Abstract
Crop rotation affects soil properties and soil microbial diversity and structure. Currently, it is not well understood how soil microbial diversity changes following different crop rotation systems of industrial hemp, an ancient and economically important crop. Therefore, these changes were analyzed in this study. Our results showed that different rotation systems significantly affected the wilt disease incidence, plant height, yield, soil physicochemical properties and soil microbial communities in the greenhouse. The rotation systems used in this study significantly reduced the plant mortality and increased the yield compared with a monoculture system. The levels of alkaline hydrolysis and available phosphorus in the soil decreased significantly compared with a monoculture cropping system. Using MiSeq high-throughput sequencing, we showed that the soil diversity and number of bacteria and fungi were significantly higher for rotation systems and controls compared to the monoculture system. The relative abundance of pathogens increased with a monoculture system. Redundancy analysis suggests that soil properties may also affect the soil microbial composition. Taken together, different rotation systems used in this study significantly decreased the disease incidence, increased plant yields and increased soil microbial diversity compared with monoculture for industrial hemp. We believe that applying these rotation systems is an efficient and eco-friendly approach to control soil borne pathogenic diseases and increase floral yields.
1. Introduction
Soil microbes are a critical component of the terrestrial soil ecosystem. They participate in the synthesis, decomposition, and transformation of many nutrients, which are important for plant growth. The number and diversity of soil microorganisms are important indicators of soil fertility and the microecological status [1]. The soil microbial community structure and its changes reflect soil productivity and stability and act as an early indicator of ecosystem change [2,3,4]. Beneficial microorganisms in the soil promote plant growth and improve plant resistance to various diseases. Under normal circumstances, the species and number of beneficial microorganisms present in the soil, including antagonistic bacteria, nitrogen-fixing bacteria, and ammonia oxidizing bacteria, are far greater than that of harmful microorganisms, such as soil-borne pathogens, and there is normally a dynamic balance between beneficial and harmful microorganisms [5]. Antagonistic microorganisms in the soil can inhibit the growth, survival, or infection of plant pathogens by secreting antibiotic toxins, biosurfactants, extracellular cell-wall decomposing enzymes and other substances. They also compete with pathogens for nutrition and mineral elements [6].
Originating in Central Asia, industrial hemp (Cannabis sativa L.) is an important herbaceous plant that has been used for centuries in medicine, as a source of textile fiber, and as a recreational drug [7,8]. In recent years, there has been continuous development and application of the medicinal uses of industrial hemp. In particular, the extract cannabidiol (CBD), which comes from the flowers and leaves of the plant, has been used to treat pain, depression, anxiety, and other related diseases [9,10,11]. Due to its health benefits, the production of industrial hemp has become an important industry.
Industrial hemp is dioecious and mainly relies on wind for its pollination. It is, therefore, difficult to maintain the chemical profile and purity of its products [12]. The use of greenhouses to grow industrial hemp becomes an effective and feasible way to prevent pollen mixing and increase the quality of industrial hemp. However, previous studies showed that continuous cropping in greenhouse results in crop yield decline, poor quality, and increased occurrence of diseases and insect pests [13,14,15]. Our previous study found that fusarium wilt occurred in industrial hemp after continuous cropping, and the pathogen Fusarium oxysporum was identified [16]. The pathogen Fusarium oxysporum could lead to monoculture watermelon decreases in crop yield and quality [17]. In recent years, more studies have found that continuous cropping is the main factor in rhizosphere microecological imbalance [18]. Guo et al. [19] revealed that the relative abundance of beneficial bacteria was significantly reduced, while the potentially harmful bacteria increased, resulting in changes in the bacterial community structure under industrial hemp monoculture system.
After continuous monocropping, the main reason for the disease occurrence is that a certain type of pathogenic bacteria becomes the dominant microbe in the soil microbial community, which increases the incidence of plant diseases. Studies have shown that a rational rotation system could make pathogenic microorganisms lose their original hosts, change the microbial community and diversity in soil, reduce the number of harmful pathogens, and thus reduce the occurrence of diseases [20]. For example, strawberry rotation with broccoli and lettuce could effectively control verticillium wilt and increase strawberry quality and yield [21]; barley–ryegrass–potato rotations inhibited the incidence of potato Rhizoctonia solani and Streptomyces scabies by 18.0 to 33.0% [22]; a black pepper–vanilla system harbored a lower abundance of Fusarium oxysporum in the vanilla rhizosphere soil and increased the putatively plant-beneficial fungal groups such as Trichoderma and Penicillium genus [23].
In the present work, we found that plant mortality increased, growth potential became weaker, and the yield of floral and leaf yields decreased in monoculture compared with rotation systems of industrial hemp. We hypothesized that rotation systems could decrease the incidence of industrial hemp fusarium wilt disease by increasing plant-beneficial microorganisms and inhibiting the pathogen population or changing the composition of industrial hemp rhizosphere bacterial and fungal communities. In addition, the effect varies between different rotation systems. To test this hypothesis, the microbial community compositions of 15 soil samples from the industrial hemp monoculture system, 3 rotation systems and control (no crop planted) in 2021 at harvest stages were analyzed using Illumina MiSeq targeted amplicon sequencing using the bacterial 16S rRNA gene and the fungal internal transcribed spacer (ITS) gene of industrial hemp rhizosphere soil. This study provides insights to soil micro-ecological environment for industrial hemp rotation systems, which is important to the sustainable development of industrial hemp.
2. Materials and Methods
2.1. Experimental Location and Design
Experiments were conducted in four greenhouses at the Modern Agriculture Demonstration Area (45°49′44″ N, 126°48′55″ E), located at the southern bank of the Songhua River, Harbin City, Heilongjiang Province. The greenhouses were filled with black soil and the soil layer is more than 60 cm thick. The soil chemical properties were reported as the following: pH 6.7–6.9; organic matter (OM) 31.9–34.1 g·kg−1; total potassium 2.68–2.93%; total nitrogen 0.158–0.167%; available phosphorus (AP) 28.5–29.75 mg·kg−1; available potassium (AK) 2.80–3.07 g·kg−1; alkaline hydrolysis (AN) 147.9–151.9 g·kg−1.
