Simultaneous Selection of Sweet-Waxy Corn Ideotypes Appealing to Hybrid Seed Producers, Growers, and Consumers in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Crossing
2.2. Experimental Design
2.3. Data Collection
2.4. Sample Preparation and Sensory Evaluation
2.5. Data Analysis
3. Results
3.1. Combined Analysis of Variance (ANOVA)
3.2. Genetic Parameters and Genetic Gains
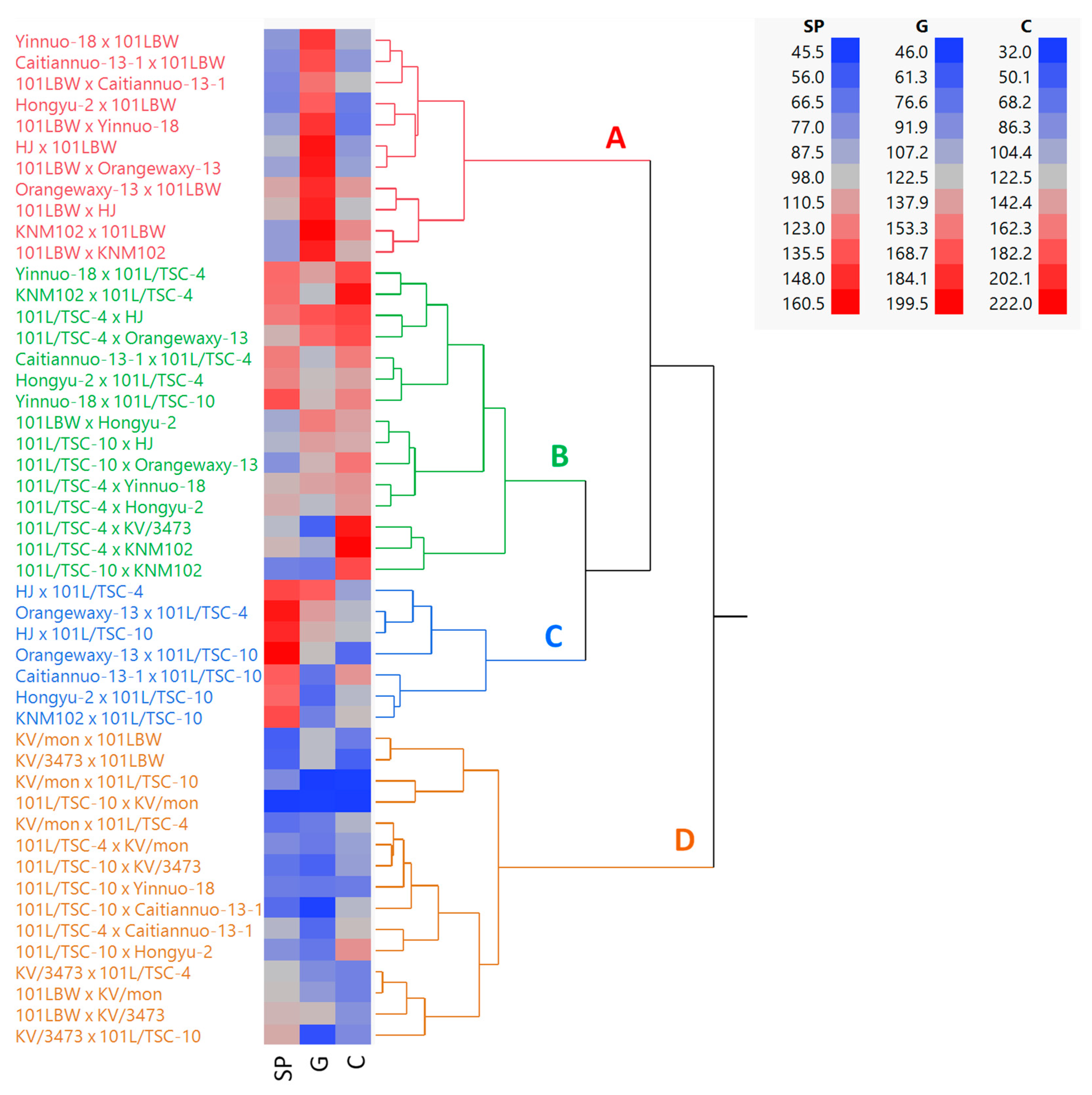
3.3. Simultaneous Selection
4. Discussion
4.1. Combined Analysis of Variance (ANOVA)
4.2. Genetic Gains Reflect the Effectiveness of Simultaneous Selection
4.3. Candidates of Sweet-Waxy Corn Hybrids and the Expected Ideotypes for Three Groups of Preferences
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lertrat, K.; Thongnarin, N. Novel approach to eating quality improvement in local waxy corn: Improvement of sweet taste in local waxy corn variety with mixed kernels from supersweet corn. Acta Hortic. 2008, 769, 145–150. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer Science & Business Media: New York, NY, USA, 2010. [Google Scholar]
- MacRobert, J.F.; Setimela, P.; Gethi, J.; Regasa, M.W. Maize Hybrid Seed Production Manual; CIMMYT: Mexico City, Mexico, 2014. [Google Scholar]
- Worku, M.; Makumbi, D.; Beyene, Y.; Das, B.; Mugo, S.; Pixley, K.; Bänziger, M.; Owino, F.; Olsen, M.; Asea, G.; et al. Grain yield performance and flowering synchrony of CIMMYT’s tropical maize (Zea mays L.) parental inbred lines and single crosses. Euphytica 2016, 211, 395–409. [Google Scholar] [CrossRef]
- Morris, L.; Bellon, M. Participatory plant breeding research: Opportunities and challenges for the international crop improvement system. Euphytica 2004, 136, 21–35. [Google Scholar] [CrossRef]
- Fergason, V. High amylose and waxy corns. In Specialty Corns, 2nd ed.; Hallauer, A.R., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2001. [Google Scholar]
- Azanza, F.; Klein, B.P.; Juvik, J.A. Sensory characterization of sweet corn lines differing in physical and chemical composition. J. Food Sci. 1996, 61, 253–257. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, S.L.; Moon, H.G.; Son, B.Y.; Kim, S.J.; Kim, S.K. Major characteristics related on eating quality and classification of inbred lines of waxy corn. Korean J. Breed. Sci. 2005, 50, 161–166. [Google Scholar]
- Hellyer, E.; Fraser, I.; Haddock-Fraser, J. Food choice, health information and functional ingredients: An experimental auction employing bread. Food Policy 2012, 37, 232–245. [Google Scholar] [CrossRef]
- Abadassi, J. Maize agronomic traits needed in tropical zone. Int. J. Environ. Sci. Technol. 2015, 4, 371–392. [Google Scholar]
