Ecosystem Services Provided by Cover Crops and Biofumigation in Sunflower Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Crop-Management Systems
2.2. Cover Crop and Soil Sampling
2.2.1. Cover Crop Sampling and Partial Land Equivalent Ratio Calculation
2.2.2. Soil Sampling
2.3. Sunflower Sampling and Disease Assessment
2.3.1. Assessment of Sunflower Nutrition Status
2.3.2. Assessment of Sunflower Verticillium Wilt Severity
2.3.3. Sunflower Yield Estimation
2.4. Statistical Analysis
3. Results
3.1. Cover Crop Production and Characterisation
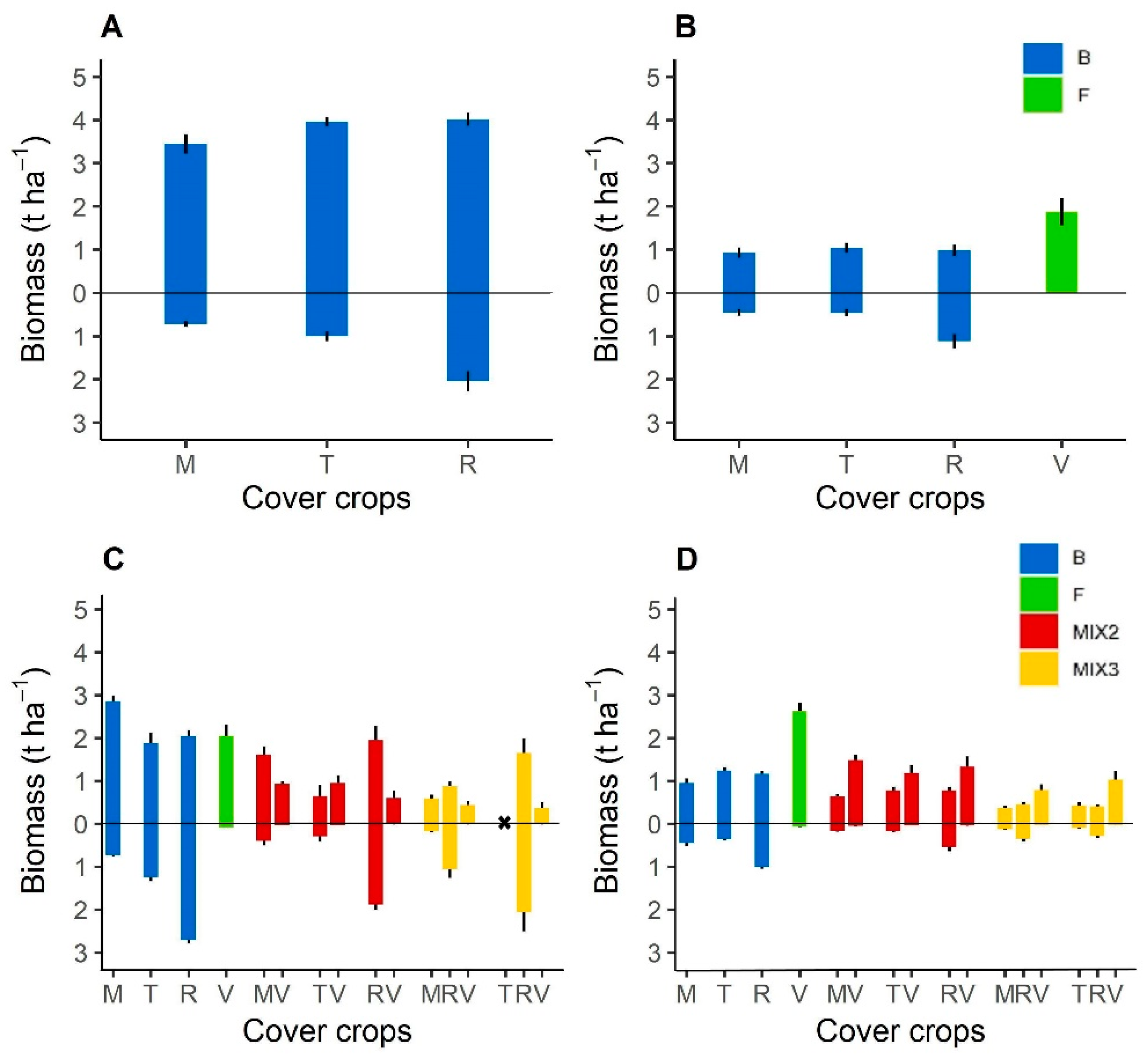
3.1.1. Cover Crop Biomass Production
3.1.2. Cover Crop C:N Ratio
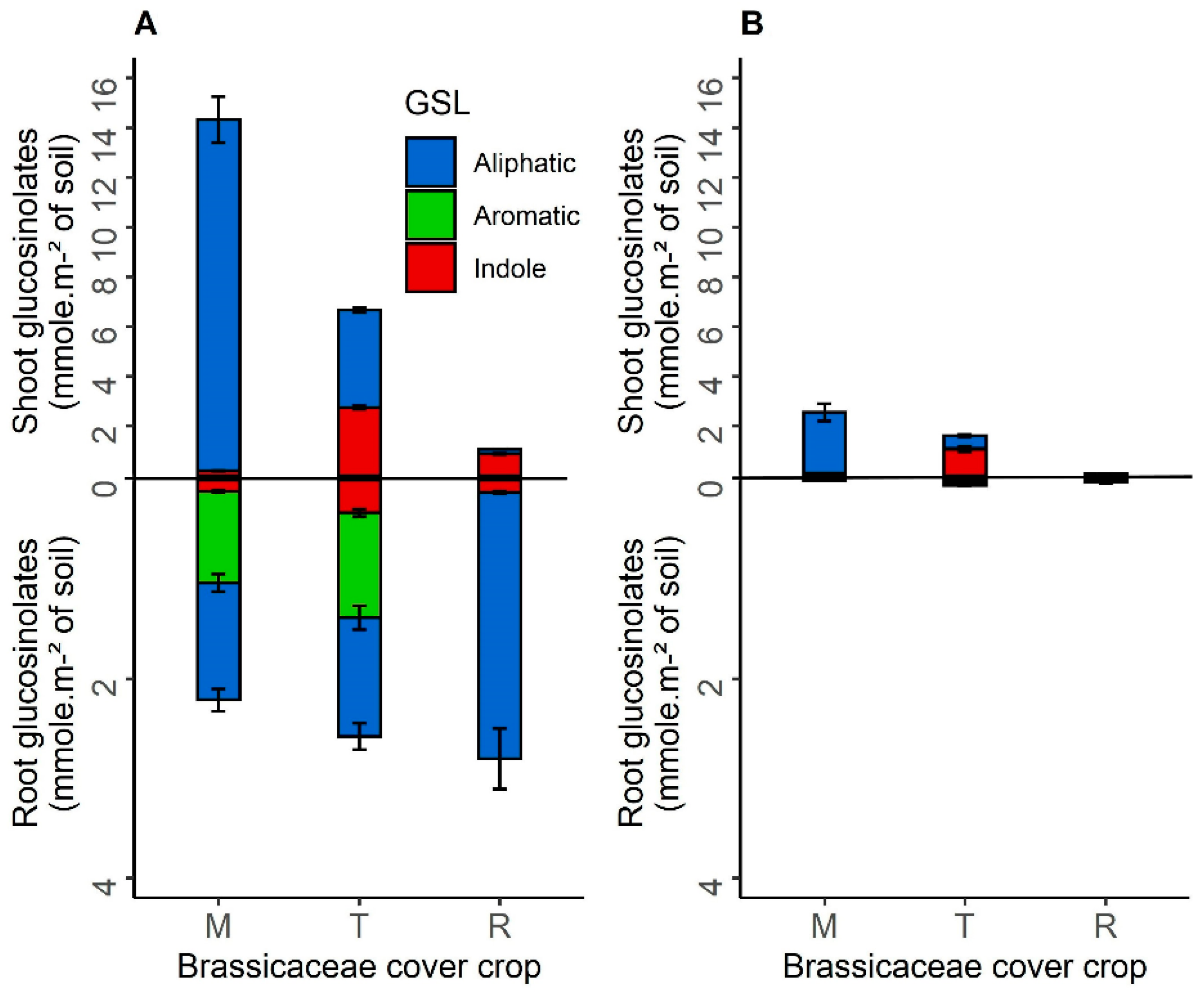
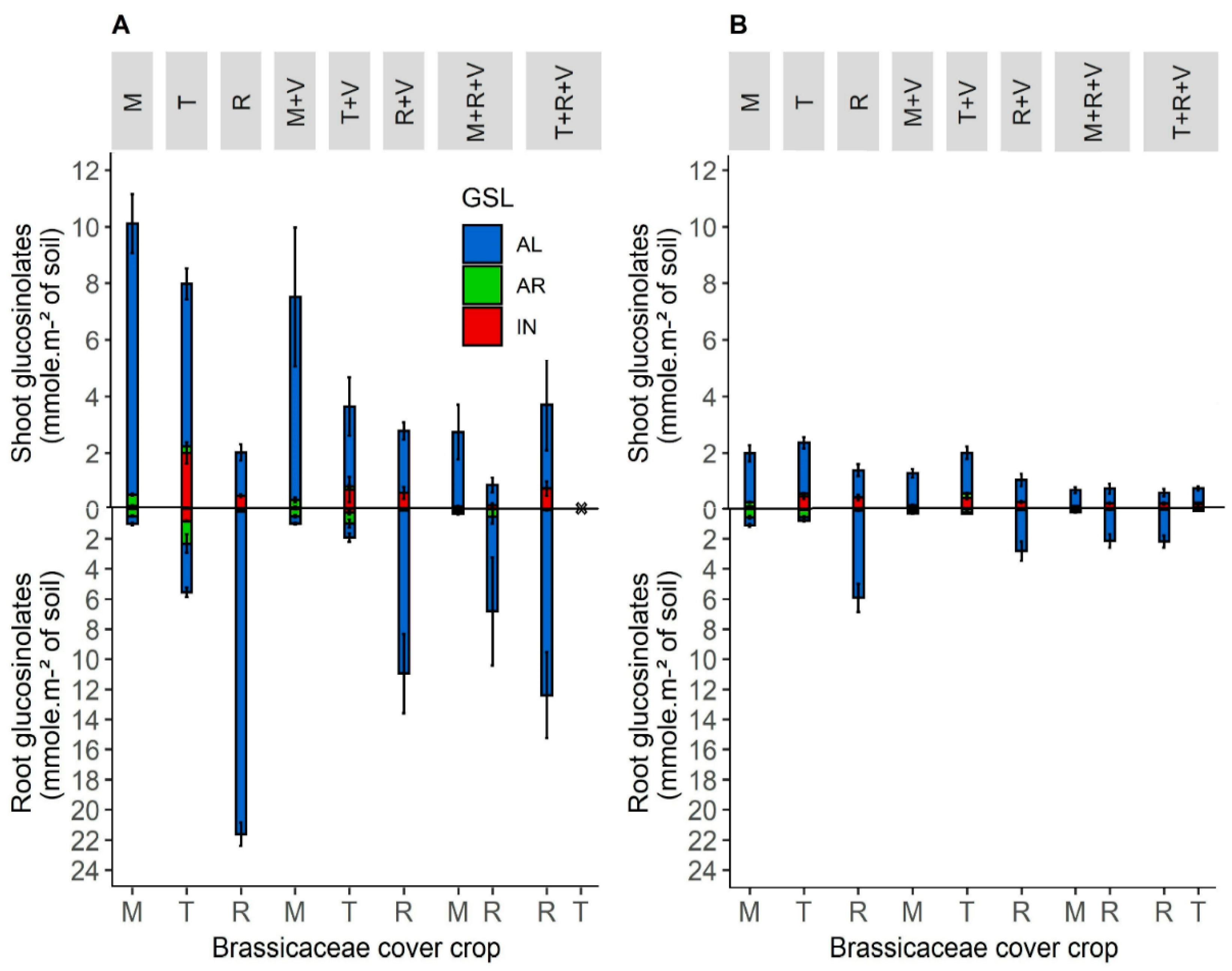
3.1.3. Glucosinolates Production of Brassicaceae Cover Crops
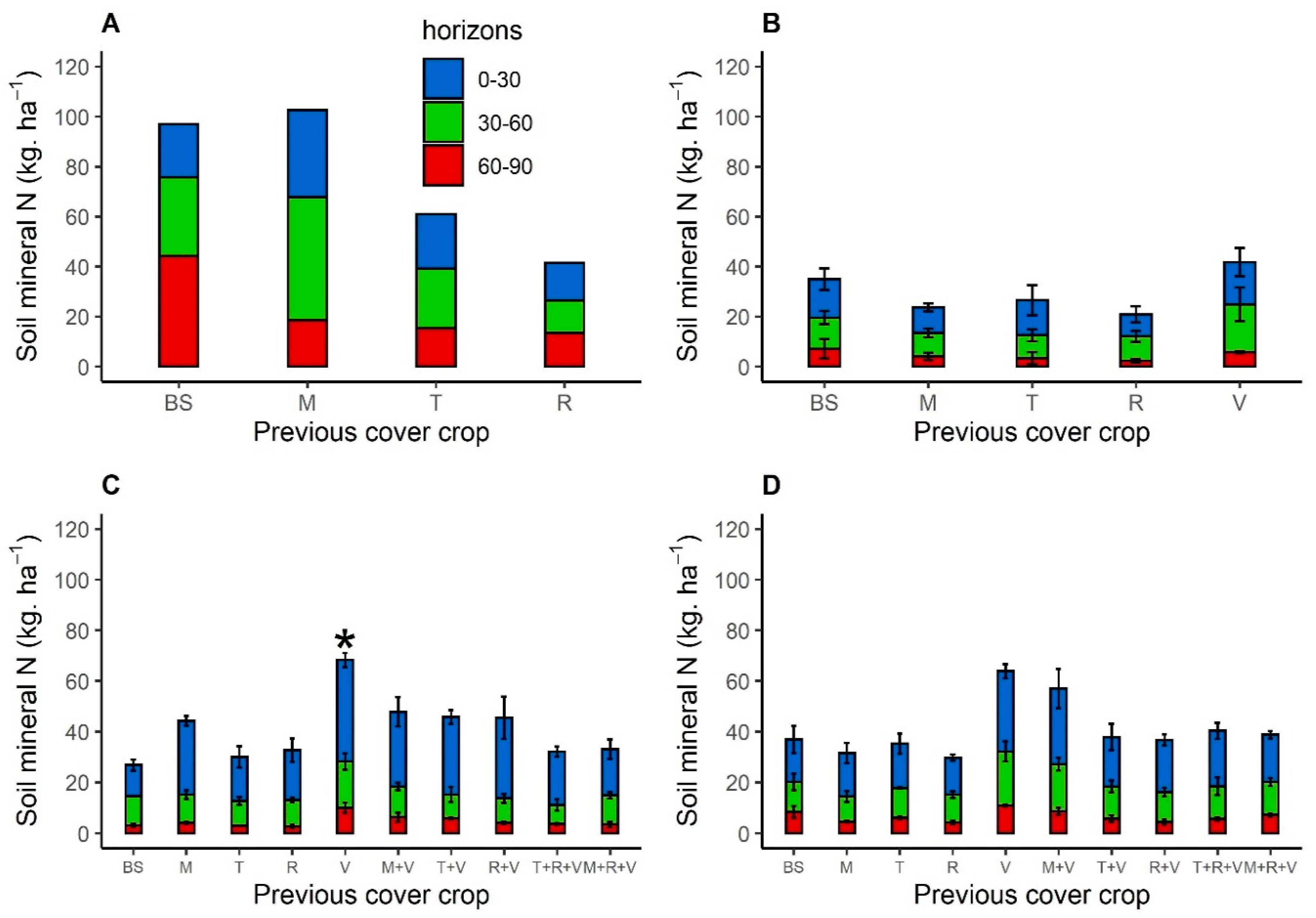
3.2. Soil Mineral Nitrogen Available for Sunflower before Sowing
3.3. Effects of Cover Crops and Biofumigation on Sunflower Verticillium Wilt
3.4. Effects of Individual Factors on Sunflower Yield
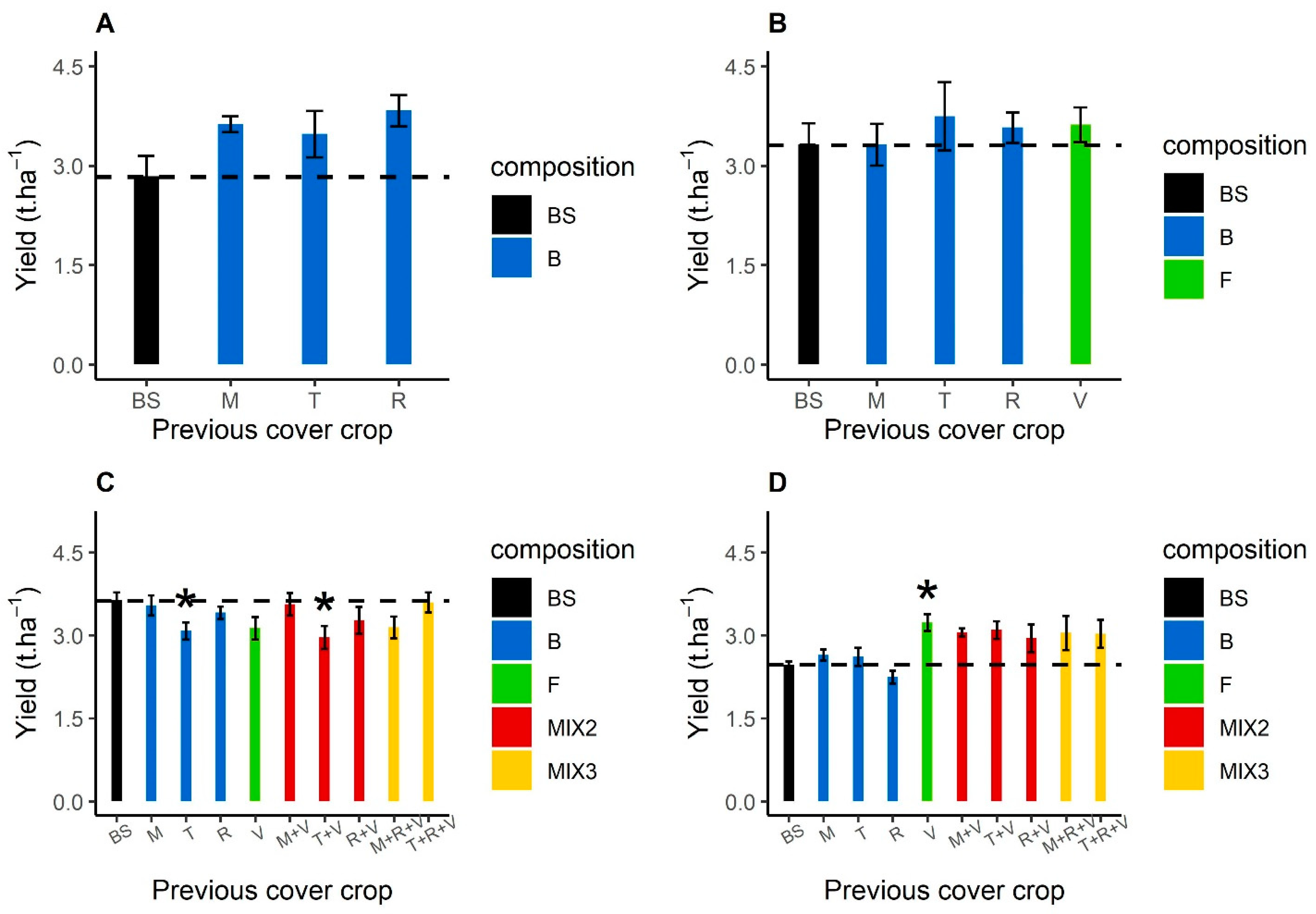
3.4.1. Previous Cover Crop
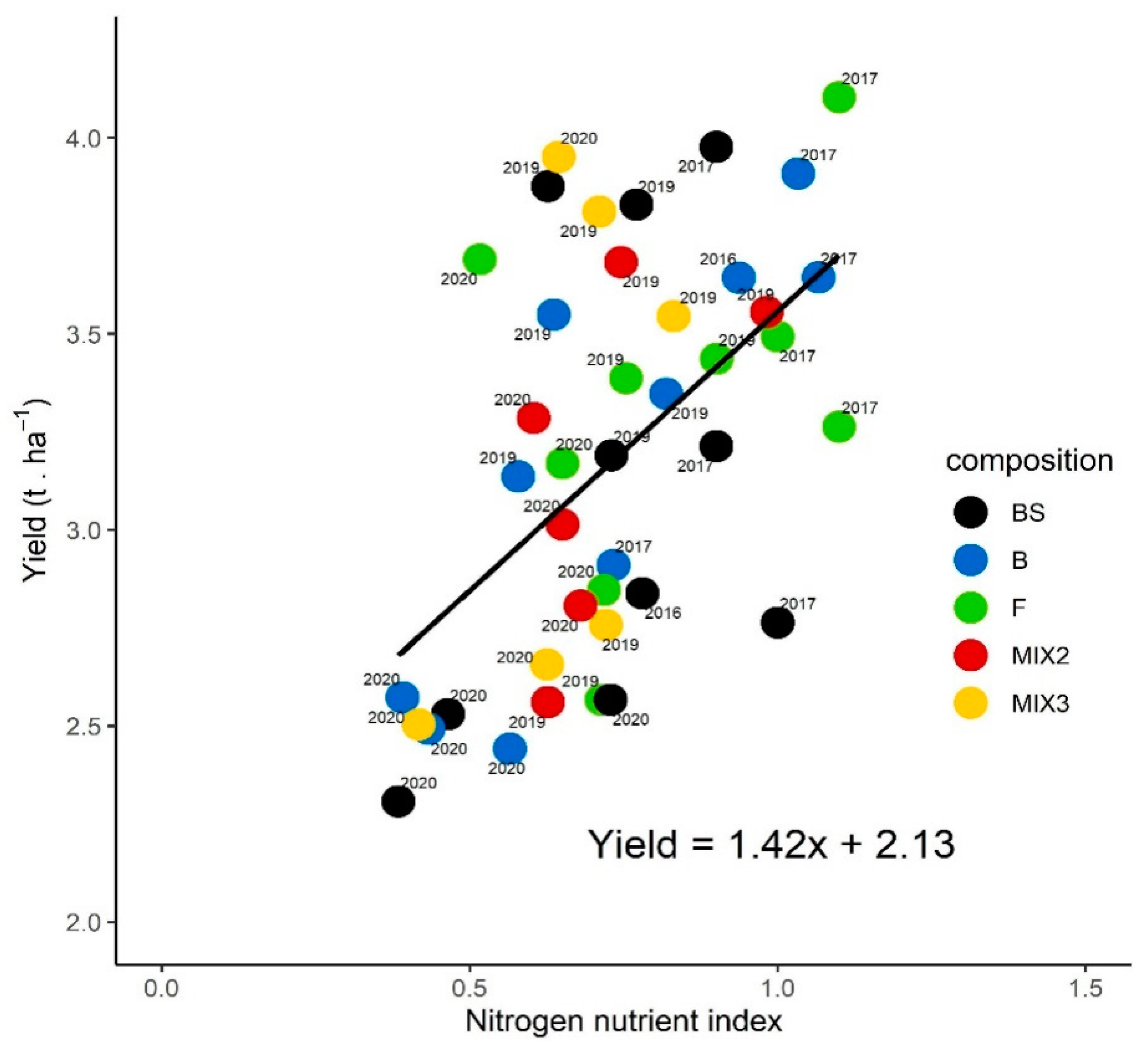
3.4.2. Nitrogen Nutrient index at Flowering and Yields
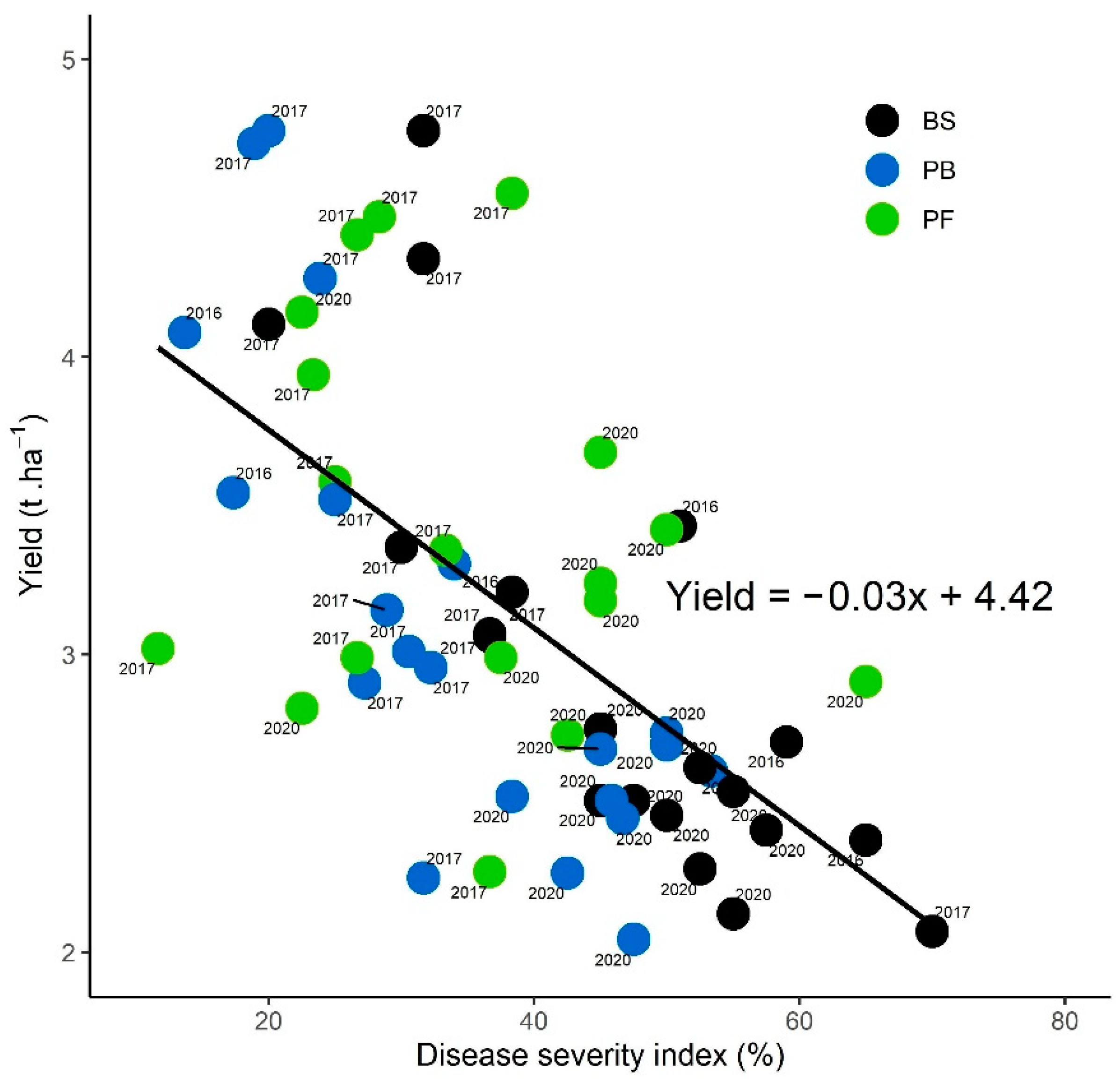
3.4.3. Disease Severity Index at Maturity and Yields
4. Discussion
4.1. Cover Crop Effects on Soil Mineral Nitrogen Available for Sunflower
4.2. Cover Crops and Biofumigation Effects on Sunflower Verticillium Wilt
4.3. Sunflower Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Cover Crops | Biomass (t ha−1) | SE | Significance of the Comparison between the Biomass of the Cover Crop in Sole Crop and in a Mixture |
