Phytoremediation of Heavy Metal Contaminated Soils Using Safflower
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
- -
- -
- mAI (modified Accumulation Index) was calculated to evaluate the plant’s ability to uptake the heavy metal from the soil [9]:
- -
- mBCF (modified bioconcentration factor) was used to evaluate the ability of the plant to extract and accumulate the metal in the aerial or radical fraction of the biomass. The content of the bioavailable metal in the soil, determined by EDTA extraction, represents the amount of metal potentially bioavailable for the plant. Therefore, this factor can represent more realistically the translocation capacity of the metal in plants [9,26,27]:
- -
3. Results
3.1. Soil Characterization
3.2. Biomass Production
3.3. Heavy Metal Uptake per Plant and Amount of Heavy Metal Extracted
3.4. Tolerance Index, Translocation Factor, and Modified Accumulation Index and Bioconcentration Factor of the Aboveground and the Belowground
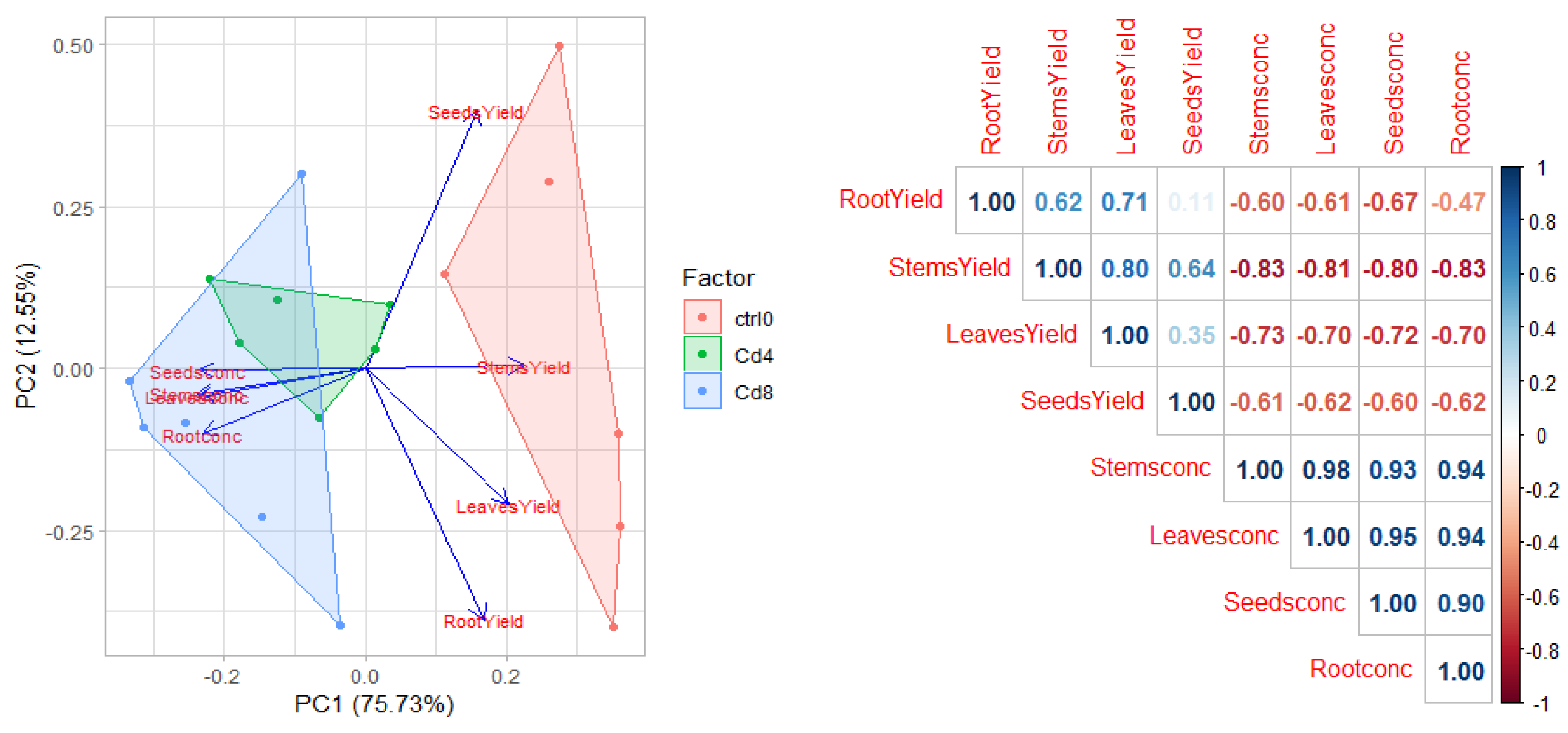
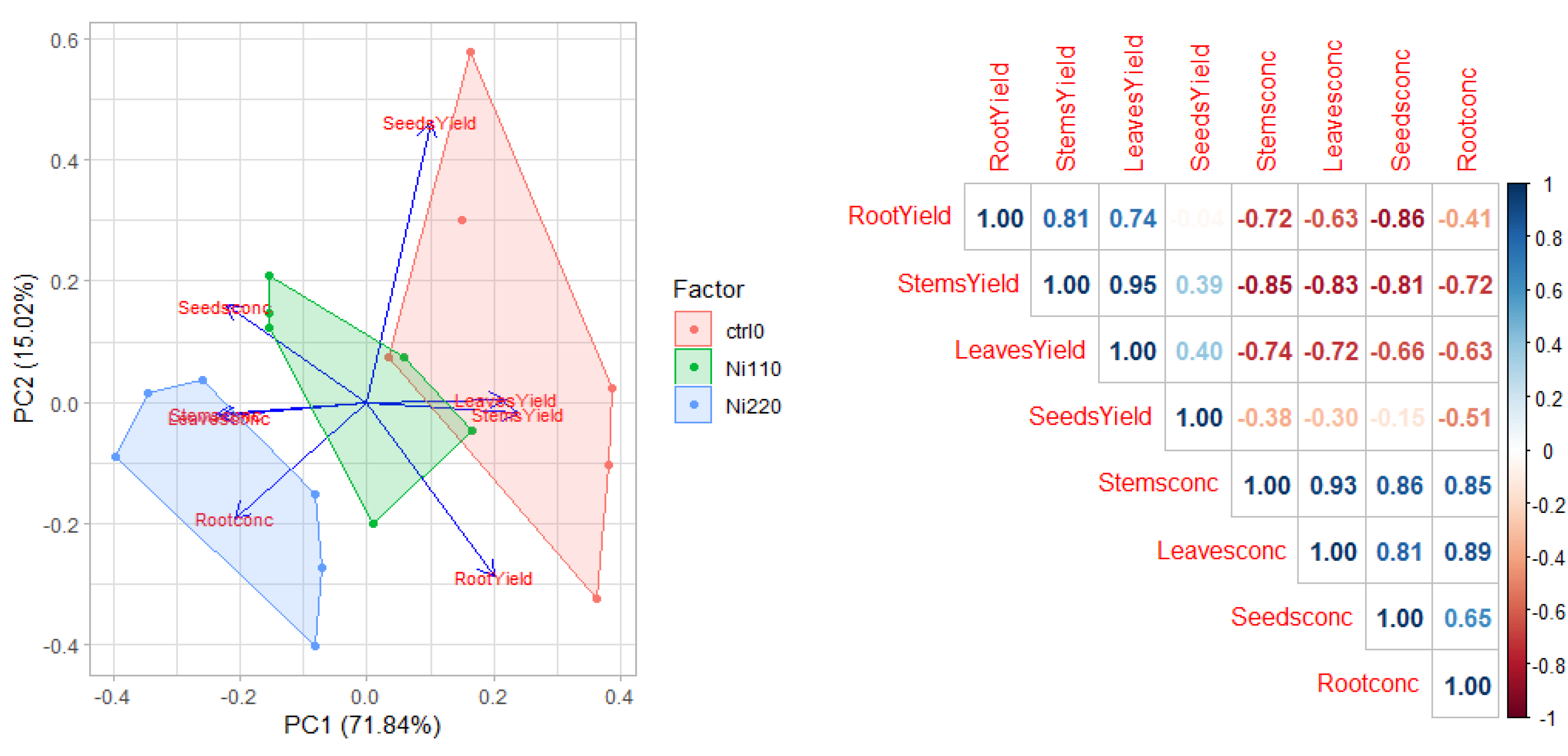
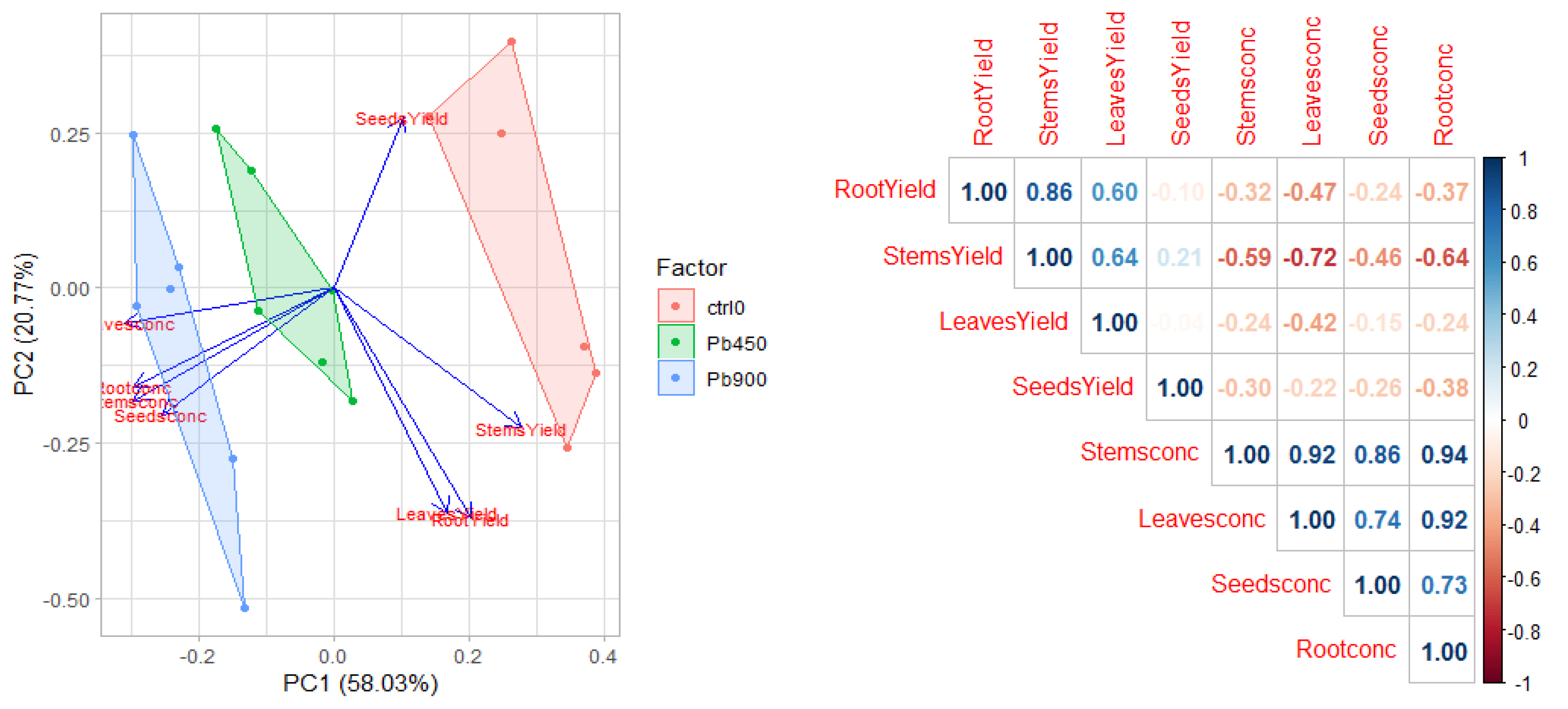
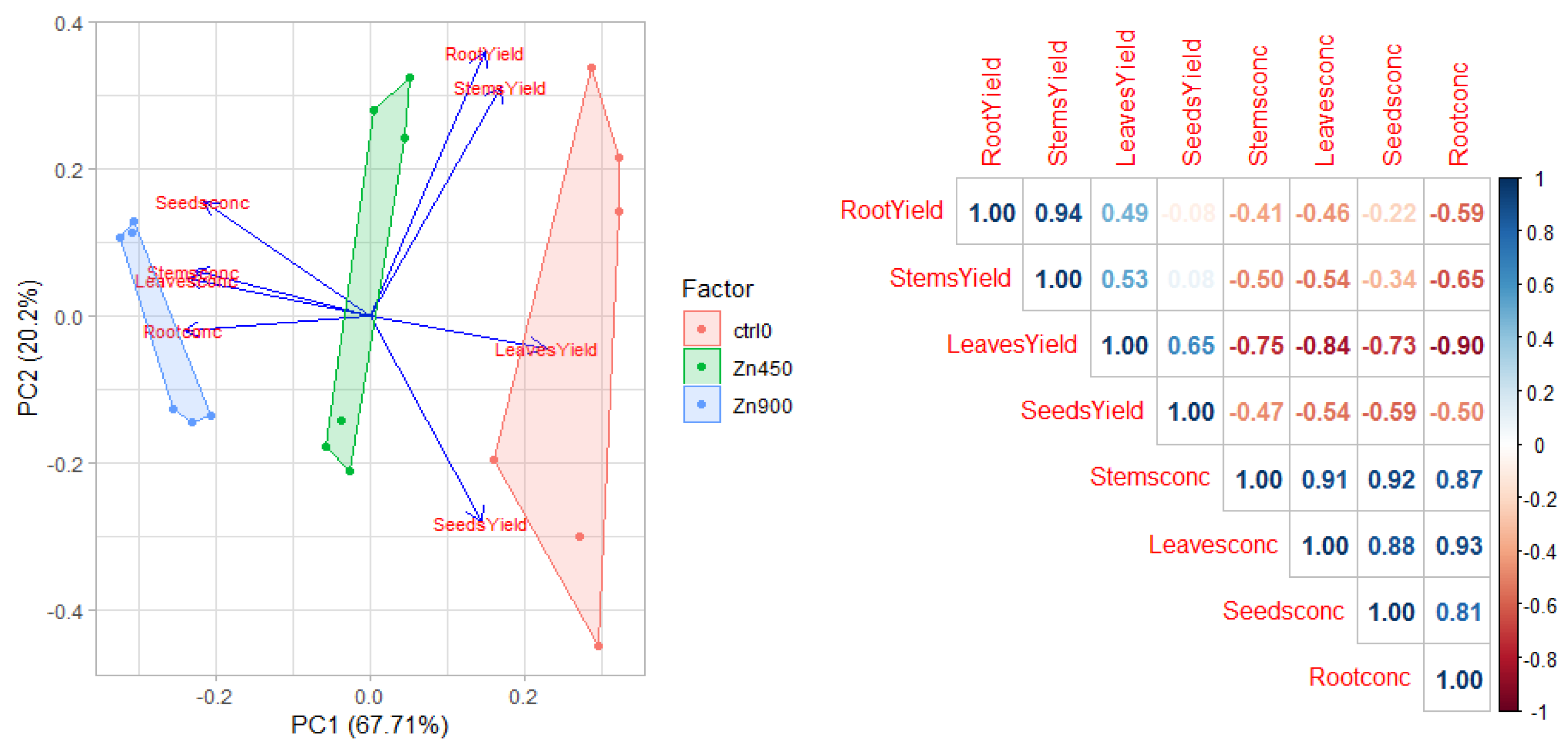
3.5. Tolerance and Phytoextraction Traits Evaluated with Principal Components Analysis and Multivariate Analysis
3.6. Trend of the Four Heavy Metals Tested in Safflower
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy Metals in Agricultural Soils of the European Union with Implica-Tions for Food Safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Worsfold, P.J. Heavy Metals in Soils: B.J. Alloway (Ed.)., 2nd Edn. Blackie, Glasgow, 1995 (ISBN 0-7514-0198-6). Anal. Chim. Acta 1995, 309, 408–409. [Google Scholar] [CrossRef]
