The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Fungal Identification
2.3. Growth Evaluation
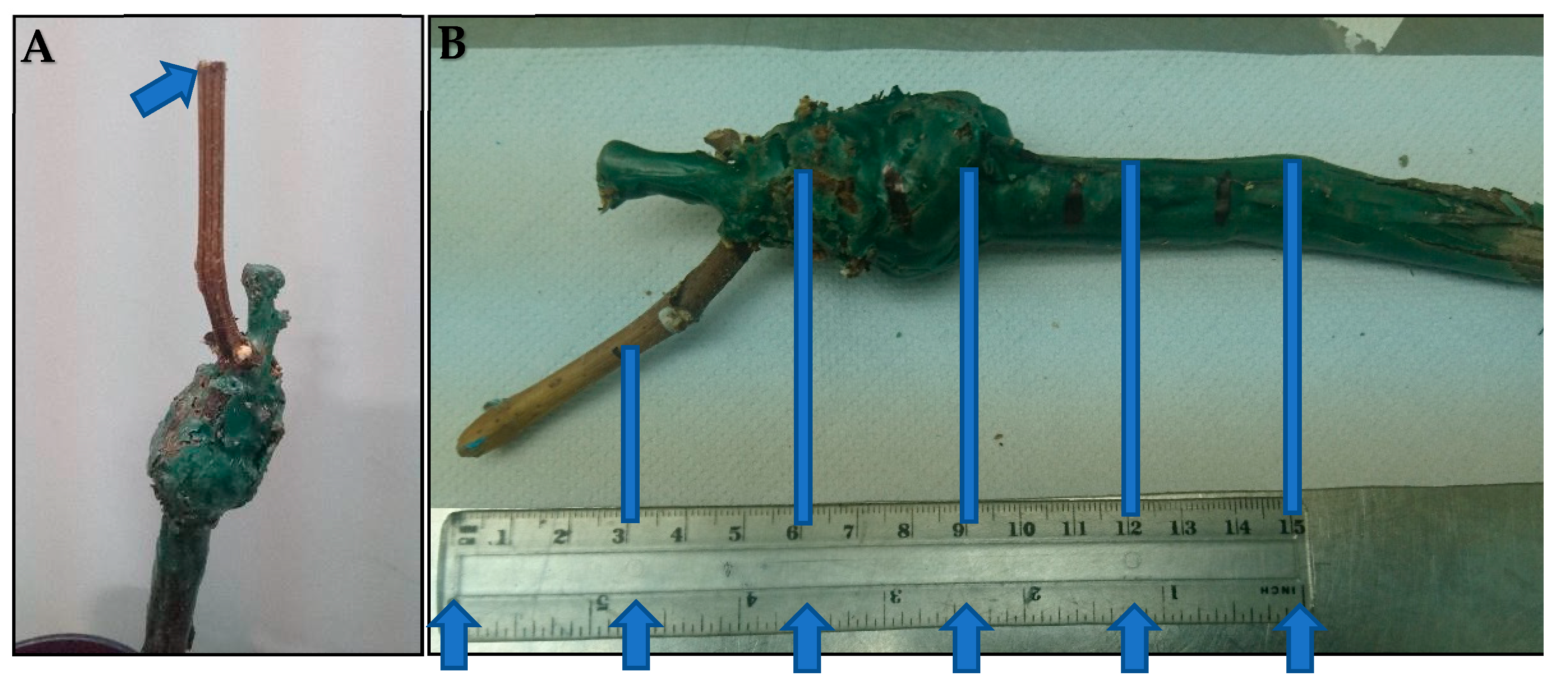
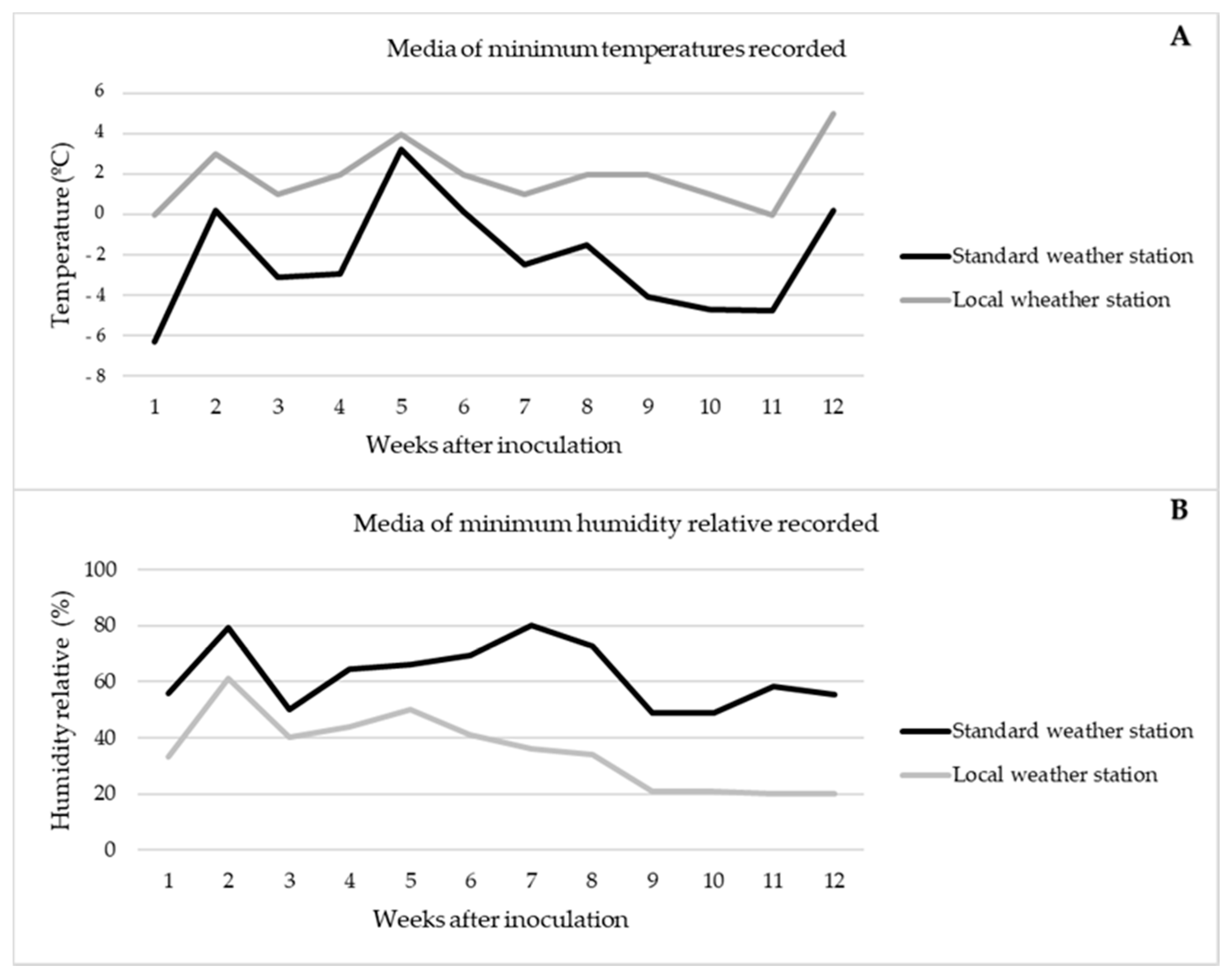
2.4. Evaluation of Trichoderma Survival over Pruning Wounds in Planta
2.5. Statistical Data Analysis
3. Results
3.1. Evaluation of Fungal Growth
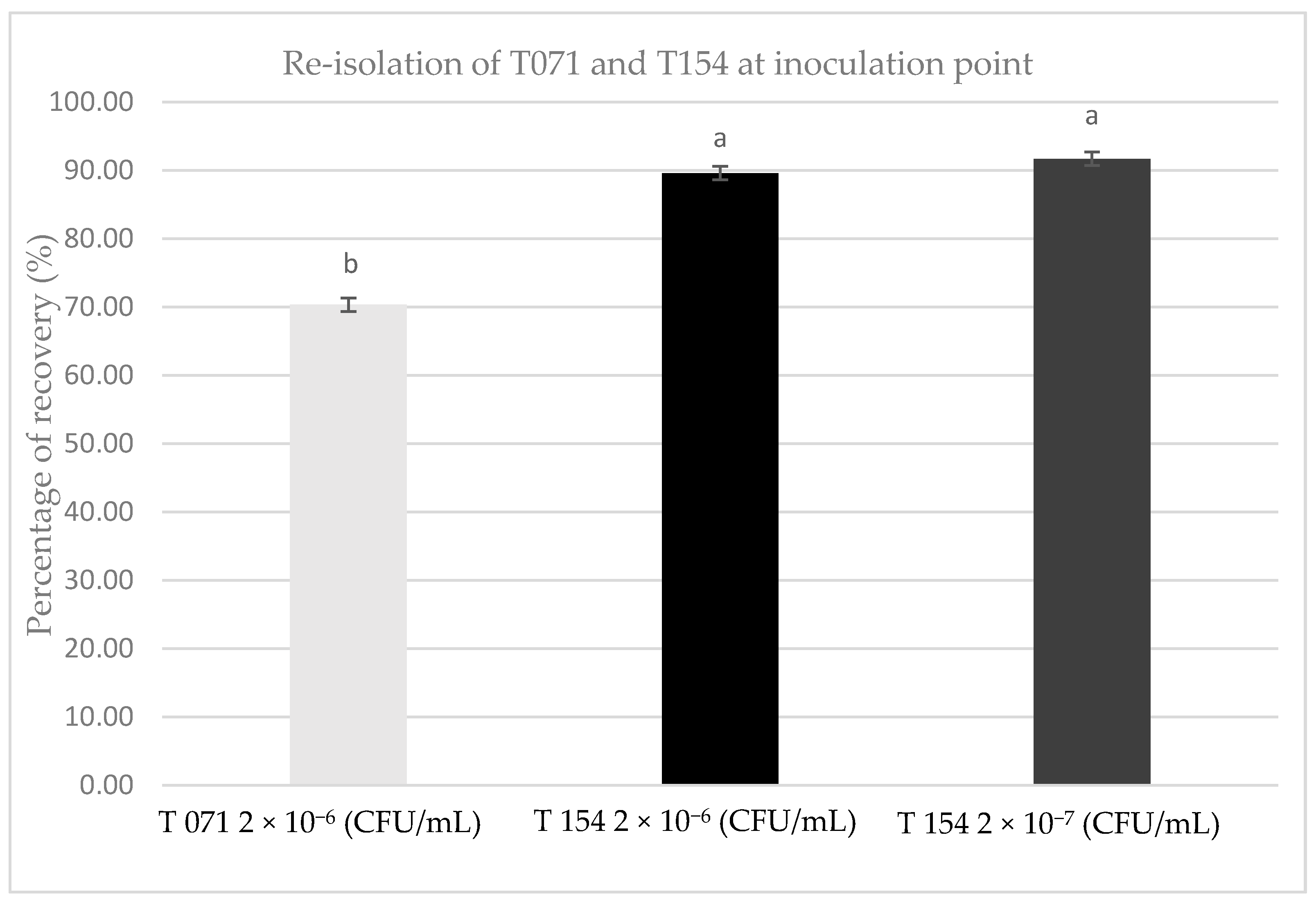
3.2. Evaluation of Survival over Pruning Wounds in Planta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on Trichoderma: From ’omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based products and their widespread use in agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine trunk diseases: A review of fifteen years of trials for their control with chemicals and biocontrol agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertot, I.; Giovannini, O.; Benanchi, M.; Caffi, T.; Rossi, V.; Mugnai, L. Combining biocontrol agents with different mechanisms of action in a strategy to control Botrytis cinerea on grapevine. Crop Prot. 2017, 97, 85–93. [Google Scholar] [CrossRef]
- Bigot, G.; Sivilotti, P.; Stecchina, M.; Lujan, C.; Freccero, A.; Mosetti, D. Long-term effects of Trichoderma asperellum and Trichoderma gamsii on the prevention of esca in different vineyards of Northeastern Italy. Crop Prot. 2020, 137, 105264. [Google Scholar] [CrossRef]
- Surico, G. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 2009, 48, 5–10. [Google Scholar] [CrossRef]
- Perazzolli, M.; Roatti, B.; Bozza, E.; Pertot, I. Trichoderma harzianum T39 induces resistance against downy mildew by priming for defense without costs for grapevine. Biol. Control 2011, 58, 74–82. [Google Scholar] [CrossRef]
- Mutawila, C.; Vinale, F.; Halleen, F.; Lorito, M.; Mostert, L. Isolation, production and in vitro effects of the major secondary metabolite produced by Trichoderma species used for the control of grapevine trunk diseases. Plant Pathol. 2015, 65, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing grapevine trunk diseases with respect to etiology and epidemiology: Current strategies and future prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramaje, D.; Armengol, J. Fungal trunk pathogens in the grapevine propagation process: Potential inoculum sources, detection, identification, and management strategies. Plant Dis. 2011, 95, 1040–1055. [Google Scholar] [CrossRef] [Green Version]
- Gramaje, D.; Mostert, L.; Groenewald, J.Z.; Crous, P.W. Phaeoacremonium: From esca disease to phaeohyphomycosis. Fungal Biol. 2015, 119, 759–783. [Google Scholar] [CrossRef]
