Pistil Biology of ‘WA 38’ Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set
Abstract
:1. Introduction
2. Materials and Methods
2.1. EPP (2019)
2.1.1. Stigmatic Receptivity
2.1.2. Pollen Tube Growth
2.1.3. Ovule Longevity
2.2. EPP (2020)
2.3. Paternal Effect on Pollen Tube Growth in ‘WA 38’ Styles
2.3.1. Year 1 (2019)
2.3.2. Year 2 (2020)
2.4. Paternal Effect on ‘WA 38’ Fruit Set and Seed Set
2.4.1. Year 1 (2019)
2.4.2. Year 2 (2020)
2.5. Statistics
3. Results
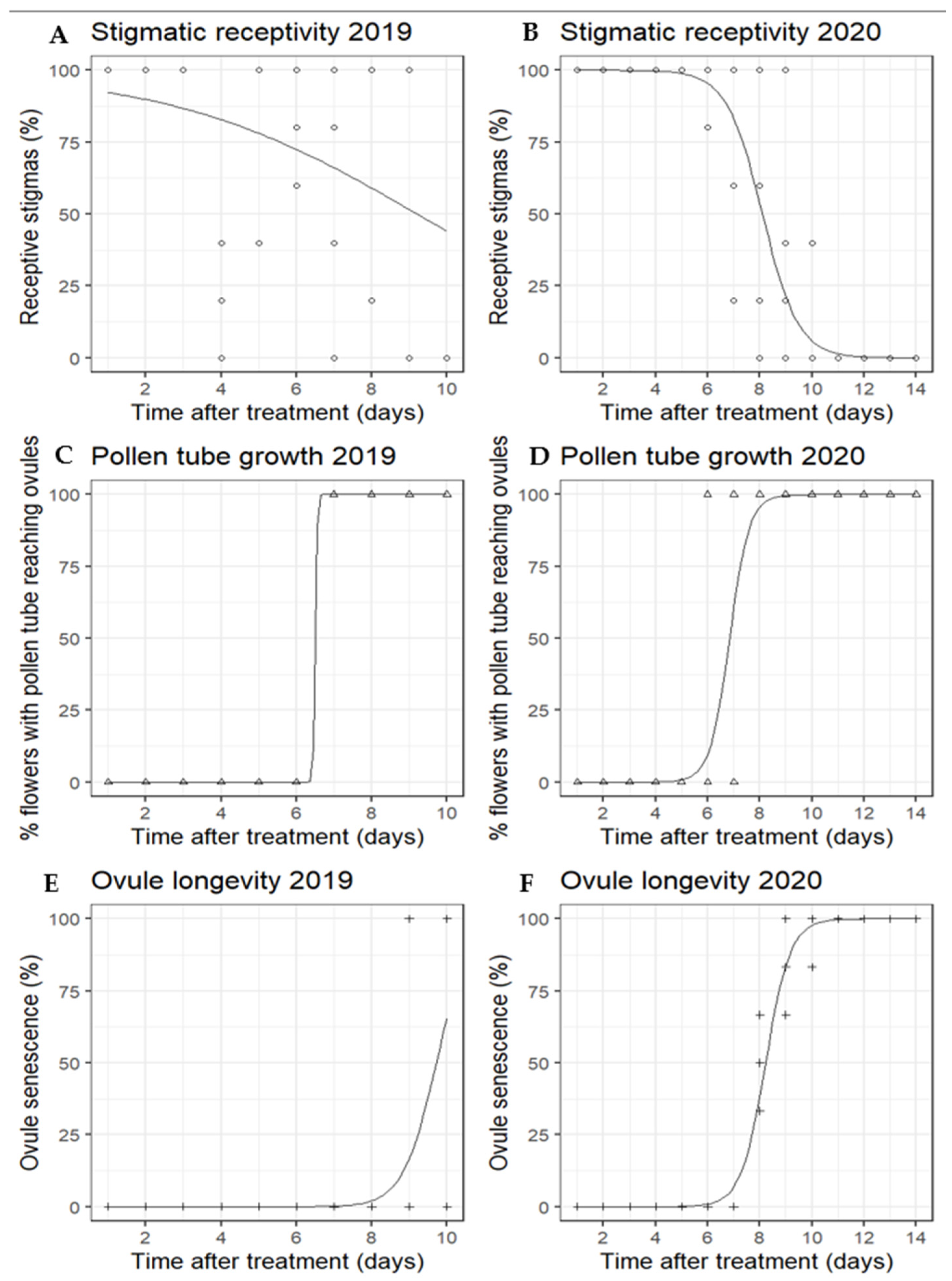
3.1. EPP
3.1.1. Stigmatic Receptivity
3.1.2. Pollen Tube Growth
3.1.3. Ovule Longevity
3.2. Paternal Effect on Pollen Tube Growth in ‘WA 38’
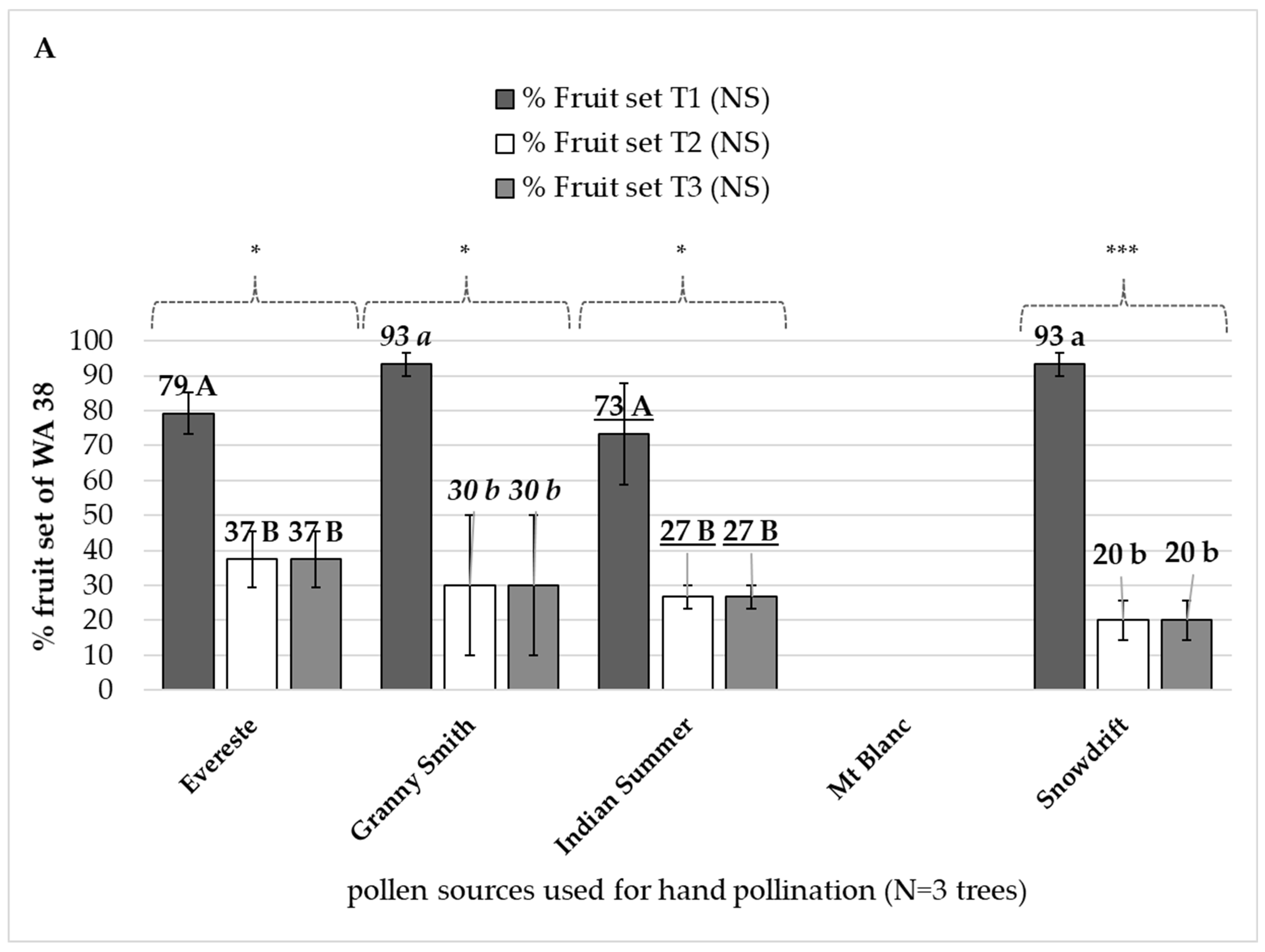
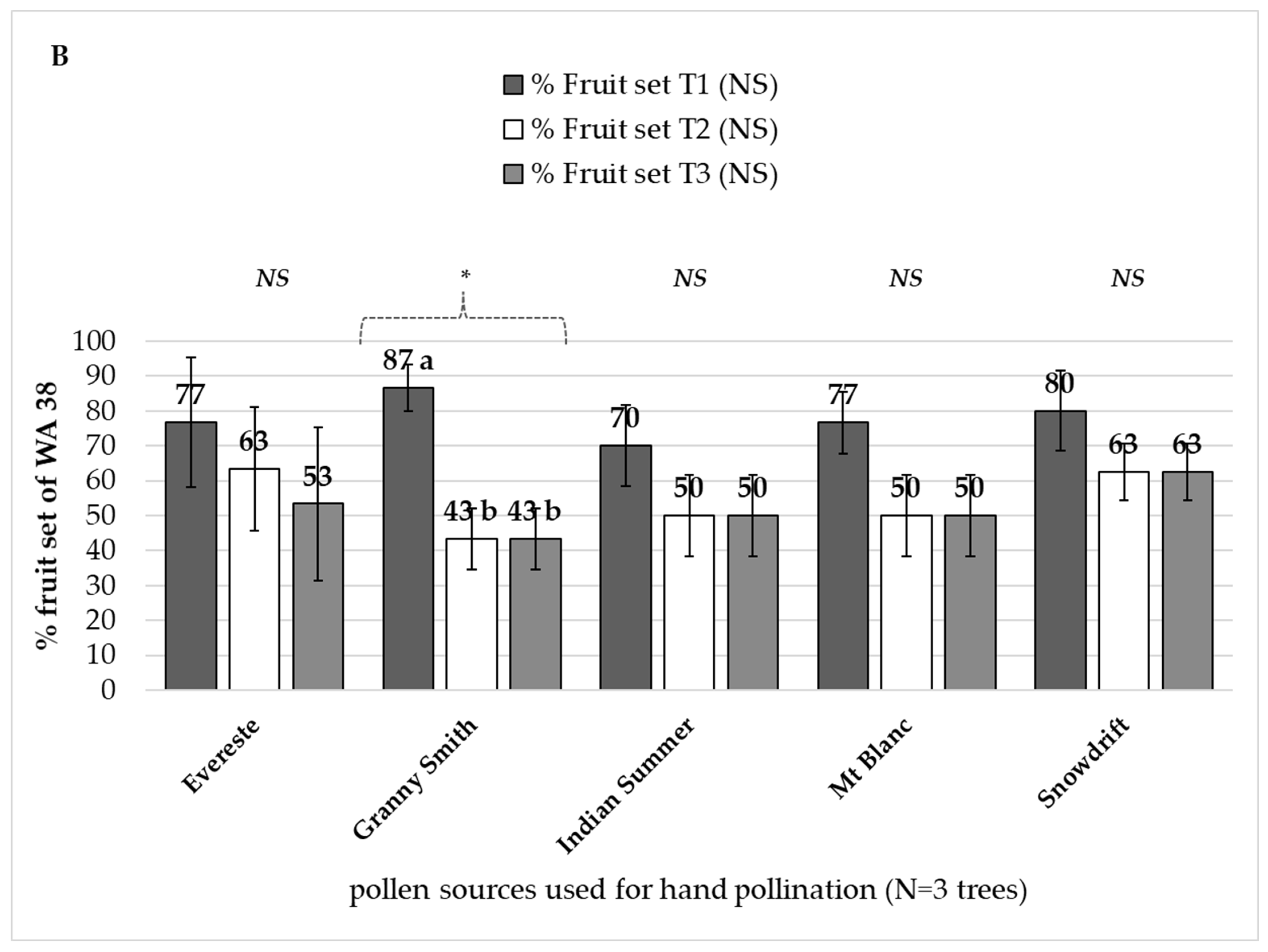
3.3. Paternal Effect on ‘WA 38’ Fruit Set and Seed Set
3.3.1. Paternal Effect on ‘WA 38’ Fruit Set
3.3.2. Paternal Effect on ‘WA 38’ Seeds Set
4. Discussion
4.1. Effective Pollination Period of ‘WA 38’
4.2. Paternal Effect on Pollen Tube Growth in ‘WA 38’ Styles
4.3. Paternal Effect on ‘WA 38’ Fruit Set and Seed Set
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anthony, B.M.; Serra, S.; Musacchi, M. Optimizing crop load for new apple cultivar: “WA 38”. Agronomy 2019, 9, 107. [Google Scholar] [CrossRef] [Green Version]
- Cosmic Crisp Apple ‘Has Potential to Become Top-Five Variety at This Time Next Year’. Available online: https://www.freshfruitportal.com/news/2021/04/26/cosmic-crisp-apple-has-potential-to-become-top-five-variety-at-this-time-next-year (accessed on 12 November 2021).
- Evans, K.S.; Barritt, B.H.; Konishi, B.S.; Brutcher, L.J.; Ross, C.F. ‘WA 38’ apple. Hortscience 2012, 47, 1177–1179. [Google Scholar] [CrossRef] [Green Version]
- Musacchi, S.; Evans, K.; Ross, C.; Serra, S. ‘WA38’ Fruit Size and Dry Matter for Fruit Quality/Consumer Preference; Final Project Report for WTFRC Project # AP-17-108; 2020-AHP-Continuing-and-Final-Reports; Washington Tree Fruit Research Commission: Wenatchee, WA, USA, 2020; pp. 137–147. [Google Scholar]
