Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Material and Site Description
2.2. Trial Management
2.3. Data Collection and Statistical Analysis
3. Results and Discussion
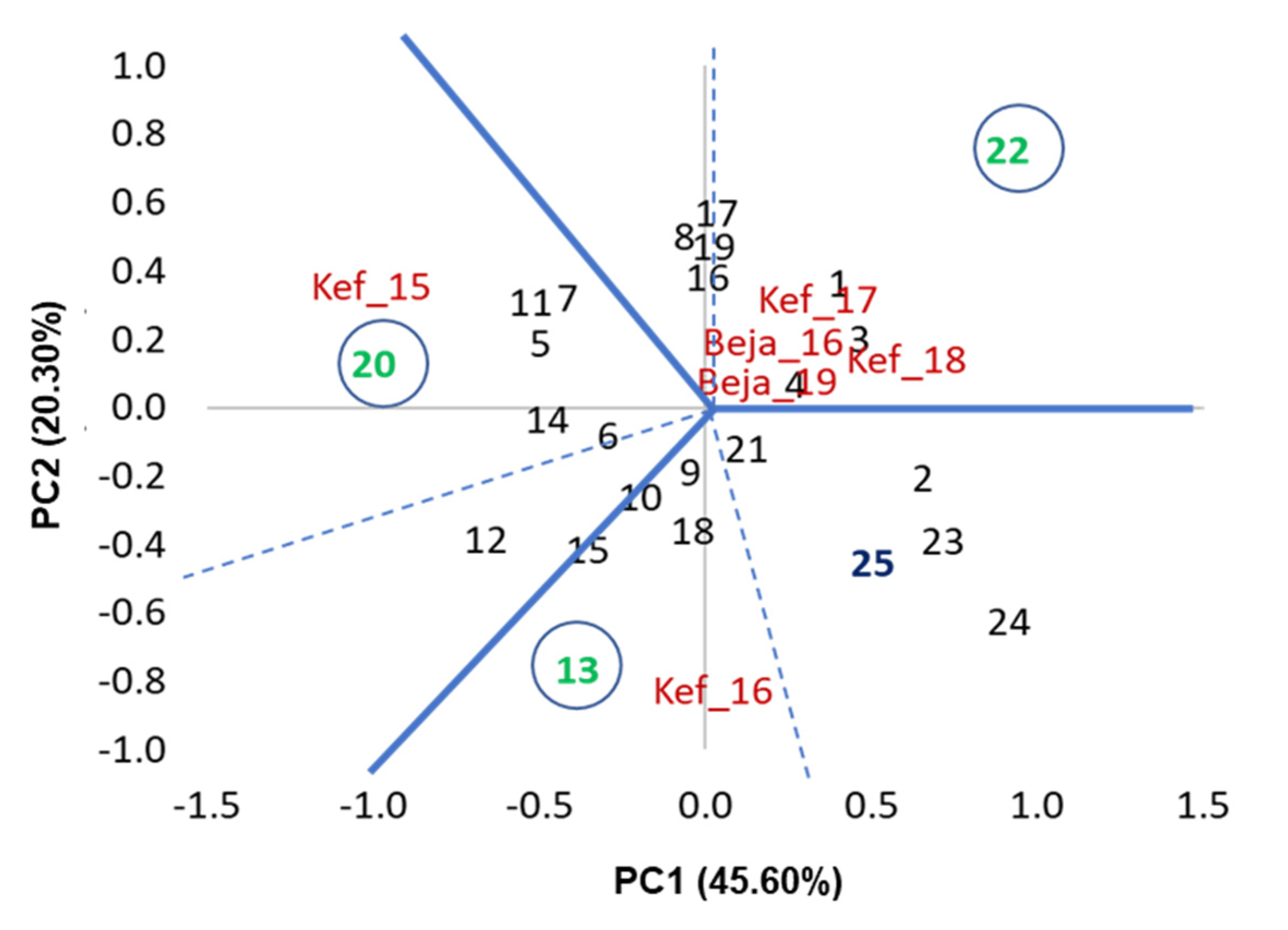
3.1. Variance and AMMI Analysis
3.2. Performance of Grain Yield and Stability of Genotypes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahl, C. Global Durum Outlook 2017. Available online: http://www.italmopa.com/wp-content/uploads/2017/05/144_all_1.pdf. (accessed on 21 October 2019).
