Bactrocera oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthetic Compounds
2.2. Field Bioassay
2.3. Measurement of Volatile Release
2.4. Statistical Analysis
2.4.1. Analysis of Variance
2.4.2. Pearson Correlation Coefficient
3. Results and Discussion
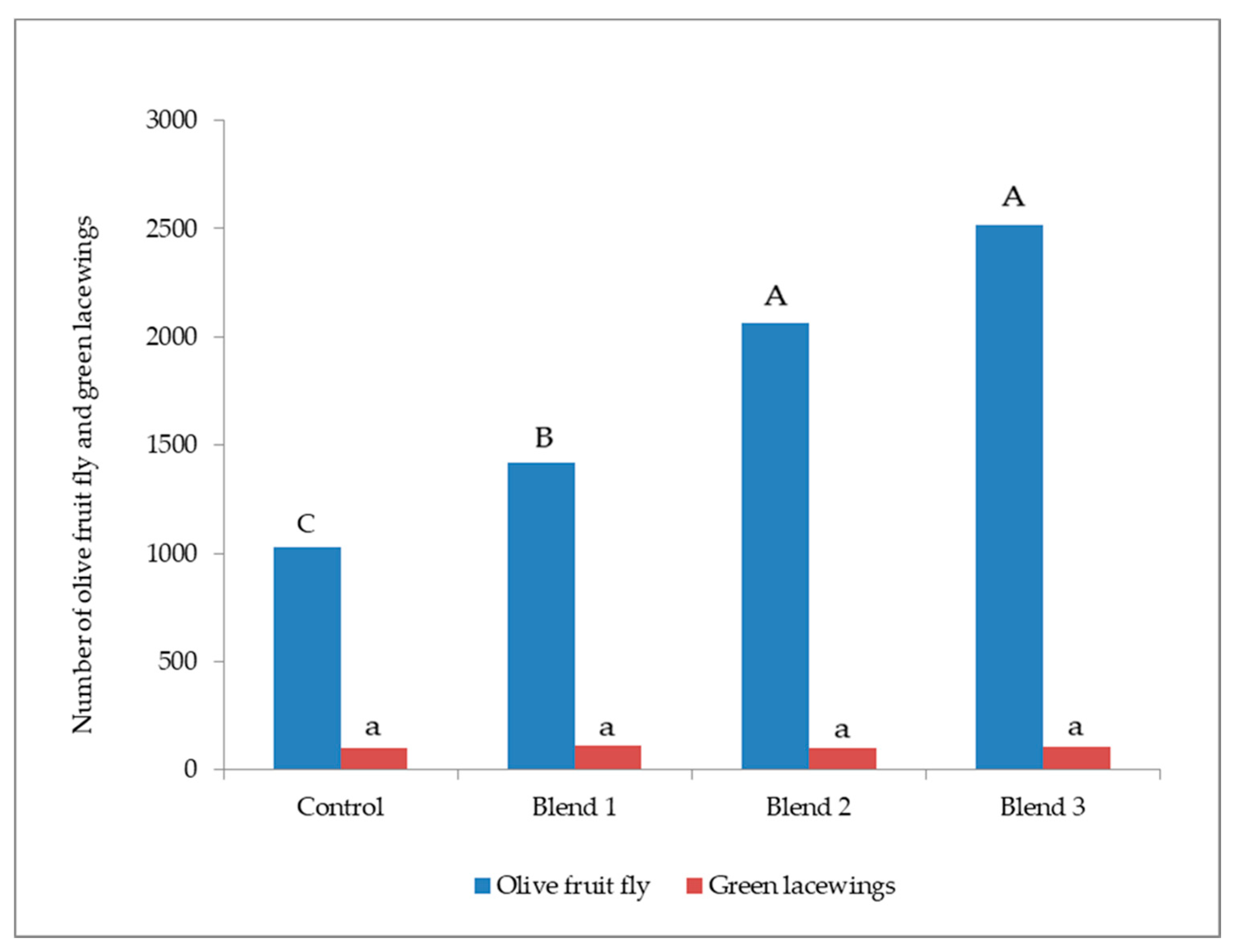
3.1. Bactrocera Oleae and Green Lacewings Attraction to Three Blends of OFF-Associated Yeast Volatile Compounds in Olive Orchard
