Spatial and Temporal Variability of the Microbiological and Chemical Properties of Soils under Wheat and Oilseed Rape Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Objectives
2.2. Soil Sampling
2.3. Enzymatic Activity
2.4. Biomass of Soil Microorganisms
2.5. Determinationof Physico-Chemical Properties
2.6. Plant Analyses
2.7. Statistics
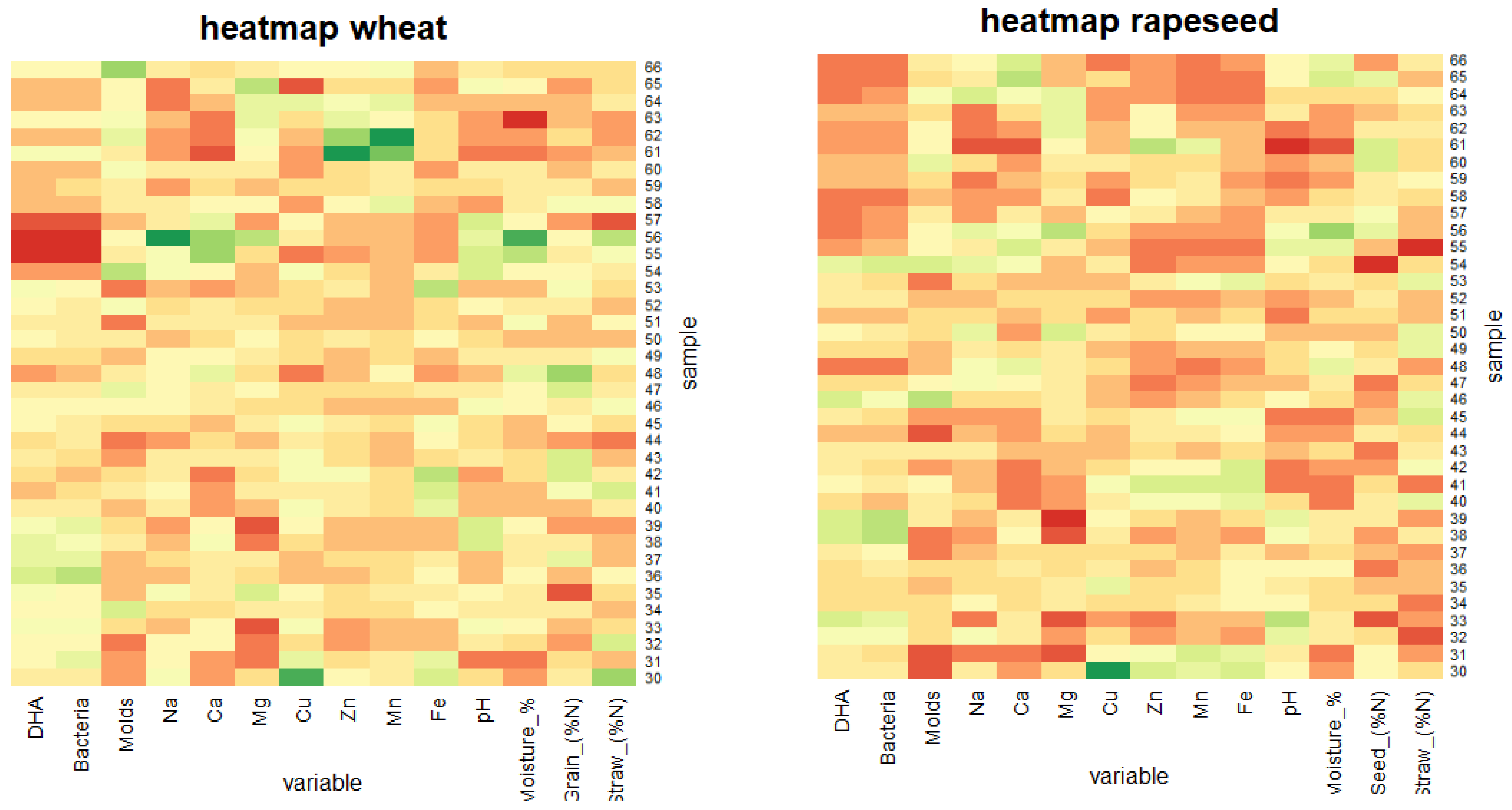
3. Results
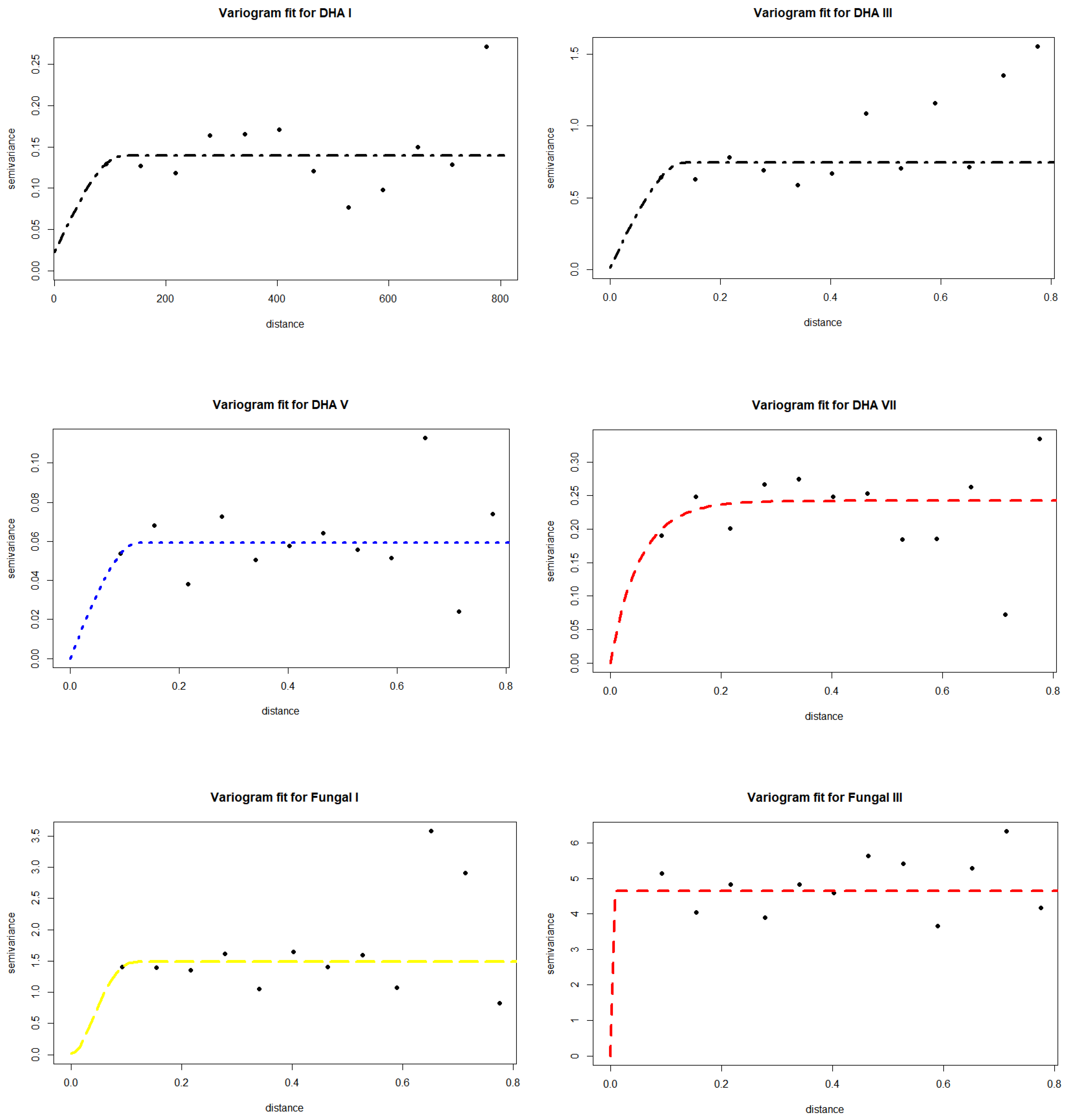
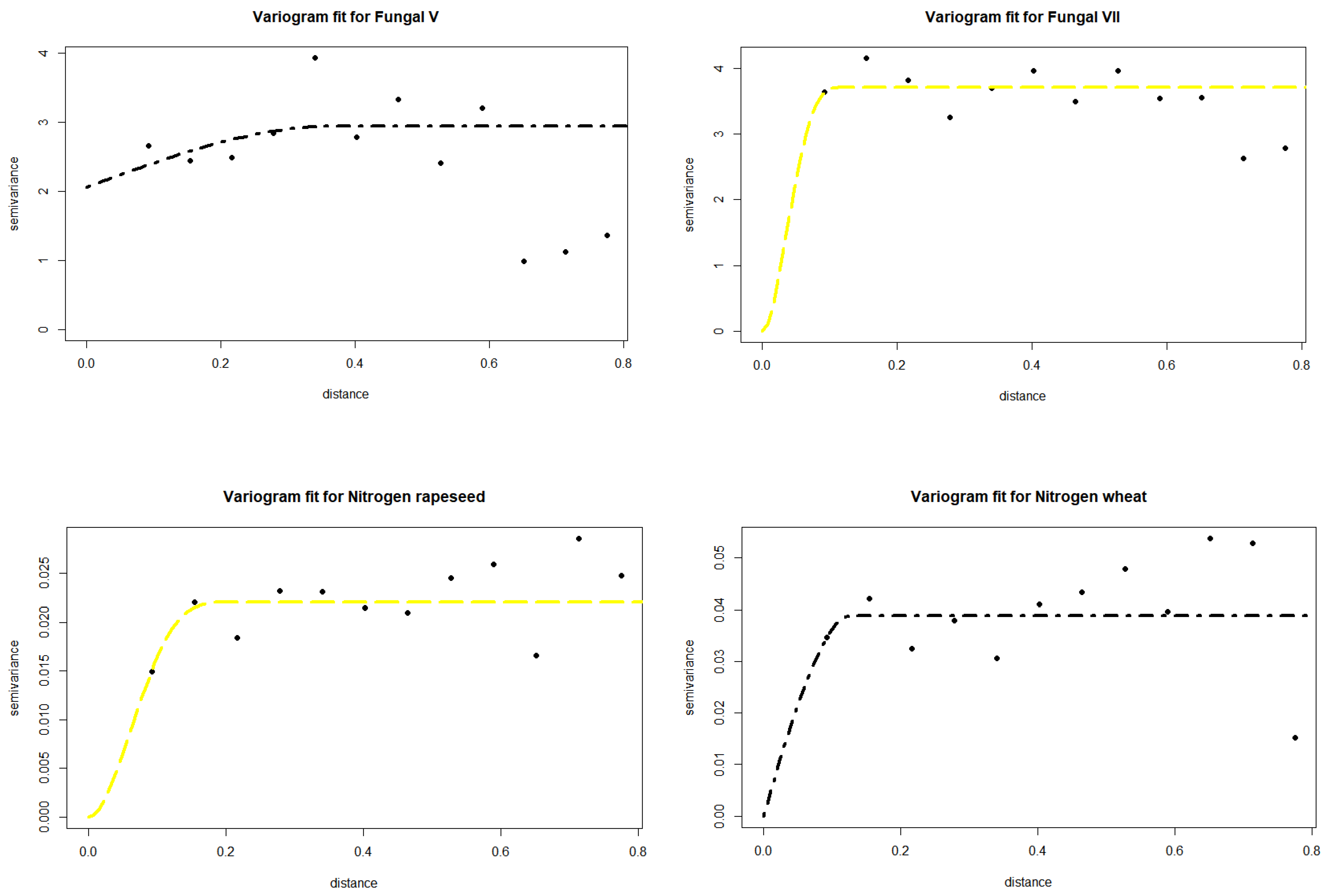
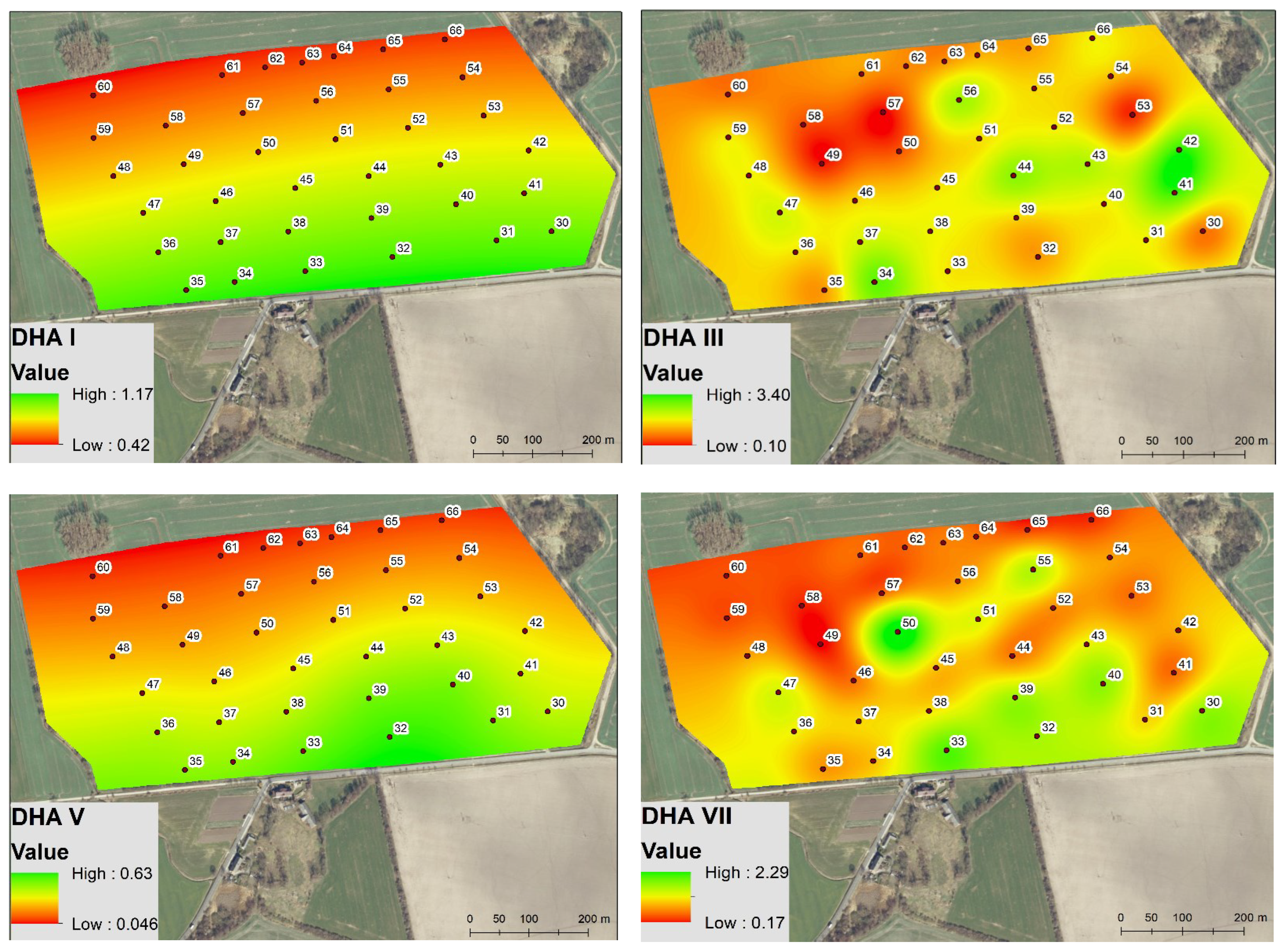
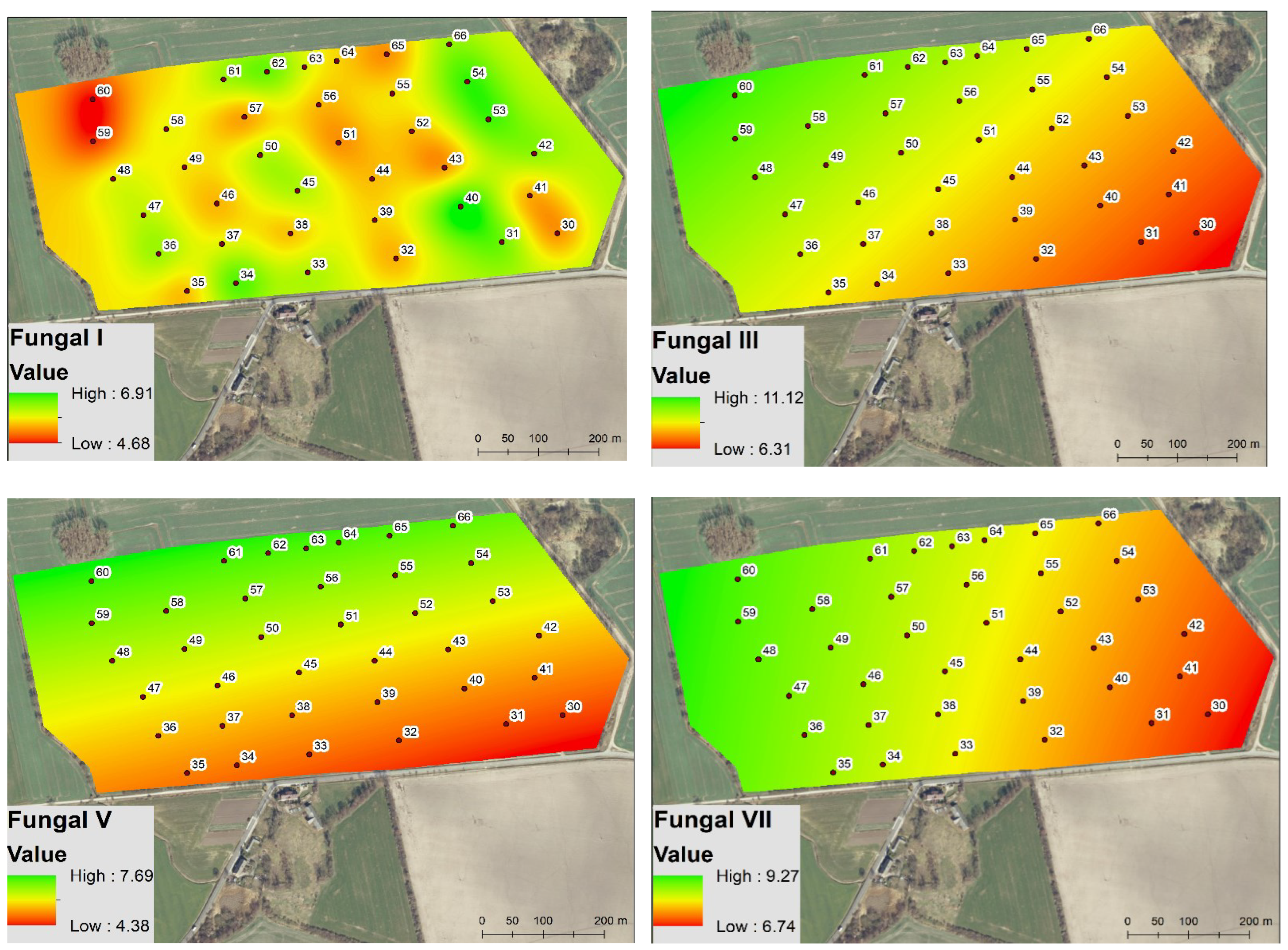
3.1. Spatio-Temporal Variability of Soil Biochemical Activity
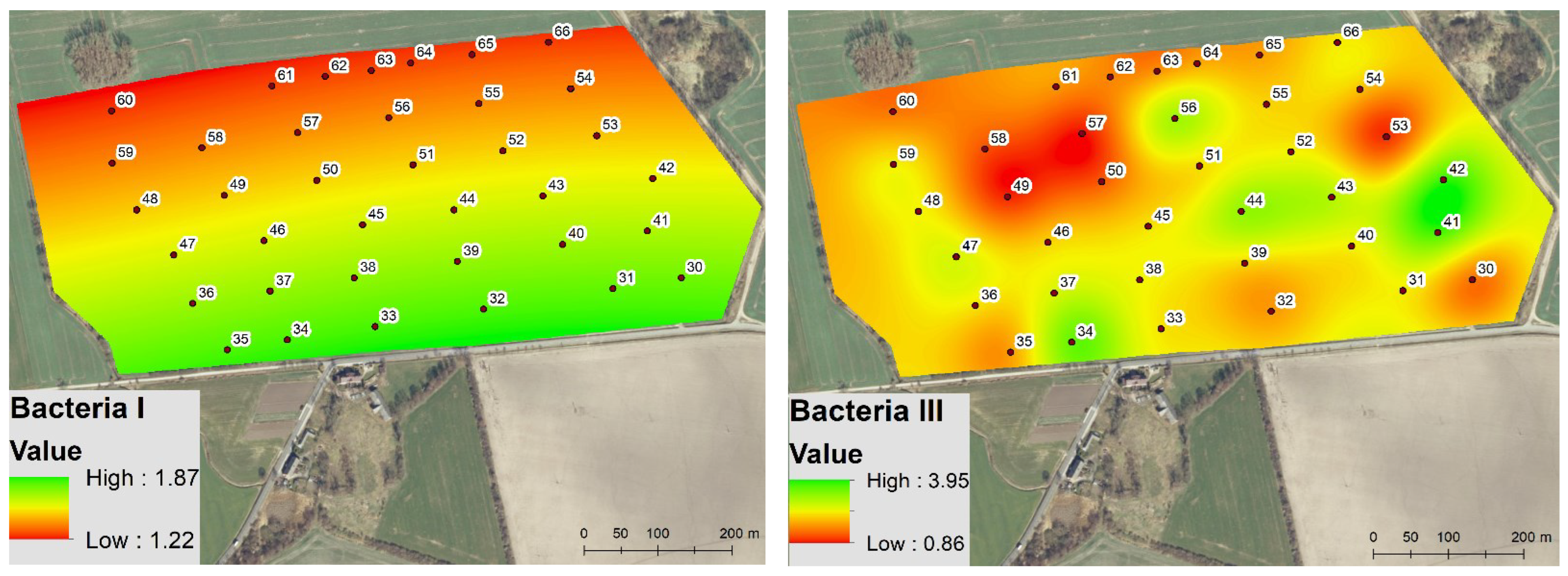
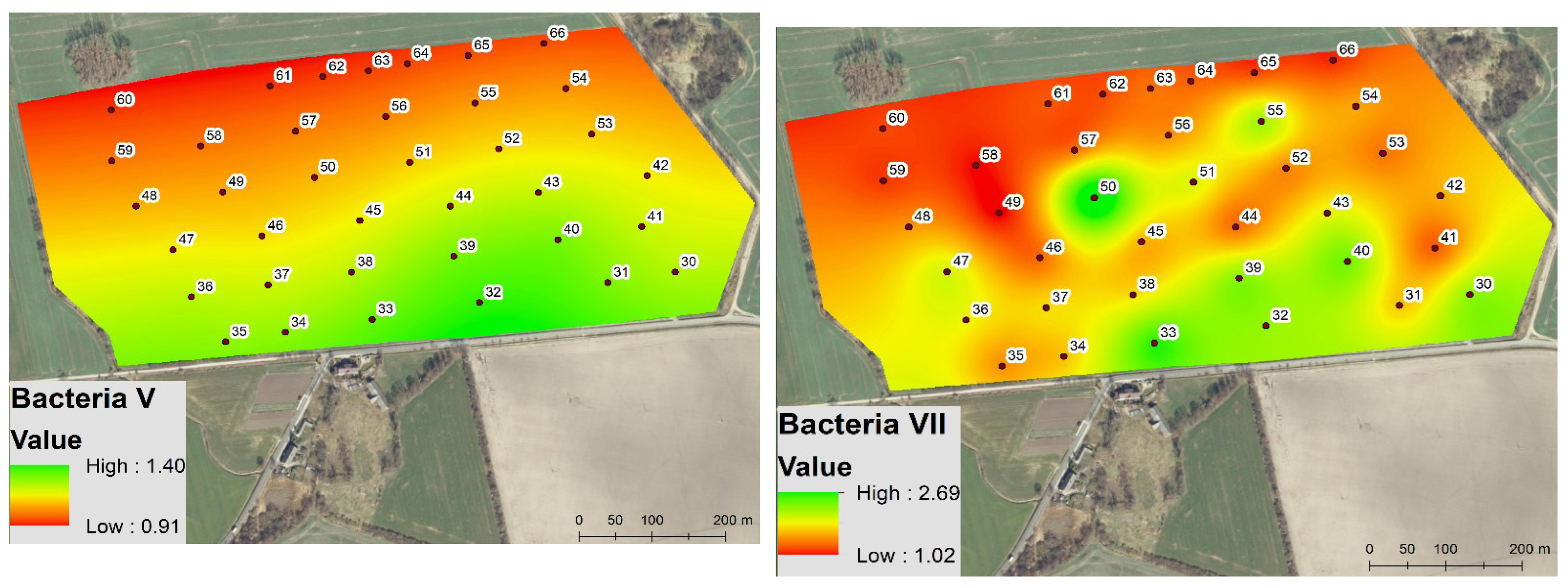
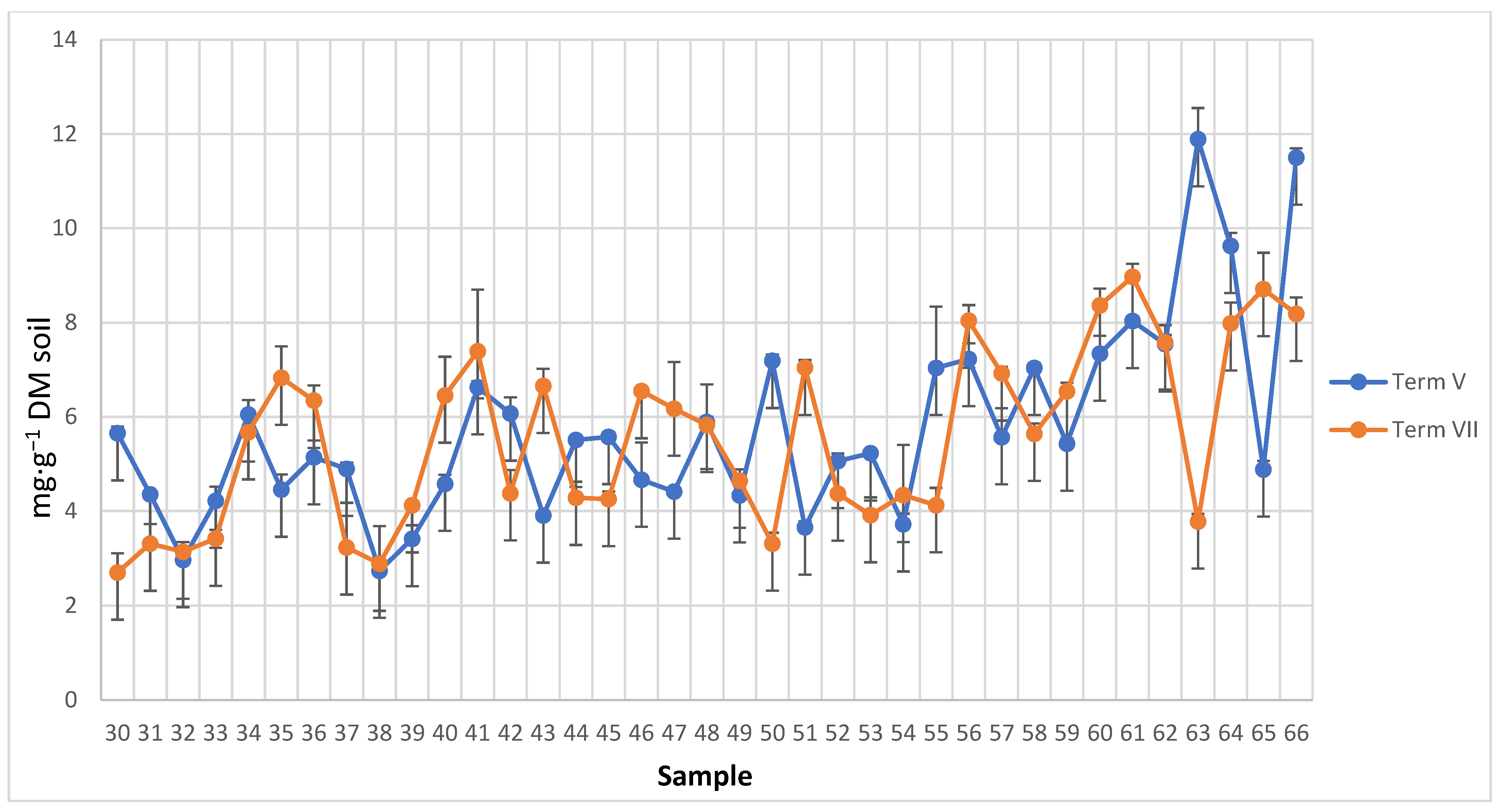
3.2. Spatio-Temporal Variability of the Soil Microbial Biomass
3.3. Spatio-Temporal Variability of Micro- and Macroelements
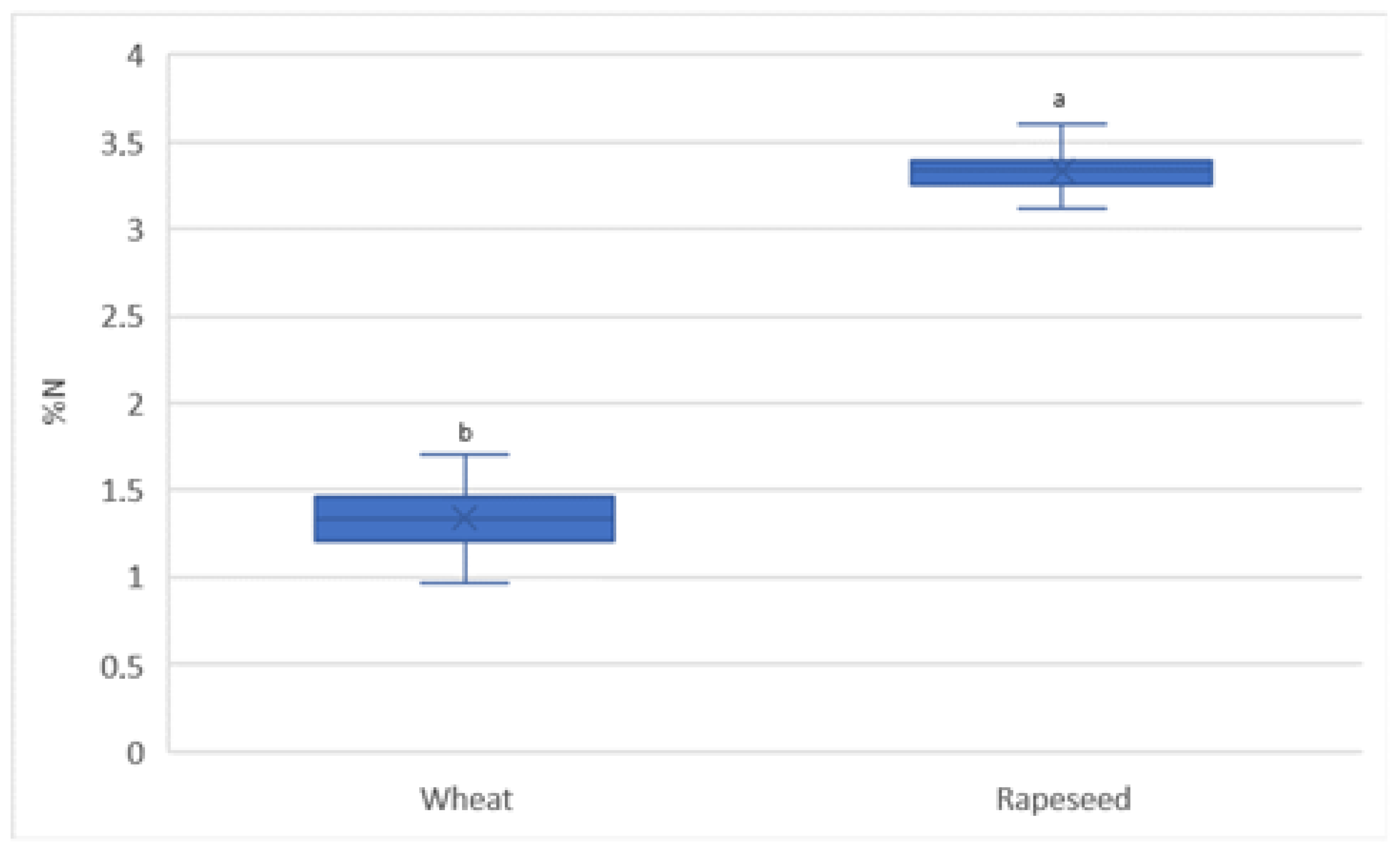
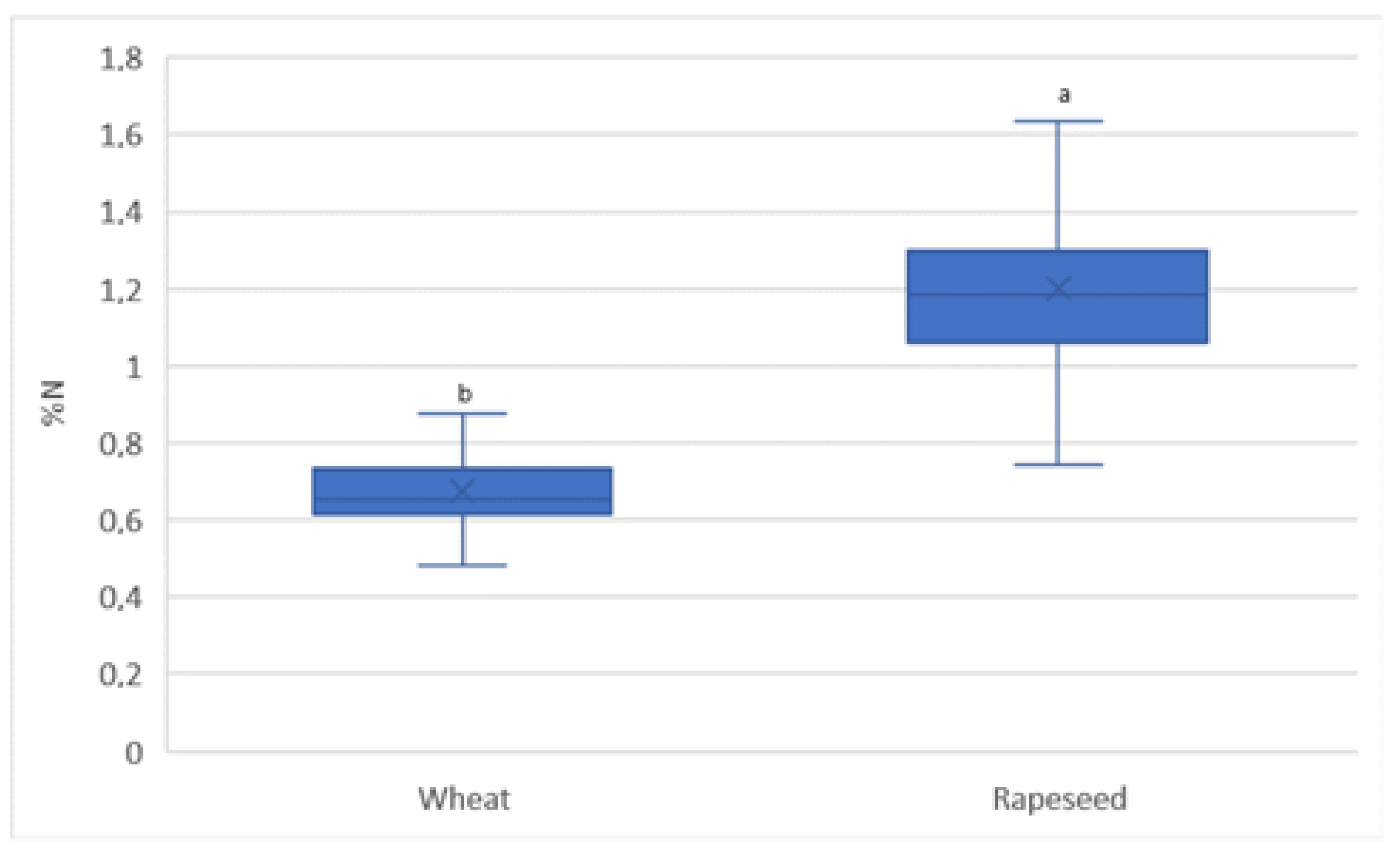
3.4. Spatial Variability in Nitrogen Content in the Crop Yield
4. Discussion
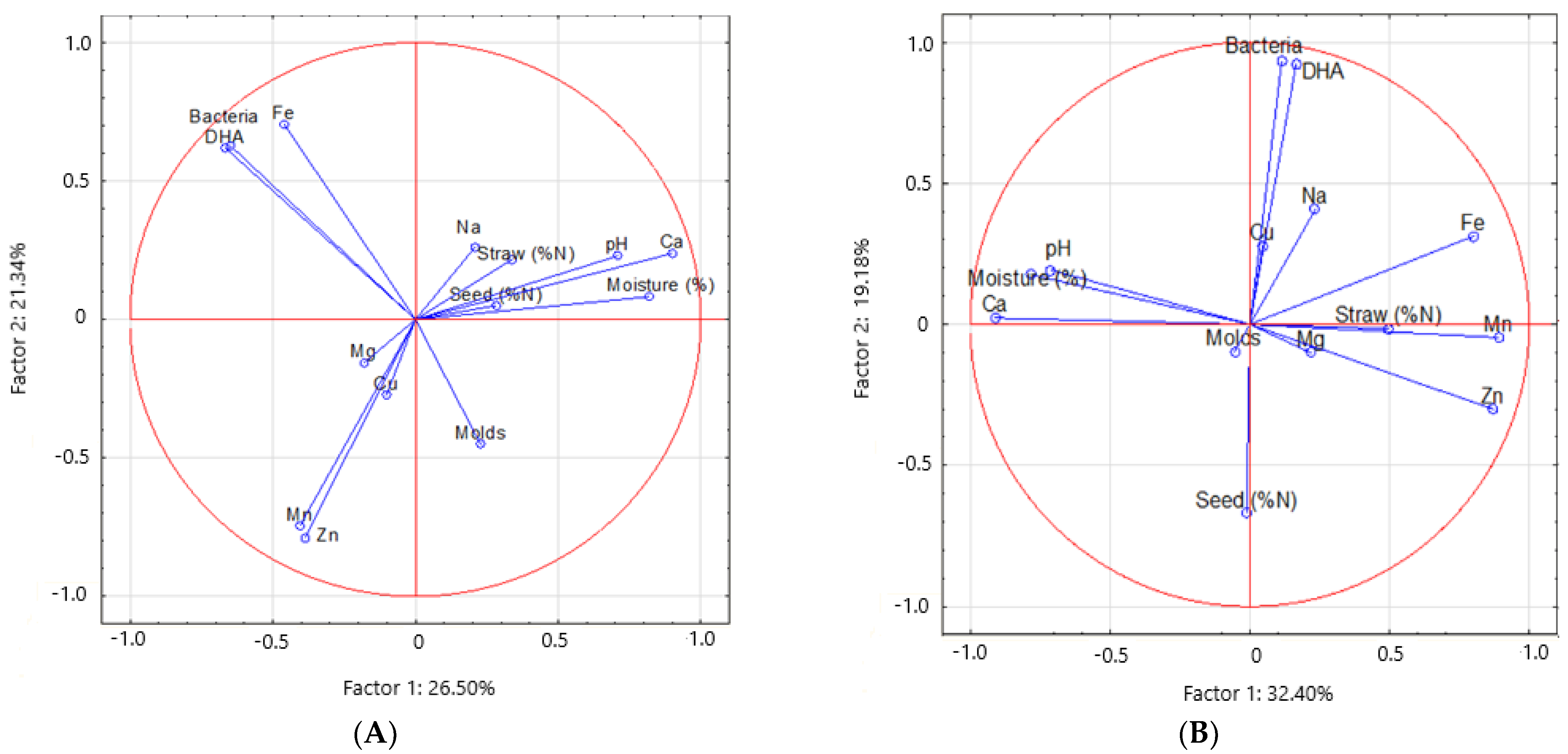
4.1. Spatio-Temporal Variability of Enzyme Activity and Soil Microbial Biomass
4.2. Spatio-Temporal Variability of Micro- and Macroelements
4.3. Spatial Variability of N Content in the Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| DHA | Bacteria | Fungi | Na | Ca | Mg | Cu | Zn | Mn | Fe | pH | Moisture (%) | Seed (%N) | Straw (%N) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA | 1.00 * | −0.27 * | −0.07 | −0.35 | 0.10 | −0.16 | −0.21 * | −0.14 | 0.62 * | −0.31 * | −0.37 * | −0.14 | −0.16 | |
| Bacteria | 1.00 * | −0.27 * | −0.07 | −0.33 | 0.10 | −0.17 | −0.22 * | −0.15 | 0.61 * | −0.30 * | −0.34 * | −0.13 | −0.15 | |
| Fungi | −0.27 * | −0.27 * | −0.004 | 0.07 | 0.29 * | −0.09 | 0.17 | 0.22 * | −0.38 * | 0.02 | 0.23 * | 0.23 * | −0.13 | |
| Na | −0.07 | −0.07 | −0.004 | 0.19 * | 0.08 | −0.20 * | −0.14 | −0.24 * | 0.17 | 0.03 | 0.08 | −0.05 | 0.46 * | |
| Ca | −0.35 * | −0.33 * | 0.07 | 0.19 | −0.15 | −0.18 | −0.52 * | −0.48 * | −0.36 * | 0.71 * | 0.82 * | 0.18 | 0.29 * | |
| Mg | 0.10 | 0.10 | 0.29 * | 0.08 | −0.15 | −0.12 | 0.20 * | 0.14 | −0.02 | −0.31 * | −0.01 | −0.02 | 0.12 | |
| Cu | −0.16 | −0.17 | −0.09 | −0.20 | −0.18 | −0.12 | 0.35 * | 0.01 | −0.005 | 0.08 | −0.24 * | −0.001 | −0.05 | |
| Zn | −0.21 * | −0.22 * | 0.17 | −0.14 | −0.52 * | 0.20 * | 0.35 * | 0.70 * | −0.35 * | −0.45 * | −0.35 * | −0.09 | −0.15 | |
| Mn | −0.14 | −0.15 | 0.22 * | −0.24 | −0.48 * | 0.14 | 0.01 | 0.70 * | −0.36 * | −0.50 * | −0.31 * | −0.16 | −0.19 * | |
| Fe | 0.62 * | 0.61 * | −0.38 * | 0.17 | −0.36 * | −0.02 | −0.005 | −0.35 * | −0.36 * | −0.19 | −0.35 * | 0.11 | 0.11 | |
| pH | −0.31 * | −0.30 * | 0.02 | 0.03 | 0.71 * | −0.31 * | 0.08 | −0.45 * | −0.50 * | −0.19 | 0.43 * | −0.04 | 0.06 | |
| Moisture (%) | −0.37 * | −0.34 * | 0.23 * | 0.08 | 0.82 * | −0.01 | −0.24 * | −0.35 * | −0.31 * | −0.35 * | 0.43 * | 0.36 * | 0.27 * | |
| Seed (%N) | −0.14 | −0.13 | 0.23 * | −0.05 | 0.18 | −0.02 | 0.002 | −0.09 | −0.16 | 0.11 | −0.04 | 0.36 * | 0.19 | |
| Straw (%N) | −0.16 | −0.15 | −0.13 | 0.46 | 0.29 * | 0.12 | −0.05 | −0.15 | −0.19 * | 0.11 | 0.06 | 0.27 * | 0.19 |
| DHA | Bacteria | Fungi | Na | Ca | Mg | Cu | Zn | Mn | Fe | pH | Moisture (%) | Seed (%N) | Straw (%N) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHA | 1.00 * | −0.03 | 0.22 * | −0.18 | −0.15 | 0.22 * | −0.09 | 0.13 | 0.30 * | 0.05 | −0.05 | −0.51 * | −0.02 | |
| Bacteria | 1.00 * | −0.03 | 0.23 * | −0.13 | −0.15 | 0.22 * | −0.14 | 0.09 | 0.27 * | 0.08 | 0.01 | −0.51 * | −0.03 | |
| Fungi | −0.03 | −0.03 | −0.05 | 0.01 | −0.01 | −0.05 | 0.00 | −0.13 | −0.26 * | −0.17 | −0.01 | −0.06 | 0.06 | |
| Na | 0.22 * | 0.23 * | −0.05 | −0.01 | 0.57 * | 0.18 | 0.00 | 0.12 | 0.52 * | −0.14 | 0.20 * | −0.17 | 0.29 * | |
| Ca | −0.18 | −0.13 | 0.01 | −0.01 | −0.08 | −0.01 | −0.79 * | −0.75 * | −0.63 * | 0.64 * | 0.84 * | 0.05 | −0.38 * | |
| Mg | −0.15 | −0.15 | −0.01 | 0.57 * | −0.08 | −0.01 | 0.20 * | 0.10 | 0.17 | −0.01 | −0.05 | 0.14 | 0.35 * | |
| Cu | 0.22 * | 0.22 * | −0.05 | 0.18 | −0.01 | −0.01 | −0.01 | 0.02 | 0.16 | −0.01 | 0.06 | 0.11 | −0.12 | |
| Zn | −0.09 | −0.14 | 0.00 | 0.00 | −0.79 * | 0.20 * | −0.01 | 0.82 * | 0.54 * | −0.63 * | −0.72 * | 0.19 * | 0.37 * | |
| Mn | 0.13 | 0.09 | −0.13 | 0.12 | −0.75 * | 0.10 | 0.02 | 0.82 * | 0.73 * | −0.59 * | −0.63 * | 0.10 | 0.35 * | |
| Fe | 0.30 * | 0.27 * | −0.26 * | 0.52 * | −0.63 * | 0.17 | 0.16 | 0.54 * | 0.73 * | −0.47 * | −0.44 * | −0.18 | 0.40 * | |
| pH | 0.05 | 0.08 | −0.17 | −0.14 | 0.64 * | −0.01 | −0.01 | −0.63 * | −0.59 * | −0.47 * | 0.51 * | −0.11 | −0.16 | |
| Moisture (%) | −0.05 | 0.01 | −0.01 | 0.20 * | 0.84 * | −0.05 | 0.06 | −0.72 * | −0.63 * | −0.44 * | 0.51 * | −0.001 | −0.26 * | |
| Seed (%N) | −0.51 * | −0.51 * | −0.06 | −0.17 | 0.05 | 0.14 | 0.11 | 0.19 * | 0.10 | −0.18 | −0.11 | −0.001 | −0.09 | |
| Straw (%N) | −0.02 | −0.03 | 0.06 | 0.29 * | −0.38 * | 0.35 * | −0.12 | 0.37 * | 0.35 * | 0.40 * | −0.16 | −0.26 * | −0.09 |
| DHA | Bacteria | Fungi | Na | Ca | Mg | Cu | Zn | Mn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| Constant term | 1000.25 | 1630.71 | 25,060.00 | 2800.40 | 1,058,395.55 | 27,103.54 | 0.65 | 0.18 | 37.67 | 152.26 |
| Term | 103.00 | 58.70 | 886.53 | 156.68 | 1980.91 | 87.89 | 0.035 | 0.009 | 15.16 | 24.47 |
| Sampling point | 2.88 | 1.77 | 27.82 | 1.93 | 1983.37 | 84.22 | 0.0003 | 0.0010 | 1.86 | 1.74 |
| Term*sampling point | 1.99 | 1.22 | 11.32 | 1.00 | 20.92 | 3.48 | 0.0002 | 0.0001 | 0.74 | 0.59 |
| Error | 0.09 | 0.06 | 0.03 | 0.01 | 2.18 | 0.21 | 0.0001 | 0.0000 | 0.01 | 0.01 |
| DHA | Bacteria | Fungi | Na | Ca | Mg | Cu | Zn | Mn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|
| Constant term | 235.47 | 764.77 | 24,339.16 | 2222.15 | 782,950.07 | 24,074.79 | 0.21 | 0.22 | 0.45 | 639.32 |
| Term | 29.87 | 15.39 | 750.16 | 81.24 | 14,870.48 | 381.71 | 0.00273 | 0.01934 | 0.02 | 14.46 |
| Sampling point | 1.43 | 0.95 | 29.64 | 0.66 | 857.19 | 50.92 | 0.00038 | 0.00043 | 0.00309 | 7.74 |
| Term*sampling point | 0.36 | 0.23 | 16.14 | 0.76 | 406.56 | 17.16 | 0.00021 | 0.00027 | 0.00134 | 2.80 |
| Error | 0.03 | 0.02 | 0.03 | 0.06 | 7.14 | 0.51 | 0.00007 | 0.00001 | 0.00008 | 0.08 |
| YEAR | 2019 | 2020 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TERM | I | II | III | IV | V | VI | VII | VIII | ||||||||
| X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | |
| Na | ||||||||||||||||
| 30 | 4.16 | 0.02 | 3.11 | 0.11 | 2.62 | 0.12 | 2.41 | 0.06 | 2.35 | 0.35 | 2.74 | 0.03 | 2.98 | 0.09 | 1.42 | 0.02 |
| 31 | 4.18 | 0.02 | 2.59 | 0.01 | 1.99 | 0.06 | 2.37 | 0.02 | 1.43 | 0.27 | 2.86 | 0.17 | 2.76 | 0.09 | 0.96 | 0.05 |
| 32 | 4.02 | 0.02 | 3.27 | 0.07 | 2.54 | 0.06 | 1.83 | 0.00 | 2.23 | 0.14 | 3.11 | 0.38 | 2.84 | 0.03 | 2.17 | 0.03 |
| 33 | 3.65 | 0.02 | 2.80 | 0.04 | 1.60 | 0.05 | 1.31 | 0.01 | 1.55 | 0.23 | 2.83 | 0.03 | 2.46 | 0.03 | 0.91 | 0.02 |
| 34 | 3.38 | 0.01 | 3.11 | 0.08 | 1.67 | 0.07 | 1.55 | 0.01 | 1.43 | 0.19 | 3.76 | 0.20 | 2.99 | 0.08 | 1.89 | 0.01 |
| 35 | 4.61 | 0.03 | 3.11 | 0.03 | 2.51 | 0.06 | 1.53 | 0.00 | 1.60 | 0.16 | 3.33 | 0.19 | 2.90 | 0.03 | 1.36 | 0.01 |
| 36 | 3.00 | 0.01 | 3.20 | 0.06 | 1.71 | 0.06 | 1.58 | 0.00 | 1.49 | 0.36 | 3.19 | 0.23 | 2.89 | 0.06 | 1.58 | 0.01 |
| 37 | 3.71 | 0.03 | 3.01 | 0.08 | 1.90 | 0.05 | 1.39 | 0.00 | 1.72 | 0.15 | 3.25 | 0.23 | 2.74 | 0.07 | 1.15 | 0.00 |
| 38 | 3.54 | 0.03 | 3.08 | 0.04 | 1.14 | 0.05 | 1.38 | 0.01 | 1.69 | 0.04 | 2.79 | 0.08 | 2.60 | 0.05 | 1.21 | 0.03 |
| 39 | 3.14 | 0.02 | 2.65 | 0.10 | 1.43 | 0.06 | 1.51 | 0.00 | 1.45 | 0.25 | 3.77 | 0.42 | 2.58 | 0.04 | 0.98 | 0.03 |
| 40 | 3.83 | 0.05 | 3.04 | 0.12 | 2.06 | 0.08 | 1.97 | 0.02 | 1.77 | 0.15 | 3.11 | 0.00 | 2.78 | 0.05 | 1.25 | 0.02 |
| 41 | 4.64 | 0.04 | 3.85 | 0.22 | 1.60 | 0.06 | 1.40 | 0.00 | 1.40 | 0.23 | 2.97 | 0.08 | 2.70 | 0.08 | 1.69 | 0.01 |
| 42 | 3.20 | 0.03 | 4.67 | 0.06 | 1.19 | 0.03 | 1.59 | 0.02 | 1.34 | 0.05 | 3.23 | 0.04 | 3.06 | 0.10 | 1.19 | 0.05 |
| 43 | 3.92 | 0.02 | 3.40 | 0.19 | 1.49 | 0.05 | 2.16 | 0.01 | 1.60 | 0.09 | 2.91 | 0.20 | 3.04 | 0.07 | 2.14 | 0.08 |
| 44 | 3.10 | 0.05 | 2.83 | 0.02 | 1.46 | 0.03 | 1.13 | 0.00 | 1.30 | 0.19 | 3.22 | 0.48 | 3.11 | 0.06 | 1.09 | 0.07 |
| 45 | 3.74 | 0.05 | 3.81 | 0.05 | 1.42 | 0.04 | 1.08 | 0.01 | 0.65 | 0.26 | 3.31 | 0.16 | 3.48 | 0.12 | 0.84 | 0.06 |
| 46 | 4.97 | 0.08 | 2.78 | 0.08 | 1.18 | 0.04 | 2.27 | 0.02 | 1.62 | 0.21 | 2.96 | 0.12 | 2.85 | 0.03 | 1.50 | 0.03 |
| 47 | 4.25 | 0.08 | 3.38 | 0.09 | 1.92 | 0.06 | 1.48 | 0.00 | 1.38 | 0.30 | 4.31 | 0.12 | 3.08 | 0.08 | 1.50 | 0.02 |
| 48 | 3.81 | 0.05 | 3.56 | 0.07 | 1.70 | 0.05 | 2.05 | 0.00 | 1.80 | 0.22 | 3.52 | 0.09 | 3.11 | 0.07 | 1.99 | 0.01 |
