Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Experimentation
2.2. Germination of Plant Material
2.3. Morphological Evaluation
2.4. Total Seed Protein Analysis
2.5. Data Analysis
3. Results
3.1. Morphological Characterization
3.1.1. Diversity in Qualitative Traits
3.1.2. Diversity in Quantitative Traits
3.2. Correlation Analysis
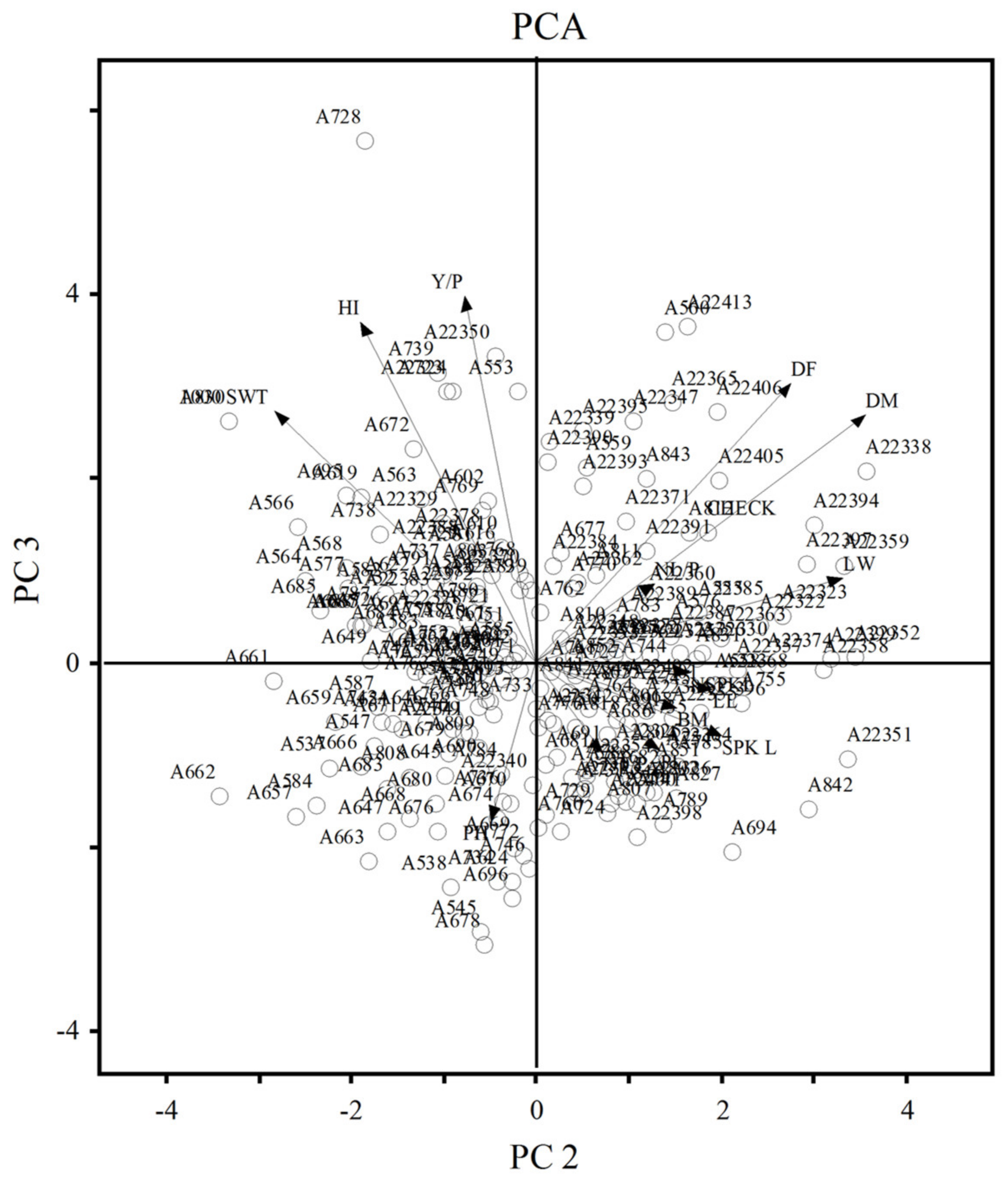
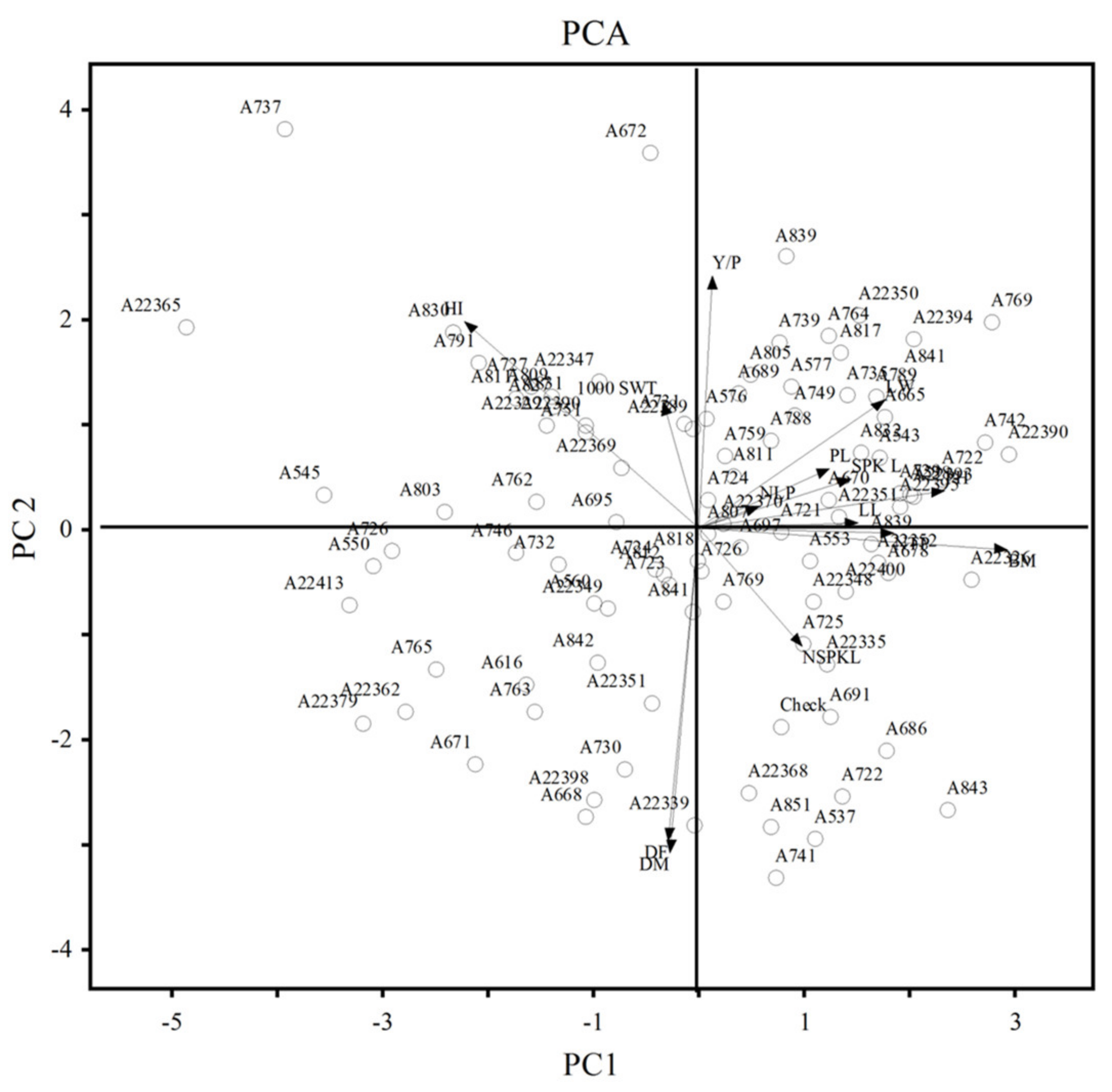
3.3. Principal Component Analysis (PCA)
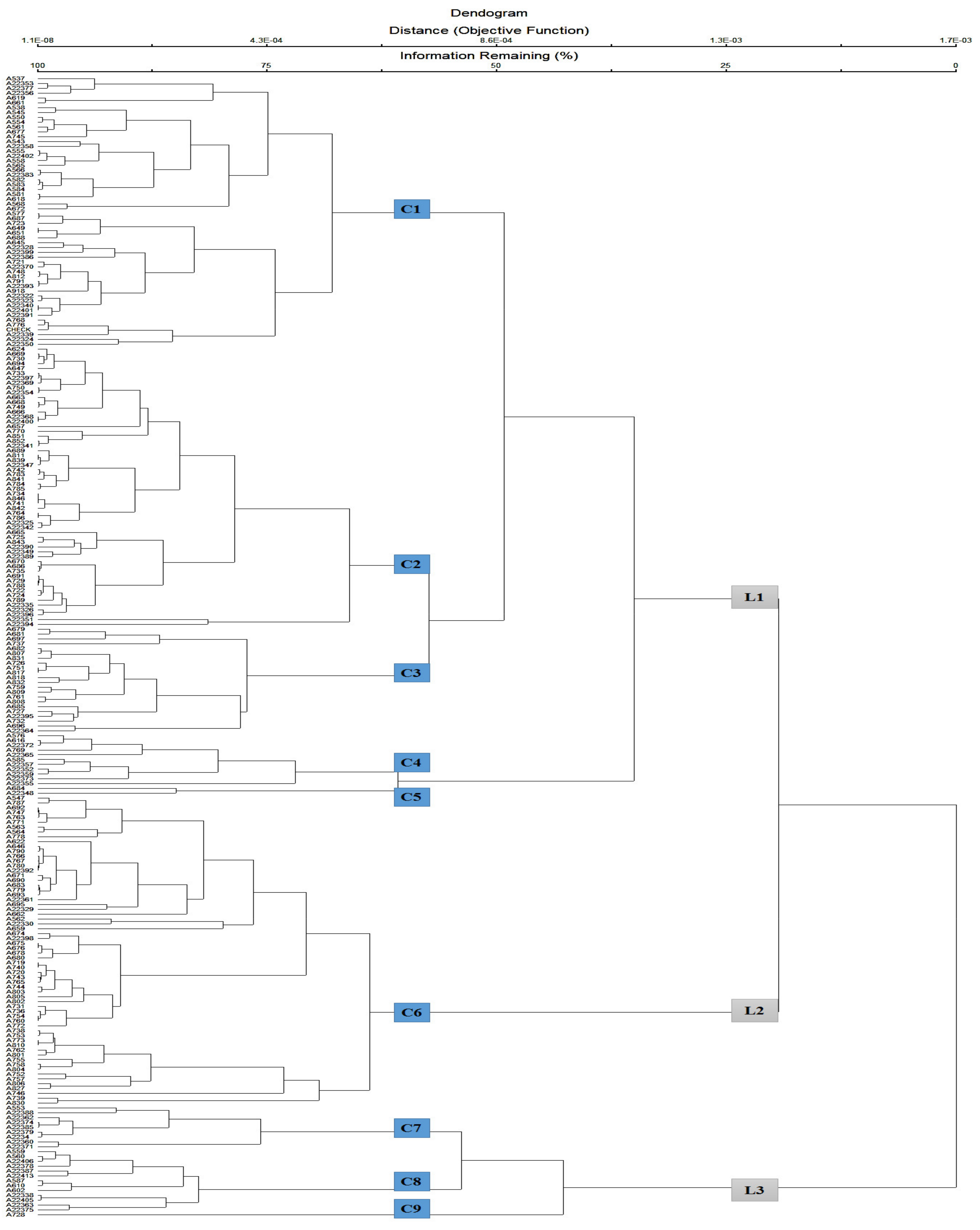
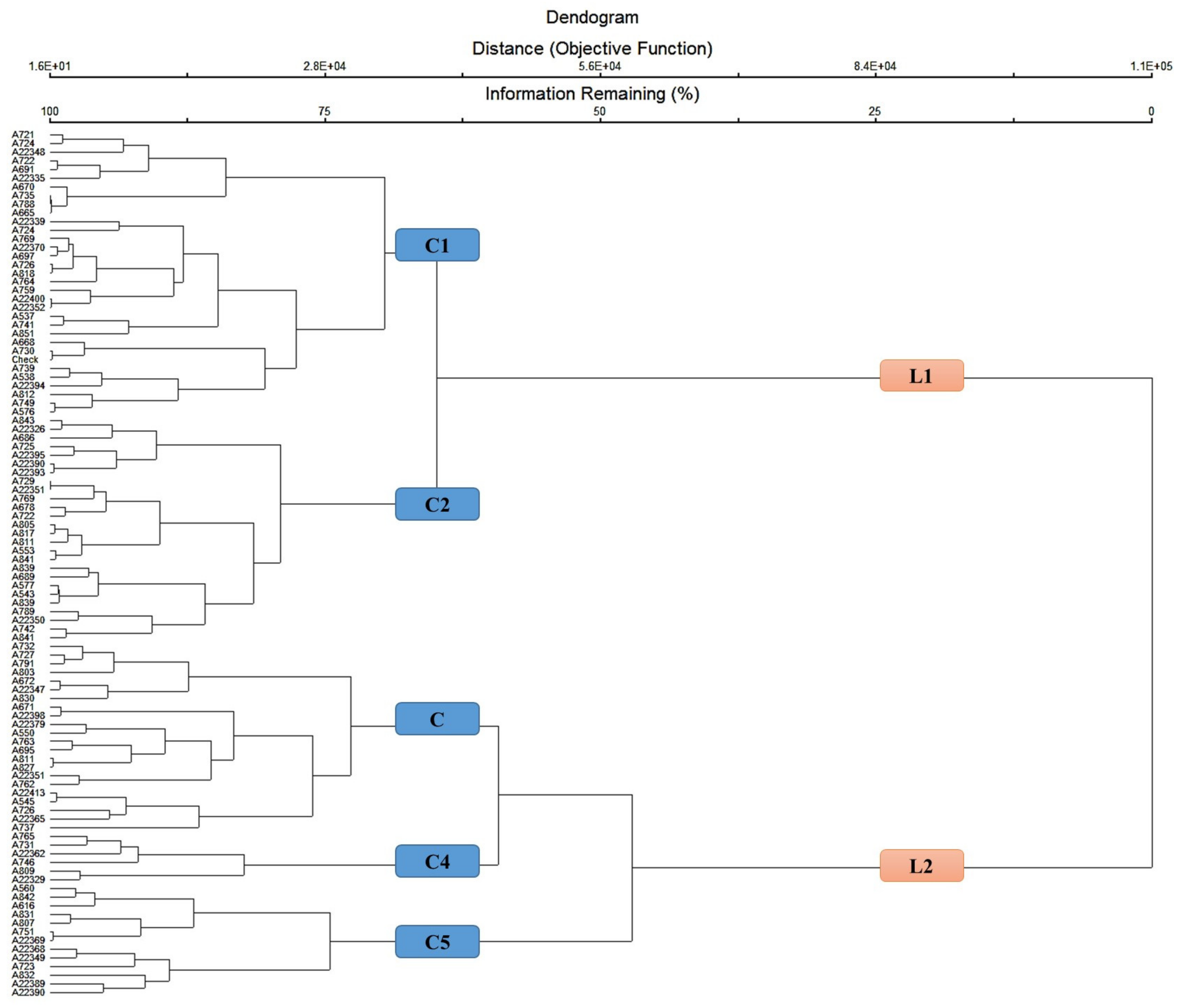