- Abera, W.; Hussein, S.; Derera, J.; Worku, M.; Laing, M.D. Preferences and constraints of maize farmers in the development and adoption of improved varieties in the mid-altitude, sub-humid agro-ecology of Western Ethiopia. Afr. J. Agric. Res. 2013, 8, 1245–1254. [Google Scholar]
- Derera, J.; Tongoona, P.; Langyintuo, A.; Laing, M.D.; Vivek, B. Farmer perceptions on maize cultivars in the marginal eastern belt of Zimbabwe and their implications for breeding. Afr. Crop Sci. J. 2006, 14, 1–15. [Google Scholar]
- Sibiya, J.; Tongoona, P.; Derera, J.; Makanda, I. Farmers’s desired traits and selection criteria for maize varieties and their implications for maize breeding: A case study from KwaZulu-Natal Province, South Africa. J. Agric. Rural Dev. Trop. Subtrop. 2013, 114, 39–49. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Simla, S.; Lertrat, K.; Suriharn, B. Combination of multiple genes controlling endosperm characters in relation to maximum eating quality of vegetable waxy corn. Sabrao J. Breed. Genet 2016, 48, 210–218. [Google Scholar]
- Dermail, A.; Suriharn, B.; Chankaew, S.; Sanitchon, J.; Lertrat, K. Hybrid prediction based on SSR-genetic distance, heterosis, and combining ability on agronomic traits and yields in sweet and waxy corn. Sci. Hortic. 2020, 259, 108817. [Google Scholar] [CrossRef]
- Fuengtee, A.; Dermail, A.; Simla, S.; Lertrat, K.; Sanitchon, J.; Chankaew, S.; Suriharn, B. Combining ability for carbohydrate components associated with consumer preferences in tropical sweet and waxy corn derived from exotic germplasm. Turk. J. Field Crops 2020, 25, 147–155. [Google Scholar] [CrossRef]
- Smith, H.F. A discriminant function for plant selection. Ann. Eugen. 1936, 7, 240–250. [Google Scholar] [CrossRef]
- Elston, R.C. A weight-free index for the purpose of ranking or selection with respect to several traits at a time. Biometrics 1963, 19, 85–97. [Google Scholar] [CrossRef]
- Simonne, E.; Simonne, A.; Boozer, R. Yield, ear characteristics, and consumer acceptance of selected white sweet corn varieties in the Southeastern United States. Horttechnology 1999, 9, 289–293. [Google Scholar] [CrossRef]
- Sharma, R.C.; Duveiller, E. Selection index for improving Helminthosporium leaf blight resistance, maturity, and kernel weight in spring wheat. Crop Sci. 2003, 43, 2031–2036. [Google Scholar] [CrossRef]
- Hazel, L.N.; Lush, J.L. The efficiency of three methods of selection. J. Hered. 1942, 33, 393–399. [Google Scholar] [CrossRef]
- dos Candido, W.S.; Silva, C.M.; Costa, M.L.; de Silva, B.E.A.; de Miranda, B.L.; Pinto, J.F.N.; dos Reis, E.F. Selection indexes in simultaneous increment of yield components in top-cross hybrids of green maize. Pesqui. Agropecu. Bras. 2020, 55, 1–8. [Google Scholar]
- Crevelari, J.A.; Pereira, M.G.; Azevedo, F.H.V.; Vieira, R.A.M. Genetic improvement of silage maize: Predicting genetic gain using selection indexes and best linear unbiased prediction. Rev. Ciênc. Agron. 2019, 50, 197–204. [Google Scholar] [CrossRef]