The experiment was conducted over three years (2019–2021) in greenhouses built at the experimental site. Each greenhouse was 12 m wide and 60 m long, and the area was 720 m2; the roof was 3.4 m at its highest point and 1.2 m at the lowest point of the arched plastic shed. The greenhouse used natural light and the temperature was between 25 °C and 35 °C. The relative humidity was 50~70%. The seeds used in this experiment were industrial hemp (Cannabis sativa L.), watermelon (Citrullus lanatus (Thunb.) Mansfeld and Nakai), potato (Ralstonia solanacearum) and bean (Phaseolus vulgaris L.). The source, growth conditions and quantity of these plants are listed in Table S1. The different rotation systems in our experiment were that industrial hemp (H) was cultivated in the first year (2019), followed with watermelon (W), potato (P), or bean (B) in the second year (2020), then industrial hemp (H) again in the third year (2021), that is, HWH-industrial hemp (year 1), watermelon (year 2) and industrial hemp (year 3); HPH-industrial hemp (year 1), potato (year 2) and industrial hemp (year 3); HBH-industrial hemp (year 1), bean (year 2) and industrial hemp (year 3) (Table S2). The monoculture system in our experiment was HHH-industrial hemp in all three years. The control was no crop planted in all three years (empty spaces on either side of each greenhouse without any crops). The plants were subjected to typical management procedures. No pest or disease controls and manual weeding were applied during the whole experiment. The base fertilizer was N:P:K = 15:15:15, 25 kg per greenhouse. The fertilizer was mixed with soil before planting the crops. No additional fertilizer was used in the crop-growing stage. Sub-membrane irrigation was used for planting. Rational irrigation was carried out according to the actual water demand of different crops.
2.2. Measurement of Wilt Disease Incidence, Crop Height, and Yield of Industrial Hemp
To evaluate the growth of industrial hemp under different rotation systems (HWH, HPH and HBH) and a monoculture system (HHH), plant height was measured at 1, 2 and 4 months after cultivation in year 1 and year 3. In October of year 1 and year 3, flowers and leaves were harvested, respectively. The yields of flowers and leaves were compared among different cultivation methods. The wilt disease incidence was measured throughout the planting season in year 1 and year 3. Wilt disease incidence was expressed as the proportion of symptomatic plants out of the total number of plants. The disease index (DI) denotes healthy plants as “0” and fusarium wilt plants as “1”. Each treatment was administered to 40 plants and was repeated three times.
2.3. Soil Sample Collection
Soil attached to plant roots of industrial hemp was collected at the harvest stage of year 3. The planting area of each greenhouse was evenly divided into six parts. Five plants were randomly selected from each part, and the roots were completely removed. The rhizosphere soil of industrial hemp was obtained by strongly vibrating root and brushing the roots with a brush. Rhizosphere soils from two parts were randomly selected as a sample with three replicates per treatment. Collected soil samples from different plants were mixed and sieved through a 2 mm mesh to thoroughly homogenize and remove roots, plant residue and rocks. Each soil sample was divided into two parts and placed in individual plastic bags, sealed, and transferred to the laboratory. Half of the samples were stored at −80 °C for DNA extraction; the other half was stored at −4 °C for chemical analysis.
2.4. Soil Physicochemical Properties Analysis
The intrinsic physical and chemical characteristics of the soil were analyzed as previously described [24]. Briefly, the soil pH was measured using a glass electrode in a soil water suspension (1:2.5 w/v). Available potassium, available phosphorus and alkali-hydrolyzed nitrogen were extracted with 2 M KCl, 0.5 M NH4+ OAc (pH = 7) and 1 M NaHCO3 (pH = 8.5), respectively. Soil filtrates were measured using a Continuous Flow Analyser (SAN++, Skalar, Breda, The Netherlands). Contents of the soil organic matter were determined using the potassium dichromate oxidation method. The experiment was repeated three times using three biological replicates.
2.5. DNA Extraction and MiSeq Sequencing
Total DNA was extracted using the E.Z.N.A. stool DNA Isolation Kit (Omega Biotek, Norcross, GA, USA), according to the manufacturer’s instructions. DNA concentration and purity were determined with a NanoDrop 2000 Spectrophotometer (ThermoFisher Scientific, Waltham, MA, USA). The V3–V4 hypervariable region of bacterial 16S rRNA gene was amplified by PCR using the following primer pairs, forward: 5′-CCTACGGGNGGCWGCAG-3′; reverse: 5′-GGACTACHVGGGTATCTAAT-3′. The fungal ITS region was amplified with the following primer pairs, forward: 5′-GATGAAGAACGYAGYRAA-3′; reverse: 5′-GGACTACHVGGGTATCT AAT-3′.
PCR amplification was performed in 50 μL reaction solution consisting of 5 μL 10× Buffer, 5 μL dNTPs (2.5 mM), 1.5 μL forward and reverse primers (5 μM), 1 μL polymerase (2 U/μL), and 100 ng template DNA. The PCR amplification condition was: initial denaturation at 95 °C for 2 min, followed by 35 cycles at 98 °C for 15 s, 63 °C for 30 min, 68 °C for 30 s, and a final extension at 72 °C for 10 min for the V3–V4 region of the 16S rRNA gene; 3 min of initial denaturation step at 95 °C, followed by 35 cycles at 94 °C for 30 s, 55 °C for 30 s and 72 °C for 45 s, and a final extension at 72 °C for 10 min for the ITS genes. PCR products were detected by electrophoresis using 2% agarose gel (ThermoFisher Scientific) and recovered using an AxyPrep DNA Gel kit (Axygen Biosciences, Union City, CA, USA). The quality and concentration of the PCR products were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies Co., Ltd., Santa Clara, CA, USA) and KAPA Library quantitative kit (KAPA Biosystems, Wilmington, CA, USA), respectively. The amplified products were purified and normalized to form a sequencing library. Illumina MiSeq high-throughput sequencing was performed by Shanghai Shenggong Biological Company (Shanghai, China).
2.6. Sequencing Data Analysis
Raw sequencing data were analyzed using the QIIME software (v1.9.0, J Gregory Caporaso, Denver, CO, USA) and the UPARSE pipeline as previously described [25]. Quality control was carried out on the raw data based on the following criteria: (1) removing reads with more than 10% of unknown nucleotides; (2) removing reads that have a Q20 below 80%; (3) removing reads with a mismatch error rate up to 2% of the readings using FLASH 55 (v1.2.11, Tanja Mago ˇc∗ and Steven L. Salzberg, Baltimore, MD, USA); (4) removing redundant sequences using QIIME56 (V1.9.1) [26,27,28]. The raw data were deposited in the NCBI database (https://www.ncbi.nlm.nih.gov, accessed on 25 May 2022) with the deposition number PRJNA841395.