- Jenkins, G.N. Trace Elements. J. R. Soc. Med. 1969, 62, 1316–1320. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.B.A.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A Novel Strategy for the Removal of Toxic Metals from the Environment Using Plants. Bio/Technology 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Liu, Y.N.; Xiao, X.Y.; Guo, Z.H. Identification of Indicators of Giant Reed (Arundo donax L.) Ecotypes for Phytoremediation of Metal-Contaminated Soil in a Non-Ferrous Mining and Smelting Area in Southern China. Ecol. Indic. 2019, 101, 249–260. [Google Scholar] [CrossRef]
- Nalepa, R.A.; Bauer, D.M. Marginal Lands: The Role of Remote Sensing in Constructing Landscapes for Agrofuel Development. J. Peasant Stud. 2012, 39, 403–422. [Google Scholar] [CrossRef]
- Von Cossel, M.; Wagner, M.; Lask, J.; Magenau, E.; Bauerle, A.; Von Cossel, V.; Warrach-Sagi, K.; Elbersen, B.; Staritsky, I.; van Eupen, M.; et al. Prospects of Bioenergy Cropping Systems for a More Social-Ecologically Sound Bioeconomy. Agronomy 2019, 9, 605. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Yang, J.; Sun, L.; Tan, X.; Peng, C.; Ali, I.; Naz, I. A Critical Review on the Phytoremediation of Heavy Metals from Environment: Performance and Challenges. Chemosphere 2022, 291, 132979. [Google Scholar] [CrossRef]
- Barbosa, B.; Boléo, S.; Sidella, S.; Costa, J.; Duarte, M.P.; Mendes, B.; Cosentino, S.L.; Fernando, A.L. Phytoremediation of Heavy Metal-Contaminated Soils Using the Perennial Energy Crops Miscanthus spp. and Arundo donax L. Bioenergy Res. 2015, 8, 1500–1511. [Google Scholar] [CrossRef]
- Fernando, A.L.; Duarte, M.P.; Vatsanidou, A.; Alexopoulou, E. Environmental Aspects of Fiber Crops Cultivation and Use. Ind. Crops Prod. 2015, 68, 105–115. [Google Scholar] [CrossRef]
- European Commission. EU Directive (EU) 2018/2001 of the European Parliament and of the Council on the Promotion of the Use of Energy from Renewable Sources; European Commision: Brussels, Belgium, 2018; pp. 82–209. [Google Scholar]
