Responses of the Lodging Resistance of Summer Maize with Different Gene Types to Plant Density
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Site and Experimental Design
2.2. External Morphology
2.3. Maximum Bending Resistance and Field Lodging Rate
2.4. Rind Penetration Strength and Bending Strength
2.5. Dry Weight and Dry Weight per Unit of Internode
2.6. Micro-Structure of the Stem
2.7. Biological Yield and Grain Yield
2.8. Statistical Analysis
3. Results
3.1. Maximum Bending Strength and Field Lodging Rate
3.2. Stem Rind Penetration Strength (RPS) and Bending Strength (BS)
3.3. Plant Character
3.4. Stem Character
3.4.1. Length and Diameter of Internode
3.4.2. Dry Weight and Dry Weight per Unit of Internode
3.4.3. Stem Vascular Bundle Structure
3.5. Biological Yield and Grain Yield
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D.G.; Ma, D.Z.; Yu, Q.Q.; Sun, Y.J.; Gu, W.R.; Chai, M.Z.; Zhang, Q. Researchi progress on influencing factors of lodging and lodging resistance in maize. J. China Agric. Univ. 2020, 25, 28–38. [Google Scholar]
- Xue, J.; Gou, L.; Zhao, Y.S.; Yao, M.N.; Yao, H.S.; Tian, J.S.; Zhang, W.F. Effects of light intensity within the canopy on maize lodging. Field Crop. Res. 2016, 188, 133–141. [Google Scholar] [CrossRef]
- Gou, L.; Huang, J.J.; Sun, R.; Ding, Z.S.; Dong, Z.Q.; Zhao, M. Variation characteristic of stalk penetration strength of maize with different density-tolerance varieties. Trans. CSAE 2010, 26, 156–162. [Google Scholar]
- Yang, Y.; Yang, J.Y.; Li, S.M.; Zhang, X.D.; Zhu, D.H.; Liu, Z.; Mi, C.Q.; Xiao, K.N. Spatial regression analysis on influence factors of maize lodging stress. Trans. CSAE 2011, 27, 244–249. [Google Scholar]
- Dai, J.R. The review and prospects of maize genetics and breeding. In Prospects of Maize Genetics an Breeding for the 21st Century-Paper Collection of International Maize Genetics an Breeding Symposium; China Agricultural Science and Technology Press: Changchun, China, 2000; Volume 9, pp. 1–7. [Google Scholar]
- Li, S.K.; Wang, C.T. Evolution and Development of Maize Production Techniques in China. Sci. Agric. Sin. 2009, 13, 1941–1951. [Google Scholar]
- Ren, B.Z.; Liu, W.; Zhang, J.W.; Dong, S.T.; Liu, P.; Zhao, B. Effects of plant density on the photosynthetic and chloroplast characteristics of maize under high-yielding conditions. Sci. Nat. 2017, 104, 12. [Google Scholar] [CrossRef]