| 49 | 4.22 | 0.08 | 4.18 | 0.07 | 1.39 | 0.05 | 1.70 | 0.01 | 1.65 | 0.16 | 2.95 | 0.04 | 2.82 | 0.02 | 1.98 | 0.02 |
| 50 | 3.72 | 0.07 | 3.05 | 0.03 | 1.28 | 0.04 | 1.63 | 0.02 | 1.57 | 0.19 | 3.19 | 0.06 | 4.53 | 0.38 | 1.65 | 0.01 |
| 51 | 5.18 | 0.10 | 2.80 | 0.12 | 1.05 | 0.04 | 1.63 | 0.01 | 1.71 | 0.23 | 2.91 | 0.09 | 2.86 | 0.21 | 1.40 | 0.04 |
| 52 | 4.55 | 0.09 | 2.59 | 0.01 | 1.24 | 0.04 | 1.55 | 0.01 | 1.27 | 0.33 | 3.63 | 0.46 | 2.63 | 0.06 | 1.15 | 0.05 |
| 53 | 3.80 | 0.08 | 2.77 | 0.08 | 1.08 | 0.04 | 1.56 | 0.02 | 1.51 | 0.36 | 3.44 | 0.33 | 2.80 | 0.06 | 1.41 | 0.09 |
| 54 | 4.32 | 0.08 | 2.89 | 0.04 | 1.24 | 0.02 | 3.70 | 0.03 | 1.82 | 0.07 | 4.05 | 0.92 | 3.15 | 0.45 | 1.90 | 0.01 |
| 55 | 4.32 | 0.06 | 3.36 | 0.10 | 2.00 | 0.06 | 2.50 | 0.00 | 1.60 | 0.30 | 3.12 | 0.06 | 2.53 | 0.09 | 2.09 | 0.03 |
| 56 | 7.91 | 0.31 | 4.26 | 0.19 | 2.40 | 0.08 | 2.70 | 0.02 | 3.52 | 0.34 | 3.17 | 0.09 | 3.27 | 0.18 | 0.77 | 0.01 |
| 57 | 4.67 | 0.05 | 2.91 | 0.06 | 1.43 | 0.07 | 1.89 | 0.03 | 2.07 | 0.41 | 3.10 | 0.19 | 2.18 | 0.03 | 1.03 | 0.04 |
| 58 | 5.13 | 0.12 | 2.50 | 0.10 | 1.86 | 0.06 | 1.40 | 0.00 | 1.89 | 0.31 | 2.90 | 0.01 | 2.30 | 0.08 | 1.17 | 0.02 |
| 59 | 3.14 | 0.05 | 2.50 | 0.00 | 1.79 | 0.54 | 1.22 | 0.01 | 1.45 | 0.25 | 3.27 | 0.03 | 2.17 | 0.03 | 1.03 | 0.03 |
| 60 | 4.01 | 0.06 | 3.07 | 0.06 | 1.76 | 0.07 | 1.80 | 0.00 | 1.79 | 0.50 | 3.01 | 0.02 | 2.97 | 0.25 | 1.29 | 0.06 |
| 61 | 3.27 | 0.05 | 2.65 | 0.05 | 1.22 | 0.05 | 1.13 | 0.00 | 1.10 | 0.27 | 3.15 | 0.09 | 2.18 | 0.01 | 0.98 | 0.04 |
| 62 | 3.36 | 0.05 | 2.52 | 0.06 | 1.17 | 0.03 | 1.27 | 0.01 | 1.30 | 0.22 | 3.24 | 0.32 | 2.44 | 0.05 | 0.79 | 0.03 |
| 63 | 3.27 | 0.06 | 2.42 | 0.15 | 2.13 | 0.06 | 1.25 | 0.01 | 1.26 | 0.25 | 3.00 | 0.10 | 2.40 | 0.06 | 1.20 | 0.01 |
| 64 | 2.74 | 0.03 | 2.50 | 0.02 | 1.23 | 0.05 | 1.67 | 0.02 | 1.40 | 0.27 | 6.01 | 0.81 | 2.53 | 0.03 | 1.36 | 0.01 |
| 65 | 2.96 | 0.05 | 2.42 | 0.08 | 1.44 | 0.07 | 1.38 | 0.00 | 2.33 | 0.20 | 3.01 | 0.10 | 2.76 | 0.38 | 1.31 | 0.00 |
| 66 | 3.95 | 0.07 | 3.60 | 0.03 | 1.42 | 0.04 | 1.70 | 0.01 | 1.51 | 0.39 | 3.80 | 0.48 | 2.64 | 0.18 | 2.06 | 0.01 |
| Ca | ||||||||||||||||
| 30 | 41.91 | 1.08 | 42.29 | 1.06 | 44.41 | 0.79 | 44.88 | 0.97 | 48.40 | 0.45 | 42.24 | 1.77 | 28.51 | 3.11 | 45.84 | 3.19 |
| 31 | 42.82 | 1.47 | 37.45 | 0.56 | 38.26 | 0.13 | 35.50 | 0.37 | 40.76 | 0.28 | 48.77 | 11.69 | 23.85 | 2.92 | 38.32 | 3.06 |
| 32 | 68.68 | 1.79 | 60.51 | 0.75 | 61.98 | 0.28 | 60.98 | 0.32 | 66.57 | 0.45 | 60.26 | 4.82 | 40.69 | 1.91 | 62.29 | 3.42 |
| 33 | 74.96 | 2.21 | 65.13 | 1.38 | 61.41 | 0.79 | 65.08 | 0.47 | 66.38 | 0.12 | 52.34 | 1.21 | 43.46 | 3.50 | 64.40 | 3.72 |
| 34 | 60.63 | 0.90 | 51.23 | 1.11 | 50.64 | 0.40 | 54.08 | 0.11 | 57.80 | 0.23 | 52.82 | 0.28 | 36.66 | 3.84 | 56.53 | 4.16 |
| 35 | 58.98 | 2.22 | 53.74 | 0.64 | 52.20 | 1.77 | 59.87 | 0.05 | 59.47 | 0.74 | 51.68 | 1.00 | 39.28 | 3.59 | 59.14 | 2.84 |
| 36 | 64.53 | 1.66 | 58.21 | 1.20 | 58.70 | 1.04 | 56.72 | 0.63 | 60.65 | 0.54 | 50.69 | 1.10 | 37.16 | 2.06 | 64.20 | 4.66 |
| 37 | 62.57 | 1.55 | 54.46 | 0.26 | 56.03 | 1.47 | 54.86 | 0.03 | 57.65 | 0.69 | 59.65 | 2.12 | 34.74 | 2.93 | 58.96 | 4.03 |
| 38 | 80.00 | 2.43 | 66.24 | 0.80 | 65.78 | 1.33 | 63.07 | 0.94 | 66.84 | 1.01 | 59.99 | 1.18 | 42.20 | 4.37 | 67.33 | 3.41 |
| 39 | 74.87 | 1.96 | 64.70 | 1.44 | 62.38 | 1.25 | 64.08 | 0.58 | 68.63 | 0.44 | 36.38 | 1.38 | 43.95 | 3.87 | 66.26 | 3.79 |
| 40 | 48.93 | 0.83 | 41.07 | 1.27 | 43.11 | 0.54 | 39.32 | 0.62 | 41.73 | 0.37 | 37.61 | 0.83 | 24.93 | 2.71 | 40.47 | 5.22 |
| 41 | 45.69 | 1.50 | 40.54 | 1.47 | 44.70 | 1.21 | 41.04 | 0.15 | 42.74 | 0.21 | 33.57 | 0.37 | 23.70 | 3.73 | 41.24 | 4.38 |
| 42 | 42.91 | 1.53 | 33.79 | 0.95 | 37.32 | 0.32 | 36.20 | 0.78 | 37.51 | 0.48 | 51.95 | 1.28 | 22.55 | 2.36 | 41.72 | 4.35 |
| 43 | 60.65 | 0.90 | 54.09 | 2.10 | 53.74 | 0.91 | 54.99 | 0.17 | 56.31 | 0.46 | 42.87 | 1.14 | 33.25 | 3.26 | 54.14 | 3.87 |
| 44 | 62.13 | 0.84 | 49.13 | 1.90 | 50.78 | 0.97 | 44.56 | 0.06 | 49.36 | 0.28 | 34.13 | 1.37 | 35.90 | 0.67 | 47.42 | 4.15 |
| 45 | 56.79 | 0.69 | 50.37 | 0.30 | 52.27 | 1.93 | 38.03 | 0.40 | 39.79 | 0.24 | 54.52 | 2.05 | 23.28 | 1.99 | 40.26 | 4.39 |
| 46 | 63.45 | 1.17 | 58.76 | 1.50 | 61.44 | 0.36 | 59.75 | 0.32 | 60.64 | 0.45 | 49.99 | 11.43 | 35.86 | 1.95 | 58.25 | 3.25 |
| 47 | 67.15 | 1.27 | 55.49 | 1.38 | 57.60 | 0.82 | 58.00 | 0.26 | 61.83 | 0.12 | 70.32 | 1.17 | 36.21 | 3.46 | 67.96 | 4.95 |
| 48 | 79.41 | 1.65 | 73.43 | 2.11 | 72.18 | 1.95 | 70.35 | 0.24 | 77.84 | 0.12 | 60.36 | 2.47 | 46.00 | 10.38 | 85.44 | 5.62 |
| 49 | 69.91 | 1.86 | 62.80 | 1.57 | 64.65 | 1.61 | 62.52 | 0.48 | 65.58 | 0.24 | 40.15 | 1.87 | 41.18 | 2.33 | 63.96 | 4.08 |
| 50 | 55.88 | 1.62 | 45.80 | 0.29 | 49.33 | 1.05 | 46.51 | 0.45 | 44.39 | 0.29 | 45.03 | 0.93 | 27.33 | 2.06 | 49.25 | 3.86 |
| 51 | 66.04 | 2.80 | 57.20 | 0.48 | 53.60 | 0.83 | 55.01 | 0.33 | 53.45 | 0.34 | 38.59 | 0.72 | 32.03 | 2.03 | 53.70 | 3.45 |
| 52 | 67.54 | 2.08 | 58.00 | 0.96 | 59.44 | 1.38 | 50.64 | 0.02 | 54.67 | 0.20 | 69.84 | 4.49 | 30.54 | 2.93 | 51.24 | 2.28 |
| 53 | 45.23 | 1.21 | 38.35 | 0.88 | 41.48 | 0.01 | 35.96 | 0.18 | 42.20 | 0.41 | 82.94 | 1.47 | 21.38 | 2.62 | 37.34 | 3.97 |
| 54 | 74.02 | 3.05 | 66.09 | 2.30 | 65.59 | 2.52 | 52.39 | 0.87 | 52.21 | 0.74 | 80.67 | 3.07 | 32.32 | 2.12 | 103.40 | 6.86 |
| 55 | 105.12 | 1.94 | 89.64 | 2.89 | 89.93 | 3.06 | 85.99 | 0.47 | 84.64 | 0.37 | 56.24 | 0.23 | 55.79 | 2.49 | 96.26 | 6.60 |
| 56 | 99.29 | 2.83 | 88.01 | 3.74 | 89.85 | 2.02 | 81.86 | 1.45 | 89.59 | 0.36 | 38.69 | 1.38 | 59.09 | 3.34 | 63.09 | 4.39 |
| 57 | 81.69 | 1.77 | 71.25 | 2.80 | 69.00 | 0.65 | 72.67 | 0.16 | 81.57 | 0.27 | 32.71 | 0.86 | 48.76 | 2.36 | 54.60 | 3.65 |
| 58 | 81.32 | 1.31 | 62.68 | 1.39 | 64.16 | 2.17 | 53.54 | 0.10 | 53.91 | 0.46 | 29.36 | 0.93 | 32.50 | 3.27 | 49.88 | 3.29 |
| 59 | 57.17 | 0.31 | 48.20 | 2.44 | 51.91 | 1.86 | 45.45 | 0.14 | 48.50 | 0.10 | 52.37 | 2.64 | 25.99 | 2.12 | 61.90 | 3.71 |
| 60 | 60.23 | 0.80 | 54.49 | 2.54 | 55.24 | 1.03 | 60.15 | 0.13 | 59.60 | 0.06 | 42.04 | 2.30 | 34.28 | 3.09 | 28.20 | 2.48 |
| 61 | 34.65 | 0.36 | 29.11 | 0.80 | 30.53 | 0.91 | 30.38 | 0.05 | 30.66 | 0.25 | 51.40 | 1.39 | 16.77 | 1.81 | 34.10 | 2.49 |
| 62 | 32.29 | 0.30 | 31.23 | 0.89 | 34.16 | 0.05 | 34.27 | 0.20 | 34.05 | 0.85 | 67.71 | 2.84 | 20.29 | 1.85 | 40.39 | 3.32 |
| 63 | 35.55 | 0.37 | 32.74 | 0.04 | 35.78 | 0.40 | 40.01 | 1.71 | 41.73 | 0.73 | 67.21 | 1.09 | 24.12 | 2.33 | 64.69 | 2.73 |
| 64 | 46.29 | 0.04 | 44.25 | 1.34 | 44.97 | 0.30 | 58.43 | 0.63 | 59.39 | 0.30 | 75.38 | 2.90 | 35.99 | 3.32 | 91.98 | 5.36 |
| 65 | 53.63 | 0.56 | 50.78 | 0.97 | 52.01 | 0.38 | 83.59 | 2.20 | 80.52 | 0.42 | 80.27 | 0.72 | 58.47 | 1.65 | 92.53 | 7.69 |
| 66 | 42.05 | 0.23 | 35.25 | 0.62 | 39.86 | 0.21 | 86.52 | 0.41 | 83.68 | 0.14 | 50.94 | 1.61 | 60.38 | 2.39 | 96.92 | 6.09 |
| Mg | ||||||||||||||||
| 30 | 5.07 | 0.05 | 9.24 | 0.33 | 8.02 | 0.71 | 8.48 | 0.50 | 10.15 | 0.64 | 7.64 | 0.36 | 6.81 | 1.30 | 7.88 | 0.09 |
| 31 | 5.15 | 0.03 | 5.36 | 0.08 | 5.21 | 0.42 | 5.18 | 0.24 | 6.55 | 0.38 | 4.28 | 1.18 | 4.46 | 0.64 | 5.22 | 0.05 |
| 32 | 4.61 | 0.11 | 4.18 | 0.19 | 3.51 | 0.43 | 6.78 | 0.57 | 9.50 | 0.45 | 5.17 | 1.52 | 6.04 | 0.55 | 6.67 | 0.23 |
| 33 | 4.89 | 0.10 | 3.67 | 0.12 | 4.56 | 0.39 | 3.18 | 0.09 | 3.46 | 0.48 | 8.79 | 0.49 | 2.63 | 0.21 | 3.18 | 0.15 |
| 34 | 9.36 | 0.13 | 9.15 | 0.37 | 9.43 | 0.54 | 8.87 | 0.68 | 11.41 | 0.21 | 8.86 | 0.08 | 8.43 | 1.20 | 8.62 | 0.06 |
| 35 | 17.66 | 0.00 | 13.94 | 0.08 | 12.49 | 0.76 | 8.52 | 0.63 | 11.48 | 0.21 | 7.79 | 0.07 | 7.46 | 0.70 | 8.61 | 0.09 |
| 36 | 8.49 | 0.30 | 8.97 | 0.24 | 8.55 | 0.61 | 7.85 | 0.69 | 9.49 | 0.49 | 8.42 | 0.44 | 6.97 | 0.52 | 8.40 | 0.10 |
| 37 | 10.47 | 0.21 | 9.27 | 0.21 | 8.92 | 0.51 | 7.78 | 0.66 | 12.44 | 0.45 | 5.14 | 0.17 | 7.03 | 0.86 | 8.28 | 0.09 |
| 38 | 5.37 | 0.16 | 5.40 | 0.09 | 4.30 | 0.35 | 5.92 | 0.35 | 6.13 | 0.11 | 2.89 | 0.07 | 4.52 | 0.61 | 6.90 | 0.35 |
| 39 | 4.80 | 0.12 | 3.84 | 0.06 | 4.13 | 0.36 | 3.28 | 0.12 | 4.36 | 2.18 | 6.13 | 0.26 | 2.71 | 0.31 | 2.80 | 0.10 |
| 40 | 6.39 | 0.01 | 7.20 | 0.39 | 6.38 | 0.55 | 6.35 | 0.38 | 8.57 | 0.11 | 5.54 | 0.27 | 4.75 | 0.61 | 6.26 | 0.07 |
| 41 | 11.82 | 0.03 | 9.88 | 0.19 | 9.65 | 0.32 | 6.96 | 0.61 | 7.44 | 0.05 | 7.58 | 0.27 | 4.51 | 0.91 | 5.55 | 0.06 |
| 42 | 7.93 | 0.22 | 8.54 | 0.36 | 6.61 | 0.52 | 7.95 | 0.61 | 10.42 | 0.31 | 7.76 | 0.62 | 5.92 | 0.93 | 6.98 | 0.07 |
| 43 | 9.43 | 0.21 | 9.34 | 0.58 | 9.07 | 0.49 | 7.87 | 0.68 | 10.44 | 0.15 | 6.76 | 0.30 | 6.70 | 0.91 | 7.05 | 0.07 |
| 44 | 7.00 | 0.19 | 7.11 | 0.43 | 7.11 | 0.69 | 7.04 | 0.65 | 9.37 | 0.41 | 8.23 | 0.56 | 8.75 | 0.30 | 5.82 | 0.07 |