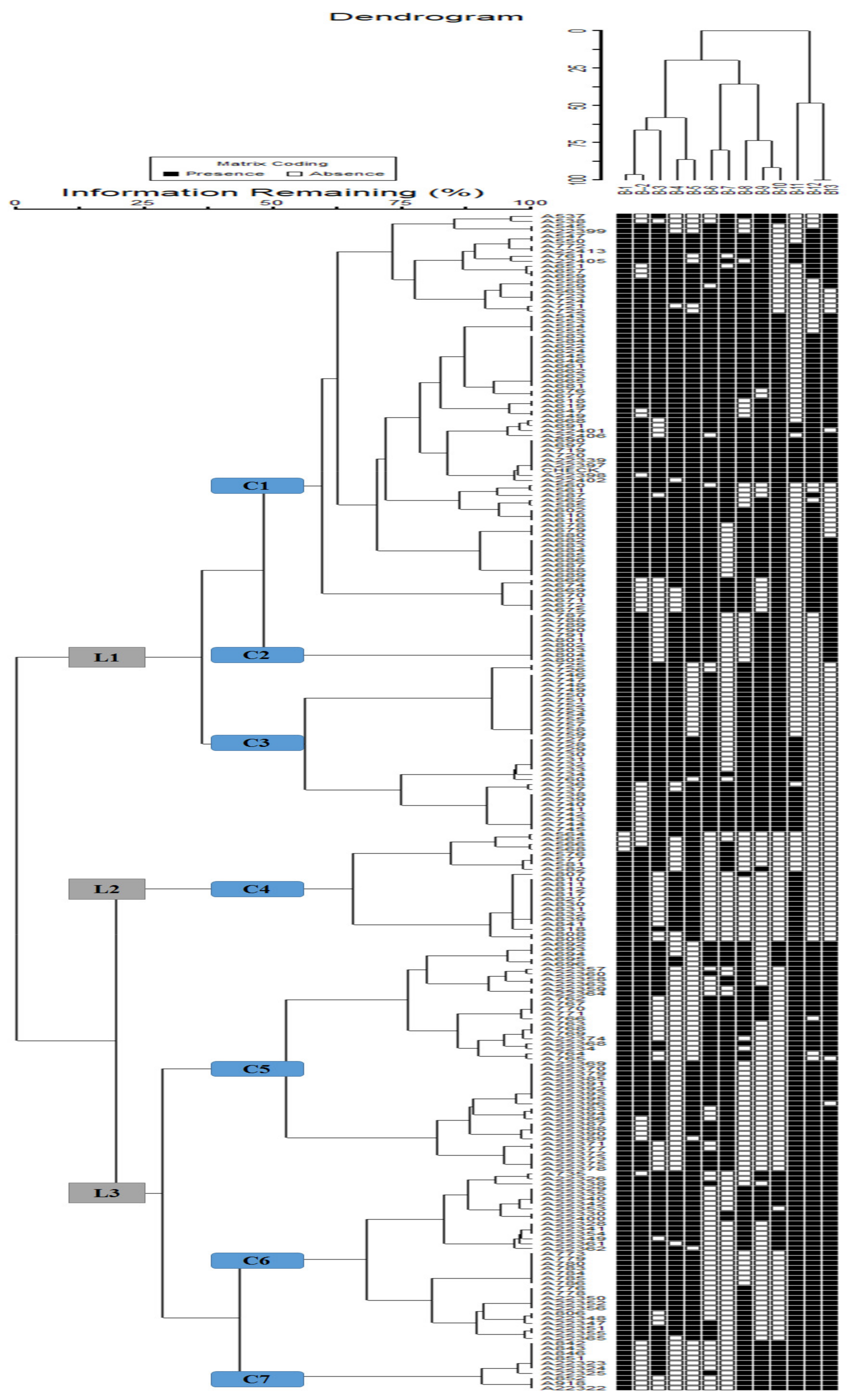
3.4. Cluster Analysis
3.5. Total Seed Storage Protein
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boczkowska, M.; Nowosielski, J.; Nowosielska, D.; Podyma, W. Assessing genetic diversity in 23 early Polish oat cultivars based on molecular and morphological studies. Genet. Resour. Crop. Evol. 2014, 61, 927–941. [Google Scholar] [CrossRef][Green Version]
- FAOSTAT, F. FAOSTAT. 20 December. Available online: http://faostat3.fao.Org/home (accessed on 1 December 2015).