- Manvelian, J.; Weisany, W.; Tahir, N.A.; Jabbari, H. Industrial Crops & Products Physiological and Biochemical Response of Safflower (Carthamus tinctorius L.) Cultivars to Zinc Application under Drought Stress. Ind. Crop. Prod. 2021, 172, 114069. [Google Scholar] [CrossRef]
- Gengmao, Z.; Yu, H.; Xing, S.; Shihui, L.; Quanmei, S.; Changhai, W. Salinity Stress Increases Secondary Metabolites and Enzyme Activity in Safflower. Ind. Crop. Prod. 2015, 64, 175–181. [Google Scholar] [CrossRef]
- Gongora, B.; Nelson, S.; De Souza, M.; Bassegio, D.; Santos, F.; Antonio, J.; Siqueira, C.; Aparecido, R.; Gurgacz, F.; Secco, D.; et al. Industrial Crops & Products Comparison of Emissions and Engine Performance of Safflower and Commercial Biodiesels. Ind. Crop. Prod. 2022, 179, 114680. [Google Scholar] [CrossRef]
- Sajad, S.; Mirmohamadsadeghi, S.; Karimi, K. Biore Fi Nery Development Based on Whole Safflower Plant. Renew. Energy 2020, 152, 399–408. [Google Scholar] [CrossRef]
- Sajad, S.; Karimi, K.; Mirmohamadsadeghi, S. Hydrothermal Pretreatment of Safflower Straw to Enhance Biogas Production. Energy 2019, 172, 545–554. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, C.; Wang, F.; Peng, L.; Zeng, Q.; Luo, S. Assessment of the Potential for Phytoremediation of Cadmium Polluted Soils by Various Crop Rotation Patterns Based on the Annual Input and Output Fluxes. J. Hazard. Mater. 2022, 423, 127183. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Y.; Wang, Z.; Gong, J.; Gao, J.; Tang, S.; Ma, S.; Duan, Z. Heavy Metal Pollution in Agricultural Soils of a Typical Volcanic Area: Risk Assessment and Source Appointment. Chemosphere 2022, 304, 135340. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 539–579. ISBN 9780891189770. [Google Scholar]
- ISO 11047:1998; Soil Quality—Determination of Cadmium, Chromium, Cobalt, Copper, lead, Manganese, Nickel and Zinc—Flame and Electrothermal Atomic Absorption Spectrometric Methods. ISO: Geneva, Switzerland, 1998.
