Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Gas Chromatography–Mass Spectrometry
2.3. Identification of Individual Compounds
2.4. Antioxidant Assay
R2 = 0.9489
2.5. Tested Microorganisms
2.6. Disc Diffusion Assay
2.7. Minimal Inhibitory Concentration
2.8. Antibiofilm Effect
2.9. Exposure of Food Models to the Vapor Phase of Lemongrass EO
2.10. Determination of Minimal Growth Inhibition
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition of LGEO
3.2. Antioxidant Activity of LGEO
3.3. In Vitro Antimicrobial Properties of LGEO
3.4. In Vitro Antifungal Properties of LGEO
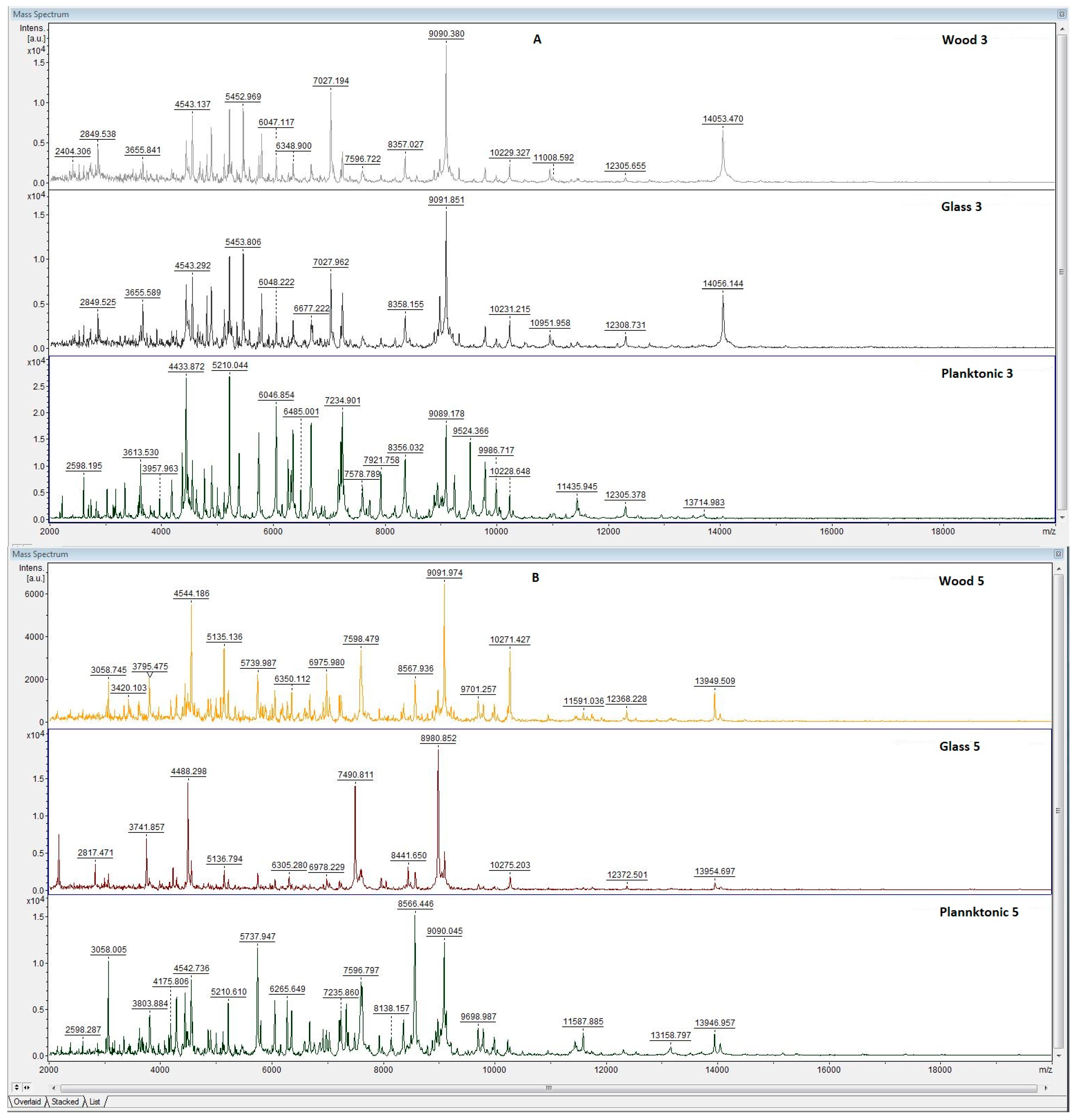
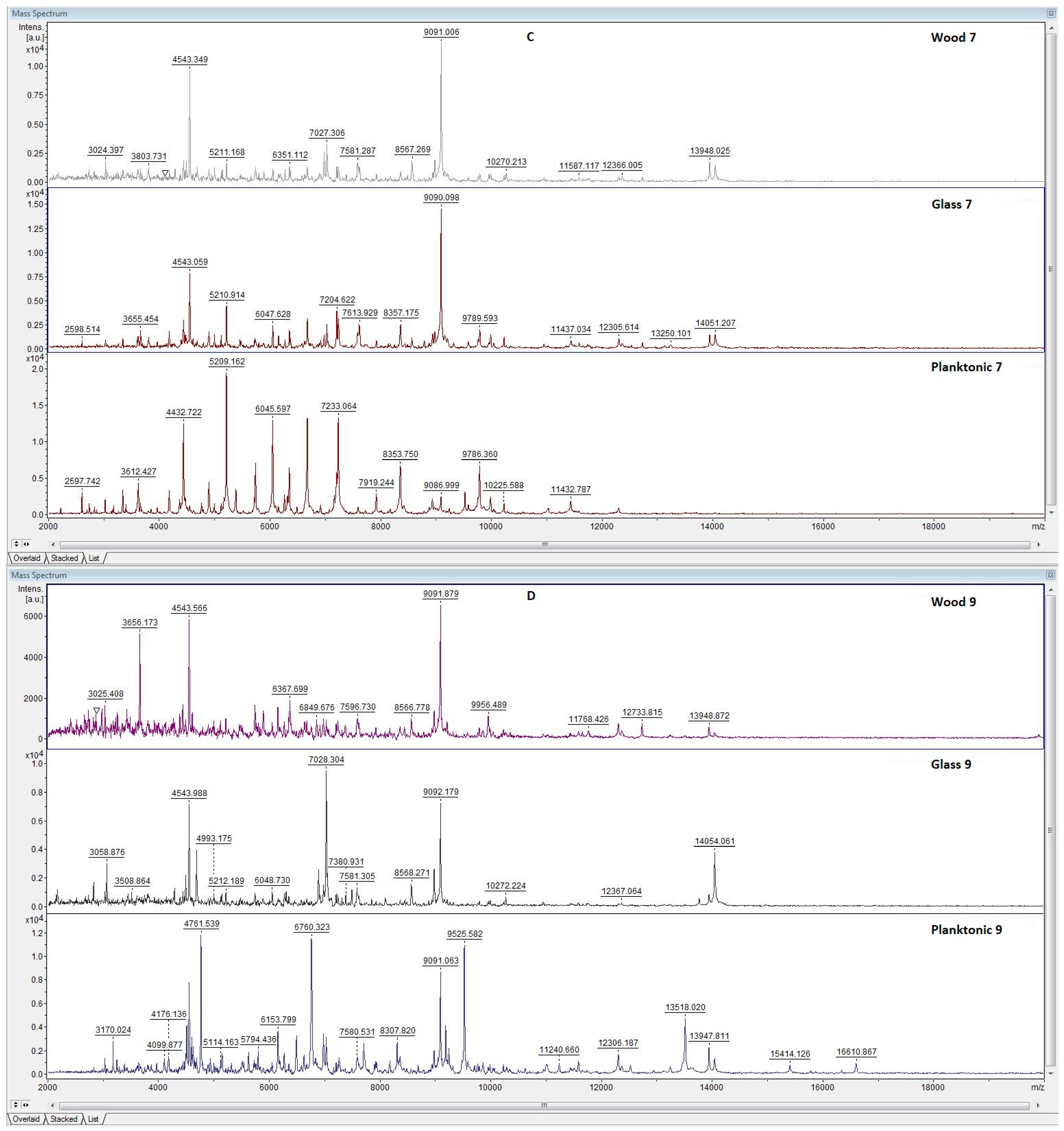
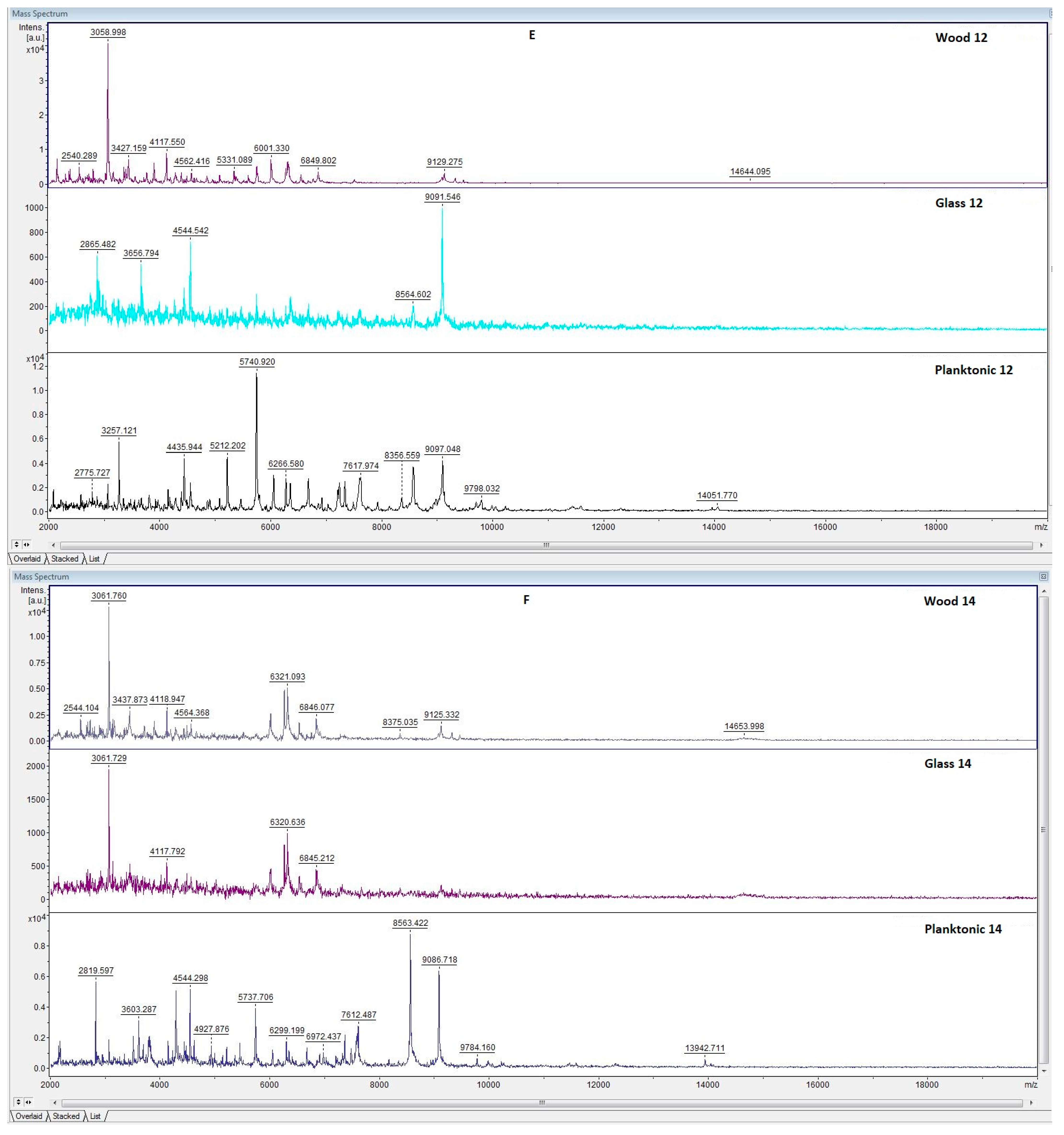
3.5. Antibiofilm Activity of LGEO
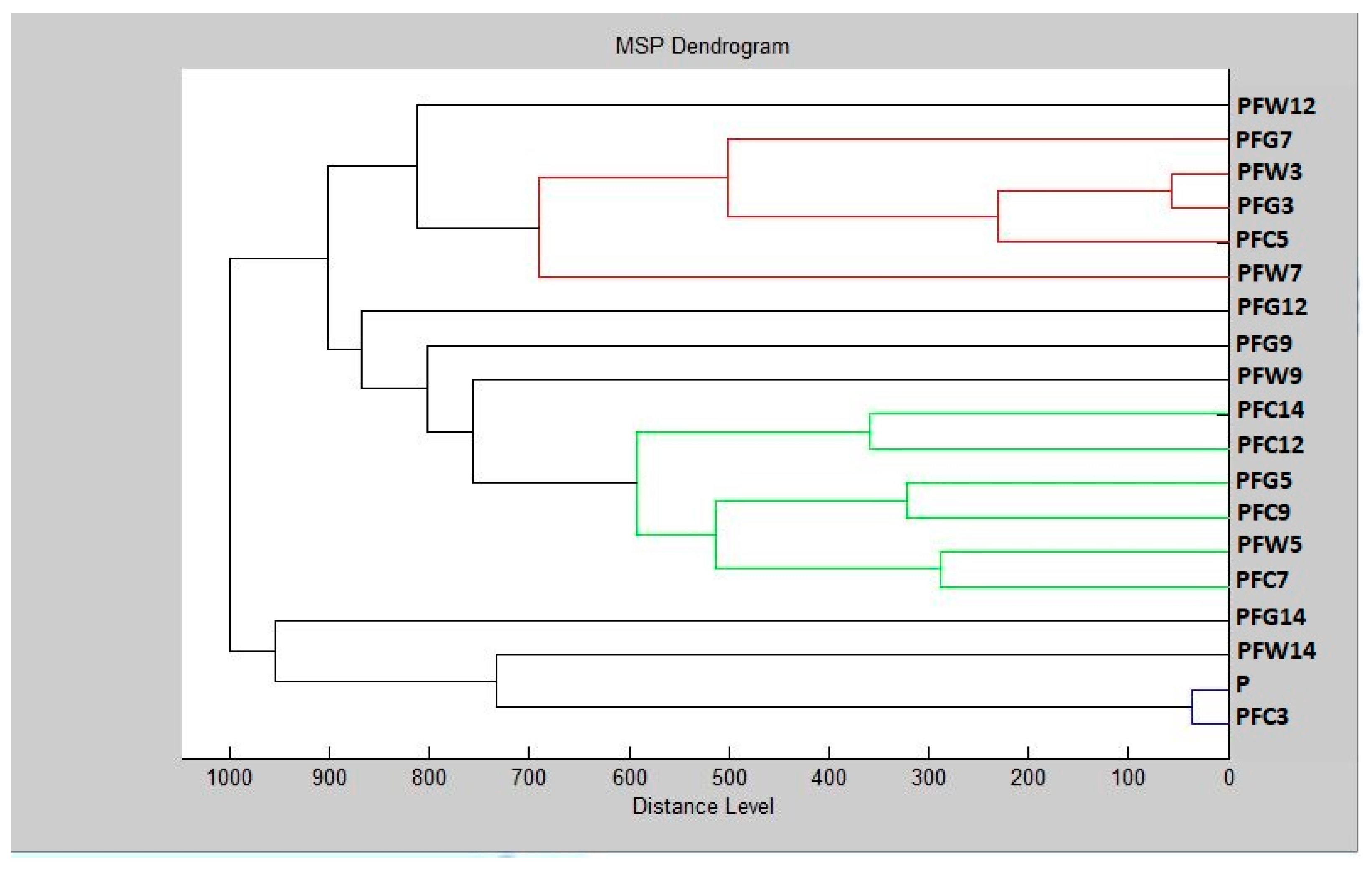
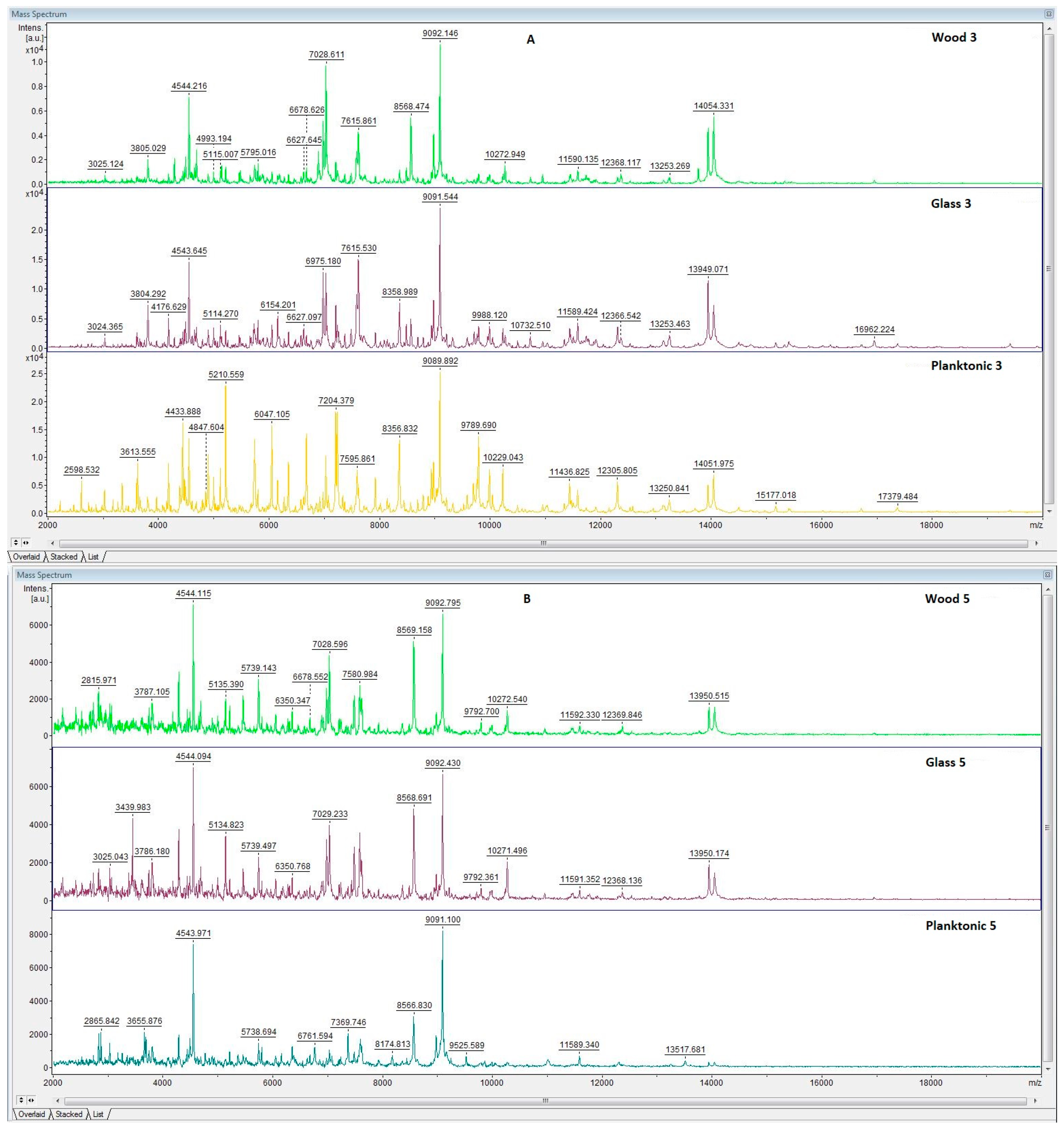
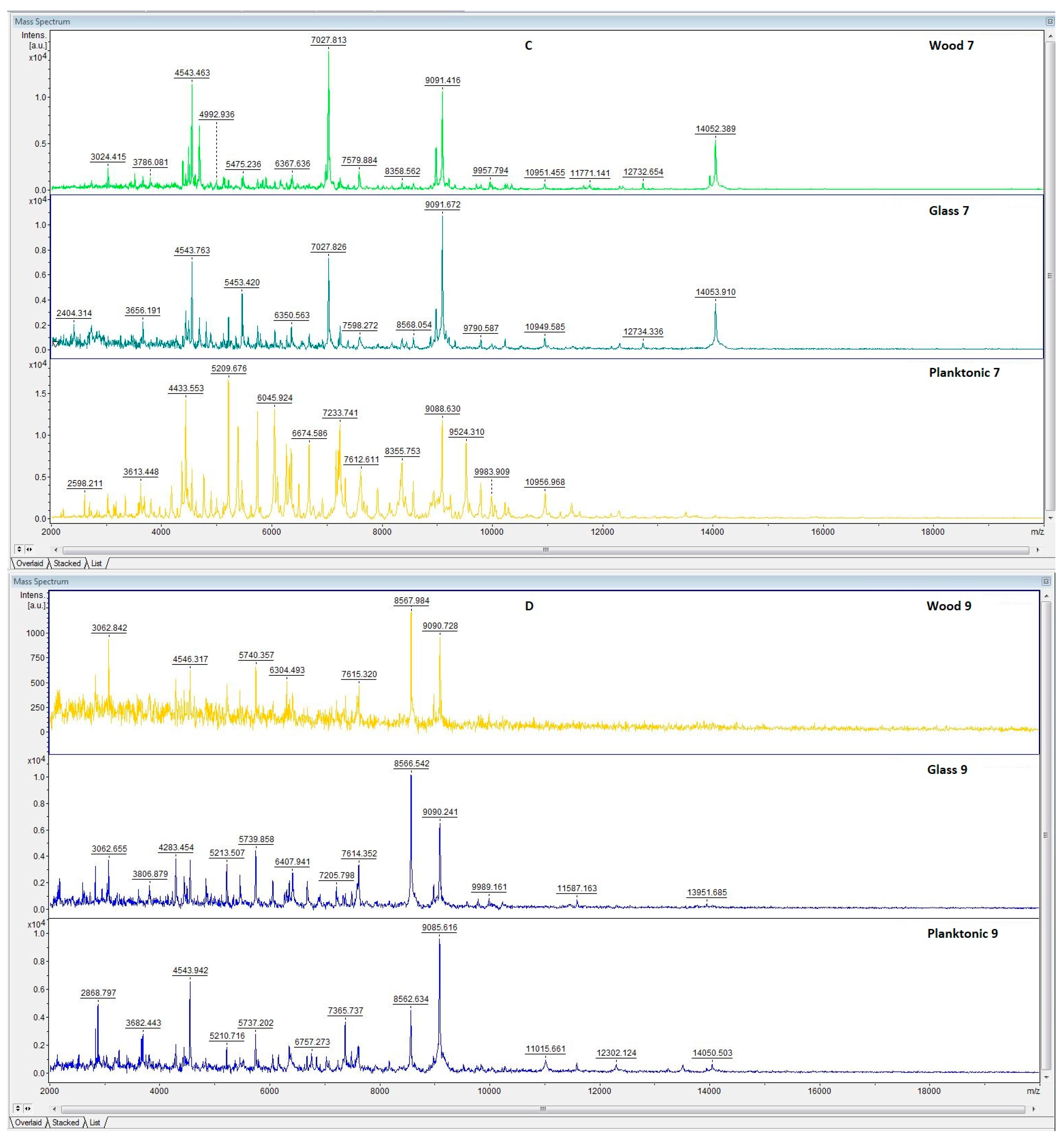
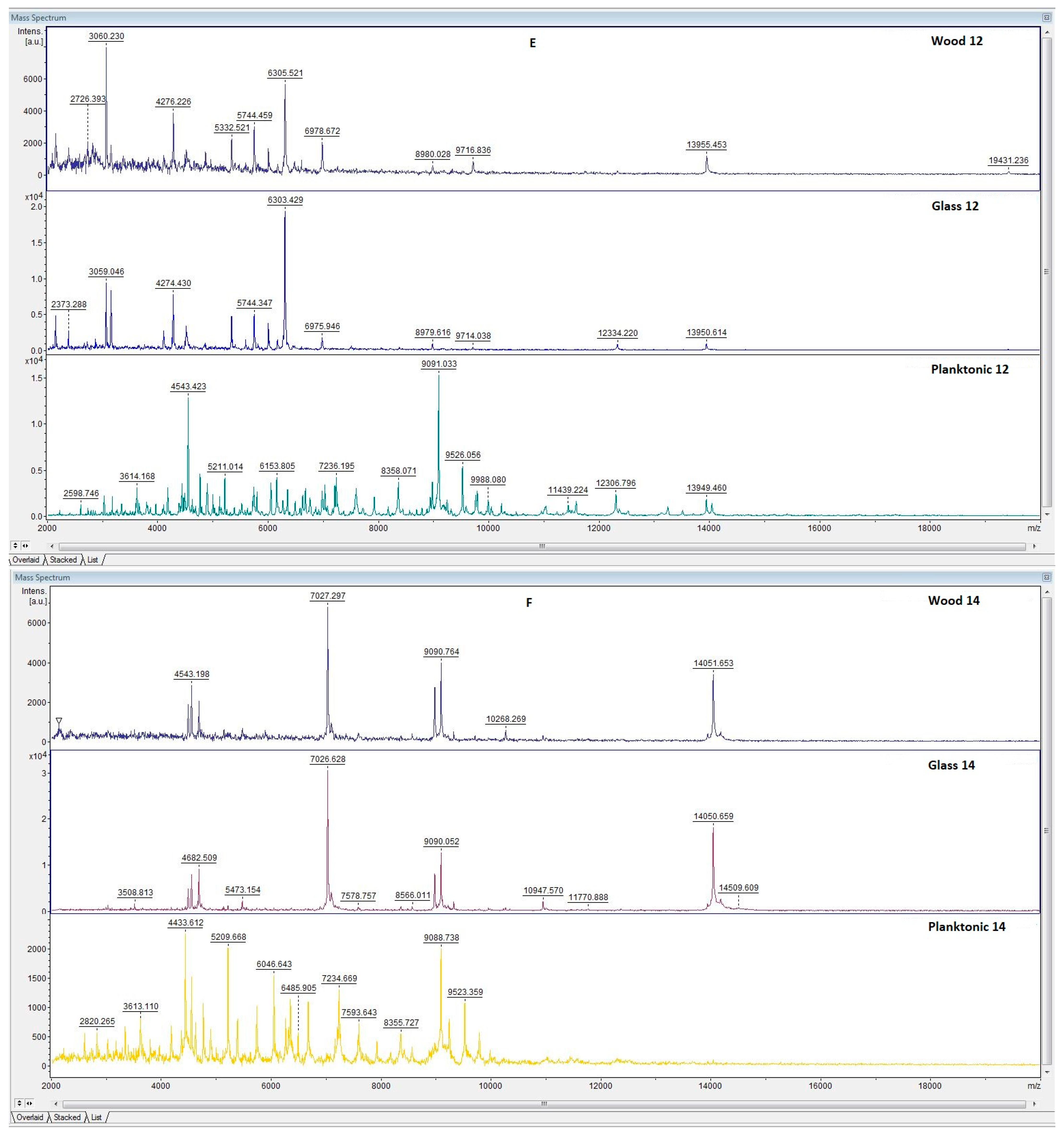
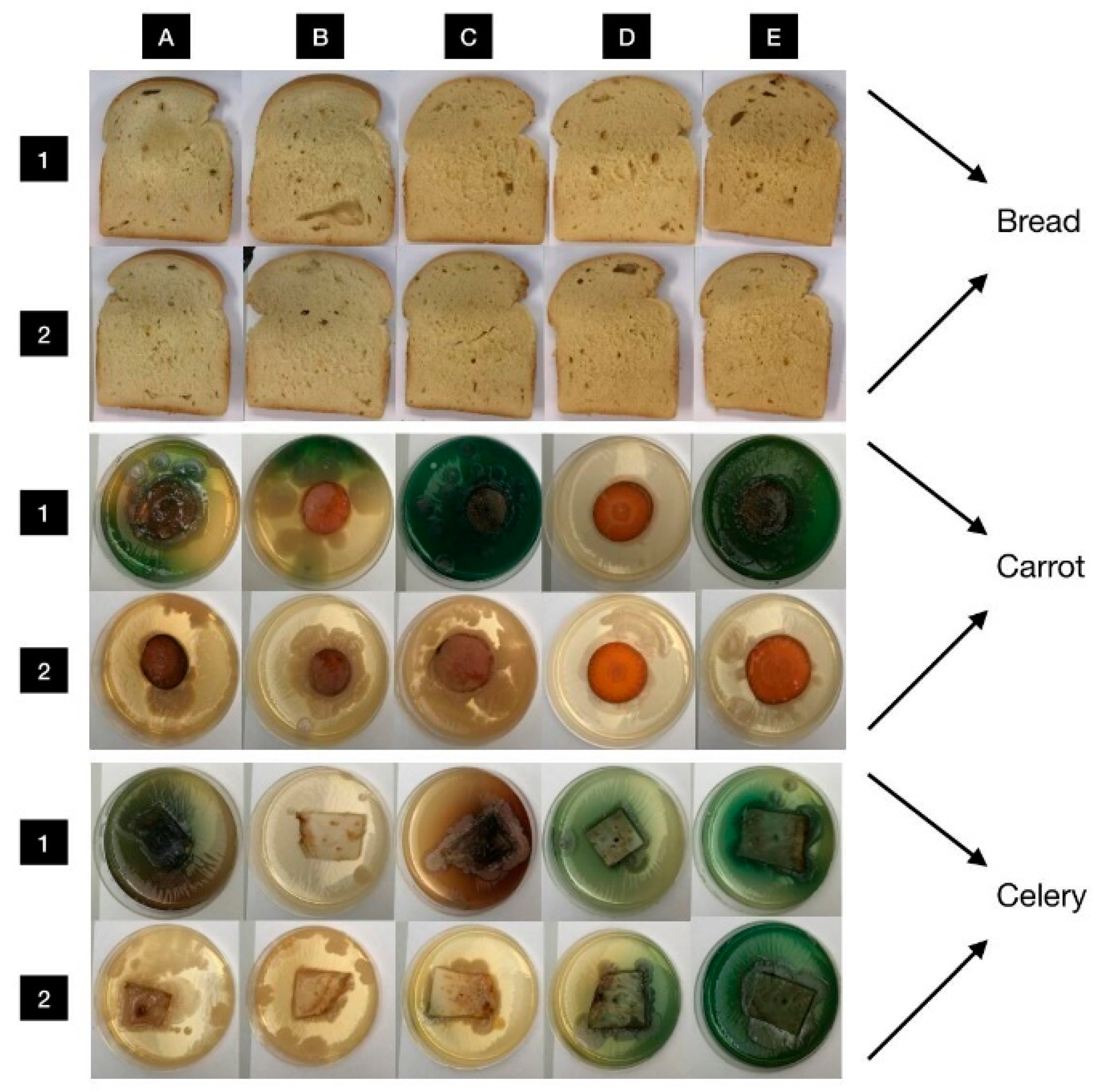
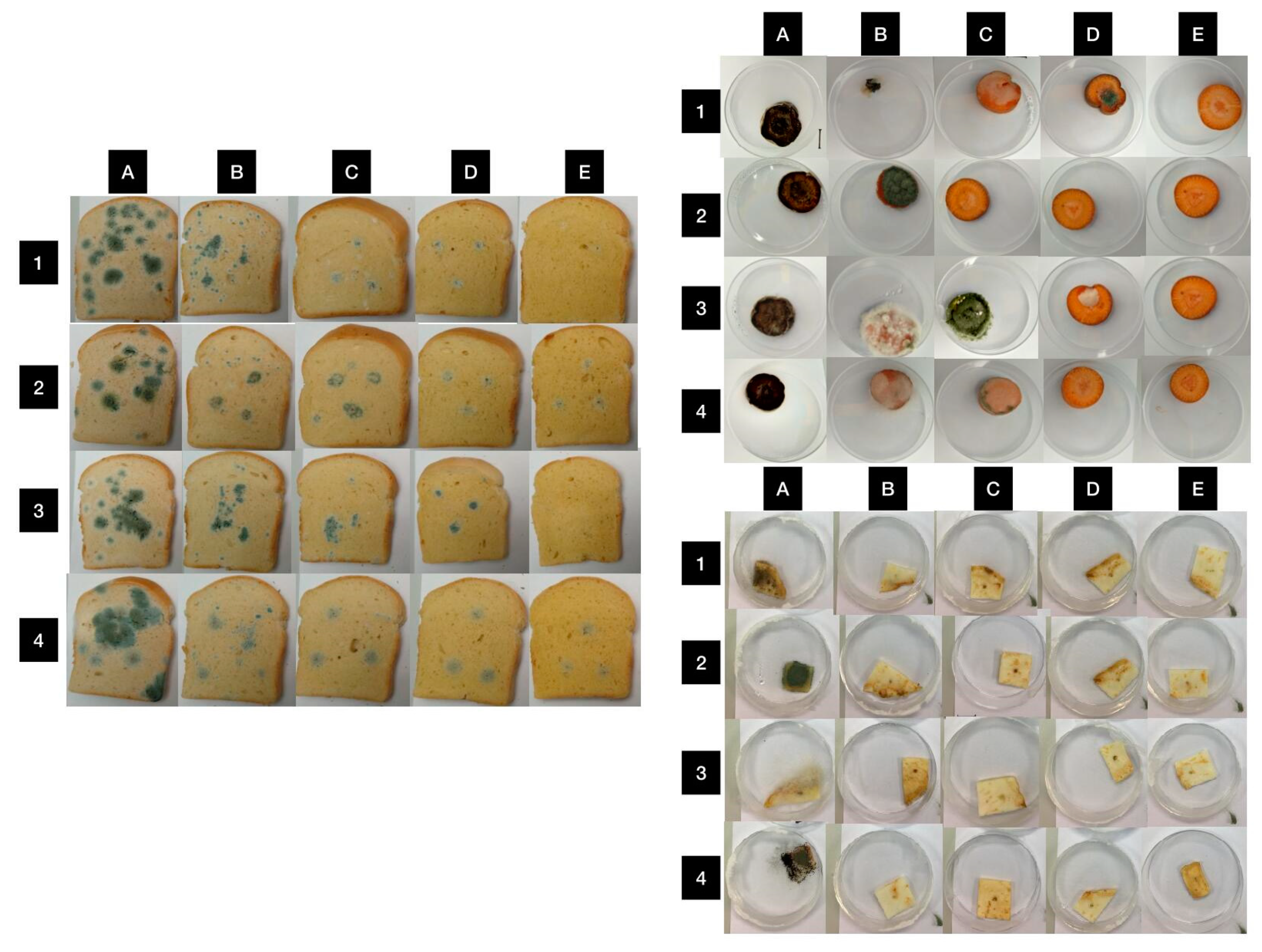
3.6. In Situ Antimicrobial Properties of LGEO
3.7. In Situ Antifungal Properties of LGEO
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinto, E.J.; Caro, I.; Villalobos-Delgado, L.H.; Mateo, J.; De-Mateo-Silleras, B.; Redondo-Del-Río, M.P. Food safety through natural antimicrobials. Antibiotics 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.A.; Gonzaga, M.L.; Bastos, M.S.; Magalhães, H.C.; Benevides, S.D.; Furtado, R.F.; Zambelli, R.A.; Garruti, D.S. Packaging with cashew gum/gelatin/essential oil for bread: Release potential of the citral. Food Packag. Shelf Life 2020, 23, 100431. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.E. Use of Cymbopogon citratus essential oil in food preservation: Recent advances and future perspectives. Crit. Rev. Food Sci. Nutr. 2017, 57, 2541–2559. [Google Scholar] [CrossRef] [PubMed]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M.; Singh, A. Biological, medicinal and toxicological significance of Eucalyptus leaf essential oil: A review. J. Sci. Food Agric. 2018, 98, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal activity of selected essential oils against Fusarium culmorum and F. graminearum and their secondary metabolites in wheat seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falleh, H.; Jemaa, M.B.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef] [PubMed]
- Elyemni, M.; Louaste, B.; Nechad, I.; Elkamli, T.; Bouia, A.; Taleb, M.; Chaouch, M.; Eloutassi, N. Extraction of essential oils of Rosmarinus officinalis L. by two different methods: Hydrodistillation and microwave assisted hydrodistillation. Sci. World J. 2019, 2019, 3659432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plata-Rueda, A.; Martínez, L.C.; da Silva Rolim, G.; Coelho, R.P.; Santos, M.H.; de Souza Tavares, W.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and repellent activities of Cymbopogon citratus (Poaceae) essential oil and its terpenoids (citral and geranyl acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bedini, S.; Flamini, G.; Ascrizzi, R.; Venturi, F.; Ferroni, G.; Bader, A.; Girardi, J.; Conti, B. Essential oils sensory quality and their bioactivity against the mosquito Aedes albopictus. Sci. Rep. 2018, 8, 17857. [Google Scholar] [CrossRef] [Green Version]