3.2. Influence of Climatic Parameters on the Abundance of Bactrocera Oleae and Green Lacewings Caught on YS Traps Containing Different Blends of OFF-Associated Yeast Volatile Compounds
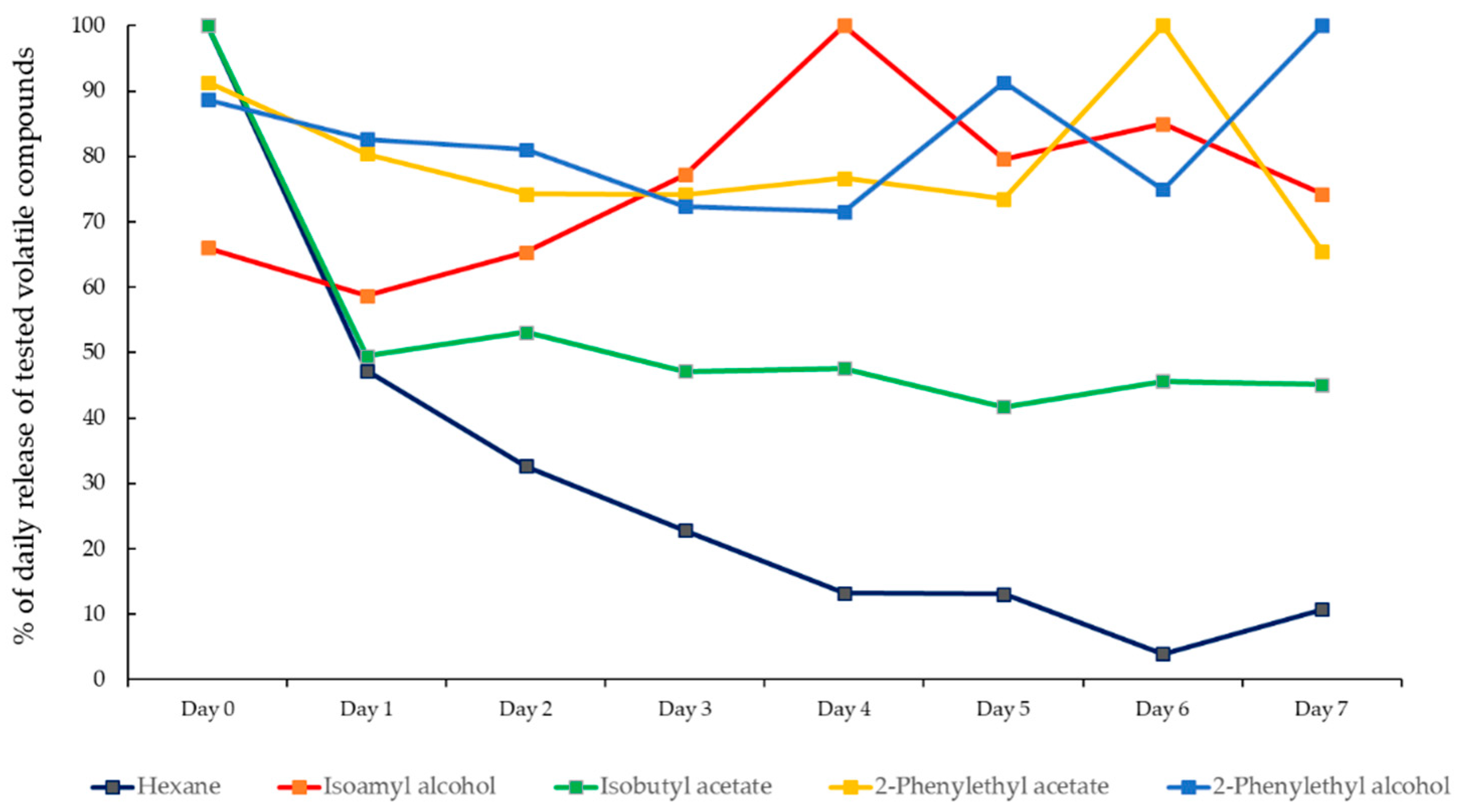
3.3. Daily Volatile Release of Investigated Blends in the Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EIP-AGRI Focus Group Pests and Diseases of the Olive Tree: Final Report. Available online: https://ec.europa.eu/eip/agriculture/en/publications/eip-agri-focus-group-pests-and-diseases-olive-tree-0 (accessed on 15 November 2021).