- Sterna, V.; Zute, S.; Brunava, L. Oat grain composition and its nutrition benefice. Agric. Agric. Sci. Procedia. 2016, 8, 252–256. [Google Scholar] [CrossRef]
- Rana, M.; Gupta, S.; Kumar, N.; Ranjan, R.; Sah, R.P.; Gajghate, R.; Dwivedi, K.K.; Ahmed, S. Genetic architecture and population structure of Oat Landraces (Avena sativa L.) using molecular and morphological descriptors. J. Tradit. Knowl. 2019, 18, 439–450. [Google Scholar]
- Al-Ajmi, M.H.; AL-Refai, S.I. Effect of early planting dates on the growth and yield of two varieties of oat (Avena sativa L.). Plant. Arch. 2020, 20, 124–127. [Google Scholar]
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Oats from farm to fork. Adv. Food Nutr. Res. 2016, 77, 1–55. [Google Scholar]
- Sarantis, S.D.; Eren, N.M.; Kowalcyk, B.; Jimenez–Flores, R.; Alvarez, V.B. Thermodynamic interactions of micellar casein and oat β-glucan in a model food system. Food Hydrocoll. 2021, 115, 106559. [Google Scholar] [CrossRef]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods-a review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the evaluation of allergenic foods and food ingredients for labelling purposes. EFSA J. 2014, 12, 3894. [Google Scholar]
- Li, X.; Cai, X.; Ma, X.; Jing, L.; Gu, J.; Bao, L.; Li, J.; Xu, M.; Zhang, Z.; Li, Y. Short-and long-term effects of wholegrain oat intake on weight management and glucolipid metabolism in overweight type-2 diabetics: A randomized control trial. Nutrients 2016, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Arabzai, M.G.; Gul, H. Application Techniques of Molecular Marker and Achievement of Marker Assisted Selection (MAS) in Three Major Crops Rice, Wheat and Maize. IJASBT 2021, 8, 82–93. [Google Scholar]
- Sohail, S.; Ansar, M.; Skalicky, M.; Wasaya, A.; Soufan, W.; Ahmad, Y.T.; El-Shehawi, A.M.; Brestic, M.; Sohidul, I.M.; Ali, R.M.; et al. Influence of Tillage Systems and Cereals–Legume Mixture on Fodder Yield, Quality and Net Returns under Rainfed Conditions. Sustainability 2021, 13, 2172. [Google Scholar] [CrossRef]
- Niazi, I.A.; Akhtar, S.; Kohli, S.; Naveed, A.; Rauf, S.; Shehzad, M. Oat (Avena sativa L.) advanced lines outperform existing cultivars for forage yield and its components under terminal heat stress. Pakistan J. Agric. Sci. 2020, 57, 327–331. [Google Scholar]
- Muhammad, U.H.; Gurmani, Z.A.; Khan, S.; Bakhsh, A. PARC Oat: A new Lodging-resistant, Late-maturing and High-yielding Variety of Oats (Avena sativa L.) for Pakistan. Pak. J. Agric. Sci. 2020, 33, 601. [Google Scholar]
- Kaur, R.; Kapoor, R.; Vikal, Y.; Kaur, K. Assessing genetic diversity in dual purpose oat (Avena sativa L.) cultivars based on morphological and quality traits. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1574–1586. [Google Scholar] [CrossRef][Green Version]