- Agriculture and Agri-Food Canada. Canada: Outlook for Principal Field Crops. Available online: https://agriculture.canada.ca/en/canadas-agriculture-sectors/crops/reports-and-statistics-data-canadian-principal-field-crops (accessed on 26 September 2019).
- Bonjeana, C.A.; Simonet, C. Are grain markets in Niger driven by speculation? Oxf. Econ. Pap. 2016, 68, 714–735. [Google Scholar] [CrossRef]
- Martínez–Moreno, F.; Solís, I.; Noguero, D.; Blanco, A.; Özberk, İ.; Nsarellah, N.; Elias, E.; Mylonas, I.; Soriano, J.M. Durum wheat in the Mediterranean Rim: Historical evolution and genetic resources. Genet. Resour. Crop Evol. 2020, 67, 1415–1436. [Google Scholar] [CrossRef]
- Merouche, A.; Debaeke, P.; Messahel, M.; Kelkouli, M. Response of durum wheat varieties to water in semiarid Algeria. Afr. J. Agric. Res. 2014, 9, 2879–2892. [Google Scholar]
- Liu, Y.; Bowman, B.C.; Hu, Y.-G.; Liang, X.; Zhao, W.; Wheeler, J.; Klassen, N.; Bockelman, H.; Bonman, J.M.; Chen, J. Evaluation of agronomic traits and drought tolerance of winter wheat accessions from the USDA-ARS national small grains collection. Agronomy 2017, 7, 51. [Google Scholar] [CrossRef]
- Mansour, M.; Hachicha, M. The vulnerability of Tunisian agriculture to climate change. In Emerging Technologies and Management of Crop Stress Tolerance—A Sustainable Approach; Ahmad, P., Rasool, S., Eds.; Elsevier: San Diego, CA, USA, 2014; Volume 2, pp. 485–500. [Google Scholar]
- Jing, R.; Daokun, S.; Liang, C.; Frank, M.Y.; Jirui, W.; Yunliang, P.; Eviatar, N.; Dongfa, S.; Ming, C.L.; Junhua, P. Genetic diversity revealed by single nucleotide pomophism markers in a worldwide germplasm collection of durum wheat. Int. J. Mol. Sci. 2013, 14, 7061–7088. [Google Scholar]
- Charmet, G. Wheat domestication: Lessons for the future. C. R. Biol. 2011, 334, 212–220. [Google Scholar] [CrossRef]
- Nyine, M.; Adhikari, E.; Clinesmith, M.; Jordan, K.W.; Fritz, A.K.; Akhunov, E. Genomic patterns of introgression in interspecific populations created by crossing wheat with its wild relative. G3 2020, 10, 3651–3661. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.H. Back into the wild −apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2016, 10, 5–24. [Google Scholar] [CrossRef]
- Rajaram, S.; Hettel, G.P. Wheat Breeding at CIMMYT: Wheat Special Report No.29; CIMMYT, Ciudad Obregon: Sonora, Mexico, 1994; pp. 21–25. [Google Scholar]
- Maccaferri, M.; Harris, N.S.; Twardziok, S.O.; Pasam, R.K.; Gundlach, H.; Spannagl, M.; Ormanbekova, D.; Lux, T.; Prade, V.M.; Milner, S.G.; et al. Durum wheat genome highlights past domestication signatures and future improvement targets. Nat. Genet. 2019, 51, 885–895. [Google Scholar] [CrossRef] [Green Version]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC Gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 2007, 58, 177–186. [Google Scholar]
- Trethowan, R.M.; Mujeeb–Kazi, A. Novel germplasm resources for improving environmental stress tolerance of hexaploid wheat. Crop Sci. 2008, 48, 1255–1265. [Google Scholar]
- Mohammadi, M.; Sharifi, P.; Karimizadeh, R. Stability analysis of durum wheat genotypes by regression parameteres in dryland conditions. Acta Univ. Agric. Silvic. 2014, 62, 1049–1056. [Google Scholar]
- Hossain, A.; Jahan, M.A.H.S.; Timsina, J.; da Silva, J.A.T.; Mahboob, M.G.; Farhad, M. Biplot yield analysis of heat-tolerant spring wheat genotypes (Triticum aestivum L.) in multiple growing environments. Open Agric. 2018, 3, 404–413. [Google Scholar]