- Lespinasse, J.M.; Chol, P.; Dupin, J.; Terenne, E. La conduite du Pommier: Types de Fructification; Incidence sur la Conduite de L’arbre; Brochure INVUFLEC: Paris, France, 1977; p. 80. [Google Scholar]
- Celton, J.M.; Kelner, J.J.; Martinez, S.; Bechti, A.; Khelifi Touhami, A.; James, M.J.; Durel, C.E.; Laurens, F.; Costes, E. Fruit self-thinning: A trait to consider for genetic improvement of apple tree. PLoS ONE 2014, 9, e91016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanami, H.; Moriya-Tanaka, Y.; Honda, C.; Wada, M.; Moriya, S.; Okada, K.; Haji, T.; Abe, K. Relationships among apple fruit abscission, source strength, and cultivar. Sci. Hortic. 2012, 146, 39–44. [Google Scholar] [CrossRef]
- Ackerman, M.; Samach, A. Doubts regarding carbohydrate shortage as a trigger toward abscission of specific Apple (Malus domestica) fruitlets. New Negat. Plant Sci. 2015, 1, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Celton, J.M.; Dheilly, E.; Guillou, M.C.; Simonneau, F.; Juchaux, M.; Costes, E.; Laurens, F.; Renou, J.P. Additional amphivasal bundles in pedicel pith exacerbate central fruit dominance and induce self-thinning of lateral fruitlets in apple. Plant Physiol. 2014, 164, 1930–1951. [Google Scholar] [CrossRef] [Green Version]
- Dal Cin, V.; Velasco, R.; Ramina, A. Dominance induction of fruitlet shedding in Malus× domestica (L. Borkh): Molecular changes associated with polar auxin transport. BMC Plant Biol. 2009, 9, 1–14. [Google Scholar] [CrossRef]
- Janick, J.; Cummins, J.N.; Brown, S.K.; Hemmat, M. Apples. In Fruit Breeding: Tree and Tropical Fruits; Janick, J., Moore, J.N., Eds.; John Wiley & Sons: New York, NY, USA, 1996; Volume 1, pp. 1–77. [Google Scholar]
- Ramírez, F.; Davenport, T.L. Apple pollination: A review. Sci. Hortic. 2013, 162, 188–203. [Google Scholar] [CrossRef]
- Williams, R.R. The effect of summer nitrogen applications on the quality of apple blossoms. J. Hortic. Sci. 1965, 40, 31–41. [Google Scholar] [CrossRef]