| 45 | 9.92 | 0.02 | 9.16 | 0.27 | 9.21 | 0.43 | 8.53 | 0.61 | 9.63 | 0.44 | 8.36 | 0.21 | 9.32 | 0.87 | 7.71 | 0.18 |
| 46 | 9.26 | 0.26 | 7.32 | 0.57 | 7.32 | 0.68 | 8.97 | 0.68 | 11.60 | 0.29 | 7.14 | 1.94 | 7.83 | 0.61 | 8.94 | 0.12 |
| 47 | 11.80 | 0.33 | 9.74 | 0.15 | 9.37 | 0.46 | 8.30 | 0.67 | 11.15 | 0.66 | 7.79 | 0.26 | 7.85 | 0.94 | 9.07 | 0.21 |
| 48 | 9.21 | 0.31 | 8.54 | 0.51 | 7.77 | 0.56 | 7.26 | 0.60 | 10.42 | 0.39 | 8.23 | 0.34 | 7.84 | 0.46 | 8.66 | 0.33 |
| 49 | 10.43 | 0.29 | 10.49 | 0.41 | 8.56 | 0.49 | 7.98 | 0.65 | 11.53 | 0.17 | 7.88 | 0.49 | 8.70 | 0.44 | 8.00 | 0.30 |
| 50 | 12.51 | 0.25 | 12.53 | 0.04 | 9.81 | 0.23 | 8.85 | 0.64 | 11.53 | 0.22 | 8.63 | 0.26 | 19.10 | 1.25 | 9.99 | 0.13 |
| 51 | 10.94 | 0.35 | 9.40 | 0.04 | 8.94 | 0.37 | 9.01 | 0.64 | 11.21 | 0.77 | 7.50 | 0.20 | 7.43 | 0.89 | 8.60 | 0.07 |
| 52 | 9.36 | 0.31 | 7.06 | 0.15 | 7.28 | 0.60 | 7.98 | 0.65 | 9.39 | 0.12 | 8.58 | 0.67 | 6.67 | 1.01 | 9.77 | 0.05 |
| 53 | 7.18 | 0.09 | 6.29 | 0.21 | 7.50 | 0.49 | 6.96 | 0.63 | 8.47 | 0.55 | 4.73 | 0.32 | 6.35 | 0.94 | 8.47 | 0.13 |
| 54 | 7.28 | 0.28 | 5.68 | 0.65 | 4.68 | 0.29 | 11.89 | 1.14 | 8.32 | 0.31 | 7.01 | 0.84 | 7.93 | 0.74 | 7.98 | 0.39 |
| 55 | 9.56 | 0.35 | 7.95 | 0.31 | 6.65 | 0.61 | 6.04 | 0.35 | 7.52 | 0.42 | 9.76 | 0.24 | 4.90 | 0.37 | 13.94 | 0.19 |
| 56 | 19.33 | 0.99 | 15.68 | 0.85 | 12.44 | 0.60 | 11.52 | 1.13 | 21.49 | 0.25 | 11.30 | 0.37 | 12.27 | 1.74 | 7.71 | 0.25 |
| 57 | 5.99 | 0.25 | 4.73 | 0.14 | 5.01 | 0.31 | 6.24 | 0.29 | 7.48 | 0.23 | 9.53 | 0.33 | 4.87 | 0.30 | 9.34 | 0.05 |
| 58 | 11.68 | 0.57 | 11.44 | 0.58 | 9.56 | 0.36 | 9.36 | 0.58 | 13.26 | 0.62 | 9.22 | 0.28 | 8.47 | 1.24 | 6.84 | 0.03 |
| 59 | 7.75 | 0.25 | 7.58 | 0.45 | 6.28 | 0.43 | 7.26 | 0.68 | 9.41 | 0.24 | 8.77 | 0.40 | 5.61 | 0.84 | 9.17 | 0.06 |
| 60 | 10.62 | 0.26 | 9.76 | 0.32 | 8.43 | 0.34 | 9.50 | 0.56 | 13.99 | 0.82 | 6.52 | 0.37 | 8.46 | 1.10 | 9.05 | 0.19 |
| 61 | 12.95 | 0.21 | 10.23 | 0.32 | 8.25 | 0.55 | 9.84 | 0.73 | 14.14 | 0.70 | 8.55 | 0.28 | 8.36 | 1.11 | 9.61 | 0.11 |
| 62 | 11.61 | 0.16 | 12.63 | 0.31 | 9.30 | 0.15 | 11.09 | 0.95 | 18.74 | 0.19 | 5.14 | 0.26 | 10.19 | 1.28 | 12.46 | 0.16 |
| 63 | 15.59 | 0.30 | 12.83 | 0.13 | 10.27 | 0.14 | 12.66 | 1.21 | 21.60 | 0.36 | 4.85 | 0.10 | 11.65 | 1.57 | 10.55 | 0.08 |
| 64 | 14.87 | 0.26 | 13.28 | 0.55 | 10.76 | 0.05 | 10.87 | 0.94 | 15.53 | 0.41 | 11.24 | 0.59 | 11.67 | 1.83 | 6.97 | 0.25 |
| 65 | 17.88 | 0.54 | 16.19 | 0.17 | 14.73 | 2.44 | 7.63 | 0.59 | 10.64 | 0.53 | 5.31 | 0.11 | 8.39 | 0.98 | 4.42 | 0.10 |
| 66 | 12.84 | 0.21 | 9.73 | 0.15 | 11.07 | 1.14 | 4.79 | 0.16 | 5.51 | 0.36 | 5.80 | 0.56 | 5.66 | 1.00 | 12.18 | 0.43 |
| YEAR | 2019 | 2020 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TERM | I | II | III | IV | V | VI | VII | VIII | ||||||||
| X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | |
| Cu | ||||||||||||||||
| 30 | 0.066 | 0.015 | 0.079 | 0.011 | 0.039 | 0.010 | 0.060 | 0.003 | 0.082 | 0.002 | 0.028 | 0.005 | 0.041 | 0.016 | 0.087 | 0.005 |
| 31 | 0.077 | 0.001 | 0.059 | 0.007 | 0.015 | 0.002 | 0.043 | 0.016 | 0.024 | 0.003 | 0.030 | 0.008 | 0.047 | 0.025 | 0.048 | 0.006 |
| 32 | 0.058 | 0.009 | 0.049 | 0.014 | 0.011 | 0.002 | 0.038 | 0.028 | 0.024 | 0.003 | 0.026 | 0.006 | 0.026 | 0.002 | 0.058 | 0.008 |
| 33 | 0.067 | 0.008 | 0.056 | 0.004 | 0.027 | 0.012 | 0.044 | 0.015 | 0.025 | 0.003 | 0.022 | 0.003 | 0.027 | 0.007 | 0.038 | 0.011 |
| 34 | 0.056 | 0.013 | 0.053 | 0.012 | 0.018 | 0.005 | 0.039 | 0.021 | 0.014 | 0.001 | 0.026 | 0.006 | 0.040 | 0.013 | 0.053 | 0.009 |
| 35 | 0.064 | 0.001 | 0.052 | 0.006 | 0.015 | 0.000 | 0.046 | 0.025 | 0.045 | 0.002 | 0.026 | 0.005 | 0.031 | 0.008 | 0.072 | 0.001 |
| 36 | 0.049 | 0.018 | 0.053 | 0.010 | 0.015 | 0.003 | 0.027 | 0.027 | 0.026 | 0.001 | 0.031 | 0.001 | 0.028 | 0.005 | 0.054 | 0.024 |
| 37 | 0.051 | 0.023 | 0.058 | 0.010 | 0.016 | 0.000 | 0.028 | 0.022 | 0.007 | 0.000 | 0.032 | 0.002 | 0.034 | 0.028 | 0.054 | 0.018 |
| 38 | 0.049 | 0.001 | 0.061 | 0.006 | 0.018 | 0.000 | 0.032 | 0.024 | 0.010 | 0.007 | 0.016 | 0.006 | 0.034 | 0.009 | 0.080 | 0.006 |
| 39 | 0.053 | 0.014 | 0.050 | 0.014 | 0.038 | 0.006 | 0.037 | 0.019 | 0.016 | 0.002 | 0.034 | 0.011 | 0.041 | 0.004 | 0.055 | 0.003 |
| 40 | 0.059 | 0.009 | 0.060 | 0.006 | 0.027 | 0.001 | 0.042 | 0.012 | 0.016 | 0.003 | 0.032 | 0.004 | 0.031 | 0.016 | 0.061 | 0.007 |
| 41 | 0.069 | 0.009 | 0.048 | 0.013 | 0.022 | 0.001 | 0.029 | 0.011 | 0.038 | 0.002 | 0.032 | 0.009 | 0.037 | 0.002 | 0.052 | 0.013 |
| 42 | 0.055 | 0.001 | 0.060 | 0.012 | 0.032 | 0.001 | 0.042 | 0.020 | 0.023 | 0.003 | 0.025 | 0.011 | 0.029 | 0.008 | 0.050 | 0.006 |
| 43 | 0.067 | 0.006 | 0.054 | 0.016 | 0.030 | 0.006 | 0.034 | 0.016 | 0.014 | 0.003 | 0.021 | 0.003 | 0.027 | 0.016 | 0.062 | 0.011 |
| 44 | 0.056 | 0.013 | 0.059 | 0.009 | 0.023 | 0.003 | 0.032 | 0.023 | 0.022 | 0.016 | 0.029 | 0.002 | 0.033 | 0.007 | 0.030 | 0.019 |
| 45 | 0.055 | 0.009 | 0.055 | 0.007 | 0.030 | 0.003 | 0.047 | 0.022 | 0.015 | 0.003 | 0.031 | 0.006 | 0.034 | 0.011 | 0.046 | 0.001 |
| 46 | 0.059 | 0.012 | 0.055 | 0.005 | 0.034 | 0.001 | 0.012 | 0.005 | 0.011 | 0.008 | 0.027 | 0.018 | 0.027 | 0.008 | 0.049 | 0.007 |
| 47 | 0.062 | 0.017 | 0.058 | 0.010 | 0.027 | 0.004 | 0.011 | 0.011 | 0.024 | 0.018 | 0.018 | 0.007 | 0.025 | 0.009 | 0.049 | 0.001 |
| 48 | 0.043 | 0.011 | 0.046 | 0.015 | 0.015 | 0.005 | 0.027 | 0.024 | 0.009 | 0.009 | 0.021 | 0.013 | 0.039 | 0.010 | 0.060 | 0.007 |
| 49 | 0.052 | 0.013 | 0.049 | 0.004 | 0.024 | 0.003 | 0.032 | 0.025 | 0.023 | 0.002 | 0.030 | 0.009 | 0.027 | 0.008 | 0.042 | 0.002 |
| 50 | 0.058 | 0.000 | 0.054 | 0.012 | 0.033 | 0.002 | 0.017 | 0.003 | 0.017 | 0.001 | 0.017 | 0.007 | 0.035 | 0.003 | 0.074 | 0.003 |
| 51 | 0.061 | 0.006 | 0.063 | 0.015 | 0.014 | 0.003 | 0.011 | 0.006 | 0.006 | 0.005 | 0.023 | 0.005 | 0.027 | 0.005 | 0.050 | 0.011 |
| 52 | 0.054 | 0.000 | 0.052 | 0.011 | 0.017 | 0.005 | 0.036 | 0.017 | 0.027 | 0.003 | 0.023 | 0.009 | 0.025 | 0.006 | 0.052 | 0.013 |
| 53 | 0.064 | 0.002 | 0.050 | 0.017 | 0.026 | 0.007 | 0.021 | 0.004 | 0.014 | 0.001 | 0.015 | 0.004 | 0.023 | 0.013 | 0.063 | 0.007 |
| 54 | 0.053 | 0.000 | 0.056 | 0.012 | 0.031 | 0.003 | 0.043 | 0.013 | 0.024 | 0.004 | 0.021 | 0.005 | 0.041 | 0.011 | 0.057 | 0.011 |
| 55 | 0.041 | 0.018 | 0.036 | 0.004 | 0.023 | 0.003 | 0.032 | 0.029 | 0.017 | 0.002 | 0.029 | 0.002 | 0.020 | 0.012 | 0.055 | 0.015 |
| 56 | 0.056 | 0.013 | 0.065 | 0.023 | 0.013 | 0.011 | 0.033 | 0.027 | 0.017 | 0.000 | 0.024 | 0.003 | 0.036 | 0.009 | 0.052 | 0.004 |
| 57 | 0.053 | 0.001 | 0.058 | 0.006 | 0.030 | 0.013 | 0.041 | 0.029 | 0.027 | 0.004 | 0.030 | 0.003 | 0.040 | 0.011 | 0.050 | 0.013 |
| 58 | 0.051 | 0.011 | 0.053 | 0.009 | 0.023 | 0.007 | 0.017 | 0.015 | 0.016 | 0.011 | 0.009 | 0.008 | 0.014 | 0.006 | 0.058 | 0.003 |
| 59 | 0.063 | 0.002 | 0.049 | 0.012 | 0.016 | 0.004 | 0.034 | 0.022 | 0.011 | 0.010 | 0.018 | 0.009 | 0.025 | 0.021 | 0.055 | 0.000 |
| 60 | 0.053 | 0.012 | 0.037 | 0.011 | 0.027 | 0.001 | 0.022 | 0.017 | 0.025 | 0.002 | 0.020 | 0.002 | 0.030 | 0.012 | 0.067 | 0.018 |
| 61 | 0.029 | 0.020 | 0.057 | 0.005 | 0.029 | 0.011 | 0.024 | 0.020 | 0.022 | 0.003 | 0.018 | 0.007 | 0.025 | 0.003 | 0.057 | 0.008 |
| 62 | 0.049 | 0.012 | 0.052 | 0.003 | 0.016 | 0.002 | 0.035 | 0.017 | 0.016 | 0.002 | 0.015 | 0.007 | 0.027 | 0.007 | 0.058 | 0.006 |
| 63 | 0.049 | 0.002 | 0.062 | 0.005 | 0.028 | 0.001 | 0.025 | 0.017 | 0.025 | 0.005 | 0.015 | 0.003 | 0.032 | 0.012 | 0.038 | 0.012 |
| 64 | 0.044 | 0.008 | 0.054 | 0.007 | 0.042 | 0.006 | 0.063 | 0.006 | 0.009 | 0.006 | 0.025 | 0.008 | 0.020 | 0.003 | 0.057 | 0.013 |
| 65 | 0.046 | 0.014 | 0.032 | 0.002 | 0.023 | 0.008 | 0.015 | 0.007 | 0.022 | 0.002 | 0.015 | 0.010 | 0.038 | 0.013 | 0.051 | 0.011 |
| 66 | 0.050 | 0.004 | 0.046 | 0.011 | 0.019 | 0.002 | 0.063 | 0.002 | 0.014 | 0.005 | 0.027 | 0.004 | 0.018 | 0.018 | 0.038 | 0.024 |
| Zn | ||||||||||||||||
| 30 | 0.028 | 0.002 | 0.021 | 0.004 | 0.036 | 0.011 | 0.036 | 0.004 | 0.069 | 0.001 | 0.030 | 0.005 | 0.022 | 0.004 | 0.035 | 0.003 |
| 31 | 0.016 | 0.000 | 0.019 | 0.003 | 0.025 | 0.012 | 0.033 | 0.005 | 0.054 | 0.007 | 0.021 | 0.017 | 0.029 | 0.003 | 0.023 | 0.003 |
| 32 | 0.013 | 0.003 | 0.006 | 0.002 | 0.021 | 0.012 | 0.020 | 0.001 | 0.046 | 0.001 | 0.009 | 0.004 | 0.010 | 0.003 | 0.015 | 0.000 |
| 33 | 0.007 | 0.002 | 0.011 | 0.002 | 0.021 | 0.010 | 0.014 | 0.001 | 0.046 | 0.002 | 0.016 | 0.002 | 0.002 | 0.002 | 0.005 | 0.000 |
| 34 | 0.016 | 0.003 | 0.019 | 0.001 | 0.031 | 0.011 | 0.023 | 0.000 | 0.046 | 0.002 | 0.017 | 0.002 | 0.017 | 0.004 | 0.019 | 0.004 |