- Tollenaar, M.; Deen, W.; Echarte, L.; Liu, W. Effect of crowding stress on dry matter accumulation and harvest index in maize. Agron. J. 2006, 98, 930–937. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E.A. Yield potential, yield stability and stress tolerance in maize. Field Crop. Res. 2002, 75, 161–169. [Google Scholar] [CrossRef]
- Zhang, S.H. The comparison between China and US in technical level of maize breeding. Seed World 2007, 4, 9–10. [Google Scholar]
- Gao, X.; Gao, J.L.; Yu, X.F.; Wang, Z.G.; Sun, J.Y.; Su, Z.J.; Hu, S.P.; Ye, J.; Wang, H.Y.; Cui, C.; et al. Stalks Lodging-resistance Characteristics and Yield Traits among Different Maize Varieties under High Close Planting. J. Maize Sci. 2012, 20, 69–73. [Google Scholar]
- Ren, B.Z.; Li, L.L.; Dong, S.T.; Liu, P.; Zhao, B.; Yang, J.S.; Wang, D.B.; Zhang, J.W. Effects of Plant Density on Stem Traits and Lodging Resistance of Summer Maize Hybrids with Different Plant Heights. Acta Agron. Sin. 2016, 42, 1864–1872. [Google Scholar] [CrossRef]
- Minami, M.; Ujihara, A. Effects of Lodging on Dry Matter Production, Grain Yield and Nutritional Composition at Different Growth Stages in Maize (Zea mays L.). Jpn. J. Crop. Sci. 2008, 60, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.L.; Du, X.; Liu, M.X.; Jin, X.L.; Cui, Y.H. Lodging of summer maize and the effects on grain yield. J. Maize Sci. 2011, 19, 105–108. [Google Scholar]
- Xu, C.L.; Gao, Y.B.; Tian, B.J.; Ren, J.H.; Meng, Q.F.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crop. Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Gou, L.; Huang, J.J.; Zhang, B.; Li, T.; Zhao, M. Effects of population density on stalk lodging resistant mechanism and agronomic characteristics of maize. Acta Agron. Sin. 2007, 10, 1688–1695. [Google Scholar]
- Novacek, M.J.; Mason, S.C.; Galusha, T.D.; Yaseen, M. Twin Rows Minimally Impact Irrigated Maize Yield, Morphology, and Lodging. Agron. J. 2013, 105, 268–276. [Google Scholar] [CrossRef]
- Norberg, O.S.; Mason, S.C.; Lowry, S.R. Ethephon Influence on Harvestable Yield, Grain Quality, and Lodging of Corn. Agron. J. 1988, 80, 768–772. [Google Scholar] [CrossRef]
- Sun, S.X.; Dai, J.Y.; Gu, W.L. Effect of nitrogen, phosphate and potash fertilizers on lodging and yield in maize. Sci. Agric. Sin. 1989, 22, 28–33, 96–97. [Google Scholar]