- Sanzol, J.; Herrero, M. The “effective pollination period” in fruit trees. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Williams, R. Pollination studies in fruit trees. III. The effective pollination period for some apple and pear varieties. Rep. Agric. Hortic. Res. Stn. Univ. Bristol. 1966, 136–138. [Google Scholar]
- Jefferies, C.J.; Brain, P. A mathematical model of pollen-tube penetration in apple styles. Planta 1984, 160, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yoder, K.; Yuan, R.; Combs, L.; Byers, R.; McFerson, J.; Schmidt, T. Effects of Temperature and the combination of liquid lime sulfur and fish oil on pollen germination, pollen tube growth, and fruit set in apples. Hortscience 2009, 44, 1277–1283. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Burgos, L. Effective pollination period as related to stigma receptivity in apricot. Sci. Hortic. 1992, 52, 77–83. [Google Scholar] [CrossRef]
- Hedhly, A.; Hormaza, J.I.; Herrero, M. The effect of temperature on stigmatic receptivity in sweet cherry (Prunus avium L.). Plant Cell Environ. 2003, 26, 1673–1680. [Google Scholar] [CrossRef] [Green Version]
- Vasilakakis, M.; Porlingis, I.C. Effect of temperature on pollen germination, pollen tube growth, effective pollination period, and fruit set of pear. Hortscience 1985, 20, 733–735. [Google Scholar]
- Robbie, F.A.; Atkinson, C.J. Wood and tree age as factors influencing the ability of apple flowers to set fruit. J. Hortic. Sci. 1994, 69, 609–623. [Google Scholar] [CrossRef]
- Robbie, F.A.; Atkinson, C.J.; Knight, J.N.; Moore, K.G. Branch orientation as a factor determining fruit set in apple trees. J. Hortic. Sci. 1993, 68, 317–325. [Google Scholar] [CrossRef]
- Green, D.W. Effect of silver nitrate, aminoethoxyvinylglycine, and giberellins A4+7 plus 6-benzylamino purine on fruit set and development of ‘Delicious’ apples. J. Am. Soc. Hortic. Sci. 1980, 105, 717–720. [Google Scholar]
- Costa, C.; Biasi, R.; Bagni, N. Effect of putrescine on fruiting performance of apple (cv. Hi Early). Acta Hortic. 1986, 179, 355–362. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Lombard, P.B.; Sugar, D.; Polito, V.S. Putrescine influences ovule senescence, fertilization time, and fruit set in ‘Comice’ pear. J. Am. Soc. Hortic. Sci. 1988, 113, 708–712. [Google Scholar]