- De Santiago, S.; de Souza Junior, C.L.; Lemos, L.B.; Môro, G.V. Prediction of genetic gain using selection indices in maize lines. Afr. J. Agric. Res. 2019, 14, 787–793. [Google Scholar]
- Crispim-Filho, A.J.; dos Santos, F.P.; Pinto, J.F.; Melo, P.G.; dos Reis, E.F.; Mendes-Resende, M.P. Dealing with multiple traits in maize: A new approach for selecting progenies. Crop Sci. 2020, 6, 3151–3165. [Google Scholar] [CrossRef]
- Berilli, A.P.C.G.; Pereira, M.G.; Trindade, R.S.; Costa, F.R.; Cunha, K.S. Response to the selection in the 11th cycle of reciprocal recurrent selection among full-sib families of maize. Acta Sci. 2013, 35, 435–441. [Google Scholar]
- Comstock, R.E.; Robinson, H.F. The components of genetic variance in populations of biparental progenies and their use in estimating the average degree of dominance. Biometrics 1948, 4, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Thai Agricultural Practice, Department of Agriculture, Thailand. Available online: http://www.doa.go.th (accessed on 5 July 2021).
- Harakotr, B.; Suriharn, B.; Tangwongchai, R.; Scott, M.P.; Lertrat, K. Anthocyanins and antioxidant activity in coloured waxy corn at different maturation stages. J. Funct. Foods 2014, 9, 109–118. [Google Scholar] [CrossRef]
- SAS Institute. SAS for Windows Version 9.0; SAS Institute: Cary, NC, USA, 2002. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedure for Agricultural Research; John Wiley and Sons: Singapore, 1984. [Google Scholar]
- Baker, R.J. Selection Indices in Plant Breeding; CRC Press: Boca Raton, FL, USA, 1986. [Google Scholar]
- Osborne, J.; Simonne, E. Data collection and statistical topics for the preparation and review of manuscripts. Horttechnology 2002, 12, 567–583. [Google Scholar] [CrossRef]
- SAS Institute. JMP Trial 15.0.0 (403428); SAS Institute: Cary, NC, USA, 2021. [Google Scholar]
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publishers: New Delhi, India, 2004. [Google Scholar]
- Souza, A.R.R.; Miranda, G.V.; Pereira, M.G.; de Souza, L.V. Predicting the genetic gain in the Brazilian white maize landrace. Ciênc. Rural 2009, 39, 19–24. [Google Scholar] [CrossRef]
- Bernardo, R. Breeding for Quantitative Traits in Plants; Stemma Press: Woodbury, MN, USA, 2020. [Google Scholar]
- Hallauer, A.R.; Miranda, J.B. Quantitative Genetics in Maize Breeding, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1988. [Google Scholar]
- Hussanun, S.; Suriharn, B.; Lertrat, K. Yield and early maturity response to four cycles of modified mass selection in purple waxy corn. Turk. J. Field Crops 2014, 19, 84–89. [Google Scholar] [CrossRef]