- Canales, F.J.; Montilla-Bascón, G.; Bekele, W.A.; Howarth, C.J.; Langdon, T.; Rispail, N.; Tinker, N.A.; Prats, E. Population genomics of Mediterranean oat (Asativa) reveals high genetic diversity and three loci for heading date. Theor. Appl. Genet. 2021, 26, 1–5. [Google Scholar]
- Ruwali, Y.; Verma, J.S.; Kumar, L. Comparative genetic diversity analysis of oat (Avena sativa L.) by microsatellite markers and morphological rainfed expressions. Afr. J. Biotechnol. 2013, 12, 3414–3424. [Google Scholar]
- Jan, M.; Nisar, M.; Ihsan, M.; Farhatullah; Jan, G.; Hanci, F. Genetic diversity in Pakistani soybean genotypes and North American ancestral lines using agro morphological and rapid markers. Fresenius Environ. Bull. 2019, 28, 2927–2936. [Google Scholar]
- Ghafoor, A.; Ahmad, Z.; Qureshi, A.S.; Bashir, M. Genetic relationship in Vigna mungo (L.) Hepper and V. radiata (L.) R. Wilczek based on morphological traits and SDS-PAGE. Euphytica 2002, 123, 367–378. [Google Scholar] [CrossRef]
- Nisar, M.; Ghafoor, A.; Khan, M.R.; Subhan, M. Genetic similarity of Pakistani pea (Pisum sativum L.) germplasm with world collection using cluster analysis and jaccard’s similarity index. J. Chem. Soc. Pak. 2009, 31, 138–144. [Google Scholar]
- Achleitner, A.; Tinker, N.A.; Zechner, E.; Buerstmayr, H. Genetic diversity among oat varieties of worldwide origin and associations of AFLP markers with quantitative traits. Theor. Appl. Genet. 2008, 117, 1041–1053. [Google Scholar] [CrossRef]
- Ahmed, S.; Roy, A.K.; Majumdar, A.B. Genetic diversity and variability analysis in oat (Avena sativa L.). Range Mgmt. Agrofor. 2011, 32, 96–99. [Google Scholar]
- Kumar, V.; Lade, S.; Yadav, H.K. Evaluation of genetic diversity in Lepidium sativum L. germplasm based on multivariate analysis. Genet. Resour. Crop. Evol. 2021, 68, 809–820. [Google Scholar] [CrossRef]
- Nikoloudakis, N.; Bladenopoulos, K.; Katsiotis, A. Structural patterns and genetic diversity among oat (Avena) landraces assessed by microsatellite markers and morphological analysis. Genet. Resour. Crop. Evol. 2016, 63, 801–811. [Google Scholar] [CrossRef]
- Gupta, K.; Mehta, A.K. Genetic variability studies of advanced generation mutant oat (Avena sativa L.) lines for yield, fodder traits and proline content. Range Mange. Agro. For. 2020, 41, 52–59. [Google Scholar]
- Wagh, V.R.; Sonone, A.H.; Damame, S.V. Assessment of genetic variability correlation and path coefficient analysis in forage oat (Avena sativa L.). Forage Res. 2018, 44, 172–175. [Google Scholar]