- Wang, X.Q.; Song, W.; Zhang, R.Y.; Chen, Y.N.; Sun, X.; Zhao, J.R. Gesearch Advances on Maize Stalk Lodging Resistance. Sci. Agric. Sin. 2021, 54, 2261–2272. [Google Scholar]
- Xue, J.; Xie, R.Z.; Zhang, W.F.; Wang, K.R.; Hou, P.; Ming, B.; Gou, L.; Li, S.K. Research progress on reduced lodging of high-yield and -density maize. J. Integr. Agric. 2017, 16, 2717–2725. [Google Scholar] [CrossRef]
- Mi, C.Q.; Zhang, X.D.; Li, S.M.; Zhu, D.H.; Yang, J.Y. Analysis of Lodging Resistance Competitiveness of Maize Cultivars in Target Growing Environments. Sens. Lett. 2013, 11, 1282–1287. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Hu, C.H. Studies on the anatomical structures of the stalks of maize with different resistance to lodging. Acta Agron. Sin. 1991, 17, 70–75. [Google Scholar]
- Yao, M.N.; Shi, Z.G.; Xue, J.; Yang, Z.W.; Gou, L.; Zhang, W.F. The Effects of different planting densities on the cortex structure of steam and lodging resistance in maize. Xinjiang Agric. Sci. 2013, 50, 2006–2014. [Google Scholar]
- Dudley, J.W. Selection for Rind Puncture Resistance in Two Maize Populations. Crop. Sci. 1994, 34, 1458–1460. [Google Scholar] [CrossRef]
- Li, S.Y.; Ma, W.; Peng, J.Y.; Chen, Z.M. Study on Yield Loss of Summer Maize Due to Lodging at the Big Flare Stage and Grain Filling Stage. Sci. Agric. Sin. 2015, 48, 3952–3964. [Google Scholar]
- Xue, J.; Wang, K.R.; Xie, R.Z.; Gou, L.; Zhang, W.F.; Ming, B.; Hou, P.; Li, S.K. Research Progress of Maize Lodging During Late Stage. Sci. Agric. Sin. 2018, 51, 1845–1854. [Google Scholar]
- Albrecht, B.; Dudley, J.W. Divergent Selection for Stalk Quality and Grain Yield in an Adapted ✕ Exotic Maize Population Cross1. Crop. Sci. 1987, 27, 487–494. [Google Scholar] [CrossRef]
- Flint-Garcia, S.A.; Darrah, L.L.; McMullen, M.D.; Hibbard, B.E. Phenotypic versus marker-assisted selection for stalk strength and second-generation European corn borer resistance in maize. Theor. Appl. Genet. 2003, 107, 1331–1336. [Google Scholar] [CrossRef]
- Sibale, E.M.; Darrah, L.L.; Zuber, M.S. Comparison of two rind penetrometers for measurement of stalk strength in maize. Maydica 1992, 37, 111–114. [Google Scholar]