- Nyomora, A.M.S.; Brown, P.H.; Pinney, K.; Polito, V.S. Foliar application of boron to almond trees affects pollen quality. J. Am. Soc. Hortic. Sci. 2000, 125, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Jahed, K.R.; Hirst, P.M. Pollen source effects on seed number, fruit quality and return bloom of apple. J. Am. Pomol. Soc. 2018, 72, 212–221. [Google Scholar]
- DeLong, C.N.; Yoder, K.S.; Combs, L.; Veilleux, R.E.; Peck, G.M. Apple pollen tube growth rates are regulated by parentage and environment. J. Am. Soc. Hortic. Sci. 2016, 141, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Robinson, T.L. V-shaped apple planting systems. Acta Hortic. 1998, 513, 337–348. [Google Scholar] [CrossRef]
- Musacchi, S.; Green, D. Innovations in apple tree cultivation to manage crop load and ripening. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2017; pp. 195–237. ISBN 9781786760326. [Google Scholar]
- Zhang, L.; Ferguson, L.; Whiting, M.D. Temperature effects on pistil viability and fruit set in sweet cherry. Sci. Hortic. 2018, 241, 8–17. [Google Scholar] [CrossRef]
- Prieto, V.M.G.; Chu, A.R.; Chacón, A.R.; Reyes, D.B.; Avitia, J.O. Effective pollination period in ‘RedChief’ and ‘Golden Delicious’ apples (Malus domestica Borkh). Span. J. Agric. Res. 2009, 7, 928–932. [Google Scholar] [CrossRef] [Green Version]
- Roeder, S.; Serra, S.; Musacchi, S. Effective Pollination Period and Parentage Effect on Pollen Tube Growth in Apple. Plants 2021, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Dorigoni, A.; Lezzer, P.; Dallabetta, N.; Serra, S.; Musacchi, S. Bi-axis: An alternative to slender spindle for apple orchards. Acta Hortic. 2011, 903, 581–588. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; De Franceschi, P. Dondini, L. Musacchi, S. Characterization of a novel self-incompatibility allele in Malus and S-genotyping of select crabapple cultivars. Sci. Hortic. 2018, 240, 186–195. [Google Scholar] [CrossRef]