- Khamphasan, P.; Lomthaisong, K.; Harakotr, B.; Scott, M.P.; Lertrat, K.; Suriharn, B. Effects of mass selection on husk and cob color in five purple corn populations segregating for purple husks. Agriculture 2020, 10, 311. [Google Scholar] [CrossRef]
- Sukto, S.; Lomthaisong, K.; Sanitchon, J.; Chankaew, S.; Scott, M.P.; Lübberstedt, T.; Lertrat, K.; Suriharn, B. Variability in prolificacy, total carotenoids, lutein, and zeaxanthin of yellow small-ear waxy corn germplasm. Int. J. Agron. 2020, 2020, 8818768. [Google Scholar] [CrossRef]
- Lu, D.L.; Sun, X.L.; Yan, F.B.; Wang, X.; Xu, R.C.; Lu, W.P. Effects of high temperature during grain filling under control conditions on the physicochemical properties of waxy maize flour. Carbohydr. Polym. 2013, 98, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Shi, Y.; Xu, R.; Lu, D.; Lu, W. Effects of shading after pollination on kernel filling and physicochemical quality traits of waxy maize. Crop J. 2016, 4, 235–245. [Google Scholar] [CrossRef]
- Miyagi, A. Influence of Japanese consumer gender and age on sensory attributes and preference (a case study on deep-fried peanuts). J. Sci. Food Agric. 2017, 97, 4009–4015. [Google Scholar] [CrossRef]
- Mora, M.; Urdaneta, E.; Chaya, C. Emotional response to wine: Sensory properties, age and gender as drivers of consumer’s preferences. Food Qual. Prefer. 2018, 66, 19–28. [Google Scholar] [CrossRef]
- Evans, M.M.S.; Kemicle, J.L. Interaction between maternal effect and zygotic effect mutations during maize seed development. Genetics 2001, 159, 303–315. [Google Scholar] [CrossRef]
- Maphumulo, S.G.; Derera, J.; Qwabe, F.; Fato, P.; Gasura, E.; Mafongoya, P. Heritability and genetic gain for grain yield and path coefficient analysis of some agronomic traits in early-maturing maize hybrids. Euphytica 2015, 206, 225–244. [Google Scholar] [CrossRef]
- Habib, S.H.; Iftekharuddaula, K.M.; Bashar, M.K.; Akter, K.; Hossain, M.K. Genetic variation, correlation and selection indices in advanced breeding lines of rice (Oryza sativa L.). Bangladesh J. Pl. Breed. Genet. 2007, 20, 25–32. [Google Scholar] [CrossRef]
- Tahir, M.; Khalil, I.H.; McCord, P.H.; Glaz, B. Character association and selection indices in sugarcane. Am. J. Exp. Agric. 2014, 4, 336–348. [Google Scholar] [CrossRef]
- Yang, Z.P.; Wu, Z.S.; Lin, Y.B. Inheritance and selection of agronomic characters in an intermating population of wheat. Acta Agric. Shanghai 1991, 7, 23–28. [Google Scholar]
- Vieira, R.A.; Rocha, R.; Scapim, C.A.; Amaral Junior, A.T. Recurrent selection of popcorn composites UEMCO1 and UEM-CO2 based on selection indices. Crop Breed. Appl. Biotechnol. 2017, 17, 266–272. [Google Scholar] [CrossRef][Green Version]
- Jahufer, M.Z.Z.; Casler, M.D. Application of the Smith-Hazel selection index for improving biomass yield and quality of switchgrass. Crop Sci. 2015, 55, 1212–1222. [Google Scholar] [CrossRef]