- Martin, S.A.; Darrah, L.L.; Hibbard, B.E. Divergent Selection for Rind Penetrometer Resistance and Its Effects on European Corn Borer Damage and Stalk Traits in Corn. Crop. Sci. 2004, 44, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Sangoi, L.; Gracietti, M.A.; Rampazzo, C.; Bianchetti, P. Response of brazilian maize hybrids from different eras to changes in plant density. Field Crop. Res. 2002, 79, 39–51. [Google Scholar] [CrossRef]
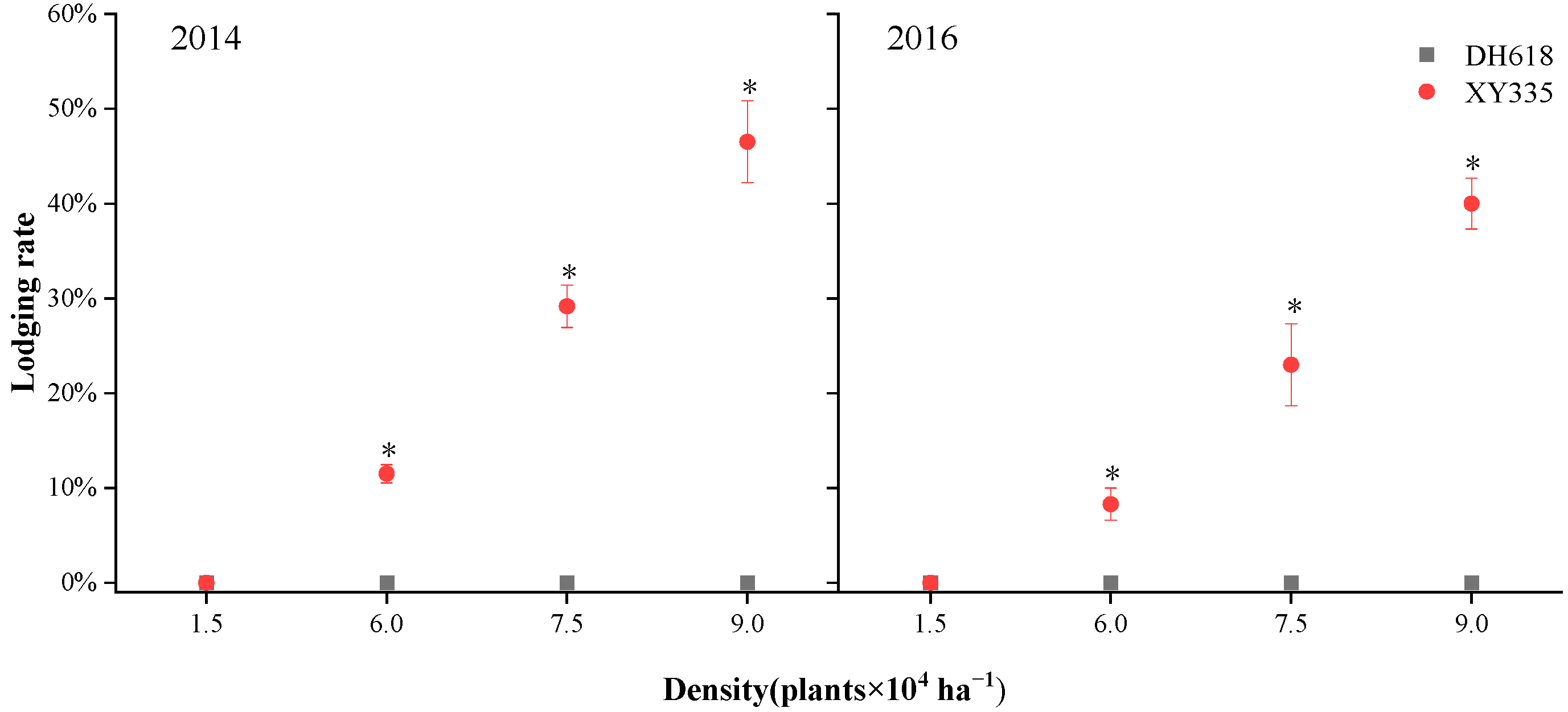

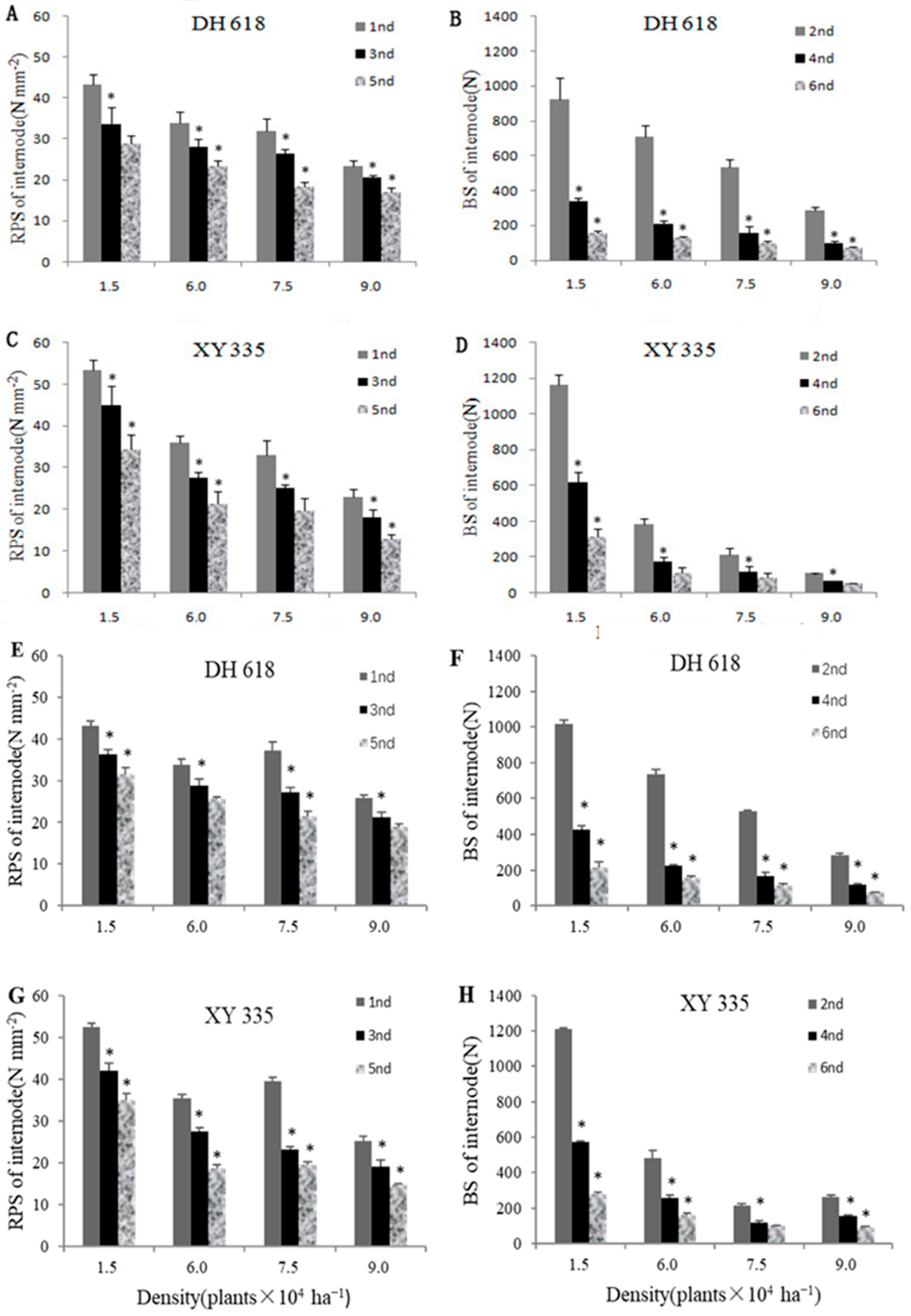
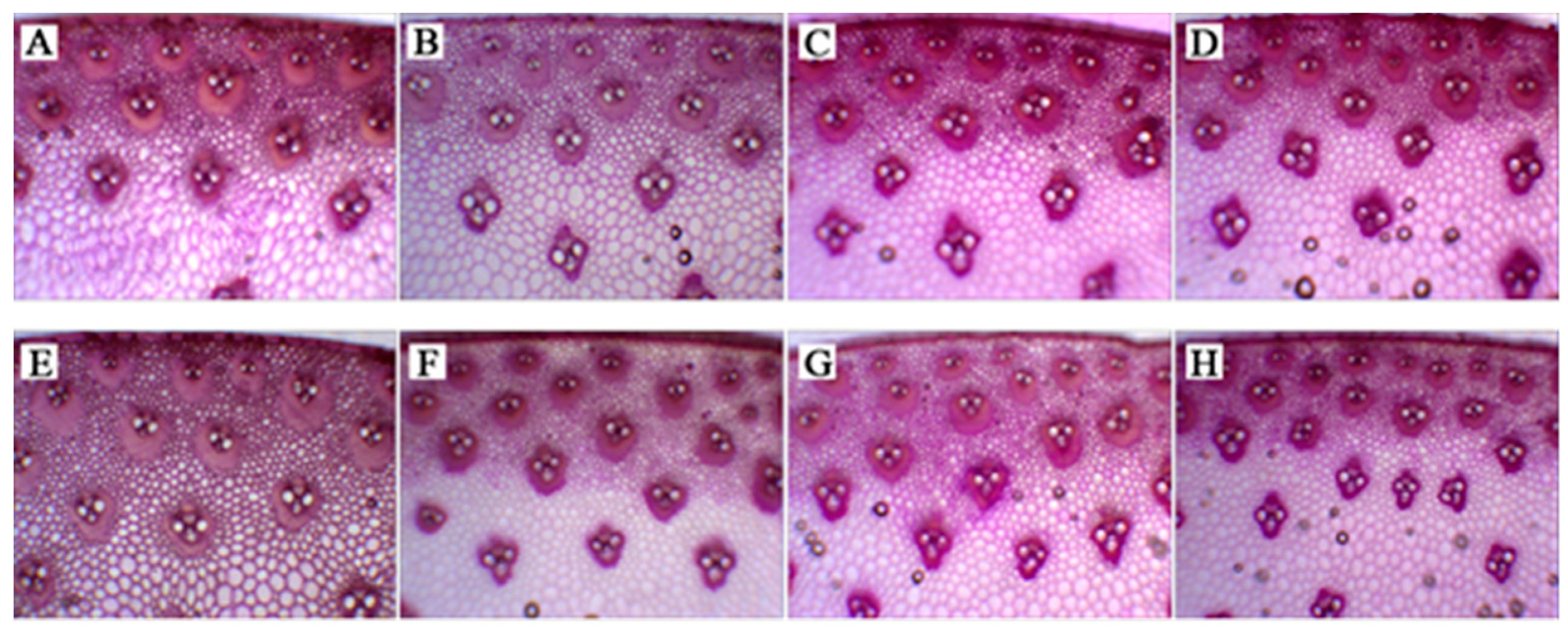

| Year | Hybrid | Plant Density (×104 ha−1) | V12 (N) | VT (N) | VT + 35 d (N) |
|---|---|---|---|---|---|
| 2014 | DH618 | 1.5 | 12.80 a | 25.93 a | 38.37 a |
| 6 | 10.87 ab | 19.91 b | 25.53 b | ||
| 7.5 | 10.00 ab | 18.60 b | 20.53 c | ||
| 9 | 8.80 b | 11.30 c | 11.57 d | ||
| XY335 | 1.5 | 19.40 a | 31.47 a | 45.53 a | |
| 6 | 9.60 b | 17.07 b | 19.07 b | ||
| 7.5 | 7.73 c | 15.17 b | 14.50 c | ||
| 9 | 7.77 c | 9.60 c | 7.83 d | ||
| 2016 | DH618 | 1.5 | 13.43 a | 30.27 a | 48.40 a |
| 6 | 11.40 b | 20.40 b | 27.20 b | ||
| 7.5 | 10.50 b | 19.17 b | 21.57 c | ||
| 9 | 10.37 b | 11.57 c | 11.23 d | ||
| XY335 | 1.5 | 20.37 a | 31.50 a | 42.20 a | |
| 6 | 10.07 b | 19.47 b | 20.90 c | ||
| 7.5 | 8.70 bc | 14.07 c | 13.03 c | ||
| 9 | 8.17 c | 10.23 d | 8.67 d | ||
| Interaction | |||||
| Hybrid (H) | * | ** | ** | ||
| Density (D) | ** | ** | ** | ||
| H × D | ** | ns | ns |
| Year | Hybrid | Plant Density (×104 ha−1) | Third Internode | Fifth Internode | ||||
|---|---|---|---|---|---|---|---|---|
| V12 (N) | VT (N) | VT + 35d (N) | V12 (N) | VT (N) | VT + 35d (N) | |||
| 2014 | DH618 | 1.5 | 13.33 a | 29.07 a | 33.67 a | 7.10 ab | 21.13 a | 29.00 a |
| 6 | 13.43 a | 20.67 b | 28.00 b | 5.53 b | 15.93 b | 23.33 b | ||
| 7.5 | 11.47 b | 20.67 b | 26.33 b | 7.43 a | 18.27 b | 18.33 c | ||
| 9 | 10.17 b | 16.05 c | 20.67 c | 6.97 ab | 15.00 b | 17.00 c | ||
| XY335 | 1.5 | 14.77 a | 30.00 a | 45.00 a | 7.40 a | 25.00 a | 34.33 a | |
| 6 | 10.70 c | 26.00 a | 27.67 b | 4.05 b | 23.00 a | 21.33 b | ||
| 7.5 | 12.40 b | 19.33 b | 25.00 b | 2.85 b | 15.67 b | 19.67 b | ||
| 9 | 9.47 c | 15.00 c | 18.00 c | 0.00 c | 11.67 b | 13.00 c | ||
| 2016 | DH618 | 1.5 | 15.33 a | 30.50 a | 36.33 a | 9.00 a | 22.67 a | 31.50 a |
| 6 | 15.17 a | 21.67 b | 28.70 b | 8.77 a | 19.97 b | 25.53 b | ||
| 7.5 | 13.43 ab | 21.70 b | 27.17 b | 8.57 a | 17.20 c | 21.43 c | ||