- ISO 707:2008; Milk and Milk Products—Guidance on Sampling. ISO: Geneva, Switzerland, 2008.
- Gomes, L.; Costa, J.; Moreira, J.; Cumbane, B.; Abias, M.; Santos, F.; Zanetti, F.; Monti, A.; Fernando, A.L. Switchgrass and Giant Reed Energy Potential When Cultivated in Heavy Metals Contaminated Soils. Energies 2022, 15, 5538. [Google Scholar] [CrossRef]
- Girdhar, M.; Sharma, N.R.; Rehman, H.; Kumar, A.; Mohan, A. Comparative Assessment for Hyperaccumulatory and Phytoremediation Capability of Three Wild Weeds. 3 Biotech 2014, 4, 579–589. [Google Scholar] [CrossRef]
- Yadav, S.K.; Juwarkar, A.A.; Kumar, G.P.; Thawale, P.R.; Singh, S.K.; Chakrabarti, T. Bioaccumulation and Phyto-Translocation of Arsenic, Chromium and Zinc by Jatropha curcas L.: Impact of Dairy Sludge and Biofertilizer. Bioresour. Technol. 2009, 100, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of Heavy Metals by Native Species Growing in a Mining Area in Sardinia, Italy: Discovering Native Flora for Phytoremediation. Int. J. Phytoremediation 2011, 13, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Mattina, M.J.I.; Lannucci-Berger, W.; Musante, C.; White, J.C. Concurrent Plant Uptake of Heavy Metals and Persistent Organic Pollutants from Soil. Environ. Pollut. 2003, 124, 375–378. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Erickson, L.; Stefanovska, T.; Popelka, J.; Hettiarachchi, G.; Davis, L.; Trögl, J. Potential Phytomanagement of Military Polluted Sites and Biomass Production Using Biofuel Crop Miscanthus x giganteus. Environ. Pollut. 2019, 249, 330–337. [Google Scholar] [CrossRef]
- Huang, J.; Hartemink, A.E. Soil and Environmental Issues in Sandy Soils. Earth-Sci. Rev. 2020, 208, 103295. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Calcagno, S.; Pulvirenti, L.; Siracusa, L. Industrial Crops & Products How Do Sowing Time and Plant Density affect the Pigments Safflomins and Carthamin in Florets of Safflower? Ind. Crops Prod. 2020, 148, 112313. [Google Scholar] [CrossRef]