| 35 | 0.016 | 0.006 | 0.012 | 0.003 | 0.025 | 0.011 | 0.025 | 0.000 | 0.046 | 0.004 | 0.021 | 0.002 | 0.015 | 0.001 | 0.020 | 0.001 |
| 36 | 0.014 | 0.003 | 0.014 | 0.000 | 0.025 | 0.010 | 0.021 | 0.003 | 0.044 | 0.003 | 0.018 | 0.001 | 0.012 | 0.002 | 0.017 | 0.001 |
| 37 | 0.020 | 0.003 | 0.015 | 0.002 | 0.023 | 0.012 | 0.024 | 0.002 | 0.055 | 0.003 | 0.010 | 0.003 | 0.018 | 0.002 | 0.020 | 0.004 |
| 38 | 0.012 | 0.001 | 0.009 | 0.001 | 0.021 | 0.011 | 0.021 | 0.000 | 0.045 | 0.002 | 0.009 | 0.001 | 0.009 | 0.001 | 0.011 | 0.000 |
| 39 | 0.009 | 0.005 | 0.013 | 0.001 | 0.028 | 0.013 | 0.019 | 0.001 | 0.043 | 0.002 | 0.029 | 0.002 | 0.007 | 0.001 | 0.015 | 0.002 |
| 40 | 0.015 | 0.003 | 0.022 | 0.001 | 0.034 | 0.009 | 0.032 | 0.006 | 0.055 | 0.002 | 0.026 | 0.002 | 0.027 | 0.001 | 0.021 | 0.007 |
| 41 | 0.017 | 0.003 | 0.022 | 0.003 | 0.030 | 0.012 | 0.038 | 0.003 | 0.053 | 0.004 | 0.035 | 0.004 | 0.033 | 0.003 | 0.034 | 0.024 |
| 42 | 0.023 | 0.000 | 0.025 | 0.001 | 0.039 | 0.009 | 0.041 | 0.003 | 0.053 | 0.002 | 0.015 | 0.002 | 0.029 | 0.003 | 0.026 | 0.020 |
| 43 | 0.019 | 0.002 | 0.014 | 0.004 | 0.034 | 0.011 | 0.021 | 0.002 | 0.043 | 0.002 | 0.019 | 0.003 | 0.013 | 0.001 | 0.026 | 0.007 |
| 44 | 0.011 | 0.000 | 0.015 | 0.002 | 0.027 | 0.010 | 0.027 | 0.001 | 0.036 | 0.003 | 0.030 | 0.003 | 0.022 | 0.002 | 0.023 | 0.013 |
| 45 | 0.018 | 0.002 | 0.017 | 0.002 | 0.032 | 0.012 | 0.040 | 0.005 | 0.035 | 0.004 | 0.015 | 0.002 | 0.031 | 0.004 | 0.034 | 0.014 |
| 46 | 0.011 | 0.001 | 0.012 | 0.003 | 0.029 | 0.012 | 0.024 | 0.003 | 0.034 | 0.004 | 0.013 | 0.001 | 0.010 | 0.002 | 0.023 | 0.005 |
| 47 | 0.019 | 0.002 | 0.018 | 0.002 | 0.031 | 0.010 | 0.022 | 0.002 | 0.028 | 0.001 | 0.009 | 0.001 | 0.013 | 0.003 | 0.022 | 0.011 |
| 48 | 0.015 | 0.000 | 0.014 | 0.002 | 0.031 | 0.012 | 0.017 | 0.003 | 0.033 | 0.002 | 0.009 | 0.001 | 0.013 | 0.001 | 0.023 | 0.009 |
| 49 | 0.015 | 0.000 | 0.013 | 0.006 | 0.027 | 0.011 | 0.016 | 0.002 | 0.026 | 0.004 | 0.021 | 0.002 | 0.013 | 0.004 | 0.021 | 0.007 |
| 50 | 0.014 | 0.003 | 0.020 | 0.004 | 0.032 | 0.011 | 0.028 | 0.001 | 0.027 | 0.002 | 0.019 | 0.001 | 0.028 | 0.002 | 0.027 | 0.009 |
| 51 | 0.010 | 0.004 | 0.016 | 0.004 | 0.028 | 0.012 | 0.022 | 0.001 | 0.036 | 0.003 | 0.020 | 0.001 | 0.014 | 0.003 | 0.029 | 0.011 |
| 52 | 0.011 | 0.003 | 0.016 | 0.002 | 0.022 | 0.010 | 0.020 | 0.002 | 0.026 | 0.004 | 0.009 | 0.002 | 0.017 | 0.003 | 0.032 | 0.017 |
| 53 | 0.009 | 0.001 | 0.024 | 0.003 | 0.034 | 0.011 | 0.037 | 0.003 | 0.035 | 0.002 | 0.007 | 0.001 | 0.036 | 0.003 | 0.034 | 0.026 |
| 54 | 0.013 | 0.001 | 0.015 | 0.001 | 0.028 | 0.012 | 0.027 | 0.001 | 0.027 | 0.001 | 0.008 | 0.002 | 0.017 | 0.006 | 0.019 | 0.009 |
| 55 | 0.004 | 0.002 | 0.012 | 0.001 | 0.026 | 0.013 | 0.013 | 0.002 | 0.027 | 0.002 | 0.011 | 0.002 | 0.005 | 0.003 | 0.020 | 0.011 |
| 56 | 0.011 | 0.000 | 0.016 | 0.004 | 0.024 | 0.012 | 0.014 | 0.001 | 0.023 | 0.005 | 0.026 | 0.002 | 0.003 | 0.002 | 0.023 | 0.010 |
| 57 | 0.006 | 0.001 | 0.015 | 0.002 | 0.027 | 0.013 | 0.013 | 0.002 | 0.036 | 0.003 | 0.036 | 0.000 | 0.008 | 0.002 | 0.025 | 0.015 |
| 58 | 0.013 | 0.001 | 0.036 | 0.004 | 0.039 | 0.009 | 0.027 | 0.003 | 0.036 | 0.002 | 0.040 | 0.003 | 0.020 | 0.003 | 0.021 | 0.011 |
| 59 | 0.014 | 0.001 | 0.019 | 0.000 | 0.027 | 0.012 | 0.027 | 0.002 | 0.034 | 0.003 | 0.013 | 0.001 | 0.033 | 0.003 | 0.023 | 0.009 |
| 60 | 0.012 | 0.004 | 0.017 | 0.002 | 0.034 | 0.012 | 0.019 | 0.000 | 0.033 | 0.001 | 0.022 | 0.000 | 0.015 | 0.001 | 0.030 | 0.012 |
| 61 | 0.046 | 0.007 | 0.060 | 0.004 | 0.077 | 0.010 | 0.059 | 0.006 | 0.055 | 0.004 | 0.020 | 0.002 | 0.049 | 0.001 | 0.040 | 0.018 |
| 62 | 0.046 | 0.004 | 0.037 | 0.002 | 0.074 | 0.009 | 0.048 | 0.004 | 0.043 | 0.002 | 0.009 | 0.002 | 0.041 | 0.003 | 0.027 | 0.017 |
| 63 | 0.027 | 0.003 | 0.038 | 0.004 | 0.055 | 0.011 | 0.033 | 0.000 | 0.055 | 0.002 | 0.006 | 0.001 | 0.024 | 0.003 | 0.030 | 0.008 |
| 64 | 0.024 | 0.001 | 0.030 | 0.003 | 0.069 | 0.010 | 0.016 | 0.001 | 0.044 | 0.003 | 0.003 | 0.003 | 0.014 | 0.006 | 0.018 | 0.016 |
| 65 | 0.016 | 0.002 | 0.021 | 0.002 | 0.038 | 0.011 | 0.013 | 0.002 | 0.043 | 0.001 | 0.004 | 0.001 | 0.009 | 0.005 | 0.026 | 0.020 |
| 66 | 0.028 | 0.001 | 0.036 | 0.003 | 0.039 | 0.012 | 0.015 | 0.001 | 0.037 | 0.002 | 0.006 | 0.000 | 0.012 | 0.004 | 0.025 | 0.013 |
| Mn | ||||||||||||||||
| 30 | 0.805 | 0.003 | 0.061 | 0.007 | 0.033 | 0.001 | 0.076 | 0.007 | 0.052 | 0.001 | 0.076 | 0.007 | 0.077 | 0.011 | 0.052 | 0.009 |
| 31 | 0.838 | 0.019 | 0.073 | 0.006 | 0.082 | 0.002 | 0.088 | 0.008 | 0.064 | 0.003 | 0.071 | 0.044 | 0.090 | 0.004 | 0.064 | 0.027 |
| 32 | 0.045 | 0.005 | 0.013 | 0.007 | 0.007 | 0.000 | 0.021 | 0.000 | 0.015 | 0.004 | 0.035 | 0.001 | 0.031 | 0.007 | 0.034 | 0.006 |
| 33 | 0.037 | 0.014 | 0.009 | 0.007 | 0.009 | 0.000 | 0.018 | 0.002 | 0.023 | 0.002 | 0.043 | 0.004 | 0.026 | 0.002 | 0.023 | 0.003 |
| 34 | 0.743 | 0.009 | 0.033 | 0.005 | 0.040 | 0.003 | 0.033 | 0.003 | 0.024 | 0.003 | 0.048 | 0.002 | 0.041 | 0.008 | 0.036 | 0.012 |
| 35 | 1.466 | 0.069 | 0.025 | 0.004 | 0.011 | 0.002 | 0.036 | 0.002 | 0.026 | 0.001 | 0.056 | 0.013 | 0.037 | 0.009 | 0.029 | 0.005 |
| 36 | 0.417 | 0.003 | 0.031 | 0.003 | 0.035 | 0.003 | 0.042 | 0.004 | 0.037 | 0.003 | 0.049 | 0.005 | 0.035 | 0.010 | 0.027 | 0.002 |
| 37 | 0.342 | 0.006 | 0.024 | 0.013 | 0.014 | 0.000 | 0.034 | 0.001 | 0.024 | 0.004 | 0.026 | 0.001 | 0.037 | 0.012 | 0.030 | 0.013 |
| 38 | 0.074 | 0.009 | 0.013 | 0.006 | 0.005 | 0.005 | 0.016 | 0.002 | 0.021 | 0.005 | 0.027 | 0.001 | 0.027 | 0.008 | 0.025 | 0.004 |
| 39 | 0.070 | 0.009 | 0.012 | 0.001 | 0.002 | 0.000 | 0.024 | 0.004 | 0.013 | 0.009 | 0.076 | 0.009 | 0.012 | 0.005 | 0.013 | 0.000 |
| 40 | 0.342 | 0.002 | 0.042 | 0.004 | 0.057 | 0.001 | 0.070 | 0.011 | 0.033 | 0.022 | 0.076 | 0.003 | 0.067 | 0.003 | 0.055 | 0.026 |
| 41 | 0.436 | 0.012 | 0.044 | 0.007 | 0.027 | 0.000 | 0.087 | 0.012 | 0.019 | 0.005 | 0.123 | 0.020 | 0.083 | 0.011 | 0.066 | 0.042 |
| 42 | 0.553 | 0.013 | 0.058 | 0.004 | 0.078 | 0.001 | 0.088 | 0.009 | 0.021 | 0.012 | 0.040 | 0.004 | 0.097 | 0.017 | 0.043 | 0.035 |
| 43 | 0.287 | 0.013 | 0.017 | 0.008 | 0.024 | 0.001 | 0.023 | 0.001 | 0.014 | 0.002 | 0.049 | 0.006 | 0.023 | 0.009 | 0.020 | 0.006 |
| 44 | 0.149 | 0.009 | 0.014 | 0.003 | 0.035 | 0.001 | 0.046 | 0.000 | 0.022 | 0.003 | 0.089 | 0.018 | 0.042 | 0.009 | 0.020 | 0.007 |
| 45 | 0.333 | 0.004 | 0.016 | 0.005 | 0.025 | 0.004 | 0.092 | 0.014 | 0.048 | 0.003 | 0.030 | 0.001 | 0.087 | 0.010 | 0.043 | 0.012 |
| 46 | 0.078 | 0.005 | 0.014 | 0.007 | 0.005 | 0.001 | 0.018 | 0.003 | 0.034 | 0.021 | 0.040 | 0.012 | 0.015 | 0.005 | 0.013 | 0.006 |
| 47 | 0.763 | 0.017 | 0.050 | 0.007 | 0.023 | 0.001 | 0.032 | 0.006 | 0.013 | 0.006 | 0.022 | 0.002 | 0.031 | 0.009 | 0.016 | 0.004 |
| 48 | 1.845 | 0.132 | 0.013 | 0.006 | 0.006 | 0.000 | 0.020 | 0.009 | 0.009 | 0.002 | 0.025 | 0.002 | 0.018 | 0.022 | 0.008 | 0.004 |
| 49 | 0.400 | 0.008 | 0.018 | 0.007 | 0.014 | 0.001 | 0.020 | 0.004 | 0.013 | 0.006 | 0.057 | 0.005 | 0.021 | 0.001 | 0.018 | 0.001 |
| 50 | 0.522 | 0.005 | 0.022 | 0.004 | 0.049 | 0.000 | 0.049 | 0.003 | 0.020 | 0.012 | 0.039 | 0.002 | 0.094 | 0.008 | 0.030 | 0.018 |
| 51 | 0.193 | 0.001 | 0.012 | 0.003 | 0.145 | 0.000 | 0.040 | 0.001 | 0.012 | 0.006 | 0.046 | 0.003 | 0.037 | 0.004 | 0.022 | 0.003 |
| 52 | 0.141 | 0.005 | 0.012 | 0.004 | 0.008 | 0.001 | 0.029 | 0.000 | 0.009 | 0.003 | 0.024 | 0.002 | 0.022 | 0.005 | 0.032 | 0.006 |
| 53 | 0.075 | 0.021 | 0.039 | 0.004 | 0.083 | 0.001 | 0.101 | 0.015 | 0.019 | 0.006 | 0.023 | 0.002 | 0.113 | 0.009 | 0.073 | 0.047 |
| 54 | 0.114 | 0.002 | 0.005 | 0.004 | 0.003 | 0.002 | 0.058 | 0.008 | 0.023 | 0.001 | 0.021 | 0.002 | 0.031 | 0.003 | 0.012 | 0.002 |
| 55 | 0.040 | 0.007 | 0.005 | 0.002 | 0.006 | 0.001 | 0.014 | 0.007 | 0.008 | 0.002 | 0.024 | 0.001 | 0.004 | 0.008 | 0.003 | 0.002 |
| 56 | 0.155 | 0.003 | 0.002 | 0.002 | 0.002 | 0.002 | 0.014 | 0.004 | 0.007 | 0.003 | 0.047 | 0.002 | 0.010 | 0.005 | 0.018 | 0.004 |
| 57 | 0.079 | 0.006 | 0.007 | 0.005 | 0.003 | 0.001 | 0.008 | 0.002 | 0.009 | 0.002 | 0.053 | 0.003 | 0.004 | 0.006 | 0.033 | 0.004 |
| 58 | 1.148 | 0.001 | 1.148 | 1.018 | 0.507 | 0.006 | 0.044 | 0.003 | 0.023 | 0.003 | 0.071 | 0.013 | 0.060 | 0.029 | 0.013 | 0.008 |
| 59 | 0.531 | 0.031 | 0.046 | 0.014 | 0.444 | 0.007 | 0.056 | 0.005 | 0.027 | 0.001 | 0.025 | 0.005 | 0.106 | 0.006 | 0.005 | 0.000 |
| 60 | 1.460 | 0.048 | 0.013 | 0.001 | 0.018 | 0.001 | 0.019 | 0.009 | 0.008 | 0.002 | 0.044 | 0.004 | 0.016 | 0.007 | 0.079 | 0.027 |
| 61 | 2.446 | 0.223 | 0.219 | 0.026 | 2.068 | 0.008 | 0.401 | 0.046 | 0.055 | 0.002 | 0.046 | 0.007 | 0.121 | 0.019 | 0.034 | 0.007 |