|---|---|---|---|---|
| AUZ16 | Brown mustard | 4.17 | 0.29 | / |
| Turnip rape | 4.96 | 0.19 | / | |
| Fodder radish | 6.06 | 0.30 | / | |
| AUZ17 | Brown mustard | 1.38 | 0.19 | / |
| Turnip rape | 1.50 | 0.19 | / | |
| Fodder radish | 2.11 | 0.29 | / | |
| Vetch | 1.88 | 0.31 | ||
| AUZ19 | Brown mustard | 3.58 | 0.13 | / |
| Turnip rape | 3.12 | 0.32 | / | |
| Fodder radish | 4.74 | 0.22 | / | |
| Vetch | 2.14 | 0.27 | / | |
| Brown mustard * Vetch | 2.02/0.96 | 0.12/0.05 | **/** | |
| Turnip rape * Vetch | 0.94/0.98 | 0.38/0.17 | ***/** | |
| Fodder radish * Vetch | 3.83/0.61 | 0.21/0.17 | ns/*** | |
| Brown mustard * Fodder radish * Vetch | 0.76/1.93/0.45 | 0.12/0.28/0.09 | ***/***/*** | |
| Turnip rape * Fodder radish * Vetch | NA/3.72/0.38 | NA/0.77 /0.13 | NA/ns/*** | |
| AUZ20 | Brown mustard | 1.39 | 0.17 | / |
| Turnip rape | 1.60 | 0.10 | / | |
| Fodder radish | 2.17 | 0.09 | / | |
| Vetch | 2.71 | 0.20 | / | |
| Brown mustard * Vetch | 0.80/1.54 | 0.08/0.12 | **/*** | |
| Turnip rape * Vetch | 0.95/1.22 | 0.10/0.18 | **/*** | |
| Fodder radish * Vetch | 1.31/1.37 | 0.19/0.25 | **/*** | |
| Brown mustard * Fodder radish * Vetch | 0.49/0.81/0.82 | 0.06/0.10/0.14 | ***/***/*** | |
| Turnip rape * Fodder radish * Vetch | 0.69/0.53/1.06 | 0.10/0.08/0.21 | ***/***/*** |
| Year | Cover Crops | C:N Ratio | SE | Significance of the Comparison of the C:N Ratios between Sole Crop and a Mixture |
|---|---|---|---|---|
| 2016 | Brown mustard | 14.64 | 0.88 | / |
| Turnip rape | 14.43 | 1.11 | / | |
| Fodder radish | 13.47 | 0.97 | / | |
| 2017 | Brown mustard | 19.61 | 1.52 | / |