- Kapatos, Ε.Τ.; Fletcher, B.S. An assessment of components of crop loss due to infestation by Dacus oleae, in Corfu. Entomol. Hell. 1983, 1, 7–16. [Google Scholar] [CrossRef][Green Version]
- Sharaf, N.S. Life history of the olive fruit fly, Dacus oleae Gmel. (Diptera: Tephritidae), and its damage to olive fruits in Tripolitania. J. Appl. Entomol. 1980, 89, 390–400. [Google Scholar] [CrossRef]
- Rice, R.E. Bionomics of the Olive Fruit Fly Bactrocera (Dacus) oleae. UC Plant Prot. Q. 2000, 10, 1–5. [Google Scholar]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Olive volatiles from Portuguese cultivars Cobrançosa, Madural and Verdeal Transmontana: Role in oviposition preference of Bactrocera oleae (Rossi) (Diptera: Tephritidae). PLoS ONE 2015, 10, e0125070. [Google Scholar] [CrossRef]
- Daane, K.M.; Johnson, M.W. Olive fruit fly: Managing an ancient pest in modern times. Annu. Rev. Entomol. 2010, 55, 151–169. [Google Scholar] [CrossRef]
- Montiel Bueno, A.; Jones, O. Alternative methods for controlling the olive fly, Bactrocera oleae, involving semiochemicals. In Proceedings of the IOBC WPRS Bulletin Pheromones and Other Semio-Chemicals in Integrated Production; Witzgall, P., Mazomenos, B., Konstantopoulou, M., Eds.; IOBC-WPRS: Samos, Greece, 2002; Volume 25, pp. 147–156. [Google Scholar]
- Collier, T.R.; Van Steenwyk, R.A. UC Agriculture & Natural Resources California Agriculture Title Prospects for integrated control of olive fruit fly are promising in California. Calif. Agric. 2003, 57, 28–32. [Google Scholar]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N.; et al. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Vitanović, E.; Ivezić, M.; Kačić, S.; Katalinić, M.; Durbešić, P.; Barčić, J.I. Arthropod communities within the olive canopy as bioindicators of different management systems. Span. J. Agric. Res. 2018, 16, e0301. [Google Scholar] [CrossRef]
- Amvrazi, E.G.; Albanis, T.A. Pesticide residue assessment in different types of olive oil and preliminary exposure assessment of Greek consumers to the pesticide residues detected. Food Chem. 2009, 113, 253–261. [Google Scholar] [CrossRef]
- Hakme, E.; Lozano, A.; Ferrer, C.; Díaz-Galiano, F.J.; Fernández-Alba, A.R. Analysis of pesticide residues in olive oil and other vegetable oils. TrAC—Trends Anal. Chem. 2018, 100, 167–179. [Google Scholar] [CrossRef]
- Lentza-Rizos, C.; Avramides, E. Pesticide residues in olive oil. Rev. Environ. Contam. Toxicol. 1995, 141, 111–134. [Google Scholar] [CrossRef]
- Generosa, C.; Omar Mohamed, A.; Perrino, E. V Correlations between organic and conventional management, on-field biodiversity and landscape diversity, in olive groves in Apulia (Italy). Adv. Plants Agric. Res. 2016, 5, 568–583. [Google Scholar] [CrossRef][Green Version]