- Amamou, H.; Ben Sassi, M.; Aouadi, H.; Khemiri, H.; Mahouachi, M.; Beckers, Y.; Hammami, H. Climate change-related risks and adaptation strategies as perceived in dairy cattle farming systems in Tunisia. Clim. Risk Manag. 2018, 20, 38–49. [Google Scholar]
- Sadok, W.; Schoppach, R.; Ghanem, M.E.; Zucca, C.; Sinclair, T.R. Wheat drought-tolerance to enhance food security in Tunisia, birthplace of the Arab Spring. Eur. J. Agron. 2019, 107, 1–9. [Google Scholar]
- Neisse, A.C.; Kirch, J.L.; Hongyu, K. AMMI and GGE Biplot for genotype × environment interaction: A medoid–based hierarchical cluster analysis approach for high–dimensional data. Biom. Lett. 2018, 55, 97–121. [Google Scholar]
- Kumar, B.; Hooda, E. GGE Biplot analysis of multi-environment yield trials for wheat in northern India Bhushan. Adv. Res. 2018, 16, 1–9. [Google Scholar]
- Chamekh, Z.; Karmous, C.; Ayadi, S.; Sahli, A.; Hammami, Z.; Belhaj Fraj, M.; Benaissa, N.; Trifa, Y.; Slim-Amara, H. Stability analysis of yield component traits in 25 durum wheat (Triticum durum Desf.) genotypes under contrasting irrigation water salinity. Agric. Water Manag. 2015, 152, 1–6. [Google Scholar]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar]
- Akbarpour, O.; Dehghani, H.; Sorkhi, B.; Gauch, H.G., Jr. Evaluation of Genotype × Environment interaction in barley (Hordeum vulgare L.) based on AMMI model using developed SAS program. J. Agr. Sci. Tech. 2014, 16, 909–920. [Google Scholar]
- Ram, K.; Munjal, R.; Kesh, H.; Suresh; Kumari, A. AMMI and GGE biplot analysis for yield stability of wheat genotypes under drought and high temperature stress. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 377–389. [Google Scholar] [CrossRef]
- Katsenios, N.; Sparangis, P.; Chanioti, S.; Giannoglou, M.; Leonidakis, D.; Christopoulos, M.V.; Katsaros, G.; Efthimiadou, A. Genotype × Environment interaction of yield and grain quality traits of maize hybrids in Greece. Agronomy 2021, 11, 357. [Google Scholar] [CrossRef]
- Gauch, H.G.; Zobel, R.W. Identifying mega-environments and targeting genotypes. Crop Sci. 1997, 37, 311–326. [Google Scholar] [CrossRef]
- Sanchez–Garcia, M.; Álvaro, F.; Martín–Sánchez, J.A.; Sillero, J.C.; Escribano, J.; Royo, C. Breeding effects on the genotype´ environment interaction for yield of bread wheat grown in Spain during the 20th century. Field Crop. Res. 2012, 126, 79–86. [Google Scholar] [CrossRef]
- Zaïm, M.; El Hassouni, K.; Gamba, F.; Filali–Maltouf, A.; Belkadi, B.; Ayed, S.; Amri, A.; Nachit, M.; Taghouti, M.; Bassi, M.F. Wide crosses of durum wheat (Triticum durum Desf.) reveal good disease resistance, yield stability, and industrial quality across Mediterranean sites. Field Crop. Res. 2017, 214, 219–227. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F.A. decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Payne, R.W.; Harding, S.A.; Murray, D.A.; Soutar, D.M.; Baird, D.B.; Welham, S.J.; Kane, A.F.; Gilmour, A.R.; Thompson, R.; Webster, R. GenStat Release 9 Reference Manual, Part 2: Directives; VSN Int.: Hemel Hempstead, UK, 2006. [Google Scholar]
- Pacheco, A.; Vargas, M.; Alvarado, G.; Rodríguez, F.; Crossa, J.; Burgueño, J.; GEA–R (Genotype × Environment Analysis with R for Windows). Version 2.0. CIMMYT. 2016. Available online: http://hdl.handle.net/11529/10203 (accessed on 20 June 2016).