High-quality reads were compared with the reference database (http://drive5.com/uchime/uchime_download.html, accessed on 21 January 2022) and pruned using the UCHIME software (http://www.drive5.com/usearch/manual/uchimealgo.html, accessed on 26 January 2022). UPARSE was used for cluster analysis. Sequences with 97% homology were combined into operational taxonomic units (OTUs). The sequence with the highest abundance score in each cluster was selected as the representative sequence.
The bacterial and fungal α-diversity estimation for Chao1 richness, ACE index, Shannon index, Simpson index, Shannon even index and coverage were calculated using QIIME [27]. Welch’s t-test and the Wilcoxon signed-rank test were used for quantitative analysis of α-diversity between groups. Both PCoA analysis and UPGMA clustering analysis were based on the Binary Jaccard algorithm to reveal the relationship of microbiome composition. Heatmap analysis was used to compare the top 50 classified genera in each sample of two growing seasons using the gplot package in R (R v.3.2.5) [29]. Redundancy analysis (RDA) was used to examine microbiome composition and microbial abundance in relation to environmental factors. RDA was also used to examine possible connections between the OTU frequencies and measured soil variables among different samples.
2.7. Statistical Analysis
One-way analysis of variance (ANOVA) was used to analyze the plant height, disease incidence and yield using SAS 9.3 software (SAS Institute Inc., Carry, NC, USA). The soil physiochemical properties, the bacterial and fungal taxa, and the microbial α-diversity indices were compared using the Student’s t-test. A p value of <0.05 was considered statistically significant.
3. Results
3.1. Wilt Disease Incidence, Crop Height, Floral and Leaf Yields of Industrial Hemp Grown in Greenhouses
To examine whether different rotation systems affect plant production of industrial hemp, we compared the wilt disease incidence, plant height, floral and leaf yields. The wilt disease incidence, plant height, floral and leaf yields of the industrial hemp were measured and compared in year 1 and year 3. Our results showed that the rotation systems (HWH, HPH and HBH) and monoculture system (HHH) had the same growth at the different growth stages, including seedling stage (1 month), rapid growth stage (2 months) and pre-flowering stage (4 months) in year 1 (Figure 1A). In year 3, the plant height of the HHH at seedling stage industrial hemp was lower than that of other treatments, but the difference was not significant. Plant height of HHH was significantly lower at 2 and 4 months than other planting methods (Figure 1A). In year 1, there was no significant difference in the wilt disease incidence all groups, while in year 3, the wilt disease incidence of HHH was significantly higher than that of the rotation systems (Figure 1B). Analysis of floral and leaf yields showed that there was no significant difference between groups in year 1, while in year 3, the floral and leaf yields per plant of HHH were significantly lower than the rotation systems (Figure 1C). The results showed that the wilt disease incidence increased, growth potential became weaker, and the yield of floral and leaf yields decreased in a monoculture compared with rotation systems of industrial hemp.
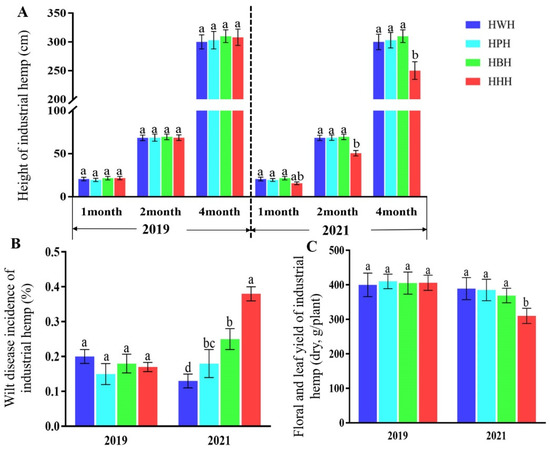
Figure 1.
Effects of monoculture or rotation systems on plant height (A), wilt disease incidence (B) and floral and leaf yield (C) of industrial hemp. Data are the mean ± standard error of 20 replicates. Letters above bars (a, b, or c) represent differences between groups according to Tukey’s test, p < 0.05 level. HWH, industrial hemp (year 1)—watermelon (year 2)—industrial hemp (year 3); HPH, industrial hemp (year 1)—potato (year 2)—industrial hemp (year 3); HBH, industrial hemp (year 1)—bean (year 2)—industrial hemp (year 3); HHH, industrial hemp in all three years (monoculture system).
3.2. Soil Physicochemical Properties
The nutrient content of soil is an indicator of soil quality and soil fertility. To examine the possible influence of different rotation systems to soil quality, we measured the soil pH, total phosphorus, total potassium, and organic matter after rotation and monoculture systems were completed. Our results showed no significant differences between the rotation systems, monoculture system and the control (CK—no crop was planted each year) in soil pH (Figure 2A), total phosphorus (Figure 2C), total potassium (Figure 2D) and organic matter (Figure 2H). The total nitrogen content in HWH was significantly lower than CK, HPH and HHH, but not significantly different from HBH (Figure 2B). The amounts of alkali-hydrolyzed nitrogen (AN) (Figure 2E), available phosphorus (AP) (Figure 2F) and available potassium (AK) (Figure 2G) in HHH were significantly lower than those in the CK, HWH, HPH and HBH. The levels of AN (Figure 2E) and AP (Figure 2F) in HWH, HPH and HHH were significantly lower than those in the CK, while the levels of AK (Figure 2G) in HWH, HPH and HHH were not significantly different from those in CK. These results indicate that the AK, AP and AN of soil were significantly reduced after monoculture cropping of industrial hemp, suggesting that a monoculture system reduces the soil quality.
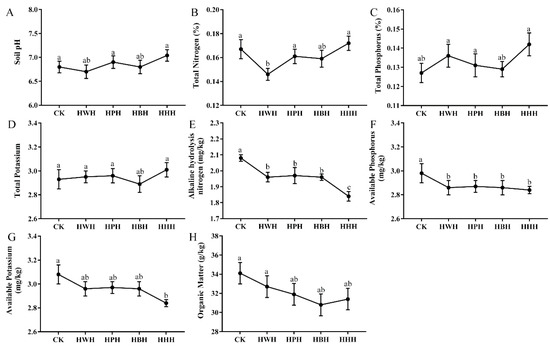
Figure 2.
Effects of soil physicochemical properties. (A) Soil pH. (B) Total nitrogen for soil. (C) Total phosphorus for soil. (D) Total potassium for soil. (E) Alkaline hydrolysis nitrogen for soil. (F) Available phosphorus for soil. (G) Available potassium for soil. (H) Organic matter for soil. HWH, industrial hemp (year 1)—watermelon (year 2)—industrial hemp (year 3); HPH, industrial hemp (year 1)—potato (year 2)—industrial hemp (year 3); HBH, industrial hemp (year 1)—bean (year 2)—industrial hemp (year 3); HHH, industrial hemp in all three years; CK, no crop planted. Data are expressed as mean ± standard error (n = 20). Letters on top of bars (a, b, or c) indicate the differences between groups according to Tukey’s test (p < 0.05).