- Lantero, E.; Matallanas, B.; Pascual, S.; Dolores Ochando, M.; Callejas, C. Phylogeography of organophosphate resistant ace alleles in spanish olive fruit fly populations: A mediterranean perspective in the global change context. Insects 2020, 11, 396. [Google Scholar] [CrossRef]
- Liscia, A.; Angioni, P.; Sacchetti, P.; Poddighe, S.; Granchietti, A.; Setzu, M.D.; Belcari, A. Characterization of olfactory sensilla of the olive fly: Behavioral and electrophysiological responses to volatile organic compounds from the host plant and bacterial filtrate. J. Insect Physiol. 2013, 59, 705–716. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Scarpati, M.L.; Verzegnassi, B.; Vita, G. Olea europaea chemicals repellent to Dacus oleae females. J. Chem. Ecol. 1994, 20, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, M.L.; Scalzo, R.L.; Vita, G. Olea europaea volatiles attractive and repellent to the olive fruit fly (Dacus oleae, Gmelin). J. Chem. Ecol. 1993, 19, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Scarpati, M.L.; Lo Scalzo, R.; Vita, G.; Gambacorta, A. Chemiotropic behavior of female olive fly (Bactrocera oleae Gmel.) on Olea europaea L. J. Chem. Ecol. 1996, 22, 1027–1036. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Baptista, P.; Pereira, J.A. A review of Bactrocera oleae (Rossi) impact in olive products: From the tree to the table. Trends Food Sci. Technol. 2015, 44, 226–242. [Google Scholar] [CrossRef]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Mosca, S.; Giunti, G.; Strano, C.P.; Palmeri, V. A Metabarcoding Survey on the Fungal Microbiota Associated to the Olive Fruit Fly. Microb. Ecol. 2017, 73, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Augustinos, A.A.; Tsiamis, G.; Cáceres, C.; Abd-Alla, A.M.M.; Bourtzis, K. Taxonomy, diet, and developmental stage contribute to the structuring of gut-associated bacterial communities in tephritid pest species. Front. Microbiol. 2019, 10, 2004. [Google Scholar] [CrossRef]
- Blow, F.; Gioti, A.; Goodhead, I.B.; Kalyva, M.; Kampouraki, A.; Vontas, J.; Darby, A.C. Functional Genomics of a Symbiotic Community: Shared Traits in the Olive Fruit Fly Gut Microbiota. Genome Biol. Evol. 2020, 12, 3778–3791. [Google Scholar] [CrossRef] [PubMed]
- Koskinioti, P.; Ras, E.; Augustinos, A.A.; Tsiamis, G.; Beukeboom, L.W.; Caceres, C.; Bourtzis, K. The effects of geographic origin and antibiotic treatment on the gut symbiotic communities of Bactrocera oleae populations. Entomol. Exp. Appl. 2019, 167, 197–208. [Google Scholar] [CrossRef]
- Vitanović, E.; Aldrich, J.R.; Boundy-Mills, K.; Čagalj, M.; Ebeler, S.E.; Burrack, H.; Zalom, F.G. Olive Fruit Fly, Bactrocera oleae (Diptera: Tephritidae), Attraction to Volatile Compounds Produced by Host and Insect-Associated Yeast Strains. J. Econ. Entomol. 2020, 113, 752–759. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Suckling, D.M.; Byers, J.A.; Jang, E.B.; Wearing, C.H. Potential of “Lure and Kill” in Long-Term Pest Management and eradication of invasive species. J. Econ. Entomol. 2009, 102, 815–835. [Google Scholar] [CrossRef] [PubMed]