- Namdjoyan, S.; Kermanian, H.; Abolhasani, A.; Modarres, S.; Nazli, T. Interactive Effects of Salicylic Acid and Nitric Oxide in Alleviating Zinc Toxicity of Safflower (Carthamus tinctorius L.). Ecotoxicology 2017, 16, 752–761. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants: Fourth Edition; CRC Taylor and Francis: Boca Raton, FL, USA, 2010; ISBN 9781420093704. [Google Scholar]
- Goodarzi, A.; Namdjoyan, S.; Soorki, A.A. Effects of Exogenous Melatonin and Glutathione on Zinc Toxicity in Safflower (Carthamus tinctorius L.) Seedlings. Ecotoxicol. Environ. Saf. 2020, 201, 110853. [Google Scholar] [CrossRef]
- Al Chami, Z.; Amer, N.; Al Bitar, L.; Cavoski, I. Potential Use of Sorghum Bicolor and Carthamus tinctorius in Phytoremediation of Nickel, Lead and Zinc. Int. J. Environ. Sci. Technol. 2015, 12, 3957–3970. [Google Scholar] [CrossRef]
- Baran, U.; Ekmekçi, Y. Physiological, Photochemical, and Antioxidant Responses of Wild and Cultivated Carthamus Species Exposed to Nickel Toxicity and Evaluation of Their Usage Potential in Phytoremediation. Environ. Sci. Pollut. Res. 2022, 29, 4446–4460. [Google Scholar] [CrossRef]
- Afzal, O.; Hassan, F.; Ahmed, M.; Shabbir, G.; Ahmed, S. Determination of Stable Safflower Genotypes in Variable Environments by Parametric and Non-Parametric Methods. J. Agric. Food Res. 2021, 6, 100233. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel Toxicity in Plants: Reasons, Toxic Effects, Tolerance Mechanisms, and Remediation Possibilities—A Review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, Z.; Namdjoyan, S.; Abolhasani, A. Exogenous Melatonin and Salicylic Acid Alleviates Cadmium Toxicity in Safflower (Carthamus tinctorius L.) Seedlings. Ecotoxicology 2021, 30, 387–401. [Google Scholar] [CrossRef]
- Namdjoyan, S.; Namdjoyan, S.; Kermanian, H. Induction of Phytochelatin and Responses of Antioxidants under Cadmium Stress in Safflower (Carthamus tinctorius) Seedlings. Turk. J. Botany 2012, 36, 495–502. [Google Scholar] [CrossRef]