3.3. Soil Microbial Diversity
The abundance and diversity of microorganisms in soil can be reflected by α-diversity analysis. In this study, MiSeq sequencing was performed on the V3–V4 region of bacterial 16S rRNA and the ITS2 highly variable region of fungal ITS from 15 samples. A total of 1,465,412 and 1,458,347 sequences were obtained from bacteria and fungi, respectively. There were 29,353 bacterial sequences and 29,834 fungal sequences per sample on average. There were at least 20,134 sequences for bacteria and 21,345 sequences for fungi. The average fragment length of bacteria and fungi was 421 bps and 348 bps, respectively. To reduce the differences caused by high-throughput sequencing, the minimum sequence number (20,134 for bacteria and 21,345 for fungi) was used for statistical analysis.
Microbial diversity is an important index for measuring the biological composition of a community. Comparative analyses of bacterial diversity indices showed that the Chao1, ACE, Shannon and Shannon even index of HHH were significantly lower than CK, HWH, HPH and HBH, while the Simpson index was significantly higher than CK, HWH, HPH and HBH (Figure 3 and Table S3). There was no significant difference in the coverage index between CK and HWH, HPH, HBH or HHH (Table S3). Similar with the bacterial diversity indices, analysis of the rhizosphere soil fungal diversity index showed that ACE, Shannon and Shannon even indices of HHH were significantly lower than CK, HWH, HPH and HBH (Figure 3 and Table S4). The Simpson index of HHH was significantly higher than CK, HWH, HPH and HBH; the Chao1 and coverage indices showed no significant differences among all samples (Figure 3 and Table S4).
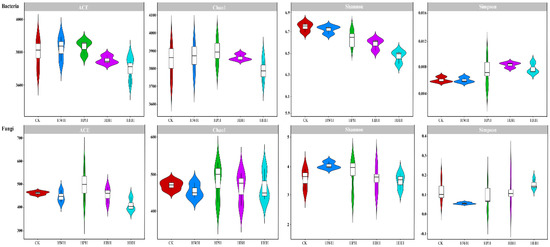
Figure 3.
The α−diversity of bacteria and fungi in the rhizosphere of industrial hemp under different cultivation patterns, displayed using with violin plots. The black line running through the violin diagram up and down represents the interval from min to max, 95% confidence interval. The lower end of the line represents the upper and lower limit, and the data beyond this range is abnormal.
3.4. Soil Microbial Composition
Principal coordinate analysis (PCoA) can be used to better understand the relationship between multiple samples. Our results showed that biological replicates were clustered together, indicating that the bacterial community structure of each planting system showed good repeatability. There were significant differences among HWH, HPH, HBH, HHH and CK (Figure 4A). Unweighted Pair Group Method with Arithmetic Mean (UPGMA) cluster analysis showed a similar result to PCoA analysis (Figure 4B). PCoA and UPGMA analyses of the fungal community composition showed a clear separation between CK and HHH but a significant overlap among HWH, HPH and HBH (Figure 4C,D).
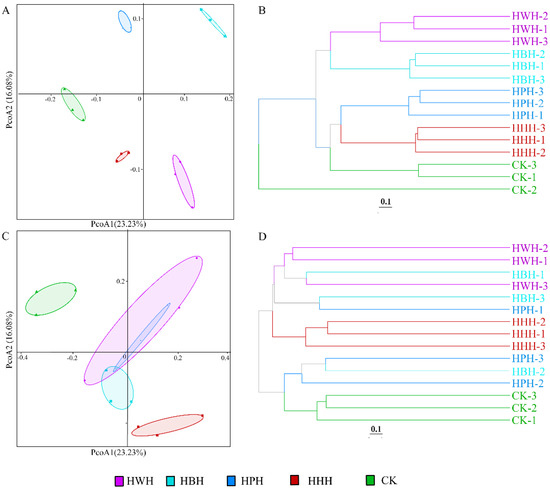
Figure 4.
PCoA and UPGMA analyses of bacterial (A,B) and fungal (C,D) community structure. Based on PCoA analysis and Unweighted Pair Group Method with Arithmetic mean (UPGMA) of the binary Jaccard algorithm.
3.5. Soil Bacterial and Fungal Abundance
A total of 13 bacterial phyla were annotated by high-throughput sequencing. The relative abundance of Proteobacteria, Acidobacteria and Bacteroidetes were higher in all groups. They accounted for 28.4–34.4%, 19.4–23.2% and 10.3–12.9% of the total soil bacteria, respectively (Figure 5A and Table S5). The relative abundance of Proteobacteria was the lowest in HHH, compared with HWH, HPH, HBH or CK. The relative abundance of Acidobacteria and Chloroflexi in CK was significantly higher than that in HWH, HPH, HHH and HWH, while no significant difference was found between HWH, HPH and HBH. The relative abundance of Bacteroidetes in HBH was significantly lower than CK, HWH, HPH and HHH. The relative abundance of Actinobacteria, Verrucomicrobia and Nitrospirae for HHH was significantly lower than CK, HWH, HPH and HBH. The relative abundances of Gemmatimonadetes in CK and HBH were significantly higher than in HWH, HPH and HHH. The relative abundance of Firmicutes was significantly higher in HBH than CK, HWH, HPH and HHH. No significant difference was found for the relative abundance of Planctomycetes, Candidatus-Saccharibacteria, candidate-division-wps1 and Cyanobacteria-Chloroplast (Figure 5A and Table S5).
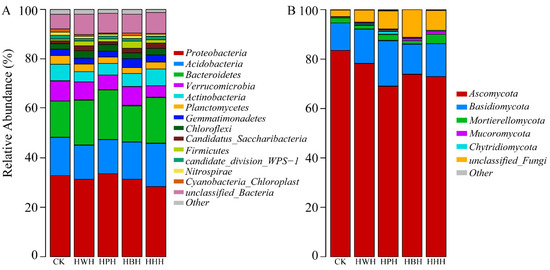
Figure 5.
The relative abundance of bacterial (A) and fungal (B) communities at the phylum level under different culture conditions.