- Zobel, R.W.; Wright, M.J.; Gauch, H.G. Statistical analysis of a yield trial. Agron. J. 1988, 80, 388–393. [Google Scholar] [CrossRef]
- Sneller, C.H.; Cilgore–Norquest, L.; Dombek, D. Repeatability of yield stability statistics in soybean. Crop Sci. 1997, 37, 383–390. [Google Scholar] [CrossRef]
- Sabaghnia, N.; Sabaghpour, S.H.; Dehghani, H. The use of an AMMI model and its parameters to analyze yield stability in multi-environment trials. J. Agric. Sci. 2008, 146, 571–581. [Google Scholar] [CrossRef]
- Bassi, F.M.; Sanchez–Garcia, M. Adaptation and stability analysis of ICARDA durum wheat elites across 18 countries. Crop Sci. 2017, 57, 2419–2430. [Google Scholar] [CrossRef]
- Kaya, Y.; Akçura, M.; Taner, S. GGE-biplot analysis of multi-environment yield trials in bread wheat. Turk. J. Agric. For. 2006, 30, 325–337. [Google Scholar]
- Subira, J.; Álvaro, F.; del Moral, L.F.G.; Royo, C. Breeding effects on the cultivar × environment interaction of durum wheat yield. Eur. J. Agron. 2015, 68, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Mohammad, F. Identifying stable bread wheat derived lines across environments through GGE biplot analysis. Sarhad J. Agric. 2018, 34, 63–69. [Google Scholar] [CrossRef]
- Thungo, Z.; Shimelis, H.; Odindo, A.O.; Mashilo, J. Genotype-by-environment interaction of elite heat and drought tolerant bread wheat (Triticum aestivum L.) genotypes under non-stressed and drought-stressed conditions. Acta Agric. Scand. B Soil Plant Sci. 2019, 69, 1651–1913. [Google Scholar] [CrossRef]
- Ndiaye, M.; Adam, M.; Ganyo, K.K.; Guissé, A.; Cissé, N.; Muller, B. Genotype-environment interaction: Trade-offs between the agronomic performance and stability of dual-purpose sorghum (Sorghum bicolor L. Moench) genotypes in Senegal. Agronomy 2019, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, R.; Farshadfar, E.; Amri, A. Interpreting genotype × environment interactions for grain yield of rainfed durum wheat in Iran. Crop J. 2015, 3, 526–535. [Google Scholar] [CrossRef] [Green Version]
- Waines, J. High temperature stress in wild wheats and spring wheats. Funct. Plant Biol. 1994, 21, 705–715. [Google Scholar] [CrossRef]
- Aberkane, H.; Amri, A.; Belkadi, B.; Filali–Maltouf, A.; Kehel, Z.; Tahir, I.S.A.; Meheesi, A.; Tsivelikas, A. Evaluation of durum wheat lines derived from interspecific crosses under drought and heat stress. Crop Sci. 2021, 61, 119–136. [Google Scholar] [CrossRef]
- Mehari, M.; Tesfay, M.; Yirga, H.; Mesele, A.; Abebe, T.; Workineh, A.; Amare, B. GGE biplot analysis of genotype-by-environment interaction and grain yield stability of bread wheat genotypes in South Tigray, Ethiopia. Commun. Biomet. Crop Sci. 2015, 10, 17–26. [Google Scholar]