| 62 | 4.717 | 0.599 | 0.110 | 0.019 | 1.622 | 0.033 | 0.135 | 0.018 | 0.025 | 0.004 | 0.019 | 0.003 | 0.047 | 0.006 | 0.010 | 0.005 |
| 63 | 1.466 | 0.059 | 0.095 | 0.011 | 0.323 | 0.000 | 0.031 | 0.000 | 0.022 | 0.000 | 0.020 | 0.004 | 0.035 | 0.001 | 0.012 | 0.001 |
| 64 | 2.146 | 0.170 | 0.044 | 0.016 | 0.503 | 0.005 | 0.015 | 0.004 | 0.015 | 0.004 | 0.020 | 0.000 | 0.009 | 0.004 | 0.006 | 0.006 |
| 65 | 0.628 | 0.004 | 0.012 | 0.002 | 0.113 | 0.005 | 0.008 | 0.003 | 0.007 | 0.005 | 0.016 | 0.004 | 0.008 | 0.008 | 0.005 | 0.002 |
| 66 | 2.157 | 0.177 | 0.142 | 0.022 | 0.354 | 0.002 | 0.013 | 0.012 | 0.008 | 0.002 | 0.025 | 0.003 | 0.002 | 0.001 | 0.007 | 0.001 |
| Fe | ||||||||||||||||
| 30 | 0.191 | 0.021 | 2.858 | 0.105 | 1.992 | 0.076 | 3.662 | 0.466 | 2.149 | 0.048 | 3.862 | 0.021 | 3.842 | 0.267 | 2.151 | 1.343 |
| 31 | 0.166 | 0.015 | 1.805 | 0.146 | 1.536 | 0.121 | 3.903 | 0.420 | 2.154 | 0.121 | 2.453 | 2.094 | 3.806 | 0.191 | 2.348 | 1.553 |
| 32 | 0.151 | 0.004 | 0.324 | 0.018 | 1.098 | 0.210 | 0.933 | 0.147 | 0.446 | 0.009 | 0.677 | 0.366 | 0.898 | 0.044 | 0.754 | 0.418 |
| 33 | 0.137 | 0.033 | 0.177 | 0.037 | 1.027 | 0.197 | 0.348 | 0.065 | 0.340 | 0.021 | 2.196 | 0.118 | 0.379 | 0.021 | 0.303 | 0.074 |
| 34 | 0.141 | 0.034 | 1.626 | 0.163 | 1.797 | 0.127 | 2.007 | 0.249 | 1.131 | 0.012 | 2.568 | 0.197 | 2.365 | 0.155 | 1.765 | 1.233 |
| 35 | 0.145 | 0.038 | 0.650 | 0.057 | 1.696 | 0.232 | 2.031 | 0.229 | 1.160 | 0.160 | 2.992 | 0.261 | 2.124 | 0.426 | 1.345 | 0.877 |
| 36 | 0.176 | 0.014 | 1.165 | 0.136 | 1.252 | 0.107 | 2.372 | 0.226 | 1.261 | 0.295 | 2.785 | 0.369 | 2.572 | 0.027 | 1.649 | 1.156 |
| 37 | 0.206 | 0.022 | 0.920 | 0.173 | 1.175 | 0.073 | 2.276 | 0.264 | 2.144 | 0.085 | 0.534 | 0.062 | 2.144 | 0.136 | 1.453 | 0.963 |
| 38 | 0.100 | 0.001 | 0.221 | 0.032 | 0.718 | 0.152 | 0.492 | 0.065 | 0.354 | 0.044 | 0.544 | 0.037 | 0.538 | 0.066 | 0.635 | 0.319 |
| 39 | 0.105 | 0.007 | 0.184 | 0.044 | 0.870 | 0.163 | 0.495 | 0.122 | 0.363 | 0.031 | 3.795 | 0.090 | 0.559 | 0.093 | 0.353 | 0.134 |
| 40 | 0.166 | 0.017 | 2.096 | 0.056 | 1.500 | 0.114 | 3.518 | 0.399 | 2.423 | 0.404 | 3.111 | 0.124 | 2.628 | 0.130 | 2.653 | 1.604 |
| 41 | 0.411 | 0.016 | 2.685 | 0.171 | 1.621 | 0.103 | 3.725 | 0.370 | 1.574 | 0.306 | 4.749 | 0.520 | 2.628 | 0.031 | 3.165 | 1.932 |
| 42 | 0.220 | 0.016 | 2.834 | 0.165 | 1.588 | 0.105 | 4.306 | 0.403 | 3.380 | 0.405 | 1.779 | 0.091 | 4.091 | 0.441 | 2.749 | 1.801 |
| 43 | 0.112 | 0.006 | 0.703 | 0.047 | 1.366 | 0.150 | 1.163 | 0.188 | 0.769 | 0.023 | 2.688 | 0.259 | 1.198 | 0.024 | 1.060 | 0.752 |
| 44 | 0.131 | 0.008 | 1.094 | 0.134 | 1.508 | 0.181 | 2.589 | 0.340 | 1.226 | 0.204 | 3.558 | 0.908 | 1.497 | 0.054 | 1.758 | 1.283 |
| 45 | 0.129 | 0.005 | 1.223 | 0.031 | 1.442 | 0.115 | 3.636 | 0.546 | 1.520 | 0.366 | 1.401 | 0.314 | 4.233 | 0.167 | 1.832 | 1.148 |
| 46 | 0.135 | 0.008 | 0.325 | 0.026 | 0.867 | 0.156 | 1.063 | 0.157 | 0.651 | 0.040 | 1.900 | 0.330 | 1.055 | 0.028 | 0.915 | 0.691 |
| 47 | 0.121 | 0.026 | 1.105 | 0.055 | 1.219 | 0.154 | 1.604 | 0.176 | 0.778 | 0.018 | 0.206 | 0.023 | 1.415 | 0.087 | 0.514 | 0.298 |
| 48 | 0.121 | 0.007 | 0.176 | 0.013 | 0.578 | 0.061 | 0.339 | 0.083 | 0.127 | 0.009 | 0.664 | 0.004 | 0.588 | 0.689 | 0.173 | 0.028 |
| 49 | 0.128 | 0.011 | 0.333 | 0.056 | 0.642 | 0.090 | 0.534 | 0.108 | 0.329 | 0.036 | 2.570 | 0.036 | 0.535 | 0.102 | 0.800 | 0.467 |
| 50 | 0.130 | 0.005 | 1.481 | 0.024 | 1.136 | 0.096 | 2.633 | 0.272 | 1.312 | 0.380 | 0.989 | 0.133 | 5.279 | 0.559 | 1.155 | 0.777 |
| 51 | 0.115 | 0.014 | 0.483 | 0.053 | 0.988 | 0.089 | 1.675 | 0.230 | 0.825 | 0.020 | 1.775 | 0.034 | 1.357 | 0.032 | 0.940 | 0.623 |
| 52 | 0.114 | 0.015 | 0.376 | 0.014 | 1.016 | 0.161 | 1.726 | 0.233 | 0.676 | 0.022 | 0.237 | 0.030 | 1.592 | 0.268 | 1.645 | 1.121 |
| 53 | 0.222 | 0.013 | 2.841 | 0.233 | 1.822 | 0.106 | 4.546 | 0.447 | 2.351 | 0.283 | 0.195 | 0.066 | 5.195 | 0.059 | 3.256 | 2.075 |
| 54 | 0.107 | 0.031 | 0.157 | 0.033 | 0.672 | 0.081 | 3.588 | 0.309 | 0.636 | 0.035 | 0.060 | 0.006 | 2.083 | 0.331 | 0.041 | 0.008 |
| 55 | 0.097 | 0.002 | 0.039 | 0.026 | 0.249 | 0.047 | 0.065 | 0.014 | 0.063 | 0.002 | 0.296 | 0.040 | 0.074 | 0.033 | 0.066 | 0.034 |
| 56 | 0.072 | 0.015 | 0.027 | 0.017 | 0.497 | 0.283 | 0.147 | 0.031 | 0.046 | 0.002 | 1.572 | 0.040 | 0.066 | 0.045 | 0.217 | 0.125 |
| 57 | 0.101 | 0.005 | 0.083 | 0.026 | 0.467 | 0.078 | 0.139 | 0.021 | 0.116 | 0.075 | 1.289 | 0.032 | 0.141 | 0.059 | 0.881 | 0.575 |
| 58 | 0.124 | 0.000 | 0.090 | 0.023 | 0.589 | 0.068 | 0.791 | 0.143 | 0.566 | 0.018 | 1.005 | 0.209 | 0.676 | 0.004 | 0.860 | 0.577 |
| 59 | 0.110 | 0.003 | 1.009 | 0.021 | 0.793 | 0.073 | 1.667 | 0.273 | 0.838 | 0.020 | 0.459 | 0.042 | 1.153 | 0.137 | 0.240 | 0.168 |
| 60 | 0.096 | 0.020 | 0.172 | 0.018 | 0.447 | 0.045 | 0.318 | 0.078 | 0.237 | 0.024 | 1.623 | 0.239 | 0.407 | 0.050 | 1.692 | 1.224 |
| 61 | 0.127 | 0.014 | 0.333 | 0.108 | 0.386 | 0.042 | 1.673 | 0.208 | 1.273 | 0.161 | 0.868 | 0.074 | 1.072 | 0.106 | 1.139 | 0.819 |
| 62 | 0.099 | 0.006 | 0.562 | 0.016 | 0.430 | 0.069 | 1.732 | 0.301 | 1.116 | 0.035 | 0.135 | 0.010 | 0.897 | 0.026 | 1.267 | 0.899 |
| 63 | 0.117 | 0.022 | 1.121 | 0.108 | 0.555 | 0.066 | 1.584 | 0.214 | 1.178 | 0.042 | 0.118 | 0.026 | 1.152 | 0.073 | 0.141 | 0.078 |
| 64 | 0.123 | 0.020 | 0.404 | 0.010 | 0.653 | 0.077 | 0.287 | 0.072 | 0.149 | 0.040 | 0.140 | 0.019 | 0.179 | 0.067 | 0.092 | 0.003 |
| 65 | 0.080 | 0.006 | 0.187 | 0.006 | 0.526 | 0.043 | 0.055 | 0.014 | 0.066 | 0.003 | 0.050 | 0.002 | 0.052 | 0.065 | 0.049 | 0.027 |
| 66 | 0.128 | 0.009 | 0.666 | 0.103 | 0.652 | 0.083 | 0.135 | 0.016 | 0.233 | 0.306 | 1.443 | 0.307 | 0.087 | 0.108 | 0.055 | 0.045 |

References
- Lan, G.; Li, Y.; Lesueur, D.; Wu, Z.; Xie, G. Seasonal Changes Impact Soil Bacterial Communities in a Rubber Plantation on Hainan Island, China. Sci. Total Environ. 2018, 626, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Atreya, K.; Sharma, S.; Bajracharya, R.M.; Rajbhandari, N.P. Developing a Sustainable Agro-System for Central Nepal Using Reduced Tillage and Straw Mulching. J. Environ. Manag. 2008, 88, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Cavigelli, M.A.; Lengnick, L.L.; Buyer, J.S.; Fravel, D.; Handoo, Z.; McCarty, G.; Millner, P.; Sikora, L.; Wright, S.; Vinyard, B. Landscape Level Variation in Soil Resources and Microbial Properties in a No-till Corn Field. Appl. Soil Ecol. 2005, 29, 99–123. [Google Scholar] [CrossRef]
- O’Brien, S.L.; Gibbons, S.M.; Owens, S.M.; Hampton-Marcell, J.; Johnston, E.R.; Jastrow, J.D.; Gilbert, J.A.; Meyer, F.; Antonopoulos, D.A. Spatial Scale Drives Patterns in Soil Bacterial Diversity. Environ. Microbiol. 2016, 18, 2039–2051. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and Fungi Can Contribute to Nutrients Bioavailability and Aggregate Formation in Degraded Soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef]
- Daniel, R. The Metagenomics of Soil. Nat. Rev. Microbiol. 2005, 3, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Yergeau, E.; Bezemer, T.M.; Hedlund, K.; Mortimer, S.R.; Kowalchuk, G.A.; Van Der Putten, W.H. Influences of Space, Soil, Nematodes and Plants on Microbial Community Composition of Chalk Grassland Soils. Environ. Microbiol. 2010, 12, 2096–2106. [Google Scholar] [CrossRef]
- Martirosyan, V.; Ehrlich, R.; Frend, Y.; Barness, G.; Steinberger, Y. Spatial Heterogeneity of a Microbial Community in a Sandy Soil Ecosystem. Pedobiologia 2013, 56, 195–203. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial Hotspots and Hot Moments in Soil: Concept & Review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- David, C.-G.; Diana, E.M.; Germán, T.; David, B.; Laurent, P.; Eulogio, J.B. Spatial Distribution of N-Cycling Microbial Communities Showed Complex Patterns in Constructed Wetland Sediments. FEMS Microbiol. 2013, 83, 340–351. [Google Scholar] [CrossRef]
- Barberán, A.; Ramirez, K.S.; Leff, J.W.; Bradford, M.A.; Wall, D.H.; Fierer, N. Why Are Some Microbes More Ubiquitous than Others? Predicting the Habitat Breadth of Soil Bacteria. Ecol. Lett. 2014, 17, 794–802. [Google Scholar] [CrossRef]
- Schulz, S.; Brankatschk, R.; Dümig, A.; Kögel-Knabner, I.; Schloter, M.; Zeyer, J. The Role of Microorganisms at Different Stages of Ecosystem Development for Soil Formation. Biogeosciences 2013, 10, 3983–3996. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, S.; Gao, Q.; Liu, S.; Ganjurjav, H.; Wang, X.; Su, X.; Wu, X. Soil Bacterial and Fungal Diversity Differently Correlated with Soil Biochemistry in Alpine Grassland Ecosystems in Response to Environmental Changes. Sci. Rep. 2017, 7, srep43077. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhao, Z.; Gong, Q.; Zhai, B.; Li, Z. Responses of Fungal–Bacterial Community and Network to Organic Inputs Vary among Different Spatial Habitats in Soil. Soil Biol. Biochem. 2018, 125, 54–63. [Google Scholar] [CrossRef]