| Turnip rape | 19.83 | 0.51 | / | |
| Fodder radish | 20.89 | 1.35 | / | |
| Vetch | 10.79 | 0.26 | / | |
| 2019 | Brown mustard | 28.31 | 1.4 | / |
| Turnip rape | 24.43 | 1.26 | / | |
| Fodder radish | 26.8 | 0.43 | / | |
| Vetch | 12.9 | 0.69 | / | |
| Brown mustard * Vetch | 16.25/12.13 | 2.26/0.19 | */ns | |
| Turnip rape * Vetch | 16.34/12.09 | 0.48/0.06 | */ns | |
| Fodder radish * Vetch | 17.83/11.98 | 1.97/0.11 | */ns | |
| Brown mustard * Fodder radish * Vetch | 18.54/19.75/12.58 | 3.30/2.96/0.25 | **/ns/ns | |
| Turnip rape * Fodder radish * Vetch | NA/21.14/11.8 | NA/1.98/0.26 | NA/ns/ns | |
| 2020 | Brown mustard | 22.62 | 0.7 | / |
| Turnip rape | 24.35 | 0.83 | / | |
| Fodder radish | 24.14 | 0.42 | / | |
| Vetch | 13.58 | 0.25 | / | |
| Brown mustard * Vetch | 19.78/13.89 | 0.47/0.19 | **/ns | |
| Turnip rape * Vetch | 21.78/14.15 | 0.55/0.24 | */ns | |
| Fodder radish * Vetch | 20.35/14.47 | 0.76/0.17 | **/ns | |
| Brown mustard * Fodder radish * Vetch | 22.81/22.3/14.24 | 0.54/0.51/0.27 | ns/ns/ns | |
| Turnip rape * Fodder radish * Vetch | 21.70/20.81/14.09 | 0.45/0.63/0.17 | **/**/ns |
| Year | Treatment | AUDPC | SE | Significance of the Comparison of the AUDPC of the Cover Crop to That of the Bare Soil |
|---|---|---|---|---|
| 2016 | Bare soil | 91.74 | 40.62 | / |
| Brown mustard | 30.72 | 32.27 | ** | |
| Turnip rape | 24.78 | 29.66 | ** | |
| Fodder radish | 25.07 | 33.84 | ** | |
| 2017 | Bare soil | 44.72 | 32.11 | / |
| Brown mustard | 39.82 | 30.21 | ns | |
| Turnip rape | 31.72 | 29.24 | * | |
| Fodder radish | 25.62 | 26.39 | ** | |
| Vetch | 32.77 | 26.24 | ns | |