- Shi, G.; Cai, Q. Cadmium Tolerance and Accumulation in Eight Potential Energy Crops. Biotechnol. Adv. 2009, 27, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kadir, M.; Cesur, C.; Aslan, V.; Yilbasi, Z. The Production of Biodiesel from Safflower (Carthamus tinctorius L.) Oil as a Potential Feedstock and Its Usage in Compression Ignition Engine: A Comprehensive Review. Renew. Sustain. Energy Rev. 2020, 119, 109574. [Google Scholar] [CrossRef]
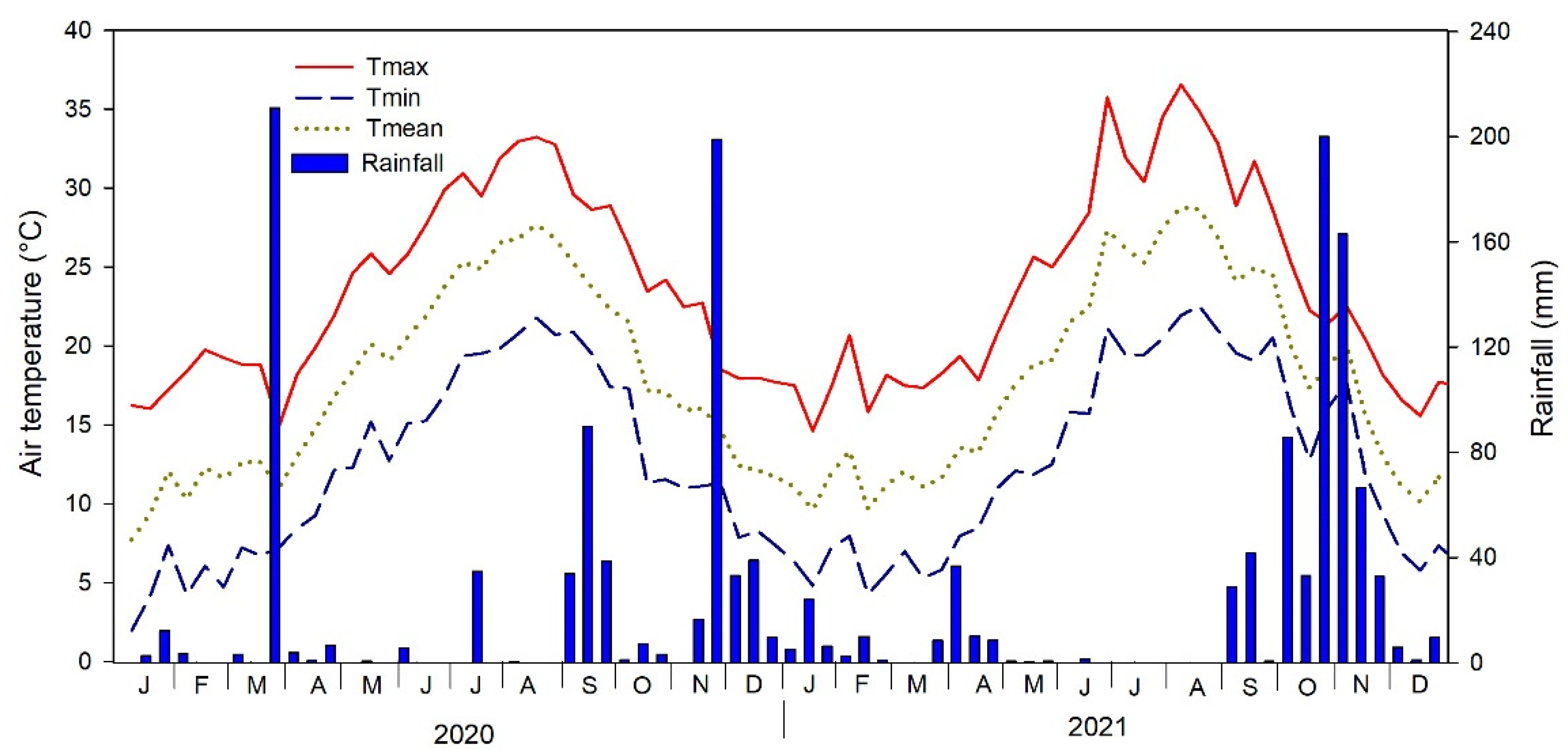


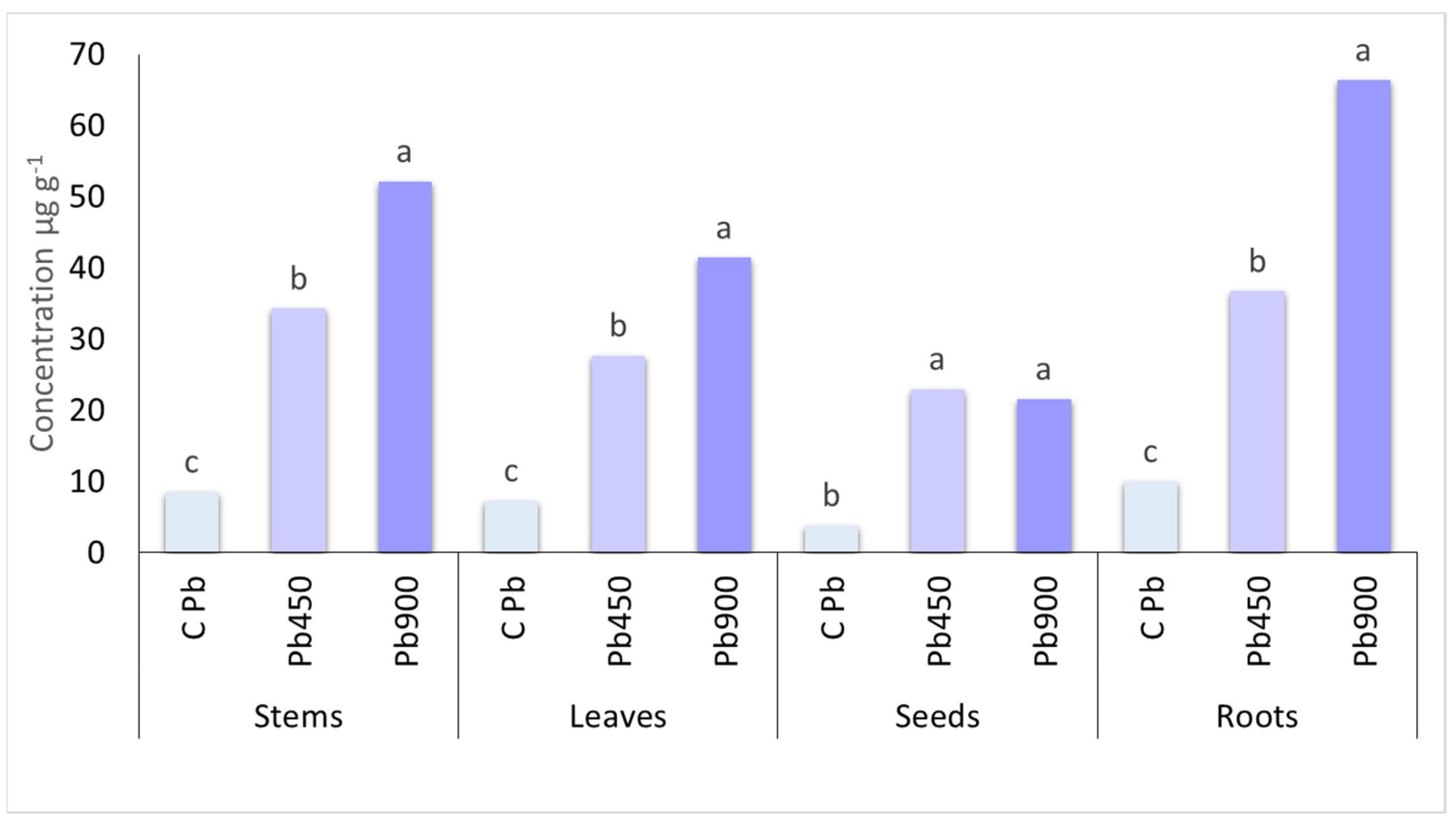
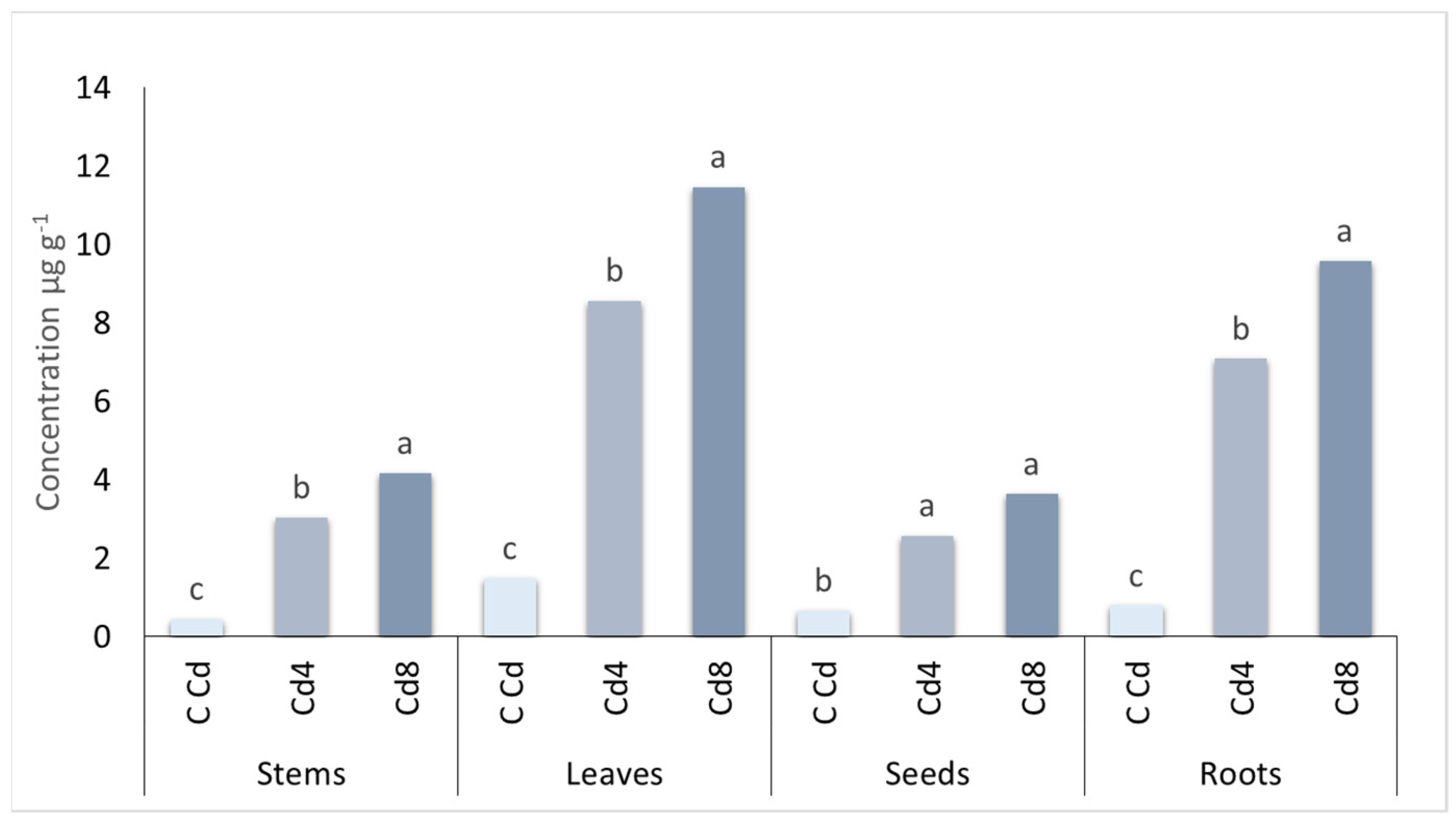
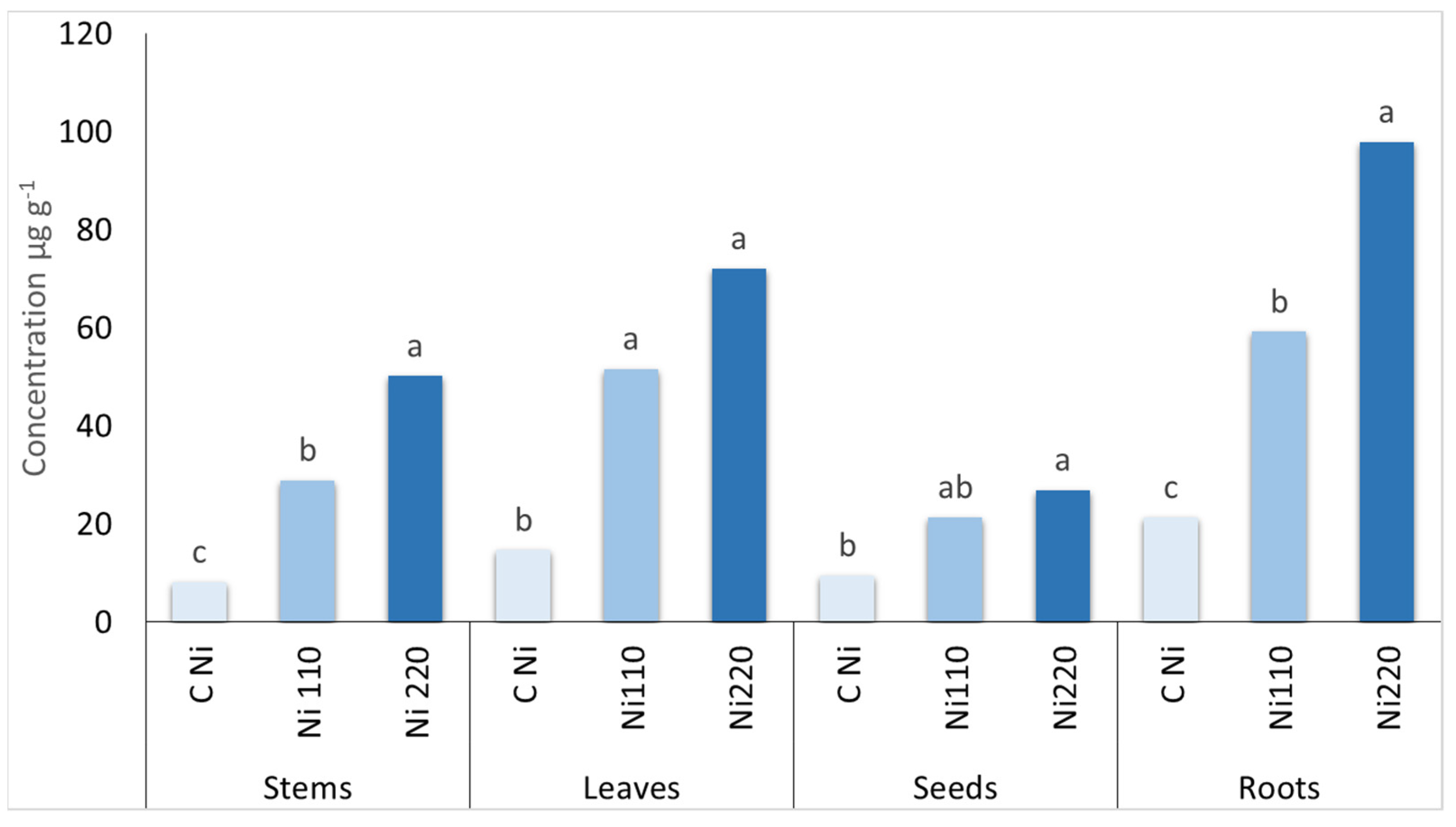
| Zinc (mg kg−1) | Cadmium (mg kg−1) | Nickel (mg kg−1) | Lead (mg kg−1) | |
|---|---|---|---|---|
| Low Level | 450 | 4 | 110 | 450 |
| High Level | 900 | 8 | 220 | 900 |
| Physical Characteristics | |
|---|---|
| Clay (%) | 3.0 |
| Silt (%) | 4.1 |
| Sand (%) | 92.9 |
| Texture | Sandy |
| Conductivity (μS/cm) | 34.2 |
| Chemical characteristics | |
| pH | 7.4 |
| Organic matter (%) | 0.86 |
| Fe (mg kg−1) | 23.6 |
| P (mg kg−1) | 7 |
| Mn (mg kg−1) | 0.1 |
| Cu (mg kg−1) | 21.8 |
| HM | Concentration (mg kg−1) | 2020 | 2021 | ||
|---|---|---|---|---|---|
| Total HM (mg kg−1) | Bioavailable HM (mg kg−1) | Total HM (mg kg−1) | Bioavailable HM (mg kg−1) | ||
| Control-Zn | - | 35.98 ± 2.72 | 9.51 ± 1.16 | 36.89 ± 10.50 | 4.57 ± 1.41 |
| Zn | 450 | 478.61 ± 5.40 | 283.18 ± 2.00 | 496.85 ± 14.01 | 324.68 ± 13.69 |
| Zn | 900 | 883.70 ± 22.33 | 544.60 ± 1.35 | 803.30 ± 45.41 | 537.35 ± 6.16 |
| Control-Pb | - | 11.01 ± 2.13 | 2.32 ± 1.16 | 17.45 ± 6.96 | 3.76 ± 1.01 |
| Pb | 450 | 447.80 ± 3.99 | 242.45 ± 1.63 | 464.96 ± 53.44 | 247.02 ± 62.63 |
| Pb | 900 | 840.13 ± 12.62 | 517.59 ± 5.85 | 876.20 ± 69.86 | 584.38 ± 31.66 |
| Control-Cd | - | 0.43 ± 0.11 | 0.11 ± 0.05 | 0.69 ± 0.10 | 0.16 ± 0.001 |
| Cd | 4 | 4.43 ± 0.22 | 2.47 ± 0.19 | 3.74 ± 0.20 | 1.47 ± 0.002 |
| Cd | 8 | 8.86 ± 0.80 | 5.80 ± 0.10 | 9.88 ± 2.12 | 5.11 ± 0.26 |
| Control-Ni | - | 33.53 ± 3.79 | 12.96 ± 1.83 | 20.92 ± 4.76 | 4.72 ± 1.82 |