- Chavarría, D.N.; Verdenelli, R.A.; Serri, D.L.; Restovich, S.B.; Andriulo, A.E.; Meriles, J.M.; Vargas-Gil, S. Effect of Cover Crops on Microbial Community Structure and Related Enzyme Activities and Macronutrient Availability. Eur.J. Soil Biol. 2016, 76, 74–82. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil Bacterial Community Dynamics Reflect Changes in Plant Community and Soil Properties during the Secondary Succession of Abandoned Farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Wu, Y.; Zhang, L.; Cheng, J.; Wei, G.; Lin, Y. Natural Revegetation of a Semiarid Habitat Alters Taxonomic and Functional Diversity of Soil Microbial Communities. Sci. Total Environ. 2018, 635, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Strickland, M.S.; Rousk, J. Considering Fungal: Bacterial Dominance in Soils–Methods, Controls, and Ecosystem Implications. Soil Biol. Biochem. 2010, 42, 1385–1395. [Google Scholar] [CrossRef]
- Grzyb, A.; Wolna-Maruwka, A.; Niewiadomska, A. Environmental Factors Affecting the Mineralization of Crop Residues. Agronomy 2020, 10, 1951. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Wang, R.; Wang, W.; Shangguan, Z. Decreased Occurrence of Carbon Cycle Functions in Microbial Communities along with Long-Term Secondary Succession. Soil Biol. Biochem. 2018, 123, 207–217. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Liu, J.; Liu, T.; Cheng, J.; Wei, G.; Lin, Y. Temporal and Spatial Succession and Dynamics of Soil Fungal Communities in Restored Grassland on the Loess Plateau in China. Land Degrad. Dev. 2019, 30, 1273–1287. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X. Long-Term Nitrogen Fertilization Decreases Bacterial Diversity and Favors the Growth of Actinobacteria and Proteobacteria in Agro-Ecosystems across the Globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.W.; Gregorich, E.G. Carbon as a Substrate for Soil Organisms. In Biological Diversity and Function in Soils; Bardgett, R., Usher, M., Hopkins, D., Eds.; Cambridge University Press: Cambridge, UK, 2005; pp. 57–79. [Google Scholar]
- McNeill, A.; Unkovich, M. The Nitrogen Cycle in Terrestrial Ecosystems. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 37–64. [Google Scholar]
- Bünemann, E.K.; Condron, L.M. Phosphorus and Sulphur Cycling in Terrestrial Ecosystems. In Nutrient Cycling in Terrestrial Ecosystems; Marschner, P., Rengel, Z., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 65–92. [Google Scholar]
- Bastida, F.; Eldridge, D.J.; García, C.; Kenny Png, G.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil Microbial Diversity–Biomass Relationships Are Driven by Soil Carbon Content across Global Biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- de Vries, F.T.; Hoffland, E.; van Eekeren, N.; Brussaard, L.; Bloem, J. Fungal/Bacterial Ratios in Grasslands with Contrasting Nitrogen Management. Soil Biol. Biochem. 2006, 38, 2092–2103. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical Composition of Dissolved Organic Matter in Agroecosystems: Correlations with Soil Enzyme Activity and Carbon and Nitrogen Mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Scharroba, A.; Dibbern, D.; Hünninghaus, M.; Kramer, S.; Moll, J.; Butenschoen, O.; Bonkowski, M.; Buscot, F.; Kandeler, E.; Koller, R. Effects of Resource Availability and Quality on the Structure of the Micro-Food Web of an Arable Soil across Depth. Soil Biol. Biochem. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef]
- Liu, J.; Yu, Z.; Yao, Q.; Hu, X.; Zhang, W.; Mi, G.; Chen, X.; Wang, G. Distinct Soil Bacterial Communities in Response to the Cropping System in a Mollisol of Northeast China. Appl. Soil Ecol. 2017, 119, 407–416. [Google Scholar] [CrossRef]
- Sharma, N.; Singhvi, R. Effects of Chemical Fertilizers and Pesticides on Human Health and Environment: A Review. IJAEB 2017, 10, 675–680. [Google Scholar] [CrossRef]
- Expósito, A.; Velasco, F. Exploring Environmental Efficiency of the European Agricultural Sector in the Use of Mineral Fertilizers. J. Clean. Prod. 2020, 253, 119971. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between Roots and Microorganisms for Nitrogen: Mechanisms and Ecological Relevance. N. Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–Microbiome Interactions: From Community Assembly to Plant Health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Schipanski, M.E.; Drinkwater, L.E. Nitrogen Fixation in Annual and Perennial Legume-Grass Mixtures across a Fertility Gradient. Plant Soil 2012, 357, 147–159. [Google Scholar] [CrossRef]
- Vasseur, C.; Joannon, A.; Aviron, S.; Burel, F.; Meynard, J.-M.; Baudry, J. The Cropping Systems Mosaic: How Does the Hidden Heterogeneity of Agricultural Landscapes Drive Arthropod Populations? Agric. Ecosys. Environ. 2013, 166, 3–14. [Google Scholar] [CrossRef]
- Khandelwal, R.A.; Olivier, B.G.; Röling, W.F.; Teusink, B.; Bruggeman, F.J. Community Flux Balance Analysis for Microbial Consortia at Balanced Growth. PLoS ONE 2013, 8, e64567. [Google Scholar] [CrossRef]
- Aon, M.A.; Colaneri, A.C., II. Temporal and Spatial Evolution of Enzymatic Activities and Physico-Chemical Properties in an Agricultural Soil. Appl. Soil Ecol. 2001, 18, 255–270. [Google Scholar] [CrossRef]
- Cirilli, M.; Bellincontro, A.; De Santis, D.; Botondi, R.; Colao, M.C.; Muleo, R.; Mencarelli, F. Temperature and Water Loss Affect ADH Activity and Gene Expression in Grape Berry during Postharvest Dehydration. Food Chem. 2012, 132, 447–454. [Google Scholar] [CrossRef]
- Levyk, V.; Maryskevych, O.; Brzezinska, M.; Wlodarczyk, T. Dehydrogenase Activity of Technogenic Soils of Former Sulphur Mines [Yavoriv and Nemyriv, Ukraine]. Int. Agrophys. 2007, 21, 255–260. [Google Scholar]
- Liang, Q.; Chen, H.; Gong, Y.; Yang, H.; Fan, M.; Kuzyakov, Y. Effects of 15 Years of Manure and Mineral Fertilizers on Enzyme Activities in Particle-Size Fractions in a North China Plain Soil. Eur. J. Soil Biol. 2014, 60, 112–119. [Google Scholar] [CrossRef]
- Sauer, D.; Kuzyakov, Y.; Stahr, K. Spatial Distribution of Root Exudates of Five Plant Species as Assessed by 14C Labeling. Plant Nutr. Soil Sci. 2006, 169, 360–362. [Google Scholar] [CrossRef]
- Al-Omran, A.M.; Al-Wabel, M.I.; El-Maghraby, S.E.; Nadeem, M.E.; Al-Sharani, S. Spatial Variability for Some Properties of the Wastewater Irrigated Soils. J. Saudi Soc. Agric. Sci. 2013, 12, 167–175. [Google Scholar] [CrossRef]
- Peigné, J.; Vian, J.-F.; Cannavacciuolo, M.; Bottollier, B.; Chaussod, R. Soil Sampling Based on Field Spatial Variability of Soil Microbial Indicators. Eur. J. Soil Biol. 2009, 45, 488–495. [Google Scholar] [CrossRef]
- Staugaitis, G.; Šumskis, D. Spatial Variability of Soil PH as Influenced by Different Soil Sampling Methods and Geostatistical Techniques. Žemdirbystė 2011, 98, 323–332. [Google Scholar]
- Piotrowska-Długosz, A.; Breza-Boruta, B.; Długosz, J. Spatial and Temporal Variability of the Soil Microbiological Properties in Two Soils with a Different Pedogenesis Cropped to Winter Rape (Brassica napus L.). Geoderma 2019, 340, 313–324. [Google Scholar] [CrossRef]
- Constancias, F.; Terrat, S.; Saby, N.P.A.; Horrigue, W.; Villerd, J.; Guillemin, J.-P.; Biju-Duval, L.; Nowak, V.; Dequiedt, S.; Ranjard, L.; et al. Mapping and Determinism of Soil Microbial Community Distribution across an Agricultural Landscape. Microbiol. Open 2015, 4, 505–517. [Google Scholar] [CrossRef]
- Vasu, D.; Singh, S.K.; Sahu, N.; Tiwary, P.; Chandran, P.; Duraisami, V.P.; Ramamurthy, V.; Lalitha, M.; Kalaiselvi, B. Assessment of Spatial Variability of Soil Properties Using Geospatial Techniques for Farm Level Nutrient Management. Soil Till. Res. 2017, 169, 25–34. [Google Scholar] [CrossRef]
- Łukowiak, R.; Grzebisz, W.; Ceglarek, J.; Podolski, A.; Kaźmierowski, C.; Piekarczyk, J. Spatial Variability of Yield and Nitrogen Indicators—A Crop Rotation Approach. Agronomy 2020, 10, 1959. [Google Scholar] [CrossRef]
- Rahimi Eichi, V.; Okamato, M.; Haefele, S.M.; Jewell, N.; Brien, C.; Garnett, T.; Langridge, P. Understanding the Interactions between Biomass, Grain Production and Grain Protein Content in High and Low Protein Wheat Genotypes under Controlled Environments. Agronomy 2019, 9, 706. [Google Scholar] [CrossRef]
- Ma, G.; Liu, W.; Li, S.; Zhang, P.; Wang, C.; Lu, H.; Wang, L.; Xie, Y.; Ma, D.; Kang, G. Determining the Optimal N Input to Improve Grain Yield and Quality in Winter Wheat with Reduced Apparent N Loss in the North China Plain. Front. Plant Sci. 2019, 10, 181. [Google Scholar] [CrossRef]