- Santana, A.I.; Gupta, M.P. Potential of Panamanian aromatic flora as a source of novel essential oils. Biodivers. Int. J. 2018, 2, 405–413. [Google Scholar]
- Sil, A.; Pramanik, K.; Samantaray, P.; Firoz, M.; Yadav, V. Essential oils: A boon towards eco-friendly management of phytopathogenic fungi. J. Entomol. Zool. Stud. 2020, 8, 1884–1891. [Google Scholar]
- Olayemi, R.F.; Jawonisi, I.O.; Samuel, J.A. Characterization and physico-chemical analysis of essential oil of Cymbopogon citratus leaves. Bayero J. Pure Appl. Sci. BAJOPAS 2018, 11, 74–81. [Google Scholar] [CrossRef]
- Zigene, Z.D.; Kassahun, B.M.; Degu, B. Agronomic Characteristics and Essential Oil Yield of Java Citronella (Cymbopogon Winterianus Jowitt) as Affected by Harvesting Age and Plant Population Density. Acad. Res. J. Agric. Sci. Res. ARJASR 2018, 6, 70–76. [Google Scholar] [CrossRef]
- Shendurse, A.M.; Sangwan, R.B.; Amit Kumar, R.V.; Patel, A.C.; Gopikrishna, G.; Roy, S.K. Phytochemical screening and antibacterial activity of lemongrass (Cymbopogon citratus) leaves essential oil. J. Pharmacogn. Phytochem. RJPP 2021, 10, 445–449. [Google Scholar]
- Martinazzo, A.P.; de Oliveira, F.D.S.; de Souza Teodoro, C.E. Antifungal activity of Cymbopogon citratus essential oil against Aspergillus flavus. Ciência Nat. 2019, 41, 20. [Google Scholar] [CrossRef] [Green Version]
- Widelska, G.; Stelmasiewicz, M.; Skalicka-Woźniak, K.; Oniszczuk, A.; Ludwiczuk, A. Antioxidant activity of lemongrass essential oil and its constituents. Facta Univ. FU Phys. Chem. Technol. 2018, 16, 132. [Google Scholar]
- Ekpenyong, C.E.; Akpan, E.E.; Daniel, N.E. Phytochemical constituents, therapeutic applications and toxicological profile of Cymbopogon citratus Stapf (DC) leaf extract. J. Pharmacogn. Phytochem. 2014, 3, 133–141. [Google Scholar]
- Majewska, E.; Kozlowska, M.; Gruszczynska-Sekowska, E.; Kowalska, D.; Tarnowska, K. Lemongrass (Cymbopogon citratus) essential oil: Extraction, composition, bioactivity and uses for food preservation-a review. Polish J. Food Nutr. Sci. 2019, 69, 4. [Google Scholar] [CrossRef]
- Soliman, W.S.; Salaheldin, S.; Amer, H.M. Chemical composition evaluation of Egyptian lemongrass, Cymbopogon citratus, essential oil. Int. J. Sci. Eng. Res. 2017, 8, 630–634. [Google Scholar]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kačániová, M. In Vitro antimicrobial activity of lavender, mint, and rosemary essential oils and the effect of their vapours on growth of Penicillium spp. in a bread model system. Molecules 2021, 26, 3859. [Google Scholar] [CrossRef] [PubMed]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.L.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Kačániová, M.; Galovičová, L.; Borotová, P.; Valková, V.; Ďúranová, H.; Kowalczewski, P.Ł.; Said-Al Ahi, H.A.H.; Hikal, W.M.; Vukic, M.; Savitskaya, T.; et al. Chemical Composition, In Vitro and In Situ Antimicrobial and Antibiofilm Activities of Syzygium aromaticum (Clove) Essential Oil. Plants 2021, 10, 2185. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food- and Airborne Fungi, 6th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2002; p. 389. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: London, UK, 2009; p. 519. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS–KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; p. 390. [Google Scholar]