- Sheick, R.; Serra, S.; Tillman, J.; Luby, J.; Evans, K.; Musacchi, S. Characterization of a novel S-RNase allele and genotyping of new apple cultivars. Sci. Hortic. 2020, 273, 109630. [Google Scholar] [CrossRef]
- Elsysy, M.; Serra, S.; Schwallier, P.; Musacchi, S.; Einhorn, T. Net enclosure of ‘Honeycrisp’ and ‘Gala’ apple trees at different bloom stages affects fruit set and alters seed production. Agronomy 2019, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Sheffield, C.S. Pollination, seed set and fruit quality in apple: Studies with Osmia lignaria (Hymenoptera: Megachiledae) in the Annapolis Valley, Nova Scotia, Canada. J. Pollinat. Ecol. 2014, 12, 120–128. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1984. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; Vienna: R Project; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 10 August 2021).
- Losada, J.M.; Herrero, M. Arabinogalactan-protein secretion is associated with the acquisition of stigmatic receptivity in the apple flower. Ann. Bot. 2012, 110, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losada, J.M.; Herrero, M. Flower strategy and stigma performance in the apple inflorescence. Sci. Hortic. 2013, 150, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Jakopic, J.; Zupan, A.; Eler, K.; Schmitzer, V.; Stampar, F.; Veberic, R. It’s great to be the king: Apple fruit development affected by the position in the cluster. Sci. Hortic. 2015, 194, 18–25. [Google Scholar] [CrossRef]
- Ferree, D.C.; Bishop, B.L.; Schupp, J.R.; Tustin, D.S.; Cashmore, W.M. Influence of flower type, position in the cluster and spur characteristics on fruit set and growth of apple cultivars. J. Hortic. Sci. Biotechnol. 2001, 76, 1–8. [Google Scholar] [CrossRef]
- Hampson, C.R.; Sanford, K.; Cline, J. Preferences of Canadian consumers for apple fruit size. Can. J. Plant Sci. 2002, 82, 165–167. [Google Scholar] [CrossRef]
- Jahed, K.R.; Hirst, P.M. Pollen tube growth and fruit set in apple. Hortscience 2017, 52, 1054–1059. [Google Scholar] [CrossRef] [Green Version]