| Ni | 110 | 121.19 ± 7.93 | 78.87 ± 14.64 | 119.39 ± 13.22 | 56.98 ± 2.86 |
| Ni | 220 | 248.20 ± 18.82 | 174.80 ± 5.13 | 207.27 ± 6.23 | 144.82 ± 7.72 |
| 2020 | 2021 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Roots (g pot−1) | Stems (g pot−1) | Leaves (g pot−1) | Seeds (g pot−1) | Total DM (g pot−1) | Roots (g pot−1) | Stems (g pot−1) | Leaves (g pot−1) | Seeds (g pot−1) | Total DM (g pot−1) | |
| Control | 1.8 a | 5.4 a | 3.7 a | 4.3 a | 15.2 a | 5.3 a | 7.0 a | 4.8 a | 3.2 a | 20.4 a |
| Zn450 | 1.0 b | 3.7 b,c | 2.9 a | 3.2 a,b | 10.7 a,b | 4.5 a,b | 5.4 a,b,c | 3.5 a,b | 2.1 a | 15.5 b |
| Zn900 | 0.8 b | 2.8 c | 2.4 a | 2.5 a,b | 8.4 b | 2.0 c | 3.6 c | 2.6 b | 2.2 a | 10.5 c |
| Pb450 | 1.1 ab | 3.7 b,c | 3.5 a | 3.2 a,b | 11.6 a,b | 3.9 a,b | 5.6 a,b | 3.5 a,b | 3.4 a | 16.5 a,b |
| Pb900 | 1.5 a,b | 3.6 b,c | 3.1 a | 2.9 a,b | 11.0 a,b | 3.0 b,c | 5.1 b,c | 4.2 a,b | 3.0 a | 15.3 b |
| Ni110 | 1.2 a,b | 3.7 b,c | 2.5 a | 3.3 a,b | 10.7 a,b | 3.5 a,b,c | 5.5 a,b,c | 3.5 a,b | 3.2 a | 15.7 b |
| Ni220 | 1.1 a,b | 3.3 b,c | 2.7 a | 2.6 a,b | 9.7 b | 3.4 b,c | 4.4 b,c | 3.0 b | 2.2 a | 13.0 b,c |
| Cd4 | 1.2 a,b | 4.3 a,b | 2.6 a | 2.2 a,b | 10.3 a,b | 3.2 b,c | 4.2 b,c | 2.7 b | 2.8 a | 12.9 b,c |
| Cd8 | 1.3 a,b | 3.2 b,c | 2.9 a | 1.7 b | 9.1 b | 3.3 b,c | 4.1 b,c | 2.9 b | 2.4 a | 12.7 b,c |
| AVG | 1.2 | 3.7 | 2.9 | 2.9 | 10.7 | 3.6 | 5.0 | 3.4 | 2.7 | 14.7 |
| Zinc | Stems | Leaves | Seeds | Root |
|---|---|---|---|---|
| Level | 0.0088 *** | 0.0479 *** | 0.0007 *** | 0.0013 *** |
| Year | 0.0010 ** | 0.2842 | 0.0002 *** | 0.0197 * |
| Level x Year | 0.0005 *** | 0.1955 | 0.0535 | 0.4405 |
| Lead | Stems | Leaves | Seeds | Roots |
|---|---|---|---|---|
| Level | 0.0001 *** | 0.0008 *** | 0.5092 | 0.0495 *** |
| Year | 0.3076 | 0.0639 | 0.0315 * | 0.1637 |
| Level: Year | 0.1376 | 0.4255 | 0.1100 | 0.6013 |
| Cadmium | Stems | Leaves | Seeds | Roots |
|---|---|---|---|---|
| Level | 0.0002 *** | 0.0023 ** | 0.0841 | 0.0041 ** |