- Valková, V.; Ďúranová, H.; Štefániková, J.; Miškeje, M.; Tokár, M.; Gabríny, L.; Kowalczewski, P.L.; Kačániová, M. Wheat bread with grape seeds micropowder: Impact on dough rheology and bread properties. Appl. Rheol. 2020, 30, 138–150. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, 104620. [Google Scholar] [CrossRef]
- Nair, E.V. Essential oil of East Indian lemongrass: Present position in India and scope of its development. Cultiv. Util. Med. Aromat. Plants 1977, 1977, 204–206. [Google Scholar]
- Hanaa, A.R.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Negrelle, R.R.B.; Gomes, E.C. Cymbopogon citratus (DC.) Stapf: Chemical composition and biological activities. Rev. Bras. Plantas Med. 2007, 9, 80–92. [Google Scholar]
- Lulekal, E.; Tesfaye, S.; Gebrechristos, S.; Dires, K.; Zenebe, T.; Zegeye, N.; Feleke, G.; Kassahun, A.; Shiferaw, Y.; Mekonnen, A. Phytochemical analysis and evaluation of skin irritation, acute and sub-acute toxicity of Cymbopogon citratus essential oil in mice and rabbits. Toxicol. Rep. 2019, 6, 1289–1294. [Google Scholar] [CrossRef]
- Gbenou, J.D.; Ahounou, J.F.; Akakpo, H.B.; Laleye, A.; Yayi, E.; Gbaguidi, F.; Baba-Moussa, L.; Darboux, R.; Dansou, P.; Moudachirou, M.; et al. Phytochemical composition of Cymbopogon citratus and Eucalyptus citriodora essential oils and their anti-inflammatory and analgesic properties on Wistar rats. Mol. Biol. Rep. 2013, 40, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, B.; Yohannes, P.G.; Dieter, R.K. Constituents of the essential oil of Ethiopian Cymbopogon citratus Stapf. J. Nat. Prod. 1983, 46, 424–426. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, N.; Wang, D.; Wang, M. Effects of essential oil citral on the growth, mycotoxin biosynthesis and transcriptomic profile of Alternaria alternata. Toxins 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wei, J.; Chen, H.; Song, Z.; Guo, H.; Yuan, Y.; Yue, T. Antibacterial activity of essential oils against Stenotrophomonas maltophilia and the effect of citral on cell membrane. LWT 2020, 117, 108667. [Google Scholar] [CrossRef]
- Diniz do Nascimento, L.; Moraes, A.A.B.D.; Costa, K.S.D.; Pereira Galúcio, J.M.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.A.; Faria, L.J.G.D. Bioactive natural compounds and antioxidant activity of essential oils from spice plants: New findings and potential applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Wang, W.; Wu, N.; Zu, Y.G.; Fu, Y.J. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008, 108, 1019–1022. [Google Scholar] [CrossRef]
- Guimarães de Lima, L.G.; Cardoso das Graças, M.; de Sousa, P.E.; de Andrade, J.; Vieira, S.S. Antioxidant and fungitoxic activities of the lemongrass essential oil and citral. Rev. Cienc. Agron. 2011, 42, 464–472. [Google Scholar]