- Naeem, M.; Chohan, M.S.M.; Khan, A.H.; Kainth, R.A. Green fodder yield performance of oats varieties under irrigated conditions. J. Agric. Res. 2006, 44, 197–201. [Google Scholar]
- Naeem, M.; Kainth, R.A.; Chohan, M.S.M.; Khan, A.H. Study on Fodder Yield Potential of Different Oats Varieties under Irrigated Conditions. Pak. J. Agric. Sci. 2005, 43, 27–31. [Google Scholar]
- Bibi, A.; Shahzad, A.N.; Sadaqat, H.A.; Tahir, M.H.N.; Fatima, B. Genetic characterization and inheritance studies of oats (Avena sativa L.) for green fodder yield. IJBPAS 2012, 1, 450–460. [Google Scholar]
- Singh, A.; Vyas, R.P.; Kumar, S.; Singh, H.C.; Deep, A.; Malik, P.; Singh, A. Genetic variability and correlation of seed yield and related characters in oat (Avena sativa L.). IJCS 2018, 6, 1532–1537. [Google Scholar]
- Ņečajeva, J.; Bleidere, M.; Jansone, Z.; Gailīte, A.; Ruņģis, D. Variability of Seed Germination and Dormancy Characteristics and Genetic Analysis of Latvian Avena fatua Populations. Plants 2021, 10, 235. [Google Scholar] [CrossRef]
- Ahmad, M.; Zaffar, G.; Dar, Z.A.; Habib, M.A. review on Oat (Avena sativa L.) as a dual-purpose crop. Sci. Res. Ess. 2014, 9, 52–59. [Google Scholar]
- Amanullah, P.S.; Zada, K.; Perveen, S. Growth characters and productivity of oat varieties at Peshawar. Sarhad J. Agric. 2004, 20, 5–10. [Google Scholar]
- Ayub, M.; Shehzad, M.; Nadeem, M.A.; Pervez, M.; Naeem, M.; Sarwar, N. Comparative study on forage yield and quality of different oat (Avena sativa L.) varieties under agro-ecological conditions of Faisalabad, Pakistan. Afr. J. Agric. Res. 2011, 6, 3388–3391. [Google Scholar]
- Sheikhehpour, S.; Bahraminejad, S.; Cheghamirza, K. Morphological and molecular genetic variations of oat genotypes grown in Kermanshah. Iran. Mol. Biol. Res. 2014, 41, 4023–4030. [Google Scholar] [CrossRef]
- Chakraborty, J.A.Y.E.E.T.A.; Arora, R.N.; Joshi, U.N.; Chhabra, A.K. Evaluation of Avena species for yield, quality attributes and disease reaction. Forage Res. 2014, 39, 179–184. [Google Scholar]
- Sumathi, S.; Balamurugan, P. Usefulness of morphological characters for varietal identification in oats (Avena sativa L.). Int. J. Plant. Sci. 2014, 9, 7–12. [Google Scholar]
- Ahmed, S.; Roy, A.K.; Majumdar, A.B. Correlation and Path Coefficient Analysis for Fodder and Grain Yield Related. Ann. Biol. 2013, 29, 75–78. [Google Scholar]
- Sahu, M.; Tiwari, A. Genetic Variability and Association Analysis of Oat (Avena sativa L.) Genotypes for Green Forage Yield and Other Components. Curr. J. Appl. Sci. Tech. 2020, 39, 133–141. [Google Scholar] [CrossRef]
- Poonia, A.T.M.A.N.; Phogat, D.S.; Pahuja, S.K.; Bhuker, A.X.A.Y.; Khatri, R.S. Variability, Character Association and Path Coefficient Analysis in fodder Oat for yield and quality traits. Forage Res. 2017, 43, 239–243. [Google Scholar]