- Mocek, A.; Drzymała, S.; Maszner, P. Geneza, Analiza i Klasyfikacja Gleb; Mocek, A., Ed.; Wydawnictwo Akademii Rolniczej: Poznań, Poland, 2006. [Google Scholar]
- Camiña, F.; Trasar-Cepeda, C.; Gil-Sotres, F.; Leirós, C. Measurement of Dehydrogenase Activity in Acid Soils Rich in Organic Matter. Soil Biol. Biochem. 1998, 30, 1005–1011. [Google Scholar] [CrossRef]
- Weyman-Kaczmarkowa, W.; Pędziwilk, Z. The Development of Fungi as Affected by PH and Type of Soil, in Relation to the Occurrence of Bacteria and Soil Fungistatic Activity. Microbiol. Res. 2000, 155, 107–112. [Google Scholar] [CrossRef]
- Zbíral, J. Determination of Plant-Available Micronutrients by the Mehlich 3 Soil Extractant–a Proposal of Critical Values. Plant Soil Environ. 2016, 62, 527–531. [Google Scholar] [CrossRef]
- Szczepaniak, W.; Nowicki, B.; Bełka, D.; Kazimierowicz, A.; Kulwicki, M.; Grzebisz, W. Effect of Foliar Application of Micronutrients and Fungicides on the Nitrogen Use Efficiency in Winter Wheat. Agronomy 2022, 12, 257. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Growth of Saprotrophic Fungi and Bacteria in Soil: Growth of Saprotrophic Fungi and Bacteria in Soil. FEMS Microbiol. 2011, 78, 17–30. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Fungal and Bacterial Growth in Soil with Plant Materials of Different C/N Ratios. FEMS Microbiol. 2007, 62, 258–267. [Google Scholar] [CrossRef]
- Vinten, A.J.; Whitmore, A.; Bloem, J.; Howard, R.; Wright, F. Factors Affecting N Immobilisation/Mineralisation Kinetics for Cellulose-, Glucose-and Straw-Amended Sandy Soils. Biol. Fertil. Soils 2002, 36, 190–199. [Google Scholar] [CrossRef]
- Koch, O.; Tscherko, D.; Kandeler, E. Temperature Sensitivity of Microbial Respiration, Nitrogen Mineralization, and Potential Soil Enzyme Activities in Organic Alpine Soils. Glob. Biogeochem. Cycles 2007, 21. [Google Scholar] [CrossRef]
- Mueller, K.E.; Tilman, D.; Fornara, D.A.; Hobbie, S.E. Root Depth Distribution and the Diversity–Productivity Relationship in a Long-Term Grassland Experiment. Ecology 2013, 94, 787–793. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Wang, R.; Wu, C. Interactions between Dicyandiamide and Periphytic Biofilms in Paddy Soils and Subsequent Effects on Nitrogen Cycling. Sci. Total Environ. 2020, 718, 137417. [Google Scholar] [CrossRef]
- Houlden, A.; Timms-Wilson, T.M.; Day, M.J.; Bailey, M.J. Influence of Plant Developmental Stage on Microbial Community Structure and Activity in the Rhizosphere of Three Field Crops. FEMS Microbiol. 2008, 65, 193–201. [Google Scholar] [CrossRef]
- Chapin, F.S.; Matson, P.A.; Vitousek, P.M. The Ecosystem Concept. In Principles of Terrestrial Ecosystem Ecology; Chapin, F.S., Matson, P.A., Mooney, H.A., Vitousek, P.M., Eds.; Springer: New York, NY, USA, 2011; pp. 3–22. [Google Scholar]
- Hinsinger, P.; Plassard, C.; Jaillard, B. Rhizosphere: A New Frontier for Soil Biogeochemistry. J. Geochem. Explor. 2006, 88, 210–213. [Google Scholar] [CrossRef]
- Galloway, A.F.; Akhtar, J.; Marcus, S.E.; Fletcher, N.; Field, K.; Knox, P. Cereal Root Exudates Contain Highly Structurally Complex Polysaccharides with Soil-Binding Properties. Plant J. 2020, 103, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Germida, J.; Siciliano, S. Taxonomic Diversity of Bacteria Associated with the Roots of Modern, Recent and Ancient Wheat Cultivars. Biol. Fertil.Soils 2001, 33, 410–415. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial Pattern of Enzyme Activities Depends on Root Exudate Composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Blagodatskaya, E.; Kuzyakov, Y. Active Microorganisms in Soil: Critical Review of Estimation Criteria and Approaches. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Yang, L.; Li, T.; Li, F.; Lemcoff, J.H.; Cohen, S. Fertilization Regulates Soil Enzymatic Activity and Fertility Dynamics in a Cucumber Field. Sci. Hortic. 2008, 116, 21–26. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef]
- Nayak, D.R.; Babu, Y.J.; Adhya, T.K. Long-Term Application of Compost Influences Microbial Biomass and Enzyme Activities in a Tropical Aeric Endoaquept Planted to Rice under Flooded Condition. Soil Biol. Biochem. 2007, 39, 1897–1906. [Google Scholar] [CrossRef]
- Wolinska, A.; Stępniewska, Z.; Pytlak, A. The Effect of Environmental Factors on Total Soil DNA Content and Dehydrogenase Activity. Arch. Biol. Sci. 2015, 67, 493–501. [Google Scholar] [CrossRef]
- Thiele-Bruhn, S.; Beck, I.-C. Effects of Sulfonamide and Tetracycline Antibiotics on Soil Microbial Activity and Microbial Biomass. Chemosphere 2005, 59, 457–465. [Google Scholar] [CrossRef]
- Feeney, D.S.; Hallett, P.D.; Rodger, S.; Bengough, A.G.; White, N.A.; Young, I.M. Impact of Fungal and Bacterial Biocides on Microbial Induced Water Repellency in Arable Soil. Geoderma 2006, 135, 72–80. [Google Scholar] [CrossRef]
- Meidute, S.; Demoling, F.; Bååth, E. Antagonistic and Synergistic Effects of Fungal and Bacterial Growth in Soil after Adding Different Carbon and Nitrogen Sources. Soil Biol. Biochem. 2008, 40, 2334–2343. [Google Scholar] [CrossRef]
- Liu, J.; Sui, Y.; Yu, Z.; Shi, Y.U.; Chu, H.; Jin, J.; Liu, X.; Wang, G. High Throughput Sequencing Analysis of Biogeographical Distribution of Bacterial Communities in the Black Soils of Northeast China. Soil Biol. Biochem. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Song, M.; Peng, W.; Zeng, F.; Du, H.; Peng, Q.; Xu, Q.; Chen, L.; Zhang, F. Spatial Patterns and Drivers of Microbial Taxa in a Karst Broadleaf Forest. Front. Microbiol. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Philippot, L.; Bru, D.; Saby, N.P.; Čuhel, J.; Arrouays, D.; Šimek, M.; Hallin, S. Spatial Patterns of Bacterial Taxa in Nature Reflect Ecological Traits of Deep Branches of the 16S RRNA Bacterial Tree. Environ. Microbiol. 2009, 11, 3096–3104. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME 2010, 4, 1340–1351. [Google Scholar] [CrossRef]
- Gomathi, S.; Ambikapathy, V.; Panneerselvam, A. Studies on Soil Mycoflora in Chilli Field of Thiruvarur District. Asian. J. Res. Pharm Sci 2011, 1, 117–122. [Google Scholar]
- Kowalska, G.; Kowalski, R.; Hawlena, J.; Rowiński, R. Seeds Of Oilseed Rape As An Alternative Source Of Protein And Minerals. J. Elem. 2020, 25, 513–522. [Google Scholar] [CrossRef]
- Ma, Q.; Luo, Y.; Wen, Y.; Hill, P.W.; Chadwick, D.R.; Wu, L.; Jones, D.L. Carbon and Sulphur Tracing from Soil Organic Sulphur in Plants and Soil Microorganisms. Soil Biol. Biochem. 2020, 150, 107971. [Google Scholar] [CrossRef]
- Grzebisz, W. Oilseed plants. In Crop Fertilization–Yield Physiology. Oilseed, Root and Legume Crops; Grzebisz, W., Ed.; PWRiL: Poznań, Poland, 2011; pp. 50–135. [Google Scholar]
- Jankowski, K.J.; Hulanicki, P.S. Yield And Quality Of Winter Oilseed Rape In Response To Different Systems Of Foliar Fertilization. J. Elem. 2016, 21, 1017–1027. [Google Scholar] [CrossRef]
- Santillano-Cázares, J.; Redmon, L.A.; Caddel, J.L.; Goad, C.L.; Rueda-Puente, E.O. Spatial and Temporal Variability of Soil Fertility in Terraced Pastures. J. Plant Nutri. 2012, 35, 2055–2066. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Functions and Deficiency and Toxicity Symptoms. Nutr. Manag. 2009, 9, 1–16. [Google Scholar]
- Huang, Z.P.; Xu, B.; Zhang, K.Q. Spatial Variability and Accumulation of Cr and Ni in Farmland Soil of Swine Wastewater Applied. Ecol. Environ. 2007, 16, 1694–1699. [Google Scholar]
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production. In Advances in Agronomy; Sparks, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 77, pp. 185–268. [Google Scholar]
- Wang, W.; Li, H.; Guénon, R.; Yang, Y.; Shu, X.; Cheng, X.; Zhang, Q. Geographical Variability of Mineral Elements and Stability of Restrictive Mineral Elements in Terrestrial Cyanobacteria Across Gradients of Climate, Soil, and Atmospheric Wet Deposition Mineral Concentration. Front. Microbiol. 2021, 11, 582655. [Google Scholar] [CrossRef]
- Bao, Z.; Wu, W.; Liu, H.; Yin, S.; Chen, H. Geostatistical Analyses of Spatial Distribution and Origin of Soil Nutrients in Long-Term Wastewater-Irrigated Area in Beijing, China. Acta Agric. Scand. B 2014, 64, 235–243. [Google Scholar] [CrossRef]
- Ayoubi, S.H.; Zamani, S.M.; Khormali, F. Spatial Variability of Some Soil Properties for Site Specific Farming in Northern Iran. Int. J.Plant Prod. 2007, 1, 225–236. [Google Scholar]
- Santra, P.; Chopra, U.K.; Chakraborty, D. Spatial Variability of Soil Properties and Its Application in Predicting Surface Map of Hydraulic Parameters in an Agricultural Farm. Curr. Sci. 2008, 95, 937–945. [Google Scholar]