- Halleen, F.; Mostert, L.; Crous, P.W. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australas. Plant Pathol. 2007, 36, 277–285. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Armengol, J. Black-foot disease of grapevine: An update on taxonomy, epidemiology and management strategies. Phytopathol. Mediterr. 2013, 52, 245–261. [Google Scholar] [CrossRef]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar] [CrossRef]
- Lombard, L.; Van der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 53, 515–532. [Google Scholar]
- Baumgartner, K.; Fujiyoshi, P.T.; Travadon, R.; Castlebury, L.A.; Wilcox, W.F.; Rolshausen, P.E. Characterization of species of Diaporthe from wood cankers of grape in eastern North American vineyards. Plant Dis. 2013, 97, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Úrbez-Torres, J.R.; Peduto, F.; Smith, R.J.; Gubler, W.D. Phomopsis dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 2013, 97, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Groenewald, J.Z.; Cheewangkoon, R.; Jami, F.; Abdollahzadeh, J.; Lombard, L.; Crous, P.W. Families, genera, and species of Botryosphaeriales. Fungal Biol. 2017, 121, 322–346. [Google Scholar] [CrossRef] [PubMed]
- Urbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, 5–45. [Google Scholar] [CrossRef]
- Pitt, W.M.; Huang, R.; Steel, C.C.; Savocchia, S. Pathogenicity and epidemiology of Botryosphaeriaceae species isolated from grapevines in Australia. Australas. Plant Pathol. 2013, 42, 573–582. [Google Scholar] [CrossRef]
- Pitt, W.M.; Huang, R.; Trouillas, F.P.; Steel, C.C.; Savocchia, S. Evidence that Eutypa lata and other diatrypaceous species occur in New South Wales vineyards. Australas. Plant Pathol. 2010, 39, 97–106. [Google Scholar] [CrossRef]
- Trouillas, F.P.; Úrbez-Torres, J.R.; Gubler, W.D. Diversity of diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 2010, 102, 319–336. [Google Scholar] [CrossRef]
- Berlanas, C.; Andrés-Sodupe, M.; López-Manzanares, B.; Maldonado-González, M.M.; Gramaje, D. Effect of white mustard cover crop residue, soil chemical fumigation and Trichoderma spp. root treatment on black-foot disease control in grapevine. Pest Manag. Sci. 2018, 74, 2864–2873. [Google Scholar] [CrossRef]
- del Martínez-Diz, M.P.; Díaz-Losada, E.; Andrés-Sodupe, M.; Bujanda, R.; Maldonado-González, M.M.; Ojeda, S.; Yacoub, A.; Rey, P.; Gramaje, D. Field evaluation of biocontrol agents against black-foot and Petri diseases of grapevine. Pest Manag. Sci. 2020, 77, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Van Jaarsveld, W.J.; Halleen, F.; Bester, M.C.; Pierron, R.J.G.; Stempien, E.; Mostert, L. Investigation of Trichoderma species colonization of nursery grapevines for improved management of black foot disease. Pest Manag. Sci. 2021, 77, 397–405. [Google Scholar] [CrossRef]