| 2019 | Bare soil | 30.31 | 21.24 | / |
| Brown mustard | 43.12 | 26.60 | ** | |
| Turnip rape | 28.00 | 20.41 | ns | |
| Fodder radish | 35.08 | 22.19 | ns | |
| Vetch | 44.06 | 29.26 | ** | |
| Brown mustard * Vetch | 40.78 | 27.42 | ns | |
| Turnip rape * Vetch | 39.73 | 22.96 | ns | |
| Fodder radish * Vetch | 34.46 | 22.96 | ns | |
| Brown mustard * Fodder radish * Vetch | 29.53 | 17.28 | ns | |
| Turnip rape * Fodder radish * Vetch | 34.18 | 23.13 | ns | |
| 2020 | Bare soil | 73.38 | 30.89 | / |
| Brown mustard | 69.18 | 26.13 | ns | |
| Turnip rape | 67.11 | 29.77 | ns | |
| Fodder radish | 60.05 | 33.75 | ns | |
| Vetch | 53.67 | 38.64 | ** | |
| Brown mustard * Vetch | 66.02 | 30.91 | ns | |
| Turnip rape * Vetch | 67.65 | 25.49 | ns | |
| Fodder radish * Vetch | 62.77 | 33.18 | ns | |
| Brown mustard * Fodder radish * Vetch | 75.31 | 26.40 | ns | |
| Turnip rape * Fodder radish * Vetch | 65.43 | 27.62 | ns |
References
- FAO. Available online: https://www.fao.org/3/cb4479en/cb4479en.pdf (accessed on 9 November 2021).
- Le Clef, E.; Kemper, T. Sunflower seed preparation and oil extraction. In Sunflower Chemistry, Production, Processing, and Utilization, 1st ed.; Martínez-Force, E., Turgut Dunford, N., Salas, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 187–226. [Google Scholar] [CrossRef]
- Debaeke, P.; Bedoussac, L.; Bonnet, C.; Bret-Mestries, E.; Seassau, C.; Gavaland, A.; Raffaillac, D.; Tribouillois, H.; Véricel, G.; Justes, E. Sunflower crop: Environmental-friendly and agroecological. OCL Oilseeds Fats Crops Lipids 2017, 24, D304. [Google Scholar] [CrossRef] [Green Version]