- Shorey, H.H. Behavioral responses to insect pheromones. Annu. Rev. Entomol. 1973, 18, 349–380. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.C.; Sansom, C.E.; Larsen, L.; Worner, S.P.; Rostás, M.; Chapman, R.B.; Butler, R.C.; de Kogel, W.J.; Davidson, M.M.; Perry, N.B.; et al. Volatile compounds as insect lures: Factors affecting release from passive dispenser systems. N. Z. J. Crop Hortic. Sci. 2019, 47, 208–223. [Google Scholar] [CrossRef]
- Piper, A.M.; Farnier, K.; Linder, T.; Speight, R.; Cunningham, J.P. Two Gut-Associated Yeasts in a Tephritid Fruit Fly have Contrasting Effects on Adult Attraction and Larval Survival. J. Chem. Ecol. 2017, 43, 891–901. [Google Scholar] [CrossRef]
- Batista, M.R.D.; Uno, F.; Chaves, R.D.; Tidon, R.; Rosa, C.A.; Klaczko, L.B. Differential attraction of drosophilids to banana baits inoculated with Saccharomyces cerevisiae and Hanseniaspora uvarum within a Neotropical forest remnant. PeerJ 2017, 5, e3063. [Google Scholar] [CrossRef]
- Dweck, H.K.M.; Ebrahim, S.A.M.; Thoma, M.; Mohamed, A.A.M.; Keesey, I.W.; Trona, F.; Lavista-Llanos, S.; Svatoš, A.; Sachse, S.; Knaden, M.; et al. Pheromones mediating copulation and attraction in Drosophila. Proc. Natl. Acad. Sci. USA 2015, 112, 2829–2835. [Google Scholar] [CrossRef]
- Mori, B.A.; Whitener, A.B.; Leinweber, Y.; Revadi, S.; Beers, E.H.; Witzgall, P.; Becher, P.G. Enhanced yeast feeding following mating facilitates control of the invasive fruit pest Drosophila suzukii. J. Appl. Ecol. 2017, 54, 170–177. [Google Scholar] [CrossRef]
- Hamby, K.A.; Becher, P.G. Current knowledge of interactions between Drosophila suzukii and microbes, and their potential utility for pest management. J. Pest Sci. 2016, 89, 621–630. [Google Scholar] [CrossRef]
- Deutscher, A.T.; Reynolds, O.L.; Chapman, T.A. Yeast: An Overlooked Component of Bactrocera tryoni (Diptera: Tephritidae) Larval Gut Microbiota. J. Econ. Entomol. 2017, 110, 298–300. [Google Scholar] [CrossRef]
- Vitanović, E.; Lopez, J.M.; Aldrich, J.R.; Špika, M.J.; Boundy-Mills, K.; Zalom, F.G. Yeasts Associated with the Olive Fruit Fly Bactrocera oleae (Rossi) (Diptera: Tephritidae) Lead to New Attractants. Agronomy 2020, 10, 1501. [Google Scholar] [CrossRef]
- Mahzoum, A.M.; Villa, M.; Benhadi-Marín, J.; Pereira, J.A. Functional response of Chrysoperla carnea (Neuroptera: Chrysopidae) larvae on Saissetia oleae (Olivier) (Hemiptera: Coccidae): Implications for biological control. Agronomy 2020, 10, 1511. [Google Scholar] [CrossRef]
- Arambourg, Y.; Pralavorio, R. Note on certain morphological characters of Prays oleae Bern. and of Prays citri Mil. (Lep. Hyponomeutidae). Rev. Zool. Agric. Pathol. Veg. 1978, 77, 143–146. [Google Scholar]