- Halleen, F.; Fourie, P.H.; Lombard, P.J. Protection of grapevine pruning wounds against Eutypa lata by biological and chemical methods. S. Afr. J. Enol. Vitic. 2010, 31, 125–132. [Google Scholar] [CrossRef] [Green Version]
- del Martinez-Diz, M.P.; Diaz-Losada, E.; Díaz-Fernández, Á.; Bouzas-Cid, Y.; Gramaje, D. Protection of grapevine pruning wounds against Phaeomoniella chlamydospora and Diplodia seriata by commercial biological and chemical methods. Crop Prot. 2021, 143, 105465. [Google Scholar] [CrossRef]
- Brader, G.; Compant, S.; Vescio, K.; Mitter, B.; Trognitz, F.; Ma, L.-J.; Sessitsch, A. Ecology and genomic insights into plant-pathogenic and -nonpathogenic endophytes. Annu. Rev. Phytopathol. 2017, 55, 61–83. [Google Scholar] [CrossRef]
- Keane, P.; Kerr, A. Factors affecting disease development. In Plant Pathogens and Plant Diseases; Brown, J.F., Ogle, H.J., Eds.; Rockvale: Armidale, Australia, 1997; pp. 287–298. [Google Scholar]
- Kredics, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Kevei, F.; Nagy, E. Influence of environmental parameters on Trichoderma strains with biocontrol potential. Food Technol. Biotechnol. 2003, 41, 37–42. [Google Scholar]
- Fedele, G.; Bove, F.; González-Domínguez, E.; Rossi, V. A generic model accounting for the interactions among pathogens, host plants, biocontrol agents, and the environment, with parametrization for Botrytis cinerea on grapevines. Agronomy 2020, 10, 222. [Google Scholar] [CrossRef] [Green Version]
- Fedele, G.; Brischetto, C.; Rossi, V. Biocontrol of Botrytis cinerea on grape berries as influenced by temperature and humidity. Front. Plant Sci. 2020, 11, 1232. [Google Scholar] [CrossRef] [PubMed]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Masson-Delmonte, V.; Zhai, P.; Pirani, A.; Connors, S.; Péan, C.; Berger, S.; Caud, N.; Chen, Y.; Goldfarb, L.; Gomis, M.; et al. IPCC 2021: Climate Change 2021: The Physical Science Basis: Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2021; in press. [Google Scholar]
- Van Leeuwen, C.; Darriet, P. The impact of climate change on viticulture and wine quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef] [Green Version]
- Parker, A.K.; García de Cortázar-Atauri, I.; Gény, L.; Spring, J.L.; Destrac, A.; Schultz, H.; Molitor, D.; Lacombe, T.; Graça, A.; Monamy, C.; et al. Temperature-based grapevine sugar ripeness modelling for a wide range of Vitis vinifera L. cultivars. Agric. For. Meteorol. 2020, 285–286, 107902. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Pascual, I.; Gomès, E. Ultraviolet-B alleviates the uncoupling effect of elevated CO2 and increased temperature on grape berry (Vitis vinifera cv. Tempranillo) anthocyanin and sugar accumulation. Aust. J. Grape Wine Res. 2016, 22, 87–95. [Google Scholar] [CrossRef]