A total of five fungal phyla were annotated by high-throughput sequencing. The relative abundance of Ascomycota was higher in CK and HWH than in HPH, HBH and HHH. The relative abundance of Ascomycota, Basidiomycota and Mortierellomycota in the rotation systems and monoculture system were significantly lower than in CK, except that the relative abundance of Ascomycota in HWH was not significantly different from CK. The relative abundance of Mucoromycota and Chytridiomycota was not significantly different between HWH, HPH, HBH, HHH and CK (Figure 5B and Table S6).
Figure 6 demonstrated the influence of rotational systems, a monoculture system and CK at the genera level of soil bacterial and fungal communities. The relative abundance of the soil microbial communities from high to low is represented by purple to white to green. Of the bacterial community, rotational cropping and CK increased the relative abundance of Subdivision, 3 genera incertae seds, Pedobacter, Chryseolinea, Nitrospira and Terrimonas but displayed reduced the abundance of Sphingomonas and Lysobacter (Figure 6A). Regarding soil fungal genera, Cladorrhinum, Zopfiella, Fusarium, Mortierella and Setophoma had relatively high abundances in the monoculture (HHH). Plectosphaerella displayed increased abundance in the rotational cropping systems, compared with monoculture (Figure 6B).
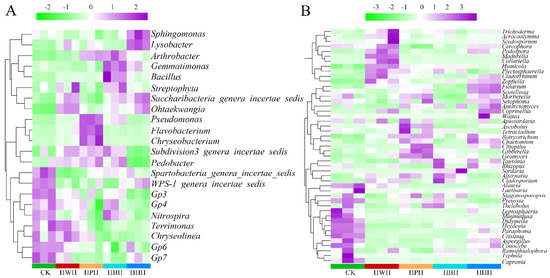
Figure 6.
Heatmap of soil bacterial (A) and fungal (B) communities at the genus level under different culture conditions.
3.6. Correlation of Physicochemical Properties with Microbial Diversity
Redundancy analysis (RDA) is used to analyze the possible influence of soil properties on changes in the soil microbial community, such as changes in structure and composition. RDA revealed that soil pH was highly correlated with disease index. The relative abundances of Flavobacterium, Gp3, Nitrospira and Gp4 were positively correlated with the disease index for bacteria (Figure 7A). The relative abundances of Aspergillus, Fusarium, Cercophora and Heydenia were positively correlated with the disease index for fungi (Figure 7B).
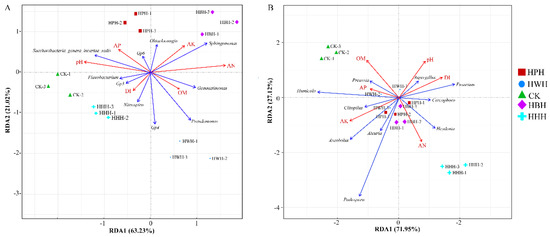
Figure 7.
Redundancy analysis (RDA) showing interactions between soil characteristics and the top 10 bacterial genera (A) or fungal genera (B). pH denotes the soil pH; AP denotes available phosphorus; AK denotes available potassium; AN denotes alkaline hydrolysis; OM organic matter; DI denotes disease index (healthy plants as “0” and disease plants as “1”).
4. Discussion
In agriculture and soil ecology, the composition and structure of soil microbe communities are central to understand the relationship between rotation systems and crop health. Crop rotation could improve both the soil microbial ecosystems and nutrients and is believed to be environmentally friendly for agricultural management [30,31,32]. In contrast, monoculture cropping of the same crop often reduces both the yield and quality of crops [33]. Monoculture cropping also increases plant-species-specific pathogens in the soil and is therefore not sustainable for long-term practice [34,35,36]. A previous study showed that the crop yield, growth condition, soil physicochemical properties and pH are improved by rotation systems [37]. In this study, we focused on the interrelationship among rotation systems, soil microbe communities, and industrial hemp fusarium wilt rate. We found that crop rotation systems (HWH, HPH, or HBH) significantly decreased industrial hemp disease, improved the plant height and increased floral and leaf yields (Figure 1). Compared with the rotation systems, monoculture cropping (HHH) caused higher plant mortality, weaker plant growth, and lower crop yields (Figure 1). These results were likely caused by improved soil physicochemical properties and microbial ecological environment in the rotation systems, which has been reported in previous studies [38]. Our results further showed that the soil AP, AK and AN content were significantly decreased in the monoculture system (HHH) (Figure 2).
Soil microorganisms are an important component of the soil, and the structure and diversity of the microbial community are important for maintaining soil ecological balance [39]. One factor that influences the structure and diversity of the microbial community is the plant species present [40]. The greater the diversity index and the more complex the structure of the microbial community, the stronger its stability and the greater its ability to cope with the environment [41]. Our current study showed that both bacterial and fungal diversity was higher in the soil with three rotation crops compared to monoculture crop. There was no significant difference between the three rotation systems and the control system (Figure 3). One of the important findings is that the community diversity was negatively correlated with industrial fusarium wilt rate. However, there was no direct relationship between biodiversity and function, but the soil with the highest biodiversity was more resistant to stress than soil with impaired biodiversity [42]. We speculated that compromised microbial diversity could lead to the soil microbial community becoming unbalanced, leading to the invasion of pathogenic bacteria. There is evidence that soil microbial diversity confers protection against soil-borne disease and is important for agricultural sustainability [43]. Therefore, our results support the hypothesis that rotation systems with high biodiversity were more resistant to infection of pathogen than monoculture cropping.
The soil microbial community is affected both by the types of soil and the types of plant species. Previous studies have shown that the microbial community is also affected by crop rotation or monoculture cropping of the same plant [44,45,46]. In this study, we found that the microbial community is significantly different between monoculture cropping and rotation systems or CK (Figure 5). The abundances of Proteobacteria and Verrucomicrobia were higher in the rotation systems and CK than with monoculture cropping (HHH), and the abundance of Actinobacteria was significantly was lower in HHH (Figure 5). Proteobacteria is important for predicting changes in soil microecology [47]. Verrucomicrobia is widely distributed in nature and can degrade refractory organic matter [48,49]. Actinobacteria secretes several secondary metabolites that promote plant growth but inhibit pathogen growth [50,51,52]. The relative abundance of Actinobacteria was higher in Rhizoctonia (root rot)-suppressive soil [53]. This can be relevant to industrial hemp health as enhanced plant vigor is important in pathogen resistance.