| 9 | 12.40 b | 18.90 c | 21.17 c | 8.10 a | 14.57 d | 18.90 d | ||
| XY335 | 1.5 | 16.83 a | 31.50 a | 42.07 a | 8.07 a | 23.17 a | 34.93 a | |
| 6 | 14.40 ab | 27.27 b | 27.57 b | 5.07 b | 25.20 a | 18.70 b | ||
| 7.5 | 12.17 bc | 20.33 c | 23.20 c | 2.90 c | 14.07 b | 19.43 b | ||
| 9 | 10.17 c | 15.77 d | 19.17 d | 1.77 c | 12.93 b | 14.67 c | ||
| Interaction | ||||||||
| Hybrid (H) | * | * | * | ** | ns | * | ||
| Density (D) | ** | ** | ** | ** | ** | ** | ||
| H × D | * | ns | ns | ns | ** | ns | ||
| Year | Hybrid | Plant Density (×104 ha−1) | Fourth Internode | Sixth Internode | ||||
|---|---|---|---|---|---|---|---|---|
| V12 (N) | VT (N) | VT + 35d (N) | V12 (N) | VT (N) | VT + 35d (N) | |||
| 2014 | DH618 | 1.5 | 203.67 a | 277.20 a | 338.67 a | 59.90 a | 153.97 a | 159.33 a |
| 6 | 110.33 b | 138.03 b | 209.67 b | 54.13 b | 106.93 b | 129.67 b | ||
| 7.5 | 94.87 c | 110.27 c | 156.67 c | 34.55 a | 81.90 c | 97.33 c | ||
| 9 | 84.57 d | 100.60 d | 100.00 d | 32.30 b | 68.30 c | 69.33 c | ||
| XY335 | 1.5 | 210.87 a | 399.00 a | 616.33 a | 60.15 a | 239.00 a | 311.33 a | |
| 6 | 83.90 b | 228.67 b | 174.00 b | 44.20 b | 128.67 b | 122.00 b | ||
| 7.5 | 78.60 b | 177.33 c | 117.67 c | 28.47 c | 95.67 c | 87.67 c | ||
| 9 | 62.93 c | 136.67 d | 61.67 d | 20.63 d | 67.33 d | 73.33 d | ||
| 2016 | DH618 | 1.5 | 213.70 a | 318.57 a | 427.77 a | 69.93 a | 209.63 a | 213.53 a |
| 6 | 115.87 b | 172.37 b | 224.43 b | 55.80 b | 135.30 b | 157.03 b | ||
| 7.5 | 99.50 b | 129.03 c | 164.70 c | 35.40 c | 95.87 c | 111.23 c | ||
| 9 | 99.67 b | 112.63 c | 117.00 d | 33.07 c | 74.57 c | 72.83 c | ||
| XY335 | 1.5 | 221.40 a | 454.87 a | 570.97 a | 60.22 a | 243.03 a | 280.37 a | |
| 6 | 88.00 b | 265.03 b | 256.13 b | 50.40 b | 155.17 b | 159.97 b | ||
| 7.5 | 82.67 b | 202.47 c | 116.23 c | 26.40 c | 99.57 c | 100.83 c | ||
| 9 | 66.17 c | 146.43 d | 154.93 d | 21.97 c | 85.90 c | 91.77 c | ||
| Interaction | ||||||||
| Hybrid (H) | ** | ** | ** | ** | ** | ** | ||
| Density (D) | ** | ** | ** | ** | ** | ** | ||
| H × D | * | ns | ns | ns | ns | ns | ||
| Year | Hybrid | Plant Density (×104 ha−1) | Plant Height (cm) | Ear Height (cm) | Height of Gravity Center (cm) | Ear Coefficient |
|---|---|---|---|---|---|---|
| 2014 | DH618 | 1.5 | 223.67 c | 65.50 c | 74.67 c | 0.29 b |