- Champolivier, L.; Debaeke, P.; Merrien, A. Pourquoi irriguer le tournesol, une culture réputée tolérante à la sécheresse? Innov. Agron. 2011, 14, 151–164. [Google Scholar]
- Velasco, L.; Fernández-Martínez, J.M.; Fernández, J. Sunflower production in the European Union. In Sunflower Chemistry, Production, Processing, and Utilization, 1st ed.; Martínez-Force, E., Turgut Dunford, N., Salas, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 555–573. [Google Scholar] [CrossRef]
- Lecomte, V.; Nolot, J.M. Place du tournesol dans le système de culture. Innov. Agron. 2011, 14, 59–76. [Google Scholar]
- Jouffret, P.; Labalette, F.; Thibierge, J. Atouts et besoins en innovations du tournesol pour une agriculture durable. Innov. Agron. 2011, 14, 1–17. [Google Scholar]
- Alberio, C.; Izquierdo, N.G.; Aguirrezábal, L.A.N. Sunflower Crop Physiology and Agronomy. In Sunflower Chemistry, Production, Processing, and Utilization, 1st ed.; Martínez-Force, E., Turgut Dunford, N., Salas, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 53–91. [Google Scholar] [CrossRef]
- Debaeke, P.; Casadebaig, P.; Flenet, F.; Langlade, N. Sunflower crop and climate change: Vulnerability, adaptation, and mitigation potential from case-studies in Europe. OCL Oilseeds Fats Crops Lipids 2017, 24, D102. [Google Scholar] [CrossRef] [Green Version]
- Harris, H.; McWilliam, J.; Mason, W. Influence of temperature on oil content and composition of sunflower seed. Aust. J. Agric. Res. 1978, 29, 1203–1212. [Google Scholar] [CrossRef]
- Franceagrimer. Available online: https://www.franceagrimer.fr (accessed on 15 June 2021).