- Baschieri, A.; Ajvazi, M.D.; Tonfack, J.L.F.; Valgimigli, L.; Amorati, R. Explaining the antioxidant activity of some common non-phenolic components of essential oils. Food Chem. 2017, 232, 656–663. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Liyana, Y.; Parveen, J. Bioactivity analysis of lemongrass (Cymbopogon citratus) essential oil. Int. Food Res. J. 2012, 19, 569. [Google Scholar]
- Hartatie, E.S.; Prihartini, I.; Widodo, W.; Wahyudi, A. Bioactive Compounds of Lemongrass (Cymbopogon citratus) essential oil from different parts of the plant and distillation methods as natural antioxidant in broiler meat. IOP Conf. Ser. Mater. Sci. Eng. 2019, 532, 012018. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Nguefack, J.; Budde, B.B.; Jakobsen, M. Five essential oils from aromatic plants of Cameroon: Their antibacterial activity and ability to permeabilize the cytoplasmic membrane of Listeria innocua examined by flow cytometry. Lett. Appl. 2004, 39, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.S.; Sumita, T.C.; Furlan, M.R.; Jorge, A.O.C.; Ueno, M. Antibacterial activity of essential oils on microorganisms isolated from urinary tract infection. Rev. Saude Publica 2004, 38, 326–328. [Google Scholar] [CrossRef]
- Onawunmi, G.O.; Yisak, W.A.; Ogunlana, E.O. Antibacterial constituents in the essential oil of Cymbopogon citratus (DC.) Stapf. J. Ethnopharmacol. 1984, 12, 279–286. [Google Scholar] [CrossRef]
- Lira, M.H.P.D.; Andrade Júnior, F.P.D.; Moraes, G.F.Q.; Macena, G.D.S.; Pereira, F.D.O.; Lima, I.O. Antimicrobial activity of geraniol: An integrative review. J. Essent. Oil Res. 2020, 32, 187–197. [Google Scholar] [CrossRef]
- Lima, T.S.; Silva, M.F.S.; Nunes, X.P.; Colombo, A.V.; Oliveira, H.P.; Goto, P.L.; Blanzat, M.; Piva, H.L.; Tedesco, A.C.; Siqueira-Moura, M.P. Cineole-containing nanoemulsion: Development, stability, and antibacterial activity. Chem. Phys. Lipids 2021, 239, 105113. [Google Scholar] [CrossRef]
- Shi, C.; Song, K.; Zhang, X.; Sun, Y.; Sui, Y.; Chen, Y.; Jia, Z.; Sun, H.; Sun, Z.; Xia, X. Antimicrobial activity and possible mechanism of action of citral against Cronobactersakazakii. PLoS ONE 2016, 11, e0159006. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.D.B.D.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Soares, M.O.; Ferreira da Vinha, A.; Barreira, S.; Coutinho, F.; Aires-Goncalves, S.; Oliveira, M.B.P.P.; Catarino, P.; Castro, A. Cymbopogon citratus EO antimicrobial activity against multi-drug resistant Gram-positive strains and non-albicans-Candida species. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 2, 1081–1086. [Google Scholar]