- Hussain, A.; Awan, M.S.; Ali, S.; Khan, S.W.; Morari, F.; Ali, S. Spatial Variability of Soil Micronutrients (Cu, Fe, Zn & Mn) and Population Dynamic of Mycoflora in Potato Fields of CKNP Region Gilgit-Baltistan Pakistan. Pak. J. Agric. Sci. 2016, 53. [Google Scholar]
- Yue, S.; Meng, Q.; Zhao, R.; Ye, Y.; Zhang, F.; Cui, Z.; Chen, X. Change in Nitrogen Requirement with Increasing Grain Yield for Winter Wheat. Agron. J. 2012, 104, 1687–1693. [Google Scholar] [CrossRef]
- Asseng, S.; Milroy, S.P. Simulation of Environmental and Genetic Effects on Grain Protein Concentration in Wheat. Eur. J. Agron. 2006, 25, 119–128. [Google Scholar] [CrossRef]
- Estrada-Campuzano, G.; Miralles, D.J.; Slafer, G.A. Genotypic Variability and Response to Water Stress of Pre-and Post-Anthesis Phases in Triticale. Eur. J. Agron. 2008, 28, 171–177. [Google Scholar] [CrossRef]
- Haberle, J.; Svoboda, P. The Effect of Water Supply during Grain Growth on the Utilization of Soil Mineral Nitrogen by Winter Wheat. Sci. Agric. Bohem. 2007, 3, 105–110. [Google Scholar]
- Semenov, M.A.; Jamieson, P.D.; Martre, P. Deconvoluting Nitrogen Use Efficiency in Wheat: A Simulation Study. Eur. J. Agron. 2007, 26, 283–294. [Google Scholar] [CrossRef]
- Triboi, E.; Triboi-Blondel, A.-M. Productivity and Grain or Seed Composition: A New Approach to an Old Problem. Eur. J. Agron. 2002, 16, 163–186. [Google Scholar] [CrossRef]
- Stepaniuk, M.; Głowacka, A. Yield of Winter Oilseed Rape (Brassica napus L. Var. Napus) in a Short-Term Monoculture and the Macronutrient Accumulation in Relation to the Dose and Method of Sulphur Application. Agronomy 2021, 12, 68. [Google Scholar] [CrossRef]
- Šidlauskas, G.; Tarakanovas, P. Factors Affecting Nitrogen Concentration in Spring Oilseed Rape (Brassica napus L.). Plant Soil Environ. 2011, 50, 227–234. [Google Scholar] [CrossRef]
- Narits, L. Effect of Nitrogen Rate and Application Time to Yield and Quality of Winter Oilseed Rape (Brassica napus L. var. oleifera subvar. biennis). Agron. Res. 2010, 8, 671–686. [Google Scholar]
- Nowosad, K.; Liersch, A.; Popławska, W.; Bocianowski, J. Genotype by Environment Interaction for Seed Yield in Rapeseed (Brassica napus L.) Using Additive Main Effects and Multiplicative Interaction Model. Euphytica 2016, 208, 187–194. [Google Scholar] [CrossRef]
- Bartomeus, I.; Gagic, V.; Bommarco, R. Pollinators, Pests and Soil Properties Interactively Shape Oilseed Rape Yield. Basic Appl.Ecol. 2015, 16, 737–745. [Google Scholar] [CrossRef]
- Cui, B.; Huang, W.; Song, X.; Ye, H.; Dong, Y. Study the Spatial-Temporal Variation of Wheat Growth under Different Site-Specific Nitrogen Fertilization Approaches. In Proceedings of the International Conference on Computer and Computing Technologies in Agriculture, Jilin, China, 12–15 August 2017; Li, D., Zhao, C., Eds.; Springer: Beijing, China, 2017; pp. 316–332. [Google Scholar]
- Welsh, J.P.; Wood, G.A.; Godwin, R.J.; Taylor, J.C.; Earl, R.; Blackmore, S.; Knight, S.M. Developing Strategies for Spatially Variable Nitrogen Application in Cereals, Part II: Wheat. Biosys. Enginee. 2003, 84, 495–511. [Google Scholar] [CrossRef]
- Basso, B.; Cammarano, D.; Grace, P.R.; Cafiero, G.; Sartori, L.; Pisante, M.; Landi, G.; De Franchi, S.; Basso, F. Criteria for Selecting Optimal Nitrogen Fertilizer Rates for Precision Agriculture. Ital. J. Agron. 2009, 4, 147–158. [Google Scholar] [CrossRef]
- Song, X.; Yang, G.; Yang, C.; Wang, J.; Cui, B. Spatial Variability Analysis of Within-Field Winter Wheat Nitrogen and Grain Quality Using Canopy Fluorescence Sensor Measurements. Remote Sens. 2017, 9, 237. [Google Scholar] [CrossRef]
- Albrizio, R.; Todorovic, M.; Matic, T.; Stellacci, A.M. Comparing the Interactive Effects of Water and Nitrogen on Durum Wheat and Barley Grown in a Mediterranean Environment. Field Crops Res. 2010, 115, 179–190. [Google Scholar] [CrossRef]
- Garrido-Lestache, E.; López-Bellido, R.J.; López-Bellido, L. Durum Wheat Quality under Mediterranean Conditions as Affected by N Rate, Timing and Splitting, N Form and S Fertilization. Eur. J. Agron. 2005, 23, 265–278. [Google Scholar] [CrossRef]
- Delin, S. Within-Field Variations in Grain Protein Content—Relationships to Yield and Soil Nitrogen and Consistency in Maps between Years. Precis. Agric. 2004, 5, 565–577. [Google Scholar] [CrossRef]





| YEAR | 2019 | |||
|---|---|---|---|---|
| TERM | I | II | III | IV |
| Winter wheat | 13.02 before the start of crop growth | 03.06 flowering of the crop | 09.07 harvest | 22.10 saturation of the soil sorption complex |
| YEAR | 2020 | |||
| TERM | V | VI | VII | VIII |
| Winter oilseed rape | 13.02 before the start of crop growth | 21.04 flowering of the crop | 07.07 harvest | 22.10 saturation of the soil sorption complex |
| Parameter | Term | Point | Interaction |
|---|---|---|---|
| WHEAT | |||
| DHA | 1109.63 ** | 31.01 ** | 21.40 ** |
| Bacteria | 986.98 ** | 29.73 ** | 20.53 ** |
| Molds | 26,071.07 ** | 818.21 ** | 332.84 ** |
| Na | 18,987.32 ** | 234.31 ** | 121.35 ** |
| Ca | 907.41 ** | 908.53 ** | 9.58 ** |
| Mg | 419.98 ** | 402.44 ** | 16.61 ** |
| Cu | 391.54 *** | 3.07 *** | 2.08 *** |
| Zn | 190.77 *** | 20.72 *** | 1.66 *** |
| Mn | 1091.73 ** | 134.31 ** | 53.39 ** |
| Fe | 3022.32 ** | 215.51 ** | 72.82 ** |
| Seed (%N) | 1322.36 *** | 343.61 *** | 19.27 *** |
| Straw (%N) | 2396.17 *** | 353.61 *** | 33.94 *** |
| RAPESEED | |||
| DHA | 876.96 ** | 41.93 ** | 10.48 ** |
| Bacteria | 674.70 ** | 41.52 ** | 10.29 ** |
| Molds | 21,934.64 ** | 866.60 ** | 471.89 ** |
| Na | 1400.50 ** | 11.33 ** | 13.02 ** |
| Ca | 2084.08 ** | 120.13 ** | 56.98 ** |
| Mg | 742.67 ** | 99.08 ** | 33.39 ** |
| Cu | 37.06 *** | 5.19 *** | 2.82 *** |
| Zn | 1967.19 ** | 44.02 ** | 27.00 ** |
| Mn | 214.79 ** | 38.29 ** | 16.62 ** |
| Fe | 175.17 ** | 93.75 ** | 33.86 ** |
| Seed (%N) | 629.87 *** | 194.86 *** | 16.21 *** |
| Straw (%N) | 691.32 *** | 171.34 *** | 20.20 *** |
| YEAR | 2019 | 2020 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TERM | I | II | III | IV | V | VI | VII | VIII | ||||||||
| X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | |
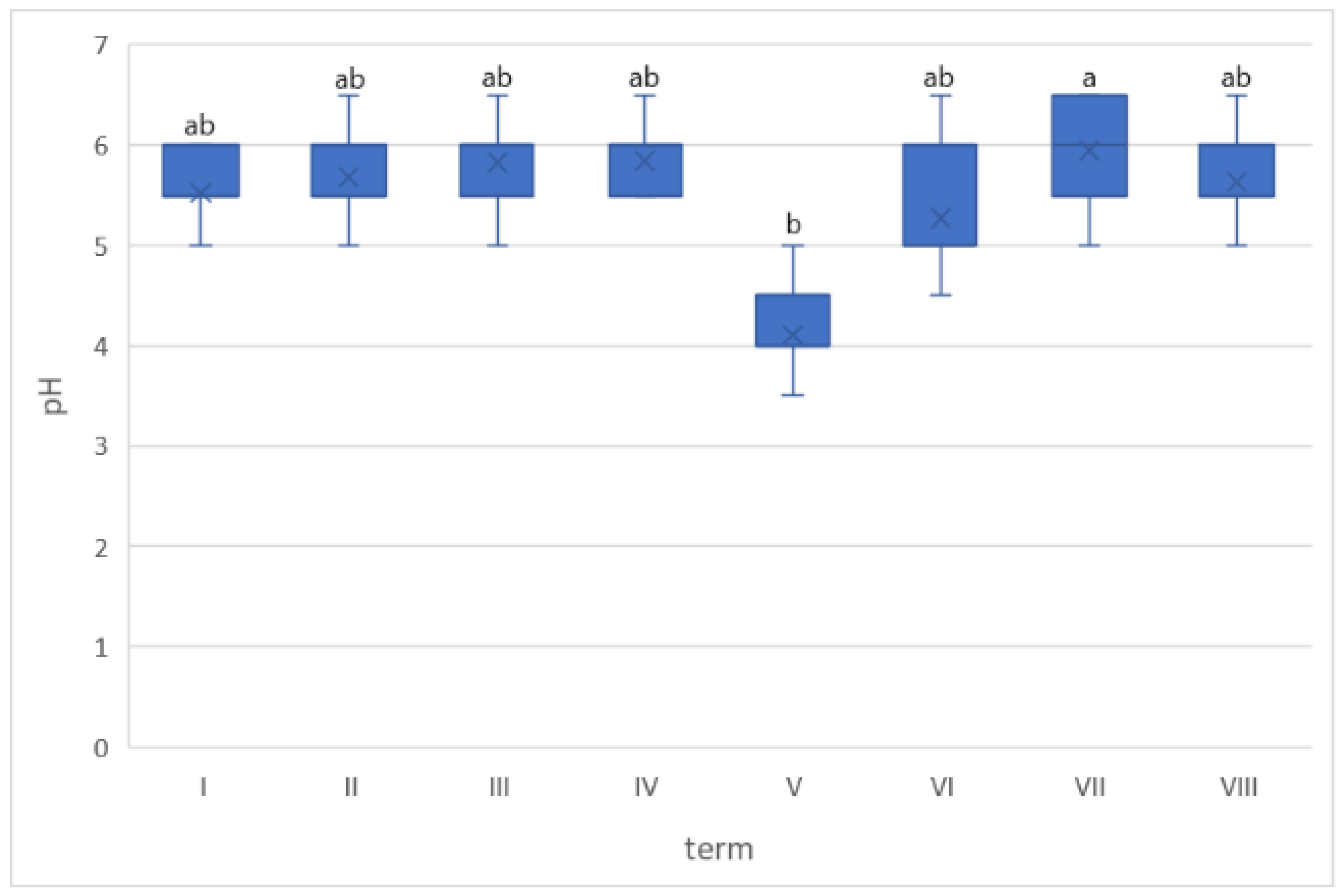
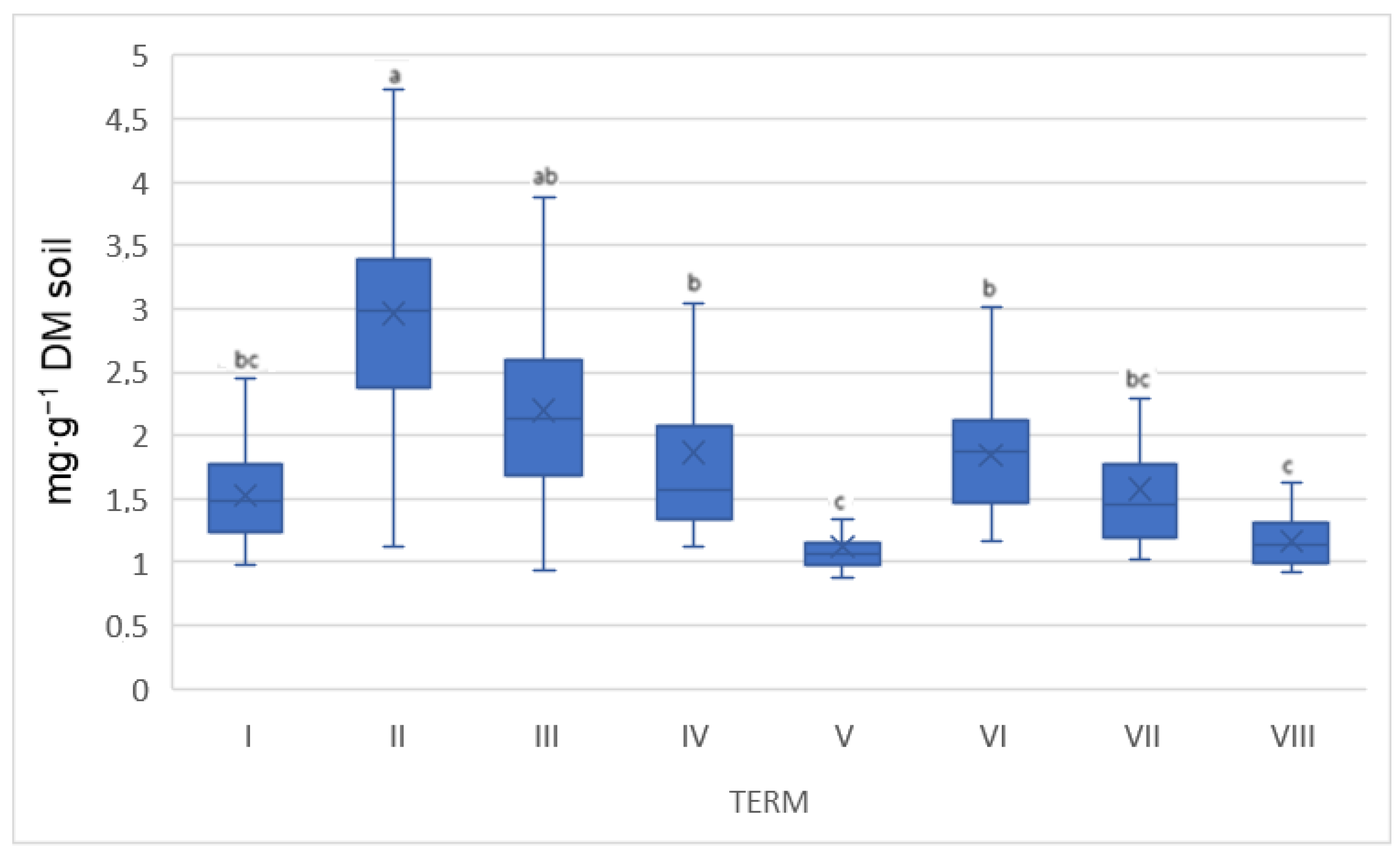
| Na | ||||||||||||||||
| 3.98 a | 0.06 | 3.09 ab | 0.07 | 1.63 c | 0.07 | 1.72 c | 0.01 | 1.64 c | 0.24 | 3.30 ab | 0.20 | 2.81 b | 0.10 | 1.39 c | 0.03 | |
| Ca | ||||||||||||||||
| 61.22 a | 1.38 | 53.42 b | 1.33 | 54.50 b | 1.05 | 55.06 b | 0.47 | 57.33 b | 0.38 | 52.75 b | 2.16 | 35.39 c | 2.88 | 59.29 ab | 4.13 | |
| Mg | ||||||||||||||||
| 9.92 ab | 0.24 | 9.01 ab | 0.30 | 8.14 ab | 0.52 | 7.96 b | 0.61 | 10.64 a | 0.42 | 7.37 b | 0.43 | 7.49 b | 0.87 | 8.04 ab | 0.15 | |
| YEAR | 2019 | 2020 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TERM | I | II | III | IV | V | VI | VII | VIII | ||||||||
| X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | X | SD | |
| Cu | ||||||||||||||||
| 0.06 a | 0.01 | 0.05 a | 0.01 | 0.02 c | 0.004 | 0.03 b | 0.02 | 0.02 c | 0.004 | 0.02 c | 0.01 | 0.03 b | 0.01 | 0.05 a | 0.01 | |
| Zn | ||||||||||||||||
| 0.02 c | 0.002 | 0.02 c | 0.002 | 0.03 b | 0.01 | 0.03 b | 0.002 | 0.04 a | 0.003 | 0.02 c | 0.002 | 0.02 c | 0.003 | 0.02 c | 0.01 | |
| Mn | ||||||||||||||||
| 0.76 a | 0.05 | 0.07 c | 0.03 | 0.18 b | 0.003 | 0.05 c | 0.01 | 0.02 c | 0.005 | 0.04 c | 0.01 | 0.04 c | 0.01 | 0.03 c | 0.01 | |
| Fe | ||||||||||||||||
| 0.14 d | 0.01 | 0.88 c | 0.07 | 1.01 b | 0.11 | 1.72 a | 0.21 | 0.97 c | 0.11 | 1.55 a | 0.20 | 1.64 a | 0.14 | 1.14 ab | 0.74 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzyb, A.; Wolna-Maruwka, A.; Łukowiak, R.; Ceglarek, J. Spatial and Temporal Variability of the Microbiological and Chemical Properties of Soils under Wheat and Oilseed Rape Cultivation. Agronomy 2022, 12, 2259. https://doi.org/10.3390/agronomy12102259
Grzyb A, Wolna-Maruwka A, Łukowiak R, Ceglarek J. Spatial and Temporal Variability of the Microbiological and Chemical Properties of Soils under Wheat and Oilseed Rape Cultivation. Agronomy. 2022; 12(10):2259. https://doi.org/10.3390/agronomy12102259
Chicago/Turabian StyleGrzyb, Aleksandra, Agnieszka Wolna-Maruwka, Remigiusz Łukowiak, and Jakub Ceglarek. 2022. "Spatial and Temporal Variability of the Microbiological and Chemical Properties of Soils under Wheat and Oilseed Rape Cultivation" Agronomy 12, no. 10: 2259. https://doi.org/10.3390/agronomy12102259
APA StyleGrzyb, A., Wolna-Maruwka, A., Łukowiak, R., & Ceglarek, J. (2022). Spatial and Temporal Variability of the Microbiological and Chemical Properties of Soils under Wheat and Oilseed Rape Cultivation. Agronomy, 12(10), 2259. https://doi.org/10.3390/agronomy12102259