- Pantaleoni, R.A.; Lentini, A.; Delrio, G. Lacewings in Sardinian olive groves. In Lacewings in the Crop Environment; McEwen, P.K., New, T.R., Whittington, A.E., Eds.; Cambridge University: Cambridge, UK, 2001; pp. 435–446. [Google Scholar]
- Gharbi, N. Effectiveness of inundative releases of Anthocoris nemoralis (Hemiptera: Anthocoridae) in controlling the olive psyllid Euphyllura olivina (Hemiptera: Psyllidae). Eur. J. Entomol. 2021, 118, 135–141. [Google Scholar] [CrossRef]
- Miller, D.R.; Rung, A.; Parikh, G. Scale Insects, edition 2, a tool for the identification of potential pest scales at U.S.A. ports-of-entry (Hemiptera, Sternorrhyncha, Coccoidea). Zookeys 2014, 431, 61. [Google Scholar] [CrossRef]
- Garratt, M.P.D.; Wright, D.J.; Leather, S.R. The effects of farming system and fertilisers on pests and natural enemies: A synthesis of current research. Agric. Ecosyst. Environ. 2011, 141, 261–270. [Google Scholar] [CrossRef]
- Broumas, T.; Haniotakis, G.; Liaropoulos, C.; Tomazou, T.; Ragoussis, N. The efficacy of an improved form of the mass-trapping method, for the control of the olive fruit fly, Bactrocera oleae (Gmelin) (Dipt., Tephritidae): Pilot-scale feasibility studies. J. Appl. Entomol. 2002, 126, 217–223. [Google Scholar] [CrossRef]
- Marubbi, T.; Cassidy, C.; Miller, E.; Koukidou, M.; Martin-Rendon, E.; Warner, S.; Loni, A.; Beech, C. Exposure to genetically engineered olive fly (Bactrocera oleae) has no negative impact on three non-target organisms. Sci. Rep. 2017, 7, 11478. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.S.; Crippen, T.L.; Hofstetter, R.W.; Tomberlin, J.K. Microbial Volatile Emissions as Insect Semiochemicals. J. Chem. Ecol. 2013, 39, 840–859. [Google Scholar] [CrossRef] [PubMed]
- Ordano, M.; Engelhard, I.; Rempoulakis, P.; Nemny-Lavy, E.; Blum, M.; Yasin, S.; Lensky, I.M.; Papadopoulos, N.T.; Nestel, D. Olive fruit fly (Bactrocera oleae) population dynamics in the Eastern Mediterranean: Influence of exogenous uncertainty on a monophagous frugivorous Insect. PLoS ONE 2015, 10, e0127798. [Google Scholar] [CrossRef]
- Becher, P.G.; Lebreton, S.; Wallin, E.A.; Hedenström, E.; Borrero, F.; Bengtsson, M.; Joerger, V.; Witzgall, P. The Scent of the Fly. J. Chem. Ecol. 2018, 44, 431–435. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Cunha, S.C.; Baptista, P.; Pereira, J.A. Identification of leaf volatiles from olive (Olea europaea) and their possible role in the ovipositional preferences of olive fly, Bactrocera oleae (Rossi) (Diptera: Tephritidae). Phytochemistry 2016, 121, 11–19. [Google Scholar] [CrossRef]
- Davis, T.S.; Landolt, P.J. A Survey of Insect Assemblages Responding to Volatiles from a Ubiquitous Fungus in an Agricultural Landscape. J. Chem. Ecol. 2013, 39, 860–868. [Google Scholar] [CrossRef]