- Roudbary, Z.; Mohammadi-Nejad, G.; Shahsavand-Hassani, H. Field screening of primary and secondary Tritipyrum genotypes using selection indices based on BLUP under saline and normal conditions. Crop Sci. 2017, 57, 1495–1503. [Google Scholar] [CrossRef]
- Itoh, Y.; Yamada, Y. Selection indices for desired relative genetic gains with inequality constraints. Theor. Appl. Genet. 1988, 75, 731–735. [Google Scholar] [CrossRef]
- Yamada, Y.; Yokouchi, K.; Nishida, A. Selection index when genetic gainsof individual traits are of primary concern. Jpn. J. Genet. 1975, 50, 33–41. [Google Scholar] [CrossRef]
- Lin, C.Y. Index selection for genetic improvement of quantitative characters. Theor. Appl. Genet. 1978, 52, 49–56. [Google Scholar] [CrossRef]
- Pešek, J.; Baker, R.J. Desired improvement in relation to selection indices. Can. J. Plant Sci. 1969, 49, 803–804. [Google Scholar] [CrossRef]
- de Siqueira Gesteira, G.; Bruzi, A.T.; Zito, R.K.; Fronza, V.; Arantes, N.E. Selection of early soybean inbred lines using multiple indices. Crop Sci. 2018, 58, 2494–2502. [Google Scholar] [CrossRef]
- Simonne, A.; Cazaux, S.; Simonne, E.; Kouri, K.; Studstill, D.; Hochmuth, R.; Stapleton, S.; Davis, W.; Taylor, M. Assessing the eating quality of muskmelon varieties using sensory evaluation. Proc. Fla. State Hortic. Soc. 2003, 116, 360–363. [Google Scholar]
- Solomon, K.F.; Zeppa, A.; Mulugeta, A.D. Combining ability, genetic diversity and heterosis in relation to F1 performance of tropically adapted shrunken (sh2) sweet corn lines. Plant Breed. 2012, 131, 430–436. [Google Scholar] [CrossRef]
- Sánchez-Toledano, B.I.; Kallas, Z.; Gil-Roig, J.M. Farmer preference for improved corn seeds in Chiapas, Mexico: A choice experiment approach. Span. J. Agric. Res. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Edwards, J. Changes in plant morphology in response to recurrent selection in the Iowa Stiff Stalk Synthetic maize population. Crop Sci. 2011, 51, 2352–2361. [Google Scholar] [CrossRef]
- Vieira, M.A.; Camargo, M.K.; Daros, E.; Zagonel, J.; Koehler, H.S. Cultivares de milho e população de plantas que afetam a produtividade de espigas verdes. Acta Sci. Agron. 2010, 32, 81–86. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, W.; Peng, J.Y.; Chen, Z.M. Study on yield loss of summer maize due to lodging at the big flare stage and grain filling stage. Sci. Agric. Sin. 2015, 19, 3952–3964. [Google Scholar]
- Lertrat, K.; Pulam, T. Breeding for increased sweetness in sweet corn. Int. J. Plant Breed. 2007, 1, 27–30. [Google Scholar]

| Parental Lines | Genotype | Source of Ancestors | Per Se Performance | |||||
|---|---|---|---|---|---|---|---|---|
| DTA 1 | PHE 1 | HYI 1 | TSU 2 | AMY 2 | TSA 2 | |||
| supersweet corn | ||||||||