- Seassau, C. Etiologie du Syndrome de Dessèchement Précoce du Tournesol: Implication de Phoma Macdonaldii et Interaction Avec la Conduite de Culture. Ph.D. Dissertation, Université Fédérale de Toulouse, Toulouse, France, 2010. [Google Scholar]
- Couëdel, A.; Alletto, L.; Tribouillois, H.; Justes, E. Cover crop crucifer-legume mixtures provide effective nitrate catch crop and nitrogen green manure ecosystem services. Agric. Ecosyst. Environ. 2018, 254, 50–59. [Google Scholar] [CrossRef]
- Poisson-Bammé, B.; Pérès, A. Survie du phoma du tournesol (Leptosphaeria lindquistii) sur les résidus de récolte. In Proceedings of the 6th International Conference on Plant Diseases, AFPP, Tours, France, 6–8 December 2000; pp. 331–338. [Google Scholar]
- Mesteries, E. Lutter contre le verticillium grâce aux variétés. Perspect. Agric. 2013, 406, 24–28. [Google Scholar]
- Uppal, A.; El Hadrami, A.; Adam, L.; Tenuta, M.; Daayf, F. Biological control of potato Verticillium wilt under controlled and field conditions using selected bacterial antagonists and plant extracts. Biol. Control 2008, 44, 90–100. [Google Scholar] [CrossRef]
- Rowe, R.C.; Powelson, M.L. Potato Early Dying: Management Challenges in a Changing Production Environment. Plant Dis. 2002, 86, 1184–1193. [Google Scholar] [CrossRef] [Green Version]
- Soesanto, L. Ecology and Biological Control of Verticillium dahliae. Ph.D. Dissertation, Wageningen University, Wageningen, The Netherlands, 2000. [Google Scholar]
- Mestries, E.; Lecomte, V. Tournesol et verticillium: État des lieux et moyens de lutte dans le Sud-Ouest. In Proceedings of the Réunion Technique Régionale CETIOM, Ondes, France, 7 December 2012; pp. 1–12. [Google Scholar]
- Wilhem, S. Longevity of the Verticillium wilt fungus in the laboratory and field. Phytopathology 1995, 45, 180–181. [Google Scholar]
- Lloyd, M.G.; McRoberts, N.; Gordon, T.R. Cryptic Infection and Systemic Colonization of Leguminous Crops by Verticillium dahliae, the Cause of Verticillium Wilt. Plant Dis. 2019, 103, 3166–3171. [Google Scholar] [CrossRef]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts, 1st ed.; CABI: Wallingford, UK, 2002; 552p. [Google Scholar] [CrossRef]
- Hartz, T.; Johnstone, P.; Miyao, E.; Davis, R. Mustard Cover Crops Are Ineffective in Suppressing Soilborne Disease or Improving Processing Tomato Yield. HortScience 2005, 40, 2016–2019. [Google Scholar] [CrossRef] [Green Version]
- Larkin, R.P.; Honeycutt, C.W.; Olanya, O.M. Management of Verticillium Wilt of Potato with Disease-Suppressive Green Manures and as Affected by Previous Cropping History. Plant Dis. 2011, 95, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Woolliams, G.E. Host range and symptomatology of Verticillium dahliae in economic, weed, and native plants in interior British Columbia. Can. J. Plant Sci. 1966, 46, 661–669. [Google Scholar] [CrossRef]
- Duniway, J.M. Status of Chemical Alternatives to Methyl Bromide for Pre-Plant Fumigation of Soil. Phytopathology 2002, 92, 1337–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villarino, M.; Larena, I.; Melgarejo, P.; De Cal, A. Effect of chemical alternatives to methyl bro-mide on soil-borne disease inci-dence and fungal populations in Spanish strawberry nurseries: A long-term study. Pest Manag. Sci. 2021, 77, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Debaeke, P.; Bret-Mestries, E.; Aubertot, J.N.; Casadebaig, P.; Champolivier, L.; Dejoux, J.F.; Seassau, C. Sunflower agronomy: 10 years of research in partnership within the “Sunflower” Technological Joint Unit (UMT) in Toulouse. OCL Oilseeds Fats Crops Lipids 2020, 27, 14. [Google Scholar] [CrossRef] [Green Version]
- Ait Kaci Ahmed, N.; Dechamp-Guillaume, G.; Seassau, C. Biofumigation to protect oilseed crops: Focus on management of soilborne fungi of sunflower. OCL Oilseeds Fats Crops Lipids 2020, 27, 59. [Google Scholar] [CrossRef]
- Kirkegaard, J.A.; Gardner, P.A.; Desmarchelier, J.M.; Angus, J.F. Biofumigation: Using Brassica species to control pests and dis-eases in horticulture and agriculture. In Proceedings of the 9th Australian Research Assembly on Brassicas, Wagga Wagga, Australia, 5–7 October 1993; pp. 1–77. [Google Scholar]
- Kirkegaard, J.A.; Matthiessen, J.N. Developing and refining the biofumigation concept. Agroindustria 2004, 3, 233–239. [Google Scholar]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J.; VanEtten, C.H. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar] [CrossRef]
- Brown, P.D.; Morra, M.J. Control of Soil-Borne Plant Pests Using Glucosinolate-Containing Plants. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1997; Volume 61, pp. 167–231. [Google Scholar]
- van Dam, N.M.; Tytgat, T.O.G.; Kirkegaard, J.A. Root and shoot glucosinolates: A comparison of their diversity, function and interactions in natural and managed ecosystems. Phytochem. Rev. 2009, 8, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Mithen, R. Glucosinolates—Biochemistry, genetics and biological activity. Plant Growth Regul. 2001, 34, 91–103. [Google Scholar] [CrossRef]
- Olivier, C.; Vaughn, S.F.; Mizubuti, E.S.G.; Loria, R. Variation in Allyl Isothiocyanate Production Within Brassica Species and Correlation with Fungicidal Activity. J. Chem. Ecol. 1999, 25, 2687–2701. [Google Scholar] [CrossRef]
- Neubauer, C.; Heitmann, B.L.; Müller, C. Biofumigation potential of Brassicaceae cultivars to Verticillium dahliae. Eur. J. Plant Pathol. 2014, 140, 341–352. [Google Scholar] [CrossRef]
- Seassau, C.; Desserr, D.; Desplanques, J.; Mestries, E.; Dechamp-Guillaume, G.; Alletto, L. Control of Verticillium dahliae causing sunflower wilt using Brassica cover crops. In Proceedings of the 19th International Sunflower Conference, Edirne, Turkey, 29 May–3 June 2016; p. 1230. [Google Scholar]
- Michel, V.V. Ten years of biofumigation research in Switzerland. In Proceedings of the 5th International Symposium of Biofumigation, Newport, UK, 9 September 2014; Volume 126, pp. 1–5. [Google Scholar]
- Matthiessen, J.N.; Kirkegaard, J. Biofumigation and Enhanced Biodegradation: Opportunity and Challenge in Soilborne Pest and Disease Management. Crit. Rev. Plant Sci. 2006, 25, 235–265. [Google Scholar] [CrossRef]
- Subbarao, K.V.; Hubbard, J.C.; Koike, S.T. Evaluation of Broccoli Residue Incorporation into Field Soil for Verticillium Wilt Control in Cauliflower. Plant Dis. 1999, 83, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthiessen, J.N.; Warton, B.; Shackleton, M.A. The importance of plant maceration and water addition in achieving high Bras-sica-derived isothiocyanate levels in soil. Agroindustria 2004, 3, 277–281. [Google Scholar]
- Gimsing, A.; Kirkegaard, J. Glucosinolate and isothiocyanate concentration in soil following incorporation of Brassica biofumigants. Soil Biol. Biochem. 2006, 38, 2255–2264. [Google Scholar] [CrossRef]
- Michel, V.V. Biofumigation – principe et application. Rev. Suisse Vitic. Arboric. Hortic. 2008, 40, 95–99. [Google Scholar]