- Hagen, K.S.; Tassan, R.L. The influence of food wheast and related Saccharomyces fragilis yeast products on the fecundity of Chrysopa carnea (Neuroptera: Chrysopidae). Can. Entomol. 1970, 102, 806–811. [Google Scholar] [CrossRef]
- Gibson, C.M.; Hunter, M.S. Reconsideration of the role of yeasts associated with Chrysoperla green lacewings. Biol. Control 2005, 32, 57–64. [Google Scholar] [CrossRef]
- Vitanović, E.; Aldrich, J.R.; Winterton, S.L.; Boundy-Mills, K.; Lopez, J.M.; Zalom, F.G. Attraction of the Green Lacewing Chrysoperla comanche (Neuroptera: Chrysopidae) to Yeast. J. Chem. Ecol. 2019, 45, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Dibo, A.; Ksantini, M. Observation of arthropod populations during outbreak of olive psyllid Euphyllura olivina in Tunisian olive groves. Tunis J. Plant Prot. 2012, 7, 35–42. [Google Scholar]
- Bjeliš, M. Zaštita Masline u Ekološkoj Proizvodnji, 2nd ed.; Own Edition: Solin, Croatia, 2009. [Google Scholar]
- Katalinić, M.; Kačić, S.; Vitanovíc, E. Štetnici i Bolesti Masline, 1st ed.; Agroknjiga: Split, Croatia, 2009. [Google Scholar]
- Wang, N.; Li, Z.H.; Wu, J.; Rajotte, E.G.; Wan, F.H.; Wang, Z.L. The potential geographical distribution of Bactrocera dorsalis (Diptera: Tephrididae) in China based on emergence rate model and ArcGIS Computer and Computing Technologies in Agriculture II. In Proceedings of the Computer and Computing Technologies in Agriculture II, Zhangjiajie, China, 19–21 October 2011; Volume 293, pp. 399–411. [Google Scholar]
- Yokoyama, V.Y. Olive fruit fly (Diptera: Tephritidae) in California: Longevity, oviposition, and development in canning olives in the laboratory and greenhouse. J. Econ. Entomol. 2012, 105, 186–195. [Google Scholar] [CrossRef][Green Version]
- Heuskin, S.; Verheggen, F.J.; Haubruge, E.; Wathelet, J.P.; Lognay, G. The use of semiochemical slow-release devices in integrated pest management strategies. Biotechnol. Agron. Soc. Environ. 2011, 15, 459–470. [Google Scholar]
- Hofmeyr, J.H.; Burger, B.V. Controlled-release pheromone dispenser for use in traps to monitor flight activity of false codling moth. J. Chem. Ecol. 1995, 21, 355–363. [Google Scholar] [CrossRef] [PubMed]

| Date | Temperature (°C) | Relative Humidity (%) | Rainfall (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Min | Max | Mean | Min | Max | Total | Min | Max | |
| 11 July 2018. | 26.3 | 25.5 | 27.6 | 48.8 | 31.0 | 70.0 | 0.8 | 0.8 | 0.8 |
| 18 July 2018. | 27.3 | 24.0 | 29 | 49.9 | 36.0 | 70.0 | 7.1 | 1.5 | 7.1 |
| 25 July 2018. | 28.1 | 25.6 | 29.2 | 42.8 | 34.0 | 60.0 | 4.1 | 1.5 | 2.6 |
| 1 August 2018. | 29.2 | 26.6 | 31.6 | 47.0 | 40.0 | 57.0 | 0.2 | 0.2 | 0.2 |
| 8 August 2018. | 29.3 | 28.6 | 30.2 | 51.1 | 44.0 | 56.0 | 1.9 | 0.3 | 1.1 |
| Adult flight of 1st gen | 28.0 | 47.9 | 14.1 | ||||||
| 15 August 2018. | 28.3 | 23.4 | 30.1 | 50.3 | 44.0 | 65.0 | 4.7 | 4.7 | 4.7 |
| 22 August 2018. | 29.3 | 28 | 30.2 | 42.4 | 36.0 | 51.0 | 0.1 | 0.1 | 0.1 |