| Year | 0.0000 *** | 0.0002 *** | 0.0016 *** | 0.0006 *** |
| Level: Year | 0.0003 *** | 0.0068 ** | 0.1370 | 0.0056 ** |
| Nickel | Stems | Leaves | Seeds | Roots |
|---|---|---|---|---|
| Level | 0.0338 * | 0.1268 | 0.2217 | 0.0017 ** |
| Year | 0.0332 *** | 0.0000 *** | 0.0002 *** | 0.0005 *** |
| Level: Year | 0.0528 | 0.3382 | 0.5072 | 0.0053 ** |
| Cont. | Conc. | TI | mAI | mBCF Abov. | mBCF Below. | TF |
|---|---|---|---|---|---|---|
| Zn | 450 | 0.73 ± 0.10 | 3.87 ± 0.33 | 0.07 ± 0.012 | 0.15 ± 0.02 | 0.51 ± 0.10 |
| Zn | 900 | 0.57 ± 0.07 | 6.01 ± 0.39 | 0.09 ± 0.001 | 0.17 ± 0.01 | 0.52 ± 0.03 |
| Pb | 450 | 0.81 ± 0.11 | 3.55 ± 0.44 | 0.12 ± 0.020 | 0.15 ± 0.02 | 0.79 ± 0.07 |
| Pb | 900 | 0.76 ± 0.11 | 4.61 ± 0.48 | 0.07 ± 0.006 | 0.12 ± 0.01 | 0.61 ± 0.08 |
| Cd | 4 | 0.66 ± 0.11 | 3.80 ± 0.56 | 2.26 ± 0.3 | 3.79 ± 0.27 | 0.63 ± 0.08 |
| Cd | 8 | 0.61 ± 0.11 | 5.21 ± 0.82 | 1.19 ± 0.1 | 1.74 ± 0.15 | 0.68 ± 0.08 |
| Ni | 110 | 0.76 ± 0.14 | 6.17 ± 0.19 | 0.48 ± 0.1 | 0.89 ± 0.19 | 0.55 ± 0.11 |
| Ni | 220 | 0.64 ± 0.10 | 7.29 ± 0.25 | 0.32 ± 0.0 | 0.62 ± 0.06 | 0.54 ± 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciaramella, B.R.; Corinzia, S.A.; Cosentino, S.L.; Testa, G. Phytoremediation of Heavy Metal Contaminated Soils Using Safflower. Agronomy 2022, 12, 2302. https://doi.org/10.3390/agronomy12102302
Ciaramella BR, Corinzia SA, Cosentino SL, Testa G. Phytoremediation of Heavy Metal Contaminated Soils Using Safflower. Agronomy. 2022; 12(10):2302. https://doi.org/10.3390/agronomy12102302
Chicago/Turabian StyleCiaramella, Barbara Rachele, Sebastiano Andrea Corinzia, Salvatore Luciano Cosentino, and Giorgio Testa. 2022. "Phytoremediation of Heavy Metal Contaminated Soils Using Safflower" Agronomy 12, no. 10: 2302. https://doi.org/10.3390/agronomy12102302
APA StyleCiaramella, B. R., Corinzia, S. A., Cosentino, S. L., & Testa, G. (2022). Phytoremediation of Heavy Metal Contaminated Soils Using Safflower. Agronomy, 12(10), 2302. https://doi.org/10.3390/agronomy12102302