- Naik, M.I.; Fomda, B.A.; Jaykumar, E.; Bhat, J.A. Antibacterial activity of lemongrass (Cymbopogon citratus) oil against some selected pathogenic bacterias. Asian Pac. J. Trop. Med. 2010, 3, 535–538. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, S.; Bouffartigues, E.; Bodilis, J.; Maillot, O.; Lesouhaitier, O.; Feuilloley, M.G.; Cornelis, P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017, 41, 698–722. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial activity and resistance: Influencing factors. Front. Pharmacol. 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, M.N.; Abee, T.; van Bokhorst-van de Veen, H. Inactivation of conidia from three Penicillium spp. isolated from fruit juices by conventional and alternative mild preservation technologies and disinfection treatments. Food Microbiol. 2019, 81, 108–114. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Economakis, C.D. Antifungal activity of lemongrass (Cympopogon citratus L.) essential oil against key postharvest pathogens. Innov. Food Sci. Emerg. Technol. 2007, 8, 253–258. [Google Scholar] [CrossRef]
- Wuryatmo, E.; Able, A.J.; Ford, C.M.; Scott, E.S. Effect of volatile citral on the development of blue mould, green mould and sour rot on navel orange. Australas. Plant Pathol. 2014, 43, 403–411. [Google Scholar] [CrossRef]
- Zheng, S.J.; Jing, G.X.; Wang, X.; Ouyang, Q.L.; Jia, L.; Tao, N.G. Citral exerts its antifungal activity against Penicillium digitatum by affecting the mitochondrial morphology and function. Food Chem. 2015, 178, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Tao, N.G.; Jia, L.; He, X.L. Use of citral incorporated in postharvest wax of citrus fruit as a botanical fungicide against Penicillium digitatum. Postharvest Biol. Technol. 2014, 90, 52–55. [Google Scholar] [CrossRef]
- Harris, R. Progress with superficial mycoses using essential oils. Int. J. Aromather. 2002, 12, 83–91. [Google Scholar] [CrossRef]
- Rajput, S.B.; Karuppayil, S.M. Small molecules inhibit growth, viability and ergosterol biosynthesis in Candida albicans. Springerplus 2013, 2, 26. [Google Scholar] [CrossRef] [Green Version]
- Jordá, T.; Puig, S. Regulation of ergosterol biosynthesis in Saccharomyces cerevisiae. Genes 2020, 11, 795. [Google Scholar] [CrossRef]
- Dincer, S.; Uslu, F.M.; Delik, A. Antibiotic resistance in biofilm. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Soto, S.; Smithson, A.; Horcajada, J.P.; Martinez, J.A.; Mensa, J.P.; Vila, J. Implication of biofilm formation in the persistence of urinary tract infection caused by uropathogenic Escherichia coli. Clin. Microbiol. Infect. 2006, 12, 1034–1036. [Google Scholar] [CrossRef] [Green Version]
- Coenye, T. Biofilms. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 335–337. [Google Scholar]
- Colagiorgi, A.; Di Ciccio, P.; Zanardi, E.; Ghidini, S.; Ianieri, A. A look inside the Listeria monocytogenes biofilms extracellular matrix. Microorganisms 2016, 4, 22. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Cole, S.; Badel-Berchoux, S.; Guillier, L.; Felix, B.; Krezdorn, N.; Hebraud, M.; Bernardi, T.; Sultan, I.; Piveteau, P. Biofilm formation of Listeria monocytogenes strains under food processing environments and pan-genome-wide association study. Front. Microbiol. 2019, 10, 2698. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri, T.; Cervantes-Huamán, B.R.; Bermúdez-Capdevila, M.; Ripolles-Avila, C.; Rodríguez-Jerez, J.J. Listeria monocytogenes biofilms in the food industry: Is the current hygiene program sufficient to combat the persistence of the pathogens? Microorganisms 2021, 9, 181. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Allen, S.C.; Phillips, C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227. [Google Scholar] [CrossRef]
