a very fine sandy loam soil. Soil Tillage Res. 2019, 189, 44–51. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Agbede, T.M.; Aboyeji, C.M.; Dunsin, O.; Simeon, V.T. Effects of biochar and poultry manure on soil characteristics and the yield of radish. Sci. Hort. 2019, 243, 457–463. [Google Scholar] [CrossRef]
- Moore, P.A.; Daniel, T.C.; Sharpley, A.N.; Wood, C.W. Poultry manure management: Environmentally sound options. J. Soil Water Conserv. 1995, 50, 321–327. [Google Scholar]
- Abd El-Kader, A.A.; Shaaban, S.M.; El-Fattah, M.S.A. Effect of irrigation levels and organic compost on okra plants (Abelmoschus esculentus) grown in sandy calcareous soil. Agric. Biol. J. N. Am. 2010, 1, 225–231. [Google Scholar] [CrossRef]
- ISO_10390; Soil Quality—Determination of ph. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO_20130; Soil Quality—Measurement of Enzyme Activity Patterns in Soil Samples Using Colorimetric Substrates in Micro-Well Plates. International Organization for Standardization: Geneva, Switzerland, 2018.
- Małachowska-Jutsz, A.; Matyja, K. Discussion on methods of soil dehydrogenase determination. Int. J. Environ. Sci. Technol. 2019, 16, 7777–7790. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Bahar, M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Zahra, M.B.; Aftab, Z.E.H.; Akhter, A.; Haider, M.S. Cumulative effect of biochar and compost on nutritional profile of soil and maize productivity. J. Plant Nutr. 2021, 44, 1664–1676. [Google Scholar] [CrossRef]
- Rehman, I.; Riaz, M.; Ali, S.; Arif, M.S.; Ali, S.; Alyemeni, M.N.; Alsahli, A.A. Evaluating the Effects of Biochar with Farmyard Manure under Optimal Mineral Fertilizing on Tomato Growth, Soil Organic C and Biochemical Quality in a Low Fertility Soil. Sustainability 2021, 13, 2652. [Google Scholar] [CrossRef]
- Mustafa, A.; Hu, X.; Abrar, M.M.; Shah, S.A.A.; Nan, S.; Saeed, Q.; Kamran, M.; Naveed, M.; Conde-Cid, M.; Hongjun, G.; et al. Long-term fertilization enhanced carbon mineralization and maize biomass through physical protection of organic carbon in fractions under continuous maize cropping. Appl. Soil Ecol. 2021, 165, 103971. [Google Scholar] [CrossRef]
- Yang, C.D.; Lu, S.G. Effects of five different biochars on aggregation, water retention and mechanical properties of paddy soil: A field experiment of three-season crops. Soil Tillage Res. 2021, 205, 104798. [Google Scholar] [CrossRef]
- Wang, J.; Xiong, Z.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and primingeffects. Global Change Biology Bioenergy. 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Amoakwah, E.; Osei, B.A.; Arthur, E. Changes in soil chemical properties and lettuce yield response following incorporation of biochar and cow dung to highly weathered acidic soils. J. Organic Agri. Environ. 2016, 4, 28–39. [Google Scholar]
- Mustafa, A.; Minggang, X.; Shah, S.A.; Abrar, M.M.; Nan, S.; Baoren, W.; Zejiang, C.; Saeed, Q.; Naveed, M.; Mehmood, K.; et al. Soil aggregation and soil aggregate stability regulate organic carbon and nitrogen storage in a red soil of southern China. J. Environ. Manag. 2020, 270, 110894. [Google Scholar] [CrossRef] [PubMed]
- Trupiano, D.; Cocozza, C.; Baronti, S. Effects of biochar and its combination with compost onlettuce (Lactuca sativa L.) growth, soil properties, and soil microbial activity and abundance. Hindawi. Int. J. Agron. 2017, 2017, 3158207. [Google Scholar] [CrossRef]
- Masto, R.E.; Ansari, M.A.; George, J.; Selvi, V.A.; Ram, L.C. Co-application of biochar and lignite fly ash on soil nutrients and biological parameters at different crop growth stages of Zea mays. Ecol. Eng. 2013, 58, 314–322. [Google Scholar] [CrossRef]
- Cao, Y.; Bai, M.; Han, B.; Impraim, R.; Butterly, C.; Hu, H.; He, J.; Chen, D. Enhanced nitrogen retention by lignite during poultry litter composting. J. Clean. Prod. 2020, 277, 10. [Google Scholar] [CrossRef]