| 101 LBW | bt2bt2Sh2Sh2wx1wx1 | USA | 53 | 170.3 | 6.8 (*) | 140.0 (*) | 38.1 | 45.4 |
| 101 L/TSC-4 | bt2bt2sh2sh2wx1wx1 | Thai/USA | 47 (*) | 119.3 (*) | 4.2 | 108.7 | 61.1 | 69.0 |
| 101 L/TSC-10 | bt2bt2sh2sh2wx1wx1 | Thai/USA | 46 (*) | 102.3 (*) | 4.9 | 87.2 (*) | 62.4 | 68.5 |
| waxy corn | ||||||||
| YINNUO 18 | Bt2Bt2Sh2Sh2wx1wx1 | China | 58 | 149.3 | 6.9 (*) | 33.8 | 150.7 | 157.5 |
| CAITIANNUO 13-1 | Bt2Bt2Sh2Sh2wx1wx1 | China | 55 | 144.2 (*) | 8.1 (*) | 39.6 | 136.6 | 145.7 |
| HONGYU 2 | Bt2Bt2Sh2Sh2wx1wx1 | China | 54 | 138.4 (*) | 7.3 | 20.3 | 143.4 | 151.7 |
| HJ | Bt2Bt2Sh2Sh2wx1wx1 | China | 61 | 143.0 | 5.2 | 39.2 | 105.8 | 121.9 |
| ORANGE WAXY 13 | Bt2Bt2Sh2Sh2wx1wx1 | China | 57 | 167.6 | 5.0 | 32.1 | 126.3 | 132.4 |
| KV/mon | Bt2Bt2Sh2Sh2wx1wx1 | Thai/USA/Vietnam | 49 (*) | 140.5 (*) | 6.9 | 33.1 | 177.5 (*) | 181.6 (*) |
| KV/3473 | Bt2Bt2Sh2Sh2wx1wx1 | Thai/USA/Vietnam | 52 (*) | 125.1 (*) | 4.7 | 30.2 | 153.7 | 165.6 |
| KNM102 | Bt2Bt2Sh2Sh2wx1wx1 | Thailand | 54 | 129.9 | 2.2 | 104.7 | 32.7 | 122.2 |
| Source | df | Mean Squares | ||||||
|---|---|---|---|---|---|---|---|---|
| ASI | PHD | EP 1 | HEL | NRE | NKR | TKE | ||
| Season | 1 | 61.4 * | 4.7 2 ns | 1.8 ns | 940.6 ** | 121.2 * | 3987.9 ** | 1016334.0 ** |
| Block/season | 4 | 5.2 ** | 0.0 ns | 0.2 ** | 6.6 * | 5.9 ** | 43.4 ** | 13652.2 ** |
| Cross | 47 | 298.9 ** | 6637.0 ** | 4.6 ** | 16.0 ** | 12.2 ** | 69.2 ** | 26897.9 ** |
| Reciprocal (rec) | 23 | 119.4 ** | 6081.8 ** | 2.7 ** | 3.9 ** | 10.5 ** | 1.7 ns | 3564.6 ** |
| Hybrid | 23 | 106.3 ** | 6081.8 ** | 3.4 ** | 26.0 ** | 13.2 ** | 125.6 ** | 44833.4 ** |
| Hybrid vs. rec | 1 | 8855.5 ** | 32173.3 * | 75.8 ns | 61.5 ns | 29.3 ns | 326.6 ns | 151048.4 ns |
| Season x cross | 47 | 12.4 ** | 284.1 ** | 0.1 ** | 7.9 ** | 0.9 ** | 24.5 ** | 5797.7 ** |
| Season x rec | 23 | 11.7 ** | 285.8 ** | 0.1 * | 8.9 ** | 0.9 ** | 28.3 ** | 5957.8 ** |
| Season x hybrid | 23 | 10.8 ** | 285.8 ** | 0.1 * | 2.4 ns | 0.7 ** | 13.6 ** | 4356.0 ** |
| Season x (hybrid vs. rec) | 1 | 63.2 ** | 206.0 ns | 0.1 ns | 109.2 ** | 3.9 ** | 187.3 ** | 35276.1 ** |
| Pooled error | 188 | 1.2 | 95.0 | 0.1 | 1.9 | 0.3 | 6.2 | 1012.9 |
| CV (%) | 12.95 | 1.18 | 5.55 | 11.27 | 4.48 | 11.33 | 11.29 | |
| Source | df | Mean Squares | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AD | SD | PH | EH | HEY | KD 1 | STC | SWT | TND | OL | ||
| Season | 1 | 483.0 ** | 70.0 ** | 161.8 ns | 87.3 ns | 326.1 ** | 82.0 ** | 4.0 ns | 31.3 ns | 0.2 ns | 35.2 ns |
| Block/season | 4 | 0.6 ns | 0.7 ns | 185.6 * | 69.7 ns | 0.1 ns | 2.5 ** | 49.2 ** | 75.3 ** | 49.7 ** | 52.0 ** |
| Cross | 47 | 34.8 ** | 35.0 ** | 2019.1 ** | 1269.3 ** | 11.7 ** | 1.0 ** | 3.2 ** | 3.9 ** | 3.0 ** | 3.1 ** |