- Kamali, E.; Jamali, A.; Ardebili, A.; Ezadi, F.; Mohebbi, A. Evaluation of antimicrobial resistance, biofilm forming potential, and the presence of biofilm-related genes among clinical isolates of Pseudomonas aeruginosa. BMC Res. Notes 2020, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H. Pseudomonas aeruginosa biofilms. Int. J. Mol. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Jara, J.; Alarcón, F.; Monnappa, A.K.; Santos, J.I.; Bianco, V.; Nie, P.; Ciamarra, M.P.; Chanales, Á.; Dinis, L.; López-Montero, I.; et al. Self-Adaptation of Pseudomonas fluorescens Biofilms to Hydrodynamic Stress. Front. Microbiol. 2021, 11, 3460. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Chen, L.; Yuk, H.G. Stress response and survival of Salmonella Enteritidis in single and dual species biofilms with Pseudomonas fluorescens following repeated exposure to quaternary ammonium compounds. Int. J. Food Microbiol. 2020, 325, 108643. [Google Scholar] [CrossRef]
- Carneiro, V.A.; Melo, R.S.; Pereira, A.M.G.; Azevedo, Á.M.A.; Matos, M.N.C.; Cavalcante, R.M.B.; Rocha, R.R.; Albuquerque, V.Q.; Guerrero, J.A.P.; Junior, F.E.A.C. Essential Oils as an Innovative Approach against Biofilm of Multidrug-Resistant Staphylococcus aureus. In Bacterial Biofilms; IntechOpen: London, UK, 2020. [Google Scholar]
- Kim, Y.M.; Farrah, S.; Baney, R.H. Structure–antimicrobial activity relationship for silanols, a new class of disinfectants, compared with alcohols and phenols. Int. J. Antimicrob. Agents 2007, 29, 217–222. [Google Scholar] [CrossRef]
- Shukla, S.K.; Khan, A.; Rao, T.S. Biofilm extracellular polymeric substances-based bioremediation of radionuclides. In Microbial and Natural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 751–773. [Google Scholar]
- Reyes-Jurado, F.; Cervantes-Rincón, T.; Bach, H.; López-Malo, A.; Palou, E. Antimicrobial activity of Mexican oregano (Lippiaberlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crops Prod. 2019, 131, 90–95. [Google Scholar] [CrossRef]
- Santamarta, S.; Aldavero, A.C.; Rojo, M.A. Essential oil of Cymbopogon martini, source of geraniol, as a potential antibacterial agent against Bacillus subtilis, a pathogen of the bakery industry. F1000 Res. 2021, 10, 1027. [Google Scholar] [CrossRef]
- Issouffou, C.; Suwansri, S.; Salaipeth, L.; Domig, K.J.; Hwanhlem, N. Synergistic effect of essential oils and enterocin KT2W2G on the growth of spoilage microorganisms isolated from spoiled banana peel. Food Control 2018, 89, 260–269. [Google Scholar] [CrossRef]
- Klein, D.; Breuch, R.; von der Mark, S.; Wickleder, C.; Kaul, P. Detection of spoilage associated bacteria using Raman-microspectroscopy combined with multivariate statistical analysis. Talanta 2019, 196, 325–328. [Google Scholar] [CrossRef]