Some potential industrial hemp fusarium wilt-suppressing bacteria were also detected in this study. Our heatmap assessment showed that the dominant taxa for the rotation systems and CK, but not the monoculture system, were Proteobacteria, Chloroflexi, Rhizobium, Bacteroides, Actinobacteria, Pseudomonas, Flavobacterium, Saccharibacteria and Nitrospirae (Figure 6). A previous study showed that Pseudomonas can inhibit the growth and infection of plant pathogens by secreting antibiotics, toxins, biosurfactants, extracellular cell-wall decomposition enzymes and other substances [54]. Firmicutes and Flavobacterium can inhibit pathogen growth [55]. Flavobacterium can inhibit the growth of the blight-causing Phytophthora capsici in pepper plants [56]. Rhizobium played an important role in plant root colonization [57]. In conclusion, the rotation systems help increase the growth of beneficial microorganisms.
Ascomycota and Basidiomycota are two large and diverse groups of fungi that play an important role in maintaining the soil ecosystem. For example, Preussia can inhibit fungal diseases, while Penicillium and Chaetomium can degrade organic matter [58,59,60]. In this study, we found that the relative abundances of Ascomycota, Basidiomycota and Mortierellomycota were significantly higher in CK than in other systems (Figure 6), suggesting that cropping, whether rotation or monoculture, can affect the overall fungal community diversity. An early study showed that certain species of the fungi genus Fusarium were pathogenic and could cause fusarium wilt disease in watermelon monoculture [61]. Fusarium oxysporum was also isolated from rhizosphere soils of industrial hemp where fusarium wilt occurred [16]. Therefore, we hypothesize that the increased disease incidence in industrial hemp after monoculture cropping was mainly due to the abundance of Fusarium oxysporum. However, other pathogens may cause wilt in industrial hemp, such as Fusarium brachygibbosum, Pythium aphanidermatum, Fusarium solani, and Fusarium equiseti [62]. Future studies will verify the pathogenicity of these strains to industrial hemp.
We conducted PCoA and RDA analyses to profile the soil microbial community structure. Our study showed that there was a significant change among rotation systems, monoculture system and CK in bacterial and fungal communities. However, the fungal community structure was also significantly altered in the different rotation systems (HWH, HPH and HBH) (Figure 4 and Figure 7). Previous studies have shown that the most prosperous growth for Fusarium oxysporum occurred at pH values between 6 and 7 [63]. The results showed that the soil AP, OM, and pH were associated with soil microbiota composition and had close relationships with DI (Figure 7). Other studies have shown that Sphingomonas could promote crop resistance against multiple pathogens [64]. The Gemmatimonas genus has been shown to be related to the formation of organic soil content and has a significant role in promoting the degradation of cellulose and enhancing pathogenic infections [65]. Our study confirmed that the rotation of industrial hemp could alleviate fusarium wilt. There was a complex interaction among industrial hemp fusarium wilt, soil physicochemical and microbial communities. Further studies are required to better understand these interactions.
5. Conclusions
Our study demonstrated that rotation systems could enhance the resistance of industrial hemp plants to pathogens by affecting the composition and function of soil bacterial and fungal communities. We believe that the application of rotation system is an efficient and eco-friendly approach to control destructive soil-borne pathogens and diseases for industrial hemp. It is also sustainable and improves the floral and leaf yield and quality while protecting the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12102293/s1, Table S1: Source of seeds and growth condition; Table S2: The time table of the cultivation during the 3 years; Table S3: Diversity and richness indexes of bacterial communities under crop rotation systems; Table S4: Diversity and richness indexes of fungal communities under crop rotation systems; Table S5: The relative abundance of bacterial communities at the phylum level; Table S6: The relative abundance of fungal communities at the phylum level.
Author Contributions
L.T. and C.F. performed the sample collection, library preparation and microbial annotation, and participated in the bioinformatics and statistical analyses; they also wrote the original draft and performed the review and editing. H.Y. and G.W. took charge of the software and methodology of the manuscript. S.Z. and J.S. oversaw project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Heilongjiang Provincial Scientific Research Institute Scientific Research Business Expense Project (CZKYF2021-2-C010).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Raw readings were submitted to the Sequence Read Archive (SRA) for the NCBI database (Accession Number: PRJNA841395).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Habiga, J.; Labuschagne, J.; Maraisc, M.; Swart, A.; Claassens, S. The effect of a medic-wheat rotational system and contrasting degrees of soil disturbance on nematode functional groups and soil microbial communities. Agric. Ecosyst. Environ. 2018, 268, 103–114. [Google Scholar] [CrossRef]
- Wang, Q.X.; Sun, H.; Xu, C.L.; Ma, L.; Li, M.J.; Shao, C.; Guan, Y.M.; Liu, N.; Liu, Z.B.; Zhang, S.N.; et al. Analysis of rhizosphere bacterial and fungal communities associated with rusty root disease of Panax ginseng. Appl. Soil Ecol. 2019, 138, 245–252. [Google Scholar] [CrossRef]
- Matsushita, Y.; Egami, K.; Sawada, A.; Saito, M.; Sano, T.; Tsushima, S.; Yoshida, S. Analyses of soil bacterial community diversity in naturally and conventionally farmed apple orchards using 16S rRNA gene sequencing. Appl. Soil Ecol. 2019, 141, 26–29. [Google Scholar] [CrossRef]
- Zhao, Q.Y.; Xiong, W.; Xing, Y.Z.; Sun, Y.; Lin, X.J.; Dong, Y.P. Long-term coffee monoculture alters soil chemical properties and microbial communities. Sci. Rep. 2018, 8, 6116–6138. [Google Scholar] [CrossRef] [PubMed]