| Reciprocal (rec) | 23 | 35.1 ** | 36.1 ** | 1804.5 ** | 1186.3 ** | 12.2 ** | 1.0 ** | 4.2 ** | 3.4 * | 3.7 ** | 3.0 ** |
| Hybrid | 23 | 35.0 ** | 28.9 ** | 2309.9 ** | 1403.4 ** | 11.7 ** | 1.0 ** | 2.1 * | 4.4 ** | 2.0 ns | 3.4 ** |
| Hybrid vs. rec | 1 | 23.9 ns | 150.2 ns | 266.6 ns | 92.0 ns | 0.3 ns | 0.1 ns | 5.2 ns | 5.4 ns | 9.2 ns | 0.4 ns |
| Season x cross | 47 | 2.3 ** | 3.8 ** | 174.4 ** | 48.1 ns | 1.6 ** | 0.4 * | 3.5 ** | 3.1 ** | 2.6 ** | 2.6 ** |
| Season x rec | 23 | 1.3 ns | 2.7 ** | 128.5 * | 28.5 ns | 0.9 ** | 0.2 ns | 4.0 ** | 3.5 * | 3.2 ** | 2.9 ** |
| Season x hybrid | 23 | 1.6 ns | 2.3 * | 110.5 ns | 61.6 * | 1.7 ** | 0.6 ** | 3.0 ** | 2.9 ns | 2.1 ns | 2.5 * |
| Season x (hybrid vs. rec) | 1 | 39.7 ** | 66.1 ** | 2698.0 ** | 188.8 * | 15.23 ** | 0.9 ns | 0.9 ns | 0.1 ns | 0.8 ns | 0.4 ns |
| Pooled error | 188 | 1.4 | 1.4 | 74.3 | 36.8 | 0.3 | 0.2 | 1.3 | 1.8 | 1.4 | 1.3 |
| CV (%) | 2.56 | 2.57 | 4.95 | 7.48 | 5.28 | 5.56 | 12.82 | 19.85 | 13.62 | 13.02 | |
| Traits | GA | RG 1 (%) | RG 2 (%) | |||
|---|---|---|---|---|---|---|
| Seed producer preference | ||||||
| Anthesis-silking interval | 44.02 | 45.24 | 0.97 | 1151.92 | −42.04 | −45.89 |
| Plant height difference | 995.78 | 1090.78 | 0.91 | 5306.50 | −39.46 | −72.09 |
| Ear position | 0.75 | 0.85 | 0.88 | 143.17 | −16.95 | −18.77 |
| Husked ear length | 0.65 | 2.55 | 0.25 | 71.64 | 8.33 | 14.71 |
| Row number per ear | 1.68 | 1.98 | 0.85 | 210.37 | 6.05 | 6.94 |
| Kernel number per row | 1.35 | 7.55 | 0.18 | 86.47 | 6.73 | 16.24 |
| Total kernel number per ear | 1921.77 | 2934.67 | 0.65 | 6243.58 | 12.26 | 22.93 |
| Grower and consumer preferences | ||||||
| Anthesis date | 5.12 | 6.52 | 0.79 | 352.77 | −5.09 | −6.33 |
| Silking date | 4.40 | 5.80 | 0.76 | 321.55 | −5.64 | −6.35 |
| Plant height | 274.08 | 348.38 | 0.79 | 2584.44 | −9.41 | −10.11 |
| Ear height | 199.77 | 236.57 | 0.84 | 2285.91 | −17.44 | −21.77 |
| Husked ear yield | 1.90 | 2.20 | 0.86 | 225.45 | 10.76 | 14.56 |
| Kernel depth | 0.03 | 0.23 | 0.14 | 12.15 | 4.35 | 3.01 |
| Stickiness | 0.32 | 1.62 | 0.19 | 43.83 | 0.11 | 0.07 |
| Sweetness | 0.35 | 2.15 | 0.16 | 42.01 | 11.11 | 2.06 |
| Tenderness | 0.27 | 1.67 | 0.16 | 36.35 | 6.12 | 2.61 |
| Overall liking | 0.30 | 1.60 | 0.19 | 41.74 | 5.96 | 0.68 |
| Cross | Parent | Female | Male | F1 Progeny | Z | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ASI | EP | TKE | PHD | AD | PH | KD | HEY | OL | ||
| Dry season | ||||||||||
| 101L/TSC-10 × KV/mon | 1 | 0.33 | 307 | 42.0 | 44 | 151.3 | 1.04 | 11.2 | 11.7 | 7.84 |
| KV/mon × 101L/TSC-10 | 2 | 0.38 | 471 | −42.0 | 43 | 153.4 | 1.03 | 12.1 | 10.7 | 6.69 |
| KV/3473 × 101LBW | 2 | 0.45 | 341 | 52.0 | 47 | 190.4 | 1.20 | 13.3 | 10.5 | 6.62 |
| 101L/TSC-10 × CAITIANNUO 13-1 | 7 | 0.33 | 307 | 52.0 | 46 | 150.2 | 1.07 | 12.8 | 9.9 | 5.84 |