- Gomès, É.; Maillot, P.; Duchêne, É. Molecular tools for adapting viticulture to climate change. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Morales, F.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Gomès, E.; Pascual, I. Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci. 2015, 236, 168–176. [Google Scholar] [CrossRef]
- Lecourieux, D.; Kappel, C.; Claverol, S.; Pieri, P.; Feil, R.; Lunn, J.E.; Bonneu, M.; Wang, L.; Gomès, E.; Delrot, S.; et al. Proteomic and metabolomic profiling underlines the stage- and time-dependent effects of high temperature on grape berry metabolism. J. Integr. Plant Biol. 2020, 62, 1132–1158. [Google Scholar] [CrossRef] [PubMed]
- Petrie, P.R.; Clingeleffer, P.R. Effects of temperature and light (before and after budburst) on inflorescence morphology and flower number of Chardonnay grapevines (Vitis vinifera L.). Aust. J. Grape Wine Res. 2005, 11, 59–65. [Google Scholar] [CrossRef]
- Valencia, E.; Gross, N.; Quero, J.L.; Carmona, C.P.; Ochoa, V.; Gozalo, B.; Delgado-Baquerizo, M.; Dumack, K.; Hamonts, K.; Singh, B.K.; et al. Cascading effects from plants to soil microorganisms explain how plant species richness and simulated climate change affect soil multifunctionality. Glob. Chang. Biol. 2018, 24, 5642–5654. [Google Scholar] [CrossRef] [PubMed]
- Campisano, A.; Albanese, D.; Yousaf, S.; Pancher, M.; Donati, C.; Pertot, I. Temperature drives the assembly of endophytic communities’ seasonal succession. Environ. Microbiol. 2017, 19, 3353–3364. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Eldridge, D.J.; Ochoa, V.; Gozalo, B.; Singh, B.K.; Maestre, F.T. Soil microbial communities drive the resistance of ecosystem multifunctionality to global change in drylands across the globe. Ecol. Lett. 2017, 20, 1295–1305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-González, Á.; Carro-Huerga, G.; Mayo-Prieto, S.; Lorenzana, A.; Gutiérrez, S.; Peláez, H.J.; Casquero, P.A. Investigations of Trichoderma spp. and Beauveria bassiana as biological control agent for Xylotrechus arvicola, a major insect pest in Spanish vineyards. J. Econ. Entomol. 2018, 111, 2585–2591. [Google Scholar] [CrossRef] [Green Version]
- Carro-Huerga, G.; Compant, S.; Gorfer, M.; Cardoza, R.E.; Schmoll, M.; Gutiérrez, S.; Casquero, P.A. Colonization of Vitis vinifera L. by the endophyte Trichoderma sp. strain T154: Biocontrol activity against Phaeoacremonium minimum. Front. Plant Sci. 2020, 11, 1170. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Steindorff, A.S.; Chenthamara, K.; Manganiello, G.; Henrissat, B.; Zhang, J.; Cai, F.; Kopchinskiy, A.G.; Kubicek, E.M.; Kuo, A.; et al. Evolution and comparative genomics of the most common Trichoderma species. BMC Genomics 2019, 20, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.T.; Cobos, R.; Martin, M.T.; Cobos, R. Identification of fungi associated with grapevine decline in Castilla y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25. [Google Scholar] [CrossRef]