- El Haddad, N.; Kabbaj, H.; Zaïm, M.; El Hassouni, K.; Tidiane Sall, A.; Azouz, M.; Rodomiro, O.; Baum, M.; Amri, A.; Gamba, F.; et al. Crop wild relatives in durum wheat breeding: Drift or thrift? Crop Sci. 2021, 61, 37–54. [Google Scholar] [CrossRef]
- Mondal, S.; Rutkoski, J.E.; Velu, G.; Singh, P.K.; Crespo–Herrera, L.A.; Guzmán, C.; Bhavani, S.; Lan, C.; He, X.; Singh, R.P. Harnessing diversity in wheat to enhance grain yield, climate resilience, disease and insect pest resistance and nutrition through conventional and modern breeding approaches. Front. Plant Sci. 2016, 7, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aberkane, H.; Belkadi, B.; Kehel, Z.; Filali–Maltouf, A.; Tahir, I.S.A.; Meheesi, S.; Amri, A. Assessment of drought and heat tolerance of durum wheat lines derived from interspecific crosses using physiological parameters and stress indices. Agronomy 2021, 11, 695. [Google Scholar] [CrossRef]
- Nachit, M.M.; Elouafi, I. Durum wheat adaptation in the mediterranean dryland: Breeding, stress physiology, and molecular markers. In CSSA Special Publications; Rao, S.C., Ryan, J., Eds.; Crop Science Society of America and American Society of Agronomy: Madison, WI, USA, 2015; pp. 203–218. ISBN 978-0-89118-611-3. [Google Scholar]

| Line | Name | Type | Pedigree |
|---|---|---|---|
| 1 | DWAyT_0204 | Wild relative-derived | Younes/TdicoAlpCol//Korifla |
| 2 | DWAyT_0214 | Wild relative-derived | Korifla/AegSpeltoidesSyr//Amedakul |
| 3 | Faraj | Commercial variety | F413J.S/3/Arthur71/Lahn//Blk2/Lahn/4/Quarmal |
| 4 | Nachit | Wild relative-derived | Amedakul1/TdicoSyrCol//Loukos |
| 5 | DWAyT_0306 | Wild relative-derived | Korifla/AegSpeltoidesSyr//Heider |
| 6 | Jabal | Wild relative-derived | Korifla/AegSpeltoidesSyr//Mrb5 |
| 7 | Waha | Commercial variety | Plc/Ruff//Gta/Rtte |
| 8 | Magrour | Wild relative-derived | Amedakul1/TdicoSyrCol//Cham1 |
| 9 | Icakassem1 | Elite | Geromtel1/Icasyr1 |
| 10 | DWAyT_0217 | Wild relative-derived | Korifla/AegSpeltoidesSyr//Loukos |
| 11 | Jabal 2 | Wild relative-derived | Korifla/AegSpeltoidesSyr//Mrb5 |
| 12 | Secondroue | Elite | Stj3//Bcr/Lks4/3/Ter3/4/Bcr/Gro1//Mgnl1 |
| 13 | DAWRyT_0208 | Wild relative-derived | Korifla/AegSpeltoidesSyr/Amedakul |
| 14 | DAWRyT0122 | Wild relative-derived | Amedakul1/TdicoJCol//Cham1 |
| 15 | DWAyT_0205 | Wild relative-derived | Younes/TdicoAlpCol//Korifla |
| 16 | Tomouh | Commercial variety | Joric69/Hau |
| 17 | DWAyT-0322 | Wild relative-derived | Heider/TAraticumMA//Mrb5 |
| 18 | Omrabi5 | Commercial variety | Joric69/Hau |
| 19 | Zagharin2 | Elite | Icasyr1/3/Gcn//Stj/Mrb3 |