- Vazirian, M.; Kashani, S.T.; Ardekani, M.R.S.; Khanavi, M.; Jamalifar, H.; Fazeli, M.R.; Toosi, A.N. Antimicrobial activity of lemongrass (Cymbopogon citratus (DC) Stapf.) essential oil against food-borne pathogens added to cream filled cakes and pastries. J. Essent. Oil Res. 2012, 24, 579–582. [Google Scholar] [CrossRef]
- Moore-Neibel, K.; Gerber, C.; Patel, J.; Friedman, M.; Ravishankar, S. Antimicrobial activity of lemongrass oil against Salmonella enterica on organic leafy greens. J. Appl. Microbiol. 2012, 112, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mani-López, E.M.; Valle-Vargas, G.P.; Palou, E.; López-Malo, A. Penicillium expansum inhibition on bread by lemongrass essential oil in vapor phase. J. Food Prot. 2018, 81, 467–471. [Google Scholar] [CrossRef]
| No | RI a | Compound b | % c |
|---|---|---|---|
| 1 | 909 | isobutyl isobutyrate | 0.1 |
| 2 | 926 | α-thujene | 0.1 |
| 3 | 938 | α-pinene | 1.9 |
| 4 | 948 | camphene | 0.7 |
| 5 | 977 | sabinene | 0.3 |
| 6 | 980 | β-pinene | 1.0 |
| 7 | 983 | 6-methyl-5-hepten-2-one | 1.0 |
| 8 | 992 | β-myrcene | 0.4 |
| 9 | 1016 | α-terpinene | 0.2 |
| 10 | 1117 | p-methyl anisole | 0.2 |
| 11 | 1023 | p-cimene | 0.9 |
| 12 | 1028 | α-limonene | 1.0 |
| 13 | 1033 | 1,8-cineole | 6.4 |
| 14 | 1047 | (E)-β-ocimene | 0.2 |
| 15 | 1060 | α-terpinene | 0.6 |
| 16 | 1087 | 4-nonanone | 0.9 |
| 17 | 1088 | α-terpinolene | 0.1 |
| 18 | 1098 | linalool | 1.7 |
| 19 | 1148 | camphor | 0.6 |
| 20 | 1152 | citronellal | 0.3 |
| 21 | 1160 | pinocarvone | 0.7 |
| 22 | 1170 | borneol | 1.2 |
| 26 | 1189 | α-terpineol | 1.5 |
| 27 | 1202 | n-decanal | 0.2 |
| 28 | 1238 | neral | 27.1 |
| 29 | 1256 | geraniol | 6.6 |
| 30 | 1266 | geranial | 34.4 |
| 31 | 1299 | geranyl formate | tr |
| 32 | 1378 | α-ylangene | 0.2 |
| 33 | 1379 | α-copaene | tr |
| 34 | 1380 | geranyl acetate | 4.3 |
| 35 | 1385 | β-bourbonene | tr |
| 36 | 1388 | β-elemene | tr |
| 37 | 1422 | (E)-caryophyllene | 1.8 |
| 38 | 1449 | (E)-isoeugenol | 0.5 |
| 39 | 1456 | α-humulene | 0.2 |
| 40 | 1483 | germacrene D | 0.2 |
| 41 | 1525 | δ-cadinene | 0.4 |
| 42 | 1542 | α-cadinene | 1.7 |
| 43 | 1566 | geranyl butanoate | 0.2 |
| total | 99.7 |
| Microorganisms | Species | Zone Inhibition (mm) | Antimicrobial Effectiveness | MIC 50 (µL/mL) | MIC 90 (µL/mL) |
|---|---|---|---|---|---|
| Gram-positive bacteria | BS | 3.67 + 0.58 af | - | 131.24 | 163.25 |
| EF | 4.33 ± 0.58 ac | - | 124.15 | 136.25 | |
| ML | 8.33 ± 0.58 be | * | 112.56 | 142.11 | |
| SA | 5.33 ± 1.15 c | * | 125.12 | 135.42 | |
| Gram-negative bacteria | PA | 4.33 ± 0.58 ac | - | 151.25 | 174.18 |
| YE | 7.68 ± 0.58 e | * | 145.18 | 156.24 | |
| SM | 2.67 ± 0.58 f | - | 162.18 | 181.37 | |
| Yeast | CA | 13.67 ± 1.53 g | ** | 212.35 | 245.18 |
| CK | 18.00 ± 2.46 h | *** | 196.28 | 211.36 | |
| CG | 13.33 ± 0.58 gi | ** | 205.26 | 221.32 | |
| CT | 16.33 ± 0.53 j | *** | 208.34 | 226.25 | |
| Biofilm-forming bacteria | SE | 2.67 ± 0.58 f | - | 98.21 | 112.36 |
| PF | 1.00 ± 0.00 d | - | 165.36 | 181.25 |
| Fungal Strains | LGEO (µL/L) | |||
|---|---|---|---|---|
| 62.5 | 125 | 250 | 500 | |
| P. aurantiogriseum | 2.18 ± 0.26 a | 2.53 ± 0.41 a | 5.20 ± 0.50 b* | 7.30 ± 0.26 c* |