- Morris, E.K.; Fletcher, R.; Veresoglou, S. Effective methods of biofumigation: A meta-analysis. Plant Soil 2020, 446, 379–392. [Google Scholar] [CrossRef]
- Booth, E.J.; Walker, K.C.; Griffiths, D.W. A time-course study of the effect of sulphur on glucosinolates in oilseed rape (Brassica napus) from the vegetative stage to maturity. J. Sci. Food Agric. 1991, 56, 479–493. [Google Scholar] [CrossRef]
- Sarwar, M.; Kirkegaard, J.A. Biofumigation potential of brassicas II: Effect of environment and ontogeny on glucosinolate production and implications for screening. Plant Soil 1998, 201, 91–101. [Google Scholar] [CrossRef]
- Motisi, N.; Doré, T.; Lucas, P.; Montfort, F. Dealing with the variability in biofumigation efficacy through an epidemiological framework. Soil Biol. Biochem. 2010, 42, 2044–2057. [Google Scholar] [CrossRef]
- Justes, E.; Mary, B.; Nicolardot, B. Quantifying and modelling C and N mineralization kinetics of catch crop residues in soil: Parameterization of the residue decomposition module of STICS model for mature and non mature residues. Plant Soil 2009, 325, 171–185. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; Quemada, M. The Kill Date as a Management Tool for Cover Cropping Success. PLoS ONE 2014, 9, e109587. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.A. Efficiency of symbiotic nitrogen fixation in legumes. Annu. Rev. Plant Physiol. 1980, 31, 29–49. [Google Scholar] [CrossRef]
- Peoples, M.B.; Brockwell, J.; Herridge, D.F.; Rochester, I.J.; Alves, B.J.R.; Urquiaga, S.; Boddey, R.M.; Dakora, F.D.; Bhattarai, S.; Maskey, S.L.; et al. The contributions of nitrogen-fixing crop legumes to the productivity of agricultural systems. Symbiosis 2009, 48, 1–17. [Google Scholar] [CrossRef]
- Tonitto, C.; David, M.; Drinkwater, L. Replacing bare fallows with cover crops in fertilizer-intensive cropping systems: A meta-analysis of crop yield and N dynamics. Agric. Ecosyst. Environ. 2006, 112, 58–72. [Google Scholar] [CrossRef]
- Massignam, A.; Chapman, S.; Hammer, G.; Fukai, S. Physiological determinants of maize and sunflower grain yield as affected by nitrogen supply. Field Crops Res. 2009, 113, 256–267. [Google Scholar] [CrossRef]
- Couëdel, A.; Kirkegaard, J.; Alletto, L.; Justes, É. Crucifer-legume cover crop mixtures for biocontrol: Toward a new multi-service paradigm. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2019; Volume 157, pp. 55–139. [Google Scholar]
- Couëdel, A.; Alletto, L.; Kirkegaard, J.; Justes, É. Crucifer glucosinolate production in legume-crucifer cover crop mixtures. Eur. J. Agron. 2018, 96, 22–33. [Google Scholar] [CrossRef]
- Rosner, K.; Bodner, G.; Hage-Ahmed, K.; Steinkellner, S. Long-term Soil Tillage and Cover Cropping Affected Arbuscular Mycorrhizal Fungi, Nutrient Concentrations, and Yield in Sunflower. Agron. J. 2018, 110, 2664–2672. [Google Scholar] [CrossRef]
- Tribouillois, H.; Cohan, J.-P.; Justes, E. Cover crop mixtures including legume produce ecosystem services of nitrate capture and green manuring: Assessment combining experimentation and modelling. Plant Soil 2016, 401, 347–364. [Google Scholar] [CrossRef]
- APC; INRAE. Crop Phenotyping and AgroEcology Experimental Facility; INRAE: Castanet-Tolosan, France, 2018. [Google Scholar] [CrossRef]
- Tribouillois, H.; Dürr, C.; Demilly, D.; Wagner, M.-H.; Justes, E. Determination of Germination Response to Temperature and Water Potential for a Wide Range of Cover Crop Species and Related Functional Groups. PLoS ONE 2016, 11, e0161185. [Google Scholar] [CrossRef]
- de Graaf, R.M.; Krosse, S.; Swolfs, A.E.; Brinke, E.T.; Prill, N.; Leimu, R.; van Galen, P.M.; Wang, Y.; Aarts, M.G.; van Dam, N.M. Isolation and identification of 4-α-rhamnosyloxy benzyl glucosinolate in Noccaea caerulescens showing intraspecific variation. Phytochemistry 2015, 110, 166–171. [Google Scholar] [CrossRef] [Green Version]
- Willey, R.W. Intercropping—Its importance and research need: Part 1. Competition and yield advantages. Field Crop Abstr. 1979, 32, 1–10. [Google Scholar]
- Bedoussac, L.; Justes, E. A comparison of commonly used indices for evaluating species interactions and intercrop efficiency: Application to durum wheat–winter pea intercrops. Field Crops Res. 2011, 124, 25–36. [Google Scholar] [CrossRef]
- Lemaire, G.; Gastal, F. N uptake and distribution in plant canopies. In Diagnosis of the Nitrogen Status in Crops, 1st ed.; Lemaire, G., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 3–43. [Google Scholar] [CrossRef]
- Debaeke, P.; Raffaillac, D. Normalized SPAD index and Nitrogen Nutrition Index (NNI): Two indicators of plant N status for sunflower crop. In Proceedings of the 9th ESA Congress, Varsovie, Poland, 4–7 September 2006; pp. 83–84. [Google Scholar]
- Li, X.; Zhang, Y.; Ding, C.; Xu, W.; Wang, X. Temporal patterns of cotton Fusarium and Verticillium wilt in Jiangsu coastal areas of China. Sci. Rep. 2017, 7, 12581. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.L.; Madden, L.V. Introduction to Plant Disease Epidemiology; John Wiley & Sons: New York, NY, USA, 1990. [Google Scholar]
- Ahrens, W.H.; Cox, D.J.; Budhwar, G. Use of the Arcsine and Square Root Transformations for Subjectively Determined Percentage Data. Weed Sci. 1990, 38, 452–458. [Google Scholar] [CrossRef]
- Zhou, Y.; Roosendaal, L.; Van Eerd, L.L. Increased nitrogen retention by cover crops: Implications of planting date on soil and plant nitrogen dynamics. Renew. Agric. Food Syst. 2020, 35, 720–729. [Google Scholar] [CrossRef]
- Sievers, T.; Cook, R.L. Aboveground and Root Decomposition of Cereal Rye and Hairy Vetch Cover Crops. Soil Sci. Soc. Am. J. 2018, 82, 147–155. [Google Scholar] [CrossRef]
- Paplomatas, E.J.; Bassett, D.M.; Broome, J.C.; DeVay, J.E. Incidence of Verticillium wilt and yield losses of cotton cultivars (Gossypium hirsutum) based on soil inoculum density of Verticillium dahliae. Phytopathology 1992, 82, 1417–1420. [Google Scholar] [CrossRef]
- Harris, D.C.; Yang, J.R. The relationship between the amount of Verticillium dahliae in soil and the incidence of strawberry wilt as a basis for disease risk prediction. Plant Pathol. 1996, 45, 106–114. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K. Are differences in root growth of nitrogen catch crops important for their ability to reduce soil nitrate-N content, and how can this be measured? Plant Soil 2001, 230, 185–195. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Catch crops and green manures as biological tools in nitrogen management in temperate zones. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2003; pp. 227–302. [Google Scholar]
- Elhakeem, A.; Bastiaans, L.; Houben, S.; Couwenberg, T.; Makowski, D.; van der Werf, W. Do cover crop mixtures give higher and more stable yields than pure stands? Field Crops Res. 2021, 270, 108217. [Google Scholar] [CrossRef]