- Qiu, Y.; Steel, C.C.; Ash, G.J.; Savocchia, S. Effects of temperature and water stress on the virulence of Botryosphaeriaceae spp. causing dieback of grapevines and their predicted distribution using CLIMEX in Australia. Acta Hortic. 2016, 1115, 171–181. [Google Scholar] [CrossRef]
- Bellée, A.; Comont, G.; Nivault, A.; Abou-Mansour, E.; Coppin, C.; Dufour, M.C.; Corio-Costet, M.F. Life traits of four Botryosphaeriaceae species and molecular responses of different grapevine cultivars or hybrids. Plant Pathol. 2017, 66, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Valtaud, C.; Larignon, P.; Roblin, G.; Fleurat-Lessard, P. Developmental and ultrastructural features of Phaeomoniella chlamydospora and Phaeoacremonium aleophilum in relation to xylem degradation in esca disease of the grapevine. J. Plant Pathol. 2009, 91, 37–51. [Google Scholar] [CrossRef]
- Mutawila, C.; Halleen, F.; Mostert, L. Optimisation of time of application of Trichoderma biocontrol agents for protection of grapevine pruning wounds. Aust. J. Grape Wine Res. 2016, 22, 279–287. [Google Scholar] [CrossRef]
- Rifai, M.A. A revision of the genus Trichoderma. Mycol. Pap. 1969, 116, 1–56. [Google Scholar]
- Butt, T.M.; Jackson, C.; Magan, N. Fungi as Biocontrol Agents Progress, Problems and Potential; CABI: Wallingford, UK, 2001; pp. 1–8. [Google Scholar]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol. Micol. 2015, 3, 1–8. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Harman, G.E. Basic biology, taxonomy and genetics. In Trichoderma and Gliocladium; CRC Press: Abingdon, UK, 1998; Volume 1, pp. 57–69. ISBN 9780748405725. [Google Scholar]
- Rinu, K.; Sati, P.; Pandey, A. Trichoderma gamsii (NFCCI 2177): A newly isolated endophytic, psychrotolerant, plant growth promoting, and antagonistic fungal strain. J. Basic Microbiol. 2014, 54, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.; Chugh, P.; Sharma, P. Cold-tolerant Trichoderma species for the management of Fusarium wilt of tomato plants. Ann. Microbiol. 2015, 65, 543–551. [Google Scholar] [CrossRef]
- Harman, G.E.; Kubicek, C.P. Enzymes, biological control and commercial applications. In Trichoderma and Gliocladium; CRC Press: Abingdon, UK, 1998; Volume 2, ISBN 9780748405725. [Google Scholar]