- Broothaerts, W. New findings in apple S-genotype analysis resolve previous confusion and request the re-numbering of some S-alleles. Theor. Appl. Genet. 2003, 106, 703–714. [Google Scholar] [CrossRef]
- Radičević, S.; Cerović, R.; Đorđević, M. Ovule senescence and unusual pollen tube growth in the ovary of sweet cherry as affected by pistilar genotype and temperature. Span. J. Agric. Res. 2018, 16, 1–17. [Google Scholar] [CrossRef]
- Stösser, R.; Anvari, S.F. On the senescence of ovules in cherries. Sci. Hortic. 1982, 16, 29–38. [Google Scholar] [CrossRef]
- Williams, R.R. The effect of nitrogen on the selffruitfulness of certain varieties of cider apples. J. Hortic. Sci. 1963, 38, 52–60. [Google Scholar] [CrossRef]
- Rodrigo, J.; Hormaza, J.I.; Herrero, M. Ovary starch reserves and flower development in apricot (Prunus armeniaca). Physiol. Plant. 2000, 108, 35–41. [Google Scholar] [CrossRef]
- Herrero, M.; Arbeloa, A. Influence of the pistil on pollen tube kinetics in peach (Prunus persica). Am. J. Bot. 1989, 76, 1441–1447. [Google Scholar] [CrossRef]
- Li, H.J.; Meng, J.G.; Yang, W.C. Multilayered signaling pathways for pollen tube growth and guidance. Plant Reprod. 2018, 31, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P. 14C-studies on apple trees: VII. The early seasonal growth in leaves, flowers, and shoots as dependent upon current photosynthesis and existing reserves. Physiol. Plant. 1971, 25, 469–473. [Google Scholar] [CrossRef]
- Loescher, W.H.; McCamant, T.; Keller, J.D. Carbohydrate reserves, translocation, and storage in woody plant roots. Hortscience 1990, 25, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Goldway, M.; Shai, O.; Yehuda, H.; Matityahu, A.; Stern, R.A. ‘Jonathan’ apple is a lower-potency pollenizer of ‘Topred’ than Golden Delicious’ due to partial S-allele incompatibility. J. Hortic. Sci. Biotechnol. 1999, 74, 381–385. [Google Scholar] [CrossRef]