| 20 | Ouassara1 | Elite | Ouasloukos1/5/Azn1/4/BEZAIZSHF//SD19539/Waha/3/Gdr2 |
| 21 | Icacube | Elite | Mgnl3/Ainzen1//Mgnl3/Ainzen1 |
| 22 | Aghramatlas | Elite | Mgnl3/Ainzen1//Mgnl3/Aghrass2 |
| 23 | Icaverve | Elite | Azeghar1/4/IcamorTA0462/3/Maamouri3//Vitron/Bidra1/5/Mgnl3/Ainzen1 |
| 24 | Icamoram 7 | Wild relative-derived | TAraraticum0472/Ammar7 |
| 25 | Salim | Local check variety | ALTAR84/FD8419-126-1-2/RAZZAK/3/KORIFLA/BALADIAHAMRA |
| Environment | Season | Location | Coordinates | Altitude (m) | Soil Texture | pH | OM (%) 1 | CaCO3 (%) 2 | N (ppm) 3 | P (ppm) 4 | K (ppm) 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kef_15 | 2014–2015 | Kef | 36°11′10″ N; 8°42′00″ E | 532 | Clay-loam | 7.91 | 1.41 | 25.67 | 98.00 | 16.53 | 510.00 |
| Kef_16 | 2015–2016 | ||||||||||
| Kef_17 | 2016–2017 | ||||||||||
| Kef_18 | 2017–2018 | ||||||||||
| Beja_16 | 2015–2016 | Beja | 36°43′ N; 9°12′ E | 161 | Vertic | 7.00 | 2.10 | 2.30 | 87.53 | 80.00 | 621.00 |
| Beja_19 | 2018–2019 |
| Environment | Total | October | Noveber | December | Janunary | February | March | April | May | June | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rainfall (mm) | Kef_15 | 362.0 | 33.0 | 43.0 | 60.0 | 70.0 | 66.0 | 66.0 | 0.0 | 22.0 | 2.0 |
| Kef_16 | 298.0 | 28.0 | 42.0 | 4.0 | 55.0 | 13.0 | 89.0 | 30.0 | 32.0 | 5.0 | |
| Kef_17 | 242.0 | 11.0 | 43.0 | 51.0 | 32.0 | 24.0 | 2.0 | 40.0 | 2.0 | 37.0 | |
| Kef_18 | 340.1 | 27.0 | 96.2 | 23.2 | 32.4 | 31.6 | 41.4 | 39.7 | 8.2 | 40.4 | |
| Beja_16 | 502.3 | 77.5 | 108.8 | 21.4 | 65.0 | 39.2 | 115.6 | 23.4 | 40.4 | 11.0 | |
| Beja_19 | 678.2 | 122.8 | 75.4 | 39.8 | 138.4 | 49.8 | 108.8 | 37.0 | 106.2 | 0.0 | |
| Average | 44.9 | 49.9 | 68.1 | 33.2 | 65.5 | 37.3 | 70.5 | 28.4 | 35.1 | 15.9 | |
| Avg. temperature (°C) | Kef_15 | 14.7 | 20.8 | 15.0 | 7.4 | 9.9 | 8.2 | 13.7 | 10.9 | 23.4 | 23.1 |
| Kef_16 | 17.6 | 22.4 | 14.6 | 9.6 | 12.6 | 11.2 | 16.2 | 18.5 | 25.3 | 28.3 | |
| Kef_17 | 15.7 | 20.8 | 14.3 | 10.1 | 6.7 | 12.5 | 12.3 | 17.0 | 21.2 | 26.5 | |
| Kef_18 | 16.5 | 16.8 | 15.5 | 10.0 | 10.8 | 10.3 | 15.7 | 18.0 | 23.0 | 28.7 | |
| Beja_16 | 10.6 | 14.1 | 11.4 | 5.1 | 6.4 | 6.5 | 6.2 | 9.6 | 12.0 | 24.3 | |
| Beja_19 | 16.8 | 18.5 | 17.1 | 15.6 | 13.4 | 12.9 | 16.6 | 13.2 | 17.3 | 26.3 | |
| Average | 15.3 | 18.9 | 14.6 | 9.6 | 9.9 | 10.3 | 13.4 | 14.5 | 20.3 | 26.2 |
| Sources of Variance | df | Sum Square | % (G + E + G × E) 1 |
|---|---|---|---|
| Genotypes (G) | 24 | 579.98 ** | 2.62 |
| Environment (E) | 5 | 16,422.55 ** | 72.05 |
| G × E | 120 | 5769.77 ** | 25.33 |
| Line | Grain Yield Converted into Ratios to Salim | AWAI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Kef_15 | Kef_16 | Kef_17 | Kef_18 | Beja_16 | Beja_19 | Mean | Groups 1 | ||