| P. expansum | 1.07 ± 0.21 a | 3.35 ± 0.37 b | 4.50 ± 0.57 c | 6.37 ± 0.15 d* |
| P. chrysogenum | 2.80 ± 0.36 a | 4.38 ± 0.28 b | 5.25 ± 0.42 c* | 7.10 ± 0.32 d* |
| P. italicum | 2.47 ± 0.26 a | 2.83 ± 0.17 a | 2.79 ± 0.19 a | 5.37 ± 0.27 b* |
| Food Model | Bacterial Strains | Bacterial Growth Inhibition (%) | |||
|---|---|---|---|---|---|
| LGEO (µL/L) | |||||
| 62.5 | 125 | 250 | 500 | ||
| Bread | M. luteus | 88.21 ± 4.29 a | 90.87± 3.28 a | 86.77 ± 4.11 a | 91.49 ± 5.65 a |
| S. marcescens | 75.33 ± 6.13 a | 80.50 ± 5.45 a | 83.24 ± 6.22 a | 82.35 ± 7.16 a | |
| Carrot | M. luteus | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 100.00 ± 0.00 b | 0.00 ± 0.00 a |
| S. marcescens | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 100.00 ± 0.00 b | 69.17 ± 6.55 c | |
| Celery | M. luteus | 98.39 ± 3.32 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| S. marcescens | 27.28 ± 6.14 a | 42.26 ± 5.91 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | |
| Food Model | Fungal Strains | Mycelial Growth Inhibition (%) | |||
|---|---|---|---|---|---|
| LGEO (µL/L) | |||||
| 62.5 | 125 | 250 | 500 | ||
| Bread | P. aurantiogriseum | 44.40 ± 4.88 a | 84.60 ± 5.27 b | 87.20 ± 4.11 b | 95.30 ± 5.39 b |
| P. expansum | 52.10 ± 3.88 a | 68.40 ± 4.17 b | 81.90 ± 4.89 c | 82.50 ± 5.99 c | |
| P. chrysogenum | 53.20 ± 5.86 a | 71.40 ± 4.29 b | 87.10 ± 4.96 c | 100.00 ± 0.00 d | |
| P. italicum | 65.30 ± 6.16 a | 87.50 ± 3.45 b | 85.90 ± 4.88 b | 89.20 ± 5.08 b | |
| Carrot | P. aurantiogriseum | 0.00 ± 0.00 a | 11.96 ± 4.07 b | 52.31 ± 6.25 c | 100.00 ± 0.00 d |
| P. expansum | 0.00 ± 0.00 a | 100.00 ± 0.00 b | 100.00 ± 0.00 b | 100.00 ± 0.00 b | |
| P. chrysogenum | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 73.12 ± 4.25 b | 100.00 ± 0.00 c | |
| P. italicum | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 100.00 ± 0.00 b | |
| Celery | P. aurantiogriseum | 96.59 ± 4.33 a | 98.92 ± 3.12 a | 97.11 ± 3.41 a | 98.33 ± 2.64 a |
| P. expansum | 97.25 ± 4.53 a | 97.87 ± 2.91 a | 98.26 ± 4.13 a | 96.45 ± 2.11 a | |
| P. chrysogenum | 97.87 ± 2.64 a | 96.97 ± 3.56 a | 95.00 ± 5.98 a | 98.46 ± 3.25 a | |
| P. italicum | 98.88 ± 2.18 a | 96.50 ± 4.26 a | 95.31 ± 5.23 a | 98.42 ± 5.27 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valková, V.; Ďúranová, H.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation. Agronomy 2022, 12, 155. https://doi.org/10.3390/agronomy12010155
Valková V, Ďúranová H, Galovičová L, Borotová P, Vukovic NL, Vukic M, Kačániová M. Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation. Agronomy. 2022; 12(1):155. https://doi.org/10.3390/agronomy12010155
Chicago/Turabian StyleValková, Veronika, Hana Ďúranová, Lucia Galovičová, Petra Borotová, Nenad L. Vukovic, Milena Vukic, and Miroslava Kačániová. 2022. "Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation" Agronomy 12, no. 1: 155. https://doi.org/10.3390/agronomy12010155
APA StyleValková, V., Ďúranová, H., Galovičová, L., Borotová, P., Vukovic, N. L., Vukic, M., & Kačániová, M. (2022). Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation. Agronomy, 12(1), 155. https://doi.org/10.3390/agronomy12010155