- Antal, Z.; Manczinger, L.; Szakacs, G.; Tengerdy, R.P.; Ferenczy, L. Colony growth, in vitro antagonism and secretion of extracellular enzymes in cold-tolerant strains of Trichoderma species. Mycol. Res. 2000, 104, 545–549. [Google Scholar] [CrossRef]
- Singh, A.; Srivastava, S.; Singh, H.B. Effect of substrates on growth and shelf life of Trichoderma harzianum and its use in biocontrol of diseases. Bioresour. Technol. 2007, 98, 470–473. [Google Scholar] [CrossRef]
- Danielson, R.M.; Davey, C.B. Non nutritional factors affecting the growth of Trichoderma in culture. Soil Biol. Biochem. 1973, 5, 495–504. [Google Scholar] [CrossRef]
- Danielson, R.M.; Davey, C.B. The abundance of Trichoderma propagules and the distribution of species in forest soils. Soil Biol. Biochem. 1973, 5, 485–494. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Jaklitsch, W.M.; Samuels, G.J.; Dodd, S.L.; Lu, B.S.; Druzhinina, I.S. Hypocrea rufa/Trichoderma viride: A reassessment, and description of five closely related species with and without warted conidia. Stud. Mycol. 2006, 55, 135–177. [Google Scholar] [CrossRef] [Green Version]
- Anees, M.; Tronsmo, A.; Edel-Hermann, V.; Hjeljord, L.G.; Héraud, C.; Steinberg, C. Characterization of field isolates of Trichoderma antagonistic against Rhizoctonia solani. Fungal Biol. 2010, 114, 691–701. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P.; Komo-Zelazowska, M.; Mulaw, T.B.; Bissett, J. The Trichoderma harzianum demon: Complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evol. Biol. 2010, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Luque, J.; Elena, G.; Garcia-Figueres, F.; Reyes, J.; Barrios, G.; Legorburu, F.J. Natural infections of pruning wounds by fungal trunk pathogens in mature grapevines in Catalonia (Northeast Spain). Aust. J. Grape Wine Res. 2014, 20, 134–143. [Google Scholar] [CrossRef]
- Martínez-Diz, M. del P.; Eichmeier, A.; Spetik, M.; Bujanda, R.; Díaz-Fernández, Á.; Díaz-Losada, E.; Gramaje, D. Grapevine pruning time affects natural wound colonization by wood-invading fungi. Fungal Ecol. 2020, 48, 100994. [Google Scholar] [CrossRef]
- Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; González-López, Ó.; Gutierrez, S.; Casquero, P.A. Influence of fungicide application and vine age on Trichoderma diversity as source of biological control agents. Agronomy 2021, 11, 446. [Google Scholar] [CrossRef]
- Mutawila, C.; Fourie, P.H.; Halleen, F.; Mostert, L. Grapevine cultivar variation to pruning wound protection by Trichoderma species against trunk pathogens. Phytopathol. Mediterr. 2011, 50, 264–276. [Google Scholar] [CrossRef]
- Mutawila, C.; Halleen, F.; Mostert, L. Development of benzimidazole resistant Trichoderma strains for the integration of chemical and biocontrol methods of grapevine pruning wound protection. BioControl 2015, 60, 387–399. [Google Scholar] [CrossRef]