- Schneider, D.; Stern, R.A.; Goldway, M.A. comparison between semi-and fully compatible apple pollinators grown under suboptimal pollination conditions. Hortscience 2005, 40, 1280–1282. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, H.C.; Salazar-Gutiérrez, M.; Chaves, B.; Hoogenboom, G. Modeling pollen tube growth of ‘Gala’ and ‘Fuji’ apples. Sci. Hortic. 2018, 240, 125–132. [Google Scholar] [CrossRef]
- Brault, A.M.; de Oliveria, D. Seed Number and an Asymmetry Index of ‘McIntosh’ Apples. Hortscience 1995, 30, 44–46. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Tateishi, N.; Tanabe, K. Pollen density on the stigma affects endogenous gibberellin metabolism, seed and fruit set, and fruit quality in Pyrus pyrifolia. J. Exp. Bot. 2010, 61, 4291–4302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erbar, C. Pollen tube transmitting tissue: Place of competition of male gametophytes. Int. J. Plant Sci. 2003, 164, S265–S277. [Google Scholar] [CrossRef] [Green Version]
- Sheffield, C.S.; Smith, R.F.; Kevan, P.G. Perfect syncarpy in apple (Malus × domestica ‘Summerland McIntosh’) and its implications for pollination, seed distribution and fruit production (Rosaceae: Maloideae). Ann. Bot. 2005, 95, 583–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pollen Sources—2019 | N Pistils (Avg 5 Styles) | Pollen Tube Length (mm) | Significance Across Days ← | |||||||
| 1 DAP | 2 DAP | 3 DAP | 4 DAP | |||||||
| Evereste | 5 | 0.45 | bD | 2.79 | bC | 4.80 | bB | 8.39 | aA | *** |
| Granny Smith | 5 | 0.81 | aD | 3.74 | aC | 5.99 | aB | 8.08 | aA | *** |
| Indian Summer | 5 | 0.70 | aC | 0.46 | dC | 1.15 | cB | 4.78 | bA | *** |
| Mt Blanc | - | - | - | - | - | - | - | - | - | - |
| Snowdrift | 5 | 0.24 | bC | 2.43 | cB | 6.43 | aA | *** | ||
| Significance across pollens ↓ | *** | *** | *** | *** | ||||||
| Pollen Sources—2020 | N Pistils (Avg 5–6 Styles) | Pollen Tube Length (mm) | Significance Across Days ← | |||||||
| 1 DAP | 2 DAP | 3 DAP | 4 DAP | |||||||
| Evereste | 5–6 | 0.96 | C | 2.66 | cB | 8.33 | A | *** | ||
| Granny Smith | 5–6 | 0.91 | C | 3.67 | bB | 7.58 | A | *** | ||
| Indian Summer | 5–6 | 0.91 | C | 4.09 | abB | 8.57 | A | *** | ||
| Mt Blanc | 5–6 | 0.83 | C | 3.81 | bB | 7.66 | A | *** | ||