- Castro-Torres, I.G.; De la O Arciniega, M.; Gallegos-Estudillo, J.; Naranjo-Rodríguez, E.B.; Domínguez-Ortíz, M. Ángel Raphanus sativus L. var niger as a source of Phytochemicals for the Prevention of Cholesterol Gallstones. Phytother. Res. 2014, 28, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, C.; Hüntemann, K.; Heitmann, B.; Müller, C. Suppression of Verticillium dahliae by glucosinolate-containing seed meal amendments. Eur. J. Plant Pathol. 2015, 142, 239–249. [Google Scholar] [CrossRef]
- Klose, S.; Ajwa, H.A.; Browne, G.T.; Subbarao, K.; Martin, F.N.; Fennimore, S.A.; Westerdahl, B.B. Dose Response of Weed Seeds, Plant-Parasitic Nematodes, and Pathogens to Twelve Rates of Metam Sodium in a California Soil. Plant Dis. 2008, 92, 1537–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthiessen, J.N.; A Shackleton, M. Biofumigation: Environmental impacts on the biological activity of diverse pure and plant-derived isothiocyanates. Pest Manag. Sci. 2005, 61, 1043–1051. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Kirkegaard, J. Glucosinolates and biofumigation: Fate of glucosinolates and their hydrolysis products in soil. Phytochem. Rev. 2009, 8, 299–310. [Google Scholar] [CrossRef]
- Seassau, C.; Dechamp-Guillaume, G.; Mestries, E.; Debaeke, P. Nitrogen and water management can limit premature ripening of sunflower induced by Phoma macdonaldii. Field Crops Res. 2010, 115, 99–106. [Google Scholar] [CrossRef]
- Ochiai, N.; Powelson, M.L.; Dick, R.P.; Crowe, F.J. Effects of Green Manure Type and Amendment Rate on Verticillium Wilt Severity and Yield of Russet Burbank Potato. Plant Dis. 2007, 91, 400–406. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Westermann, D.T.; Hafez, S.L.; Everson, D.O.; Sorensen, L.H.; Schneider, A.T. Effects of green manures on Verticillium wilt of potato. Phytopathology 1996, 86, 444–453. [Google Scholar] [CrossRef]
- Wheeler, T.A.; Bordovsky, J.P.; Keeling, J.W.; Mullinix, J.B.G. Effects of Crop Rotation, Cultivar, and Irrigation and Nitrogen Rate on Verticillium Wilt in Cotton. Plant Dis. 2012, 96, 985–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocozza, C.; Abdeldaym, E.A.; Brunetti, G.; Nigro, F.; Traversa, A. Synergistic effect of organic and inorganic fertilization on the soil inoculum density of the soilborne pathogens Verticillium dahliae and Phytophthora spp. under open-field conditions. Chem. Biol. Technol. Agric. 2021, 8, 24. [Google Scholar] [CrossRef]
- Tenuta, M.; Lazarovits, G. Ammonia and Nitrous Acid from Nitrogenous Amendments Kill the Microsclerotia of Verticillium dahliae. Phytopathology 2002, 92, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Nolte, P.; Sorensen, L.H.; Schneider, A.T. Ecological Relationships of Verticillium Wilt Suppression of Potato by Green Manures. Am. J. Potato Res. 2010, 87, 315–326. [Google Scholar] [CrossRef]
- Huber, D.; Römheld, V.; Weinmann, M. Relationship between Nutrition, Plant Diseases and Pests. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 283–298. [Google Scholar] [CrossRef]
- Parr, M.; Grossman, J.M.; Reberg-Horton, S.C.; Brinton, C.; Crozier, C. Nitrogen Delivery from Legume Cover Crops in No-Till Organic Corn Production. Agron. J. 2011, 103, 1578–1590. [Google Scholar] [CrossRef]
- Larkin, R.P.; Griffin, T.S.; Honeycutt, C.W. Rotation and Cover Crop Effects on Soilborne Potato Diseases, Tuber Yield, and Soil Microbial Communities. Plant Dis. 2010, 94, 1491–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeux, G.; Cordeau, S.; Antichi, D.; Carlesi, S.; Mazzoncini, M.; Munier-Jolain, N.; Bàrberi, P. Cover crops promote crop productivity but do not enhance weed management in tillage-based cropping systems. Eur. J. Agron. 2020, 123, 126221. [Google Scholar] [CrossRef]
- Davis, J.R.; Huisman, O.C.; Everson, D.O.; Schneider, A.T. Verticillium wilt of potato: A model of key factors related to disease severity and tuber yield in Southeastern Idaho. Am. J. Potato Res. 2001, 78, 291–300. [Google Scholar] [CrossRef]
- Zavatta, M.; Muramoto, J.; Milazzo, E.; Koike, S.; Klonsky, K.; Goodhue, R.; Shennan, C. Integrating broccoli rotation, mustard meal, and anaerobic soil disinfestation to manage verticillium wilt in strawberry. Crop Prot. 2021, 146, 105659. [Google Scholar] [CrossRef]

| Previous Crop | Field Characteristics | Cover Crop Management | Sunflower Management | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Organic Matter (g kg−1) | Soil Texture | Species | Cultivars | Sowing Date | Densities (Seeds m−2) | Irrigation (mm) | Destruction Date | Cultivar | Sowing Date | N Fertilisation (k ha−1) | Irrigation (mm) | Harvest Date | |
| Durum wheat | 12.3 | Loamy-sandy clay | Brown mustard | Etamine | 08/09/2015 | 132 | 0 | 07/12/2015 | cv.1 | 28/04/2016 | 0 | 0 | 19/09/2016 |
| Turnip rape | Chicon | 111 | |||||||||||
| Fodder radish | Anaconda | 102 | |||||||||||
| Durum wheat | 9.76 | Loamy-sandy clay | Brown mustard | Etamine | 05/09/2016 | 161 | 33 mm (3 DAS) | 07/12/2016 | cv. 1 | 20/04/2017 | 110 | 0 | 07/09/2017 |
| Turnip rape | Chicon | 212 | |||||||||||
| Fodder radish | Anaconda | 100 | |||||||||||
| Vetch | Titane | 86 | |||||||||||
| Durum wheat | 8.57 | Loamy clay | Brown mustard | Etamine | 28/08/2018 | 100 | 30 mm (8 DAS) 30 mm (24 DAS) | 07/01/2019 | cv.2 | 13/05/2019 | 60 | 30 mm (73 DAS) | 18/09/2019 |
| Turnip rape | Chicon | 80 | |||||||||||
| Fodder radish | Anaconda | 80 | |||||||||||
| Vetch | Titane | 80 | |||||||||||
| Sunflower | 8.57 | Loamy clay | Brown mustard | Etamine | 09/10/2019 | 100 | 54 mm (1 DAS) | 25/02/2020 | cv.2 | 25/05/2020 | 40 | 20 mm (3 DAS) 50 mm (58 DAS) | 19/10/2020 |
| Turnip rape | Chicon | 80 | |||||||||||
| Fodder radish | Anaconda | 80 | |||||||||||
| Vetch | Titane | 80 | |||||||||||
| Year | Cover Crops | Biofumigation | Sunflower | |||
|---|---|---|---|---|---|---|
| GDD (°C) Br 1/Fb 2 | Precipitation (mm) | Temperature (°C) | Cumulative Precipitation 7 Days (mm) | GDD (°C) 3 | Precipitation (mm) | |
| 2015–2016 | 615|1033 | 100 | 13 | 2.5 | 2228 | 296 |
| 2016–2017 | 686|1119 | 119 | 7 | 1.5 | 2212 | 267 |
| 2018–2019 | 905|1489 | 191 | 2 | 3.5 | 2147 | 248 |
| 2019–2020 | 487|1112 | 400 | 10 | 50.0 | 2319 | 257 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Kaci Ahmed, N.; Galaup, B.; Desplanques, J.; Dechamp-Guillaume, G.; Seassau, C. Ecosystem Services Provided by Cover Crops and Biofumigation in Sunflower Cultivation. Agronomy 2022, 12, 120. https://doi.org/10.3390/agronomy12010120
Ait Kaci Ahmed N, Galaup B, Desplanques J, Dechamp-Guillaume G, Seassau C. Ecosystem Services Provided by Cover Crops and Biofumigation in Sunflower Cultivation. Agronomy. 2022; 12(1):120. https://doi.org/10.3390/agronomy12010120
Chicago/Turabian StyleAit Kaci Ahmed, Neila, Benoit Galaup, Jérémy Desplanques, Grégory Dechamp-Guillaume, and Célia Seassau. 2022. "Ecosystem Services Provided by Cover Crops and Biofumigation in Sunflower Cultivation" Agronomy 12, no. 1: 120. https://doi.org/10.3390/agronomy12010120
APA StyleAit Kaci Ahmed, N., Galaup, B., Desplanques, J., Dechamp-Guillaume, G., & Seassau, C. (2022). Ecosystem Services Provided by Cover Crops and Biofumigation in Sunflower Cultivation. Agronomy, 12(1), 120. https://doi.org/10.3390/agronomy12010120