- Kotze, C.; Van Niekerk, J.; Mostert, L.; Halleen, F.; Fourie, P.; Van Niekerk, J.; Mostert, L.; Halleen, F.; Fourie, P. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 2011, 50, 247–263. [Google Scholar] [CrossRef]
- Eskalen, A.; Feliciano, A.J.; Gubler, W.D. Susceptibility of grapevine pruning wounds and symptom development in response to infection by Phaeoacremonium aleophilum and Phaeomoniella chlamydospora. Plant Dis. 2007, 91, 1100–1104. [Google Scholar] [CrossRef] [Green Version]
- John, S.; Wicks, T.J.; Hunt, J.S.; Scott, E.S. Colonisation of grapevine wood by Trichoderma harzianum and Eutypa lata. Aust. J. Grape Wine Res. 2008, 14, 18–24. [Google Scholar] [CrossRef]
- Prozil, S.O.; Evtuguin, D.V.; Lopes, L.P.C. Chemical composition of grape stalks of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Hjelford, L.G.; Stensvand, A.; Tronsmo, A. Effect of temperature and nutrient stress on the capacity of commercial Trichoderma products to control Botrytis cinerea and Mucor piriformis in greenhouse strawberries. Biol. Control 2000, 19, 149–160. [Google Scholar] [CrossRef]
- Mayo-Prieto, S.; Campelo, M.P.; Lorenzana, A.; Rodríguez-González, A.; Reinoso, B.; Gutiérrez, S.; Casquero, P.A. Antifungal activity and bean growth promotion of Trichoderma strains isolated from seed vs soil. Eur. J. Plant Pathol. 2020, 158, 817–828. [Google Scholar] [CrossRef]
- John, S.; Wicks, T.J.; Hunt, J.S.; Lorimer, M.F.; Oakey, H.; Scott, E.S. Protection of grapevine pruning wounds from infection by Eutypa lata. Aust. J. Grape Wine Res. 2005, 14, 134–142. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F. Technology in precision viticulture: A state of the art review. Int. J. Wine Res. 2015, 7, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Pertot, I.; Prodorutti, D.; Colombini, A.; Pasini, L. Trichoderma atroviride SC1 prevents Phaeomoniella chlamydospora and Phaeoacremonium aleophilum infection of grapevine plants during the grafting process in nurseries. BioControl 2016, 61, 257–267. [Google Scholar] [CrossRef]
- Di Marco, S.; Osti, F.; Cesari, A. Experiments on the control of esca by Trichoderma. Phytopathol. Mediterr. 2004, 43, 108–115. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Karimi, K.; Babai Ahari, A.; Arzanlou, M.; Amini, J.; Pertot, I. Comparison of indigenous Trichoderma spp. strains to a foreign commercial strain in terms of biocontrol efficacy against Colletotrichum nymphaeae and related biological features. J. Plant Dis. Prot. 2017, 124, 453–466. [Google Scholar] [CrossRef]