- Mark, M. Assessment and management of soil community structure for disease suppression. Annu. Rev. Phytopathol. 2004, 42, 35–59. [Google Scholar]
- Penton, C.R.; Gupta, V.V.S.R.; Tiedje, J.M.; Neate, S.M.; Ophel-Keller, K.; Gillings, M.; Harvey, P.; Pham, A.; Roget, D.K. Fungal community structure in disease suppressive soils assessed by 28S LSU gene sequencing. PLoS ONE 2014, 9, e93893. [Google Scholar] [CrossRef] [PubMed]
- Kriese, U.; Schumann, E.; Weber, W.E.; Beyer, M.; Brühl, L.; Matthäus, B. Oil content, tocopherol composition and fatty acid patterns of the seeds of 51 Cannabis sativa L. Genotypes. Euphytica 2004, 137, 339–351. [Google Scholar] [CrossRef]
- Zuardi, A.W. History of cannabis as a medicine: A review. Rev. Bras. Psiquiatr. 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Duke, J.A.; Wain, K.K. Medicinal plants of the world. In Handbook of Medicinal Herbs; Duke, J.A., Ed.; CRC Press: Boca Raton, FL, USA, 1981; pp. 305–309. [Google Scholar]
- Perucca, P.; Scheffer, I.E.; Simon Harvey, A.; James, P.A.; Lunke, S.; Thorne, N.; Gaff, C.; Regan, B.M.; Damiano, J.A.; Michael, S.; et al. Real-world utility of whole exome sequencing with targeted gene analysis for focal epilepsy. Epilepsy Res. 2017, 131, 1–8. [Google Scholar] [CrossRef]
- Desroches, J.; Beaulieu, P. Opioids and cannabinoids interactions: Involvement in pain management. Curr. Drug Targets 2010, 11, 462–473. [Google Scholar] [CrossRef]
- Choudhary, N. Floral biology and pollination biology of Cannabis sativa L. Int. J. Plant Rep. Biol. 2010, 2, 191–195. [Google Scholar]
- Martyn, R.D. Fusarium wilt of watermelon: 120 years of research. Hortic. Rev. 2014, 42, 349–442. [Google Scholar]
- Shen, Z.; Penton, C.R.; Lv, N.; Xue, C.; Yuan, X.F.; Ruan, Y.Z.; Shen, Q.R. Banana fusarium wilt disease incidence is influenced by shifts of soil microbial communities under different monoculture spans. Microb. Ecol. 2017, 75, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chi, J.L.; Ao, J.; Gao, X.M.; Liu, X.H.; Sun, Y.L.; Zhu, W. Effects of different continuous cropping years on bacterial community and diversity of cucumber rhizosphere soil in solar-greenhouse. Curr. Microbiol. 2021, 78, 2380–2390. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Fan, C.; Guo, X.Y.; Yuan, H.M.; Wu, G.W.; Zhang, S.Q. First report of fusarium wilt industrial hemp (Cannabis sativa L.) caused by Fusarium oxysporum in the northeast China. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Dong, L.L.; Li, Y.; Xu, J.; Wei, G.F.; Shen, L.; Ding, W.L.; Chen, S.L. Biofertilizers regulate the soil microbial community and enhance Panax ginseng yields. Chin. Med. 2019, 14, 20–34. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.M.; Chen, D.; Wei, H.; Saleem, M. Continuous cropping alters multiple biotic and abiotic indicators of soil health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Guo, L.; Chen, X.; Li, Z.; Wang, M.; Che, Y.; Zhang, L.; Jiang, Z.; Jie, S. Effects of continuous cropping on bacterial community and diversity in rhizosphere soil of industrial hemp: A Five-Year Experiment. Diversity 2022, 14, 250. [Google Scholar] [CrossRef]
- Alvey, S.; Yang, C.H.; Buerkert, A.; Crowley, D.E. Cereal/legume rotation effects on rhizosphere bacterial community structure in West African soils. Biol. Fert Soils 2003, 37, 73–82. [Google Scholar] [CrossRef]
- Samuelmc, N.; Zahangir, K.; Frankn, M.; Stevent, K.; Krishnav, S. Comparison of crop rotation for verticillium wilt management and effect on Pythium species in conventional and organic strawberry production. Plant Dis. 2009, 93, 519–527. [Google Scholar]
- Larkin, R.P.; Honeycutt, C.W.; Olanya, O.M.; Halloran, J.M.; He, Z. Impacts of crop rotation and irrigation on soilborne diseases and soil microbial communities. In Sustainable Potato Production: Global Case Studies; Springer: Dordrecht, The Netherlands, 2012; pp. 23–41. [Google Scholar]
- Wu, X.; Zhao, Q.Y.; Xue, C.; Xun, W.B.; Zhao, J.; Wu, H.S.; Li, R.; Shen, Q.R. Comparison of fungal community in black pepper-vanilla and vanilla monoculture systems associated with vanilla fusarium wilt disease. Front. Microbiol. 2016, 7, 117–132. [Google Scholar]
- Bao, S.D. Soil Analysis in Agricultural Chemistry, 3rd ed.; China Agricultural Press: Beijing, China, 2005. (In Chinese) [Google Scholar]
- Zhong, Y.Q.W.; Yan, W.M.; Shangguan, Z.P. Impact of long-term N additions upon coupling between soil microbial community structure and activity, and nutrient-use efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R, a Language and Environment for Statistic Computing; R Foundation for Statistical Computing: Vienna, Austria, 2006; Available online: http://www.R-project.org (accessed on 1 January 2022).
- Guo, J.J.; Ning, L.; Chen, H.; Chen, Z.; Kong, Y.L.; Wang, M.; Shen, Q.R.; Guo, S.W. Distinct divers of activity, abundance, diversity and composition of ammonia-oxidizers: Evidence form a long-term field experiment. Soil Biol. Biochem. 2017, 115, 403–414. [Google Scholar] [CrossRef]
- Jay, S.S.; Vimal, C.P.; Singh, D.P. Efficient soil microorganisms: A new dimension for sustainable agriculture and environmental development. Agric. Ecosyst. Environ. 2011, 140, 339–353. [Google Scholar]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- Wang, M.Y.; Wu, C.M.; Cheng, Z.H.; Meng, H.W.; Zhang, M.R.; Zhang, H.J. Soil chemical property changes in eggplant/garlic relay intercropping systems under continuous cropping. PLoS ONE 2014, 9, e111040. [Google Scholar] [CrossRef]
- Zhou, X.G.; Wu, F.Z. Dynamics of the diversity of fungal and Fusarium communities during continuous cropping of cucumber in the greenhouse. FEMS Microbiol. Ecol. 2012, 80, 469–478. [Google Scholar] [CrossRef]
- Cook, R.J. Toward cropping systems that enhance productivity and sustainability. Proc. Natl. Acad. Sci. USA 2006, 103, 18389–18394. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.G.; Yu, G.B.; Wu, F.Z. Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur. J. Soil Biol. 2011, 47, 279–287. [Google Scholar] [CrossRef]
- Liu, T.J.; Cheng, Z.H.; Meng, H.W.; Ahmad, I.; Zhao, H.L. Growth, yield and quality of spring tomato and physicochemical properties of medium in a tomato/garlic intercropping system under plastic tunnel organic medium cultivation. Sci. Hortic. 2014, 170, 159–168. [Google Scholar] [CrossRef]