| Snowdrift | 5–6 | 0.92 | C | 4.47 | aB | 7.96 | A | *** | ||
| Significance across pollens ↓ | NS | *** | NS | |||||||
| Pollen Sources—2019 | n Apples | Avg Apple Diameter (mm) | Avg Apple Weight (g) | Avg Number of Healthy-Mature Seeds/Apple | Avg Number of under-Developed Seeds/Apple | Avg Number of Total Seeds/Apple |
| Evereste | 11 | 77.8 | 211 | 9.64 | 0.82 | 10.45 |
| Granny Smith | 9 | 77.3 | 206 | 10.33 | 0.78 | 11.11 |
| Indian Summer | 8 | 80.8 | 241 | 8.88 | 1.75 | 10.63 |
| Mt Blanc | − | − | − | − | − | − |
| Snowdrift | 6 | 81.2 | 244 | 9.67 | 1.17 | 10.83 |
| Significance across pollens ↓ | NS | NS | NS | NS | NS | |
| Pollen Sources—2020 | n Apples | Avg Apple Diameter (mm) | Avg Apple Weight (g) | Avg Number of Healthy-Mature Seeds/Apple | Avg Number of under-Developed Seeds/Apple | Avg Number of Total Seeds/Apple |
| Evereste | 16 | 80.5 | 252 | 9.13 | 0.94 | 10.06 |
| Granny Smith | 13 | 79.4 | 230 | 9.46 | 0.46 | 9.92 |
| Indian Summer | 14 | 83.1 | 277 | 9.27 | 0.67 | 9.93 |
| Mt Blanc | 15 | 83.1 | 275 | 8.73 | 1.27 | 10.00 |
| Snowdrift | 18 | 85.4 | 287 | 9.56 | 0.50 | 10.06 |
| Significance across pollens ↓ | NS | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, S.; Roeder, S.; Sheick, R.; Musacchi, S. Pistil Biology of ‘WA 38’ Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set. Agronomy 2022, 12, 123. https://doi.org/10.3390/agronomy12010123
Serra S, Roeder S, Sheick R, Musacchi S. Pistil Biology of ‘WA 38’ Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set. Agronomy. 2022; 12(1):123. https://doi.org/10.3390/agronomy12010123
Chicago/Turabian StyleSerra, Sara, Stefan Roeder, Ryan Sheick, and Stefano Musacchi. 2022. "Pistil Biology of ‘WA 38’ Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set" Agronomy 12, no. 1: 123. https://doi.org/10.3390/agronomy12010123
APA StyleSerra, S., Roeder, S., Sheick, R., & Musacchi, S. (2022). Pistil Biology of ‘WA 38’ Apple and Effect of Pollen Source on Pollen Tube Growth and Fruit Set. Agronomy, 12(1), 123. https://doi.org/10.3390/agronomy12010123