| 6 | 262.67 b | 79.00 b | 96.33 b | 0.30 b | ||
| 7.5 | 269.00 b | 80.00 b | 97.67 b | 0.30 b | ||
| 9 | 282.00 a | 102.33 a | 108.00 a | 0.36 a | ||
| XY335 | 1.5 | 325.67 d | 118.33 c | 114.67 c | 0.37 a | |
| 6 | 334.67 c | 118.67 c | 124.67 b | 0.35 a | ||
| 7.5 | 353.00 b | 126.00 b | 133.33 a | 0.36 a | ||
| 9 | 363.33 a | 131.33 a | 137.00 a | 0.36 a | ||
| 2016 | DH618 | 1.5 | 218.67 c | 58.00 d | 67.33 c | 0.27 d |
| 6 | 246.33 b | 76.00 c | 85.67 b | 0.31 c | ||
| 7.5 | 250.67 b | 80.67 b | 90.67 b | 0.32 b | ||
| 9 | 261.00 a | 89.33 a | 98.33 a | 0.34 a | ||
| XY335 | 1.5 | 297.33 c | 89.33 c | 95.67 c | 0.30 b | |
| 6 | 302.00 bc | 106.67 b | 114.00 b | 0.35 a | ||
| 7.5 | 310.33 b | 109.00 b | 115.67 b | 0.35 a | ||
| 9 | 321.33 a | 116.00 a | 120.33 a | 0.36 a | ||
| Interaction | ||||||
| Hybrid (H) | ** | ** | ** | ** | ||
| Density (D) | ** | ** | ** | ** | ||
| H × D | ** | ns | ns | ns |
| Year | Hybrid | Plant Density (×104 ha−1) | Length of Internode (cm) | Diameter of Internode (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | |||
| 2014 | DH618 | 1.5 | 9.10 c | 12.90 c | 14.63 c | 15.20 c | 2.71 a | 2.56 a | 2.37 a | 2.20 a |
| 6 | 11.67 b | 16.27 b | 19.00 b | 20.00 b | 2.48 b | 2.38 b | 2.22 b | 2.06 b | ||
| 7.5 | 12.73 b | 16.83 b | 19.50 ac | 21.33 a | 2.20 c | 2.18 c | 2.03 c | 1.87 c | ||
| 9 | 14.57 a | 18.50 a | 20.33 a | 21.67 a | 2.12 c | 2.03 c | 1.88 d | 1.75 d | ||
| XY335 | 1.5 | 14.00 b | 16.83 b | 18.83 c | 19.53 b | 3.10 a | 2.91 a | 2.79 a | 2.61 a | |
| 6 | 17.83 a | 19.90 a | 22.73 b | 22.53 a | 2.44 b | 2.32 b | 2.29 b | 2.07 b | ||
| 7.5 | 18.33 a | 20.33 a | 23.47 a | 22.33 a | 2.24 c | 2.06 c | 1.95 c | 1.80 c | ||
| 9 | 18.50 a | 20.50 a | 23.63 a | 22.57 a | 1.84 d | 1.70 d | 1.63 d | 1.48 d | ||
| 2016 | DH618 | 1.5 | 9.31 d | 11.50 c | 13.20 b | 12.10 b | 2.83 a | 2.67 a | 2.45 a | 2.26 a |
| 6 | 10.98 c | 15.87 b | 18.48 a | 19.50 a | 2.49 b | 2.38 b | 2.31 b | 2.14 a | ||
| 7.5 | 11.98 b | 16.45 ab | 19.10 a | 20.98 a | 2.32 c | 2.22 bc | 2.09 c | 1.88 b | ||
| 9 | 13.12 a | 17.67 a | 18.12 a | 20.40 a | 2.21 d | 2.16 c | 1.93 d | 1.79 b | ||
| XY335 | 1.5 | 12.80 b | 15.68 b | 16.33 b | 17.12 c | 3.17 a | 3.07 a | 2.85 a | 2.67 a | |
| 6 | 16.21 a | 18.65 a | 21.54 a | 20.13 b | 2.63 b | 2.36 b | 2.32 b | 2.14 b | ||