- Chamberlain, L.A.; Bolton, M.L.; Cox, M.S.; Suen, G.; Conley, S.P.; Ané, J.M. Crop rotation, but not cover crops, influenced soil bacterial community composition in a corn-soybean system in southern Wisconsin. Appl. Soil Ecol. 2020, 154, 103603. [Google Scholar] [CrossRef]
- Kennedy, A.C.; Smith, K.L. Soil microbial diversity and the sustainability of agricultural soils. Plant Soil 1995, 170, 75–86. [Google Scholar] [CrossRef]
- Shin, K.; Diepen, G.; Blok, W.; van Bruggen, A.H.C. Variability of effective micro-organisms (EM) in bokashi and soil and effects on soil-borne plant pathogens. Crop Prot. 2017, 99, 168–176. [Google Scholar] [CrossRef]
- Hunter, P.R.; Gaston, M.A. Numerical index of the discriminatory ability of typing systems: An application of Simpsons index of diversity. J. Clin. Microbiol. 1988, 26, 2465–2466. [Google Scholar] [CrossRef]
- Griffiths, B.S.; Ritz, K.; Bardgett, R.D.; Cook, R.; Christensen, S.; Ekelund, F.; Nicolardot, B. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions: An examination of the biodiversity–ecosystem function relationship. Oikos 2000, 90, 279–294. [Google Scholar] [CrossRef]
- Brussaard, L.; De Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Marschner, P.; Yang, C.H.; Lieberei, R.; Crowley, D.E. Soil and plant specific effects on bacterial community composition in the rhizosphere. Soil Biol. Biochem. 2001, 33, 1437–1445. [Google Scholar] [CrossRef]
- Broeckling, C.D.; Broz, A.K.; Bergelson, J.; Manter, D.K.; Vivanco, J.M. Root exudates regulate soil fungal community composition and diversity. Appl. Environ. Microb. 2008, 74, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing- based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microb. 2009, 75, 5111–5120. [Google Scholar] [CrossRef]
- Dai, Z.M.; Su, W.Q.; Chen, H.H.; Barberán, A.; Zhao, H.C.; Yu, M.J.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X.; et al. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agroecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, H.H.; Fu, S.L.; Yao, Q. Variation in soil microbial community structure associated with different legume species is greater than that associated with different grass species. Front. Microbiol. 2017, 8, 1007. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, A.A.; Soares, T.; Rossetto, R.; van Veen, J.A.; Tsai, S.M.; Kuramae, E.E. Verrucomicrobial community structure and abundance as indicators for changes in chemical factors linked to soil fertility. Anton. Van. Leeuw. 2015, 108, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Leveau, J.; Gardener, B.B.M.; Pierson, E.A.; Pierson III, L.S.; Ryu, C.M. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microb. 2011, 77, 1548–1555. [Google Scholar] [CrossRef]
- Doumbou, C.L.; Salove, M.K.H.; Crawford, C.; Beaulieu, D.L. Actinomycetes, promising tools to control plant diseases and to promote plant growth. Phytoprotection 2002, 82, 85–102. [Google Scholar] [CrossRef]
- Tokala, R.K.; Strap, J.L.; Jung, C.M.; Crawford, D.L.; Salove, M.H.; Deobald, L.A.; Bailey, J.F.; Morra, M.J. Novel plant-microbe rhizosphere interaction involving streptomyces lydicus wyec108 and the pea plant (Pisum sativum). Appl. Environ. Microbiol. 2002, 68, 2161–2171. [Google Scholar] [CrossRef]
- Mendes, R.; Kruijt, M.; de Bruijn, I.; Dekkers, E.; van der Voort, M.; Schneider, J.H.M.; Piceno, Y.M.; DeSantis, T.Z.; Andersen, G.L.; Bakker, P.A.H.M.; et al. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science 2011, 332, 1097–1100. [Google Scholar] [CrossRef]
- E, Y.Y.; Yuan, J.; Yang, F.; Wang, L.; Ma, J.H.; Li, J.; Pu, X.W.; Raza, W.; Huang, Q.W.; Shen, Q.R. PGPR strain paenibacillus polymyxa SQR-21 potentially benefits watermelon growth by re-shaping root protein expression. AMB Express 2017, 7, 104–116. [Google Scholar] [CrossRef]
- Xiong, W.; Zhao, Q.Y.; Zhao, J.; Xun, W.B.; Li, R.; Zhang, R.F.; Wu, H.S.; Shen, Q.R. Different continuous cropping spans significantly affect microbial community membership and structure in a vanilla-grown soil as revealed by deep pyrosequencing. Microb. Ecol. 2015, 70, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.K.; Kim, K.D. The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microbiol. 2012, 113, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Roque-Malo, S.; Woo, D.K.; Kumar, P. Modeling the role of root exudation in critical zone nutrient dynamics. Water Resour. Res. 2020, 56, e2019WR026606. [Google Scholar] [CrossRef]
- Poch, G.K.; Gloer, J.B. Auranticins A and B: Two new depsidones from a mangrove isolate of the fungus Preussia aurantiaca. J. Nat. Prod. 1991, 54, 213–217. [Google Scholar] [CrossRef]
- Lakshmanan, V.; Selvaraj, G.; Bais, H.P. Functional soil microbiome: Belowground solutions to an aboveground problem. Plant Physiol. 2014, 166, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.L.; Xia, Y.; Fan, C.; Kou, J.M.; Wu, F.Z.; Li, W.H.; Pan, K. Control of fusarium wilt by wheat straw is associated with microbial network changes in watermelon rhizosphere. Sci. Rep. 2020, 10, 12736–12750. [Google Scholar] [CrossRef]
- Punja, Z.K.; Scott, C.; Chen, S. Root and crown rot pathogens causing wilt symptoms on field-grown marijuana (Cannabis sativa L.) plants. Can. J. Plant Pathol. 2018, 40, 1715–2992. [Google Scholar] [CrossRef]
- Maitlo, S.; Rajput, A.Q.; Syed, R.N.; Khanzada, M.A.; Lodhi, A.M. Influence of physiological factors on vegetative growth and sporulation of Fusarium oxysporum f. sp. ciceris. Pak. J. Bot. 2017, 49, 311–316. [Google Scholar]
- Adhikari, T.B.; Joseph, C.M.; Yang, G.P.; Phillips, D.A.; Nelson, L.M. Evaluation of bacteria isolated from rice for plant growthpromotion and biological control of seedling disease of rice. Can. J. Microbiol. 2001, 47, 916–924. [Google Scholar] [CrossRef]
- Wang, Y.H.; Yu, Z.; Wang, G.; Liu, J.; Liu, X.; Jin, J. Microbial association with the dynamics of particulate organic carbon in response to the amendment of elevated CO2-derived wheat residue into a Mollisol. Sci. Total Environ. 2017, 607–608, 972–981. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).