- Dubey, R.K.; Dubey, P.K.; Abhilash, P. Sustainable soil amendments for improving the soil quality, yield and nutrient content of Brassica juncea (L.) grown in different agroecological zones of eastern Uttar Pradesh, India. Soil Tillage Res. 2019, 195, 11. [Google Scholar] [CrossRef]
- Al-Omran, A.; Ibrahim, A.; Alharbi, A. Evaluating the impact of combined application of biochar and compost on hydro-physical properties of loamy sand soil. Commun. Soil Sci. Plant Anal. 2019, 50, 2442–2456. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Kucerik, J.; Mustafa, A.; Radziemska, M.; Kintl, A.; Malicek, O.; Baltazar, T.; Latal, O.; Brtnicky, M. Manure Maturation with Biochar: Effects on Plant Biomass, Manure Quality and Soil Microbiological Characteristics. Agriculture 2022, 12, 314. [Google Scholar] [CrossRef]
- Irmak Yilmaz, F. Impact of biochar and animal manure on some biological and chemical properties of soil. Appl. Ecol. Environ. Res. 2019, 17, 8865–8876. [Google Scholar] [CrossRef]
- Mate, C.H.; Mukherjee, I.; Das, S.K. Persistence of spiromesifen in soil: Influence of moisture, light, pH and organic amendment. Environ. Monit. Assess. 2015, 187, 1–12. [Google Scholar] [CrossRef]
- Cárdenas-Aguiar, E.; Méndez, A.; Paz-Ferreiro, J.; Gascó, G. The Effects of Rabbit Manure-Derived Biochar on Soil Health and Quality Attributes of Two Mine Tailings. Sustainability 2022, 14, 1866. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of Biochar on Manure Carbon Stabilization and Greenhouse Gas Emissions. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef]
- Häring, V.; Manka’abusi, D.; Akoto-Danso, E.K.; Werner, S.; Atiah, K.; Steiner, C.; Lompo, D.J.P.; Adiku, S.; Buerkert, A.; Marschner, B. Effects of biochar, wastewater irrigation and fertilization on soil properties in West African urban agriculture. Sci. Rep. 2017, 7, 10738. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Lehmann, J.; Thies, J.E. Soil Microbial Community Response to Amending Maize Soils with Maize Stover Charcoal. In Proceedings of the 2008 Conference of International Biochar Initiative, Newcastle, UK, 10 September 2008; pp. 8–10. [Google Scholar]

| Variant | Variant Code | pH [-] | C [%] | N [%] | C:N [-] | |
|---|---|---|---|---|---|---|
| I. | Control without manure | Control | - | - | - | - |
| II. | Manure | M | 6.5 | 20.1 | 1.1 | 18.2 |
| III. | Manure + biochar 10% | M + B10 | 6.9 | 26.4 | 1.5 | 17.4 |
| IV. | Manure + biochar 20% | M + B20 | 7.1 | 35.9 | 2.0 | 17.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, A.; Holatko, J.; Hammerschmiedt, T.; Kucerik, J.; Baltazar, T.; Kintl, A.; Malicek, O.; Havlicek, Z.; Brtnicky, M. Unveiling the Impacts of Biochar, Manure and Their Optimal Combinations on Microbiological Soil Health Indicators and Lettuce Biomass. Agronomy 2022, 12, 2307. https://doi.org/10.3390/agronomy12102307
Mustafa A, Holatko J, Hammerschmiedt T, Kucerik J, Baltazar T, Kintl A, Malicek O, Havlicek Z, Brtnicky M. Unveiling the Impacts of Biochar, Manure and Their Optimal Combinations on Microbiological Soil Health Indicators and Lettuce Biomass. Agronomy. 2022; 12(10):2307. https://doi.org/10.3390/agronomy12102307
Chicago/Turabian StyleMustafa, Adnan, Jiri Holatko, Tereza Hammerschmiedt, Jiri Kucerik, Tivadar Baltazar, Antonin Kintl, Ondrej Malicek, Zdenek Havlicek, and Martin Brtnicky. 2022. "Unveiling the Impacts of Biochar, Manure and Their Optimal Combinations on Microbiological Soil Health Indicators and Lettuce Biomass" Agronomy 12, no. 10: 2307. https://doi.org/10.3390/agronomy12102307
APA StyleMustafa, A., Holatko, J., Hammerschmiedt, T., Kucerik, J., Baltazar, T., Kintl, A., Malicek, O., Havlicek, Z., & Brtnicky, M. (2022). Unveiling the Impacts of Biochar, Manure and Their Optimal Combinations on Microbiological Soil Health Indicators and Lettuce Biomass. Agronomy, 12(10), 2307. https://doi.org/10.3390/agronomy12102307