| 7.5 | 16.33 a | 19.49 a | 22.89 a | 20.11 b | 2.28 c | 2.12 c | 2.02 c | 1.88 c | ||
| 9 | 17.88 a | 19.21 a | 21.73 a | 22.89 a | 1.91 d | 1.71 d | 1.62 d | 1.51 d | ||
| Interaction | ||||||||||
| Hybrid (H) | ** | ** | ** | ** | ** | * | ** | * | ||
| Density (D) | ** | ** | ** | ** | ** | ** | ** | ** | ||
| H × D | ns | ns | ns | ** | ns | ** | ns | ns | ||
| Year | Hybrid | Plant Density (×104 ha−1) | Dry Weight of Internode (g) | Dry Weight per Unit of Internode (g cm−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 3 | 4 | 5 | 6 | |||
| 2014 | DH618 | 1.5 | 6.68 a | 6.75 a | 7.19 a | 5.96 a | 0.73 a | 0.52 a | 0.49 a | 0.39 a |
| 6 | 5.03 b | 6.08 b | 6.46 b | 5.52 b | 0.43 b | 0.37 b | 0.34 b | 0.28 b | ||
| 7.5 | 4.18 c | 5.11 c | 5.46 c | 5.17 b | 0.33 c | 0.30 c | 0.28 c | 0.24 bc | ||
| 9 | 3.87 d | 4.37 d | 4.67 d | 4.39 c | 0.26 d | 0.24 d | 0.23 d | 0.20 c | ||
| XY335 | 1.5 | 16.71 a | 18.67 a | 18.13 a | 14.77 a | 1.19 a | 1.11 a | 0.96 a | 0.76 a | |
| 6 | 8.96 b | 9.69 b | 9.38 b | 8.37 b | 0.50 b | 0.49 b | 0.41 b | 0.37 b | ||
| 7.5 | 6.09 c | 6.43 c | 6.58 c | 5.81 c | 0.33 c | 0.32 c | 0.28 c | 0.26 c | ||
| 9 | 4.04 d | 4.30 c | 4.43 d | 3.89 d | 0.22 d | 0.21 d | 0.19 d | 0.17 d | ||
| 2016 | DH618 | 1.5 | 7.25 a | 7.78 a | 7.54 a | 5.99 a | 0.78 a | 0.68 a | 0.57 a | 0.50 a |
| 6 | 4.67 b | 6.36 b | 6.92 b | 5.64 a | 0.43 b | 0.40 b | 0.37 b | 0.29 b | ||
| 7.5 | 4.18 b | 4.65 c | 4.73 c | 4.96 a | 0.35 c | 0.28 c | 0.25 c | 0.24 b | ||
| 9 | 4.28 b | 4.09 d | 4.55 c | 5.18 a | 0.33 c | 0.23 d | 0.25 c | 0.25 b | ||
| XY335 | 1.5 | 22.67 a | 22.66 a | 19.77 a | 16.59 a | 1.77 a | 1.45 a | 1.21 a | 0.97 a | |
| 6 | 8.53 b | 9.80 b | 10.32 b | 8.12 b | 0.53 b | 0.53 b | 0.48 b | 0.40 b | ||
| 7.5 | 6.71 b | 7.42 c | 7.57 c | 6.47 c | 0.41 b | 0.38 c | 0.33 bc | 0.32 c | ||
| 9 | 4.42 c | 4.38 d | 4.63 d | 4.15 d | 0.25 c | 0.23 d | 0.21 c | 0.18 d | ||
| Interaction | ||||||||||
| Hybrid (H) | ** | ** | ** | ** | ** | ** | ** | ** | ||
| Density (D) | ** | ** | ** | ** | ** | ** | ** | ** | ||
| H × D | ** | ns | ns | ns | ns | ** | ns | ns | ||
| Hybrid | Plant Density (×104 ha−1) | Vascular Bundle Number | Density of Vascular Bundle (Number/mm2) | Cortex Thickness (μm) | Vascular Bundle Sclerenchyma Thickness (μm) |
|---|---|---|---|---|---|
| DH618 | 1.5 | 751 a | 1.31 d | 50.61 a | 49.52 a |