| 29 August 2018. | 25.7 | 22.2 | 28.0 | 51.5 | 43.0 | 64.0 | 4.3 | 4.3 | 4.3 |
| 5 September 2018. | 24.9 | 22.5 | 26.6 | 57.1 | 50.0 | 65.0 | 10.2 | 0.9 | 9.3 |
| 12 September 2018. | 25.5 | 23.9 | 26.5 | 48.1 | 40.0 | 61.0 | 4.4 | 4.4 | 4.4 |
| Adult flight of 2nd gen | 26.7 | 49.9 | 23.7 | ||||||
| 19 September 2018. | 25.2 | 24.6 | 25.8 | 56.4 | 48.0 | 69.0 | 0.1 | 0.1 | 0.1 |
| 26 September 2018. | 21.2 | 14.5 | 25.1 | 57.6 | 32.0 | 68.0 | 6.2 | 6.2 | 6.2 |
| 3 October 2018. | 18.6 | 15.2 | 20.6 | 43.9 | 28.0 | 64.0 | 11.1 | 4.4 | 6.7 |
| 10 October 2018. | 20.3 | 19.0 | 21.2 | 62.3 | 33.0 | 82.0 | 76.6 | 0.1 | 67.1 |
| 18 October 2018. | 20.4 | 19.8 | 21.0 | 51.0 | 45.0 | 59.0 | |||
| 24 October 2018. | 17.9 | 14.8 | 20.2 | 58.5 | 42.0 | 88.0 | |||
| Adult flight of 3rd gen | 20.6 | 54.9 | 94.0 | ||||||
| Climatic Parameter | Temperature (°C) | Relative Humidity (%) | Rainfall (mm) | |
|---|---|---|---|---|
| Olive fruit fly | Blend 1 | r =−0.739 | r = 0.159 | r = 0.572 |
| p = 0.001 | p = 0.557 | p = 0.041 | ||
| Blend 2 | r = −0.494 | r = 0.215 | r = 0.467 | |
| p = 0.052 | p = 0.423 | p = 0.108 | ||
| Blend 3 | r = −0.487 | r = 0.091 | r = 0.330 | |
| p = 0.056 | p = 0.737 | p = 0.270 | ||
| Control | r =−0.632 | r = 0.024 | r = 0.258 | |
| p = 0.009 | p = 0.929 | p = 0.394 | ||
| Green lacewings | Blend 1 | r = 0.434 | r = −0.068 | r = −0.429 |
| p= 0.105 | p = 0.809 | p = 0.144 | ||
| Blend 2 | r = 0.729 | r = −0.297 | r =−0.557 | |
| p = 0.002 | p = 0.282 | p = 0.048 | ||
| Blend 3 | r = 0.618 | r = −0.178 | r = −0.454 | |
| p = 0.014 | p = 0.526 | p = 0.119 | ||
| Control | r = 0.664 | r = −0.214 | r = −0.485 | |
| p = 0.007 | p = 0.443 | p = 0.093 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bego, A.; Burul, F.; Popović, M.; Jukić Špika, M.; Veršić Bratinčević, M.; Pošćić, F.; Vitanović, E. Bactrocera oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants. Agronomy 2022, 12, 72. https://doi.org/10.3390/agronomy12010072
Bego A, Burul F, Popović M, Jukić Špika M, Veršić Bratinčević M, Pošćić F, Vitanović E. Bactrocera oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants. Agronomy. 2022; 12(1):72. https://doi.org/10.3390/agronomy12010072
Chicago/Turabian StyleBego, Ana, Filipa Burul, Marijana Popović, Maja Jukić Špika, Maja Veršić Bratinčević, Filip Pošćić, and Elda Vitanović. 2022. "Bactrocera oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants" Agronomy 12, no. 1: 72. https://doi.org/10.3390/agronomy12010072
APA StyleBego, A., Burul, F., Popović, M., Jukić Špika, M., Veršić Bratinčević, M., Pošćić, F., & Vitanović, E. (2022). Bactrocera oleae (Rossi) (Diptera: Tephritidae) Response to Different Blends of Olive Fruit Fly-Associated Yeast Volatile Compounds as Attractants. Agronomy, 12(1), 72. https://doi.org/10.3390/agronomy12010072