| 101L/TSC-10 × YINNUO 18 | 10 | 0.33 | 307 | 52.5 | 48 | 146.7 | 1.11 | 12.4 | 9.8 | 5.01 |
| Grand mean 1 | 7 | 0.42 | 341 | 0.0 | 48 | 173.5 | 1.03 | 11.5 | 9.3 | |
| HSD 5% 1 | 2 | 0.03 | 62 | 17.2 | 1 | 15.9 | 0.11 | 0.3 | 1.8 | |
| Rainy season | ||||||||||
| 101L/TSC-10 × KV/mon | 0 | 0.32 | 225 | 28.7 | 42 | 162.2 | 0.97 | 10.9 | 7.8 | 7.15 |
| 101L/TSC-10 × KV/3473 | 2 | 0.32 | 225 | 23.6 | 42 | 150.8 | 0.97 | 10.5 | 8.2 | 7.13 |
| KV/mon × 101L/TSC-4 | 3 | 0.41 | 346 | −12.6 | 42 | 158.2 | 0.90 | 11.6 | 9.5 | 6.40 |
| KV/mon × 101L/TSC-10 | 4 | 0.41 | 346 | −28.7 | 42 | 150.5 | 0.95 | 10.7 | 8.7 | 5.76 |
| 101L/TSC-10 × CAITIANNUO 13-1 | 5 | 0.32 | 225 | 26.6 | 43 | 164.8 | 0.96 | 10.4 | 8.7 | 5.49 |
| Grand mean 2 | 6 | 0.44 | 223 | 0.0 | 45 | 175.0 | 0.92 | 9.43 | 8.6 | |
| HSD 5% 2 | 2 | 0.04 | 39 | 14.3 | 2 | 11.7 | 0.05 | 0.4 | 1.9 | |
| Cross | Overall Rank Sum (ORS) | ORSI 4 | |||||
|---|---|---|---|---|---|---|---|
| Seed Producer 1 | Grower 2 | Consumer 3 | |||||
| Dry | Rainy | Dry | Rainy | Dry | Rainy | ||
| 101LTSC-10 × KV/mon | 45.5 | 35.0 | 47.5 | 58.0 | 32.0 | 142.0 | 360.0 |
| KV/mon × 101L/TSC-10 | 76.0 | 85.5 | 46.0 | 45.0 | 34.0 | 126.0 | 412.5 |
| 101LTSC-10 × CAITIANNUO 13-1 | 63.5 | 59.5 | 48.5 | 48.5 | 115.0 | 106.5 | 441.5 |
| KV/mon × 101L/TSC-4 | 65.0 | 70.0 | 81.5 | 67.0 | 112.0 | 54.5 | 450.0 |
| 101LTSC-10 × KV3473 | 67.0 | 52.0 | 66.5 | 36.0 | 97.5 | 141.5 | 460.5 |
| Minimum | 45.5 | 35.0 | 46.0 | 36.0 | 32.0 | 50.0 | 360.0 |
| Maximum | 160.5 | 146.0 | 199.5 | 200.0 | 222.0 | 210.0 | 894.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dermail, A.; Fuengtee, A.; Lertrat, K.; Suwarno, W.B.; Lübberstedt, T.; Suriharn, K. Simultaneous Selection of Sweet-Waxy Corn Ideotypes Appealing to Hybrid Seed Producers, Growers, and Consumers in Thailand. Agronomy 2022, 12, 87. https://doi.org/10.3390/agronomy12010087
Dermail A, Fuengtee A, Lertrat K, Suwarno WB, Lübberstedt T, Suriharn K. Simultaneous Selection of Sweet-Waxy Corn Ideotypes Appealing to Hybrid Seed Producers, Growers, and Consumers in Thailand. Agronomy. 2022; 12(1):87. https://doi.org/10.3390/agronomy12010087
Chicago/Turabian StyleDermail, Abil, Aphakorn Fuengtee, Kamol Lertrat, Willy Bayuardi Suwarno, Thomas Lübberstedt, and Khundej Suriharn. 2022. "Simultaneous Selection of Sweet-Waxy Corn Ideotypes Appealing to Hybrid Seed Producers, Growers, and Consumers in Thailand" Agronomy 12, no. 1: 87. https://doi.org/10.3390/agronomy12010087
APA StyleDermail, A., Fuengtee, A., Lertrat, K., Suwarno, W. B., Lübberstedt, T., & Suriharn, K. (2022). Simultaneous Selection of Sweet-Waxy Corn Ideotypes Appealing to Hybrid Seed Producers, Growers, and Consumers in Thailand. Agronomy, 12(1), 87. https://doi.org/10.3390/agronomy12010087