- Mayo, S.; Gutiérrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Pérez, J.M.; González-García, S.; Cobos, R.; Olego, M.Á.; Ibañez, A.; Díez-Galán, A.; Garzón-Jimeno, E.; Coque, J.J.R. Use of endophytic and rhizosphere actinobacteria from grapevine plants to reduce nursery fungal graft infections that lead to young grapevine decline. Appl. Environ. Microbiol. 2017, 83, e01564-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
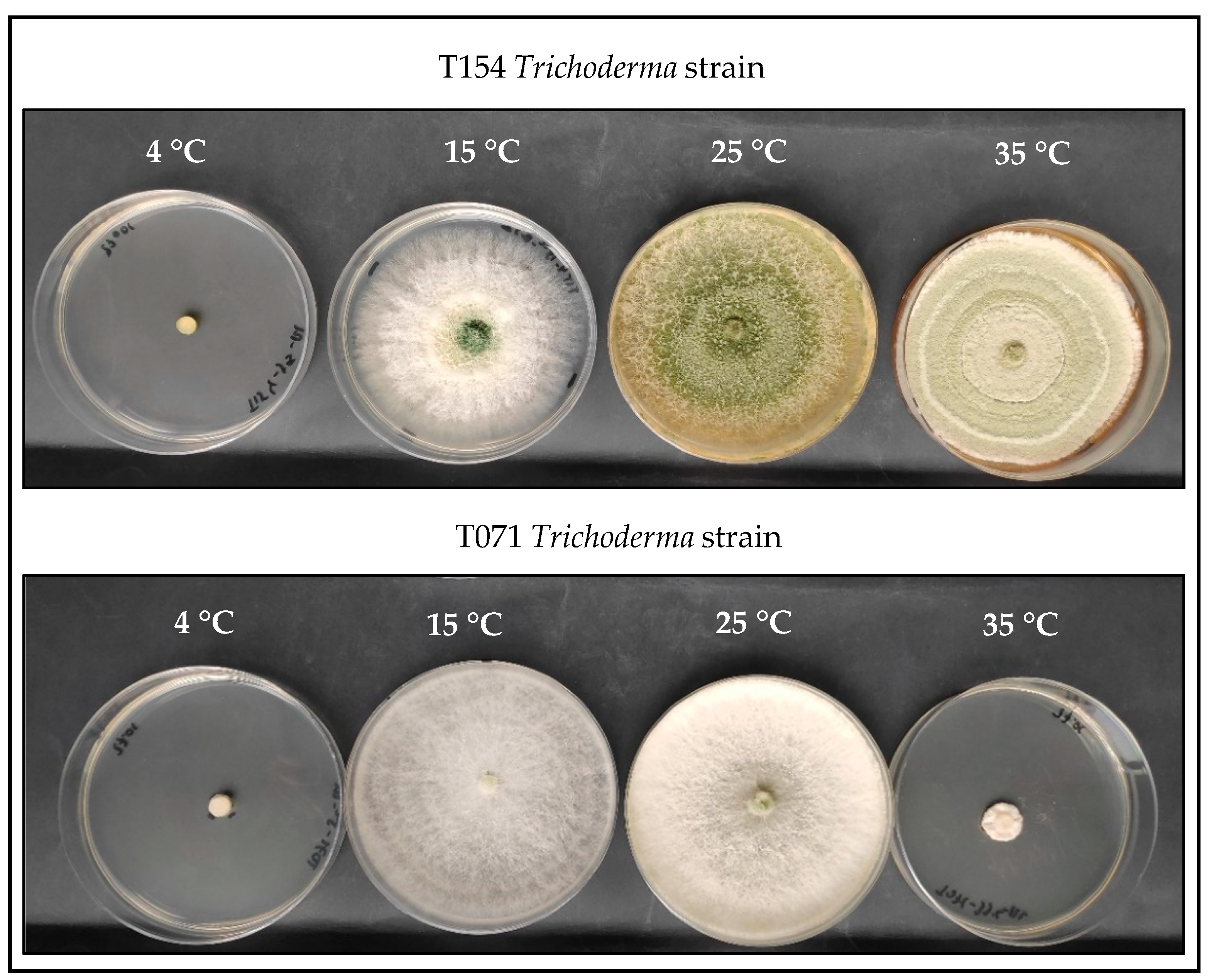
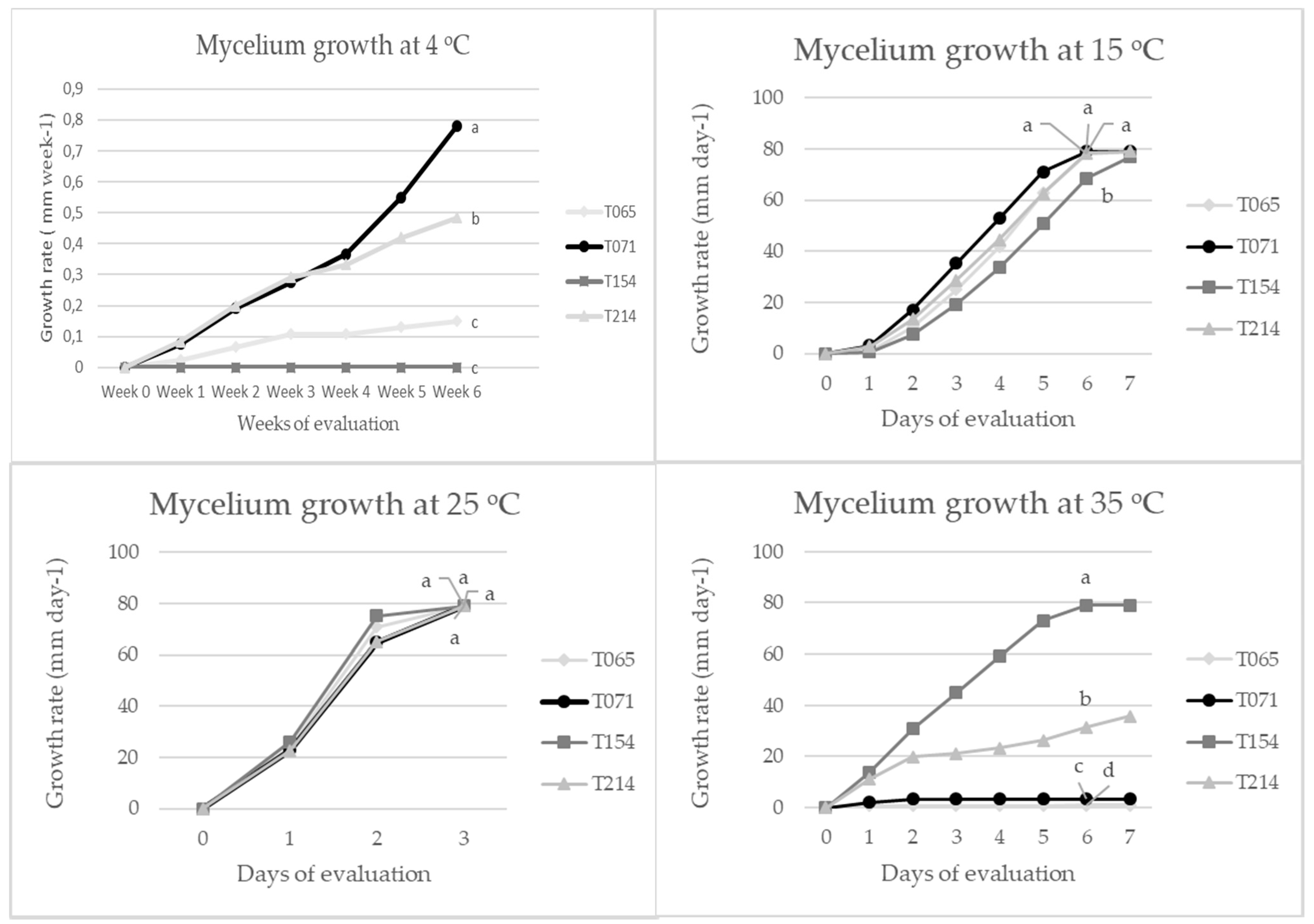
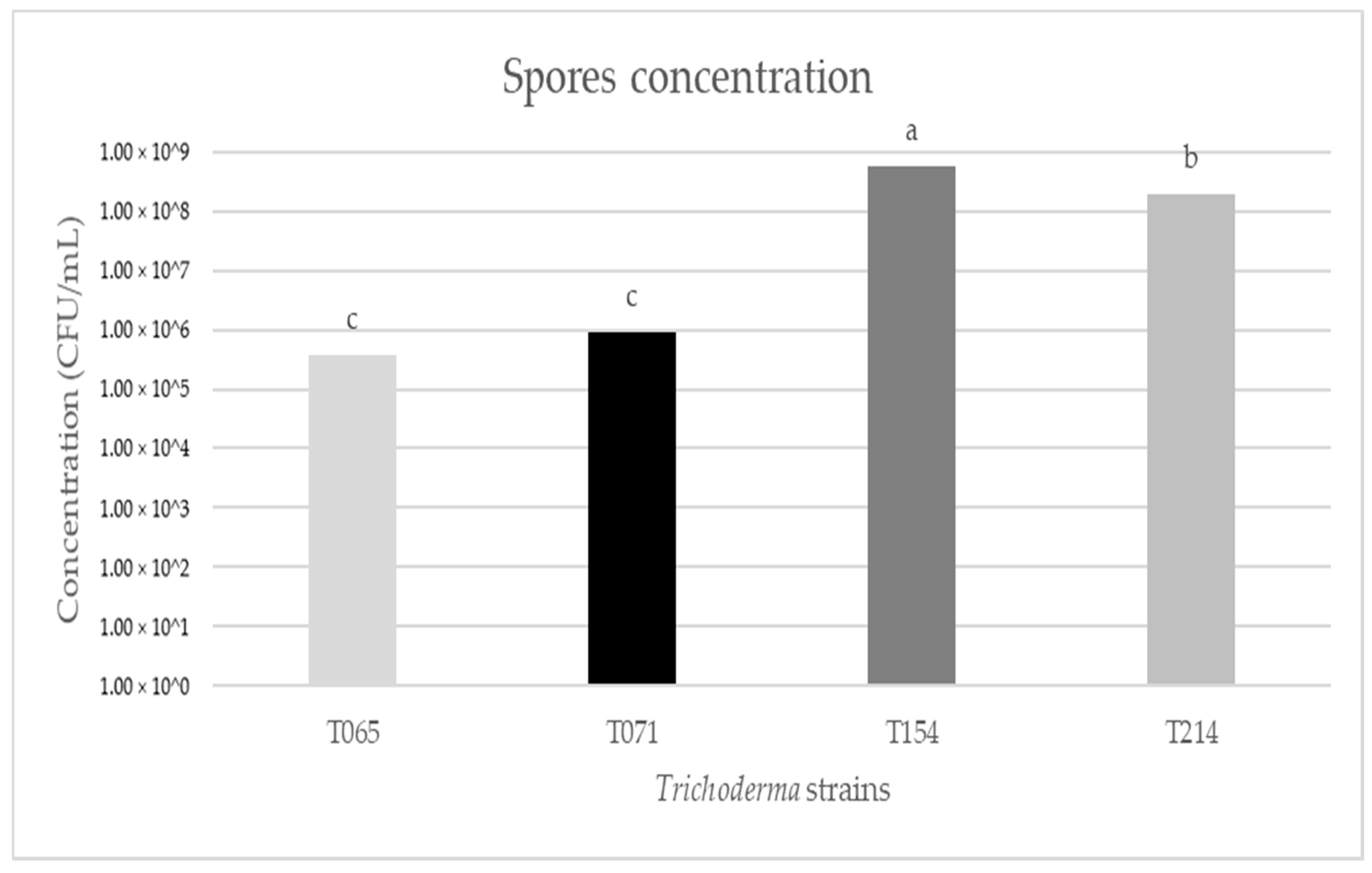
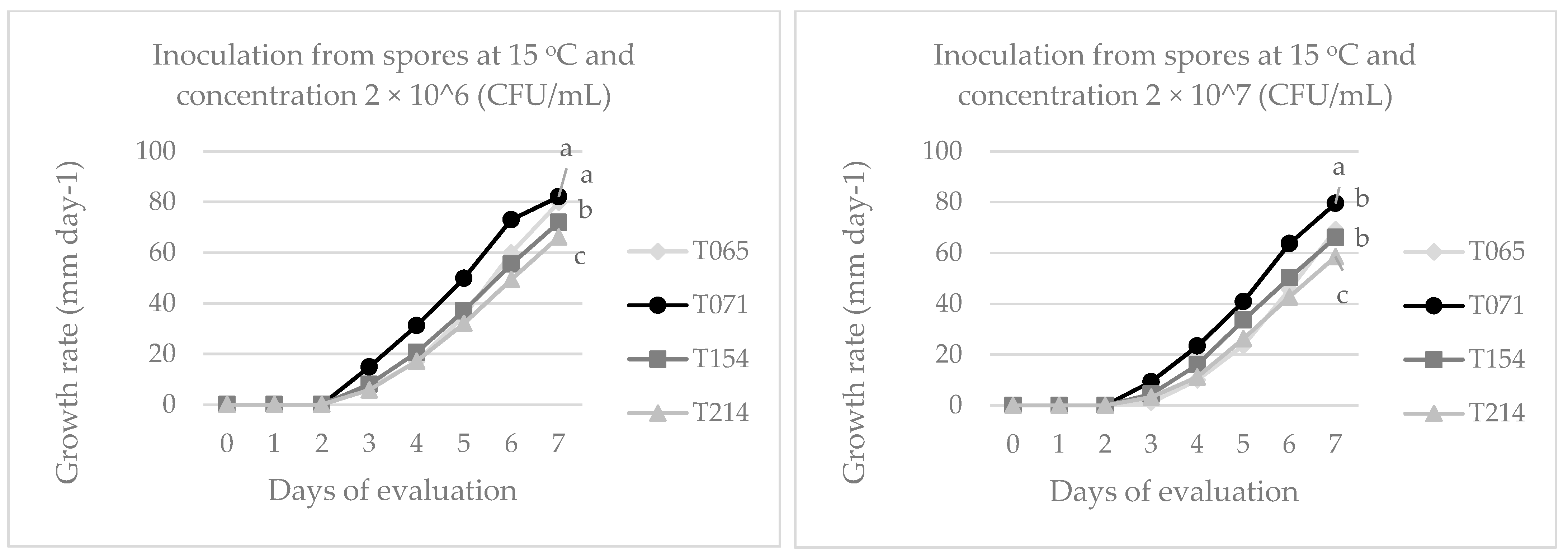
| Number Isolate | Reference 1 | Origin | Main Mechanism of Biocontrol |
|---|---|---|---|
| T065 | This study | Vineyard soil | - |
| T071 | T. gamsii (Section Trichoderma) [46]. | Vineyard soil | Mycoparasistim in insects |
| T154 | Trichoderma spp. (Clade Harzianum/Virens) [47]. | Grapevine bark | Mycoparasistim of fungi and niche exclusion |
| T214 | This study | Grapevine bark | - |
| Most Frequent Fungi Associated with GTDs Isolated in Castilla y León Region 1 | Minimal Temperature for Mycelial Growth (°C) | Maximum Temperature for Mycelial Growth (°C) | Reference of Temperature Conditions |
|---|---|---|---|
| Botryosphaeria dothidea (Moug.) Ces. and De Not | 4 | 35 | [50] |
| Botryosphaeria parva Pennycook and Samuels | 4 | 35 | [50] |
| Botryosphaeria stevensii Shoemaker | 15 | 33 | [51] |
| Diplodia seriata De Not | 4 | 35 | [50] |
| Phaeoacremonium minimum (Tul. and C. Tul.) Gramaje, L. Mostert and Crous | 10 | 30 | [52] |
| Phaeomoniella chlamydospora (W. Gams, Crous, M.J. Wingf. and Mugnai) Crous and W. Gams | 10 | 35 | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carro-Huerga, G.; Mayo-Prieto, S.; Rodríguez-González, Á.; Álvarez-García, S.; Gutiérrez, S.; Casquero, P.A. The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds. Agronomy 2021, 11, 1771. https://doi.org/10.3390/agronomy11091771
Carro-Huerga G, Mayo-Prieto S, Rodríguez-González Á, Álvarez-García S, Gutiérrez S, Casquero PA. The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds. Agronomy. 2021; 11(9):1771. https://doi.org/10.3390/agronomy11091771
Chicago/Turabian StyleCarro-Huerga, Guzmán, Sara Mayo-Prieto, Álvaro Rodríguez-González, Samuel Álvarez-García, Santiago Gutiérrez, and Pedro A. Casquero. 2021. "The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds" Agronomy 11, no. 9: 1771. https://doi.org/10.3390/agronomy11091771
APA StyleCarro-Huerga, G., Mayo-Prieto, S., Rodríguez-González, Á., Álvarez-García, S., Gutiérrez, S., & Casquero, P. A. (2021). The Influence of Temperature on the Growth, Sporulation, Colonization, and Survival of Trichoderma spp. in Grapevine Pruning Wounds. Agronomy, 11(9), 1771. https://doi.org/10.3390/agronomy11091771