| 6 | 711 b | 1.48 c | 45.73 b | 45.68 b | |
| 7.5 | 631 c | 1.67 b | 44.17 b | 43.36 c | |
| 9 | 625 c | 1.78 a | 40.10 c | 40.66 d | |
| XY335 | 1.5 | 826 a | 1.10 d | 50.58 a | 51.68 a |
| 6 | 781 b | 1.68 c | 40.84 b | 46.42 b | |
| 7.5 | 756 c | 1.93 b | 35.16 c | 43.82 c | |
| 9 | 566 d | 2.14 a | 31.64 d | 38.90 d |
| Year | Hybrid | Plant Density (×104 ha−1) | Dry Weight per Plant (g p−1) | Dry Kernel Weight per Plant (g p−1) | Grain Yield (kg ha−1) |
|---|---|---|---|---|---|
| 2014 | DH618 | 1.5 | 572.37 a | 334.83 a | 5028.00 b |
| 6 | 386.54 b | 211.52 b | 12,263.25 a | ||
| 7.5 | 327.06 c | 184.28 bc | 13,220.40 a | ||
| 9 | 289.68 c | 147.74 c | 12,493.95 a | ||
| XY335 | 1.5 | 738.21 a | 402.47 a | 5996.85 c | |
| 6 | 440.08 b | 236.39 b | 13,972.65 a | ||
| 7.5 | 361.33 c | 180.95 c | 12,912.60 a | ||
| 9 | 311.23 d | 163.3 c | 11,005.65 b | ||
| 2016 | DH618 | 1.5 | 613.94 a | 358.97 a | 5387.40 c |
| 6 | 411.59 b | 227.53 b | 13,109.85 b | ||
| 7.5 | 351.57 c | 198.14 bc | 14,424.60 a | ||
| 9 | 305.84 c | 158.31 c | 13,665.55 ab | ||
| XY335 | 1.5 | 801.92 a | 437.91 a | 6527.85 c | |
| 6 | 456.90 b | 246.06 b | 14,453.85 a | ||
| 7.5 | 388.63 bc | 190.98 b | 13,698.60 a | ||
| 9 | 347.74 c | 175.29 b | 12,004.85 b | ||
| Interaction | |||||
| Hybrid (H) | ** | * | ** | ||
| Density (D) | ** | ** | ** | ||
| H × D | ** | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Geng, W.; Zhang, J.; Ren, B.; Wang, L. Responses of the Lodging Resistance of Summer Maize with Different Gene Types to Plant Density. Agronomy 2022, 12, 10. https://doi.org/10.3390/agronomy12010010
Yang J, Geng W, Zhang J, Ren B, Wang L. Responses of the Lodging Resistance of Summer Maize with Different Gene Types to Plant Density. Agronomy. 2022; 12(1):10. https://doi.org/10.3390/agronomy12010010
Chicago/Turabian StyleYang, Jinsheng, Wenjie Geng, Jiwang Zhang, Baizhao Ren, and Lichun Wang. 2022. "Responses of the Lodging Resistance of Summer Maize with Different Gene Types to Plant Density" Agronomy 12, no. 1: 10. https://doi.org/10.3390/agronomy12010010
APA StyleYang, J., Geng, W., Zhang, J., Ren, B., & Wang, L. (2022). Responses of the Lodging Resistance of Summer Maize with Different Gene Types to Plant Density. Agronomy, 12(1), 10. https://doi.org/10.3390/agronomy12010010