| 1 | 1.13 | 0.71 | 1.11 | 0.96 | 1.01 | 1.48 | 1.04 | A | 0.72 |
| 2 | 0.88 | 0.79 | 0.64 | 0.85 | 1.01 | 1.14 | 0.89 | AB | 0.65 |
| 3 | 1.01 | 0.66 | 0.81 | 0.75 | 1.08 | 1.27 | 0.93 | AB | 0.72 |
| 4 | 1.08 | 0.77 | 1.10 | 0.68 | 1.01 | 1.07 | 0.95 | AB | 0.79 |
| 5 | 1.46 | 0.82 | 0.84 | 0.80 | 1.24 | 1.05 | 1.07 | A | 0.72 |
| 6 | 1.24 | 0.74 | 0.70 | 0.58 | 0.93 | 1.18 | 0.91 | AB | 0.83 |
| 7 | 1.34 | 0.67 | 0.84 | 0.86 | 0.95 | 1.00 | 0.96 | AB | 0.66 |
| 8 | 1.20 | 0.57 | 0.57 | 0.83 | 1.11 | 1.29 | 0.94 | AB | 0.82 |
| 9 | 1.25 | 0.96 | 0.64 | 0.84 | 1.36 | 1.29 | 1.09 | A | 0.87 |
| 10 | 1.27 | 0.93 | 0.79 | 0.73 | 1.19 | 1.11 | 1.03 | AB | 0.85 |
| 11 | 1.35 | 0.66 | 0.72 | 0.75 | 1.00 | 0.97 | 0.94 | AB | 0.64 |
| 12 | 1.41 | 0.92 | 0.45 | 0.58 | 1.13 | 1.22 | 1.00 | AB | 0.58 |
| 13 | 1.23 | 1.00 | 0.28 | 0.71 | 0.94 | 1.12 | 0.93 | AB | 0.67 |
| 14 | 1.33 | 0.75 | 0.10 | 0.80 | 1.14 | 1.39 | 0.97 | AB | 0.64 |
| 15 | 1.20 | 0.85 | 0.21 | 0.79 | 0.94 | 0.98 | 0.88 | B | 0.75 |
| 16 | 1.18 | 0.62 | 1.00 | 0.84 | 0.87 | 1.13 | 0.93 | AB | 0.83 |
| 17 | 1.14 | 0.49 | 0.48 | 0.70 | 1.12 | 1.41 | 0.90 | AB | 0.76 |
| 18 | 1.19 | 0.90 | 0.46 | 0.83 | 1.04 | 1.30 | 0.98 | AB | 0.86 |
| 19 | 1.18 | 0.64 | 0.95 | 0.80 | 1.13 | 1.05 | 0.96 | AB | 0.85 |
| 20 | 1.60 | 0.76 | 0.76 | 0.68 | 0.91 | 1.14 | 1.01 | AB | 0.46 |
| 21 | 1.17 | 0.83 | 0.86 | 0.71 | 1.07 | 1.31 | 1.00 | AB | 0.91 |
| 22 | 0.86 | 0.51 | 0.88 | 0.92 | 1.44 | 1.11 | 0.95 | AB | 0.38 |
| 23 | 1.02 | 1.01 | 1.10 | 0.75 | 1.18 | 1.33 | 1.06 | A | 0.63 |
| 24 | 0.79 | 0.93 | 0.87 | 0.64 | 1.01 | 1.30 | 0.91 | AB | 0.46 |
| 25 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | AB | 0.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayed, S.; Bouhaouel, I.; Othmani, A.; Bassi, F.M. Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia. Agronomy 2021, 11, 1782. https://doi.org/10.3390/agronomy11091782
Ayed S, Bouhaouel I, Othmani A, Bassi FM. Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia. Agronomy. 2021; 11(9):1782. https://doi.org/10.3390/agronomy11091782
Chicago/Turabian StyleAyed, Sourour, Imen Bouhaouel, Afef Othmani, and Filippo Maria Bassi. 2021. "Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia" Agronomy 11, no. 9: 1782. https://doi.org/10.3390/agronomy11091782
APA StyleAyed, S., Bouhaouel, I., Othmani, A., & Bassi, F. M. (2021). Use of Wild Relatives in Durum Wheat (Triticum turgidum L. var. durum Desf.) Breeding Program: Adaptation and Stability in Context of Contrasting Environments in Tunisia. Agronomy, 11(9), 1782. https://doi.org/10.3390/agronomy11091782