- Kadam, S.S.; Solanki, N.S.; Arif, M.; Dashora, L.N.; Mundra, S.L.; Upadhyay, B. Productivity and quality of fodder oats (Avena sativa L.) as influenced by sowing time, cutting schedules and nitrogen levels. Indian J. Anim. Nutr. 2019, 36, 179–186. [Google Scholar] [CrossRef]
- Nisar, M.; Wadood, S.F.; Iqbal, A.; Nausheen, A.; Ghafoor, A. Intra and inter specific profiling of Pakistani Quercus species growing in the hilly areas of District Dir Khyber Pakhtunkhwa. Pak. J. Bot 2016, 48, 263–270. [Google Scholar]
- Ghafoor, A.; Ahmad, Z. Diversity of agronomic traits and total seed protein in black gram Vigna mungo (L.) Hepper. Acta Biol. Crac. Ser. Bot 2005, 47, 69–75. [Google Scholar]
- Mirza, B.; Shoaib, M.; Ahmad, M.; Fu, Y.B. Genetic diversity in Pakistani population of Avena fatua revealed by seed storage protein polymorphism. Commun. Biometry Crop. Sci. 2007, 2, 41–48. [Google Scholar]
- Tahir, N.A.R. Comparison of RAPD–PCR and SDS–PAGE techniques to evaluate genetic variation among nine barley varieties (Hordeum spp). Malays. Appl. Biol. 2014, 43, 109–119. [Google Scholar]
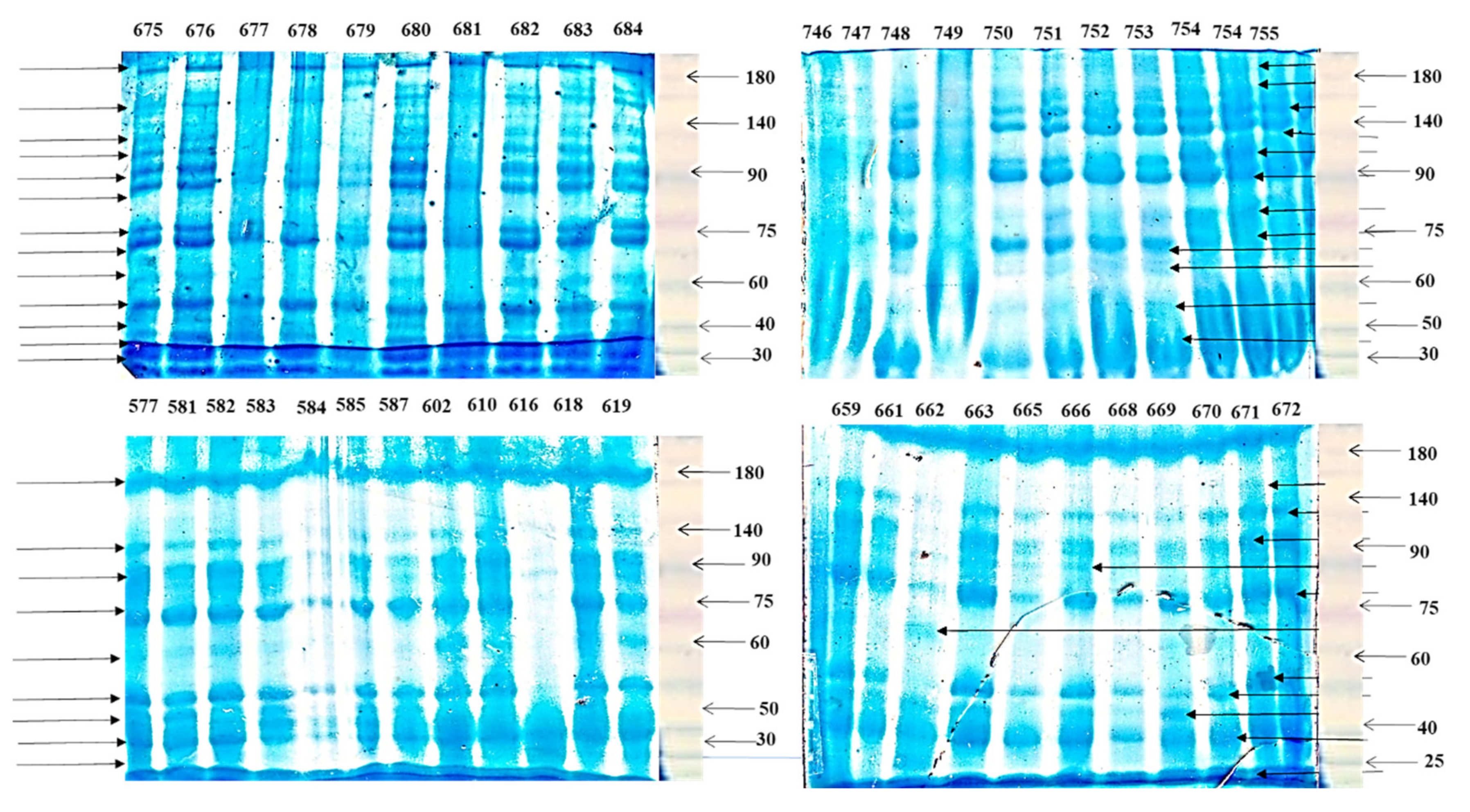
| Traits | Year | Range | Mean ± SD | CV % |
|---|---|---|---|---|
| Days to Flowering | 2019 | 142–167 | 152.56 ± 4.67 | 3.06 |
| 2020 | 144–165 | 150.55 ± 4.54 | 3.02 | |
| Plant Height (cm) | 2019 | 59.6–145.2 | 106.81 ± 18.43 | 17.25 |
| 2020 | 69.2–140.0 | 108.99 ± 19.90 | 18.26 | |
| Number of Tiller/Plant | 2019 | 3.4–14.2 | 7.42 ± 1.79 | 24.13 |
| 2020 | 4.2–11 | 7.245 ± 1.46 | 20.18 | |
| Number of Leaves/Plant | 2019 | 4.1–10 | 6.51 ± 1.30 | 19.95 |
| 2020 | 4.2–15.2 | 7.95 ± 2.01 | 25.29 | |
| Leaf Length (cm) | 2019 | 17.3–49.8 | 33.102 ± 6.10 | 18.43 |
| 2020 | 20.0–48.0 | 33.63 ± 7.18 | 21.34 | |
| Leaf Width (cm) | 2019 | 0.8–2.4 | 1.72 ± 0.27 | 15.71 |
| 2020 | 0.94–2.48 | 1.74 ± 0.31 | 17.88 | |
| Peduncle Length (cm) | 2019 | 12.4–23.6 | 17.8 6± 2.14 | 11.99 |
| 2020 | 12.8–30 | 19.34 ± 2.97 | 15.35 | |
| Spike Length (cm) | 2019 | 14.8–25.4 | 19.96 ± 2.22 | 11.13 |
| 2020 | 14.0–28.8 | 21.83 ± 3.56 | 16.31 | |
| Number of Spikelet | 2019 | 4.6–15 | 8.55 ± 1.71 | 20.00 |
| 2020 | 6–14.2 | 9 ± 1.95 | 21.72 | |
| Plant Biomass | 2019 | 51.6–145 | 95.49 ± 5.17 | 23.43 |
| 2020 | 61.2–146 | 111.59 ± 22.52 | 20.17 | |
| Days to Maturity | 2019 | 172–196 | 182.29 ± 5.04 | 2.76 |
| 2020 | 174–188 | 179.8 ± 4.60 | 2.56 | |
| Yield/Plant | 2019 | 8.8–38.2 | 20.78 ± 5.17 | 24.85 |
| 2020 | 12–38.3 | 24.844 ± 5.52 | 21.79 | |
| 1000 Seed weight | 2019 | 15.4–37.2 | 20.78 ± 4.93 | 19.89 |
| 2020 | 15.0–31.8 | 25.18 ± 4.15 | 16.48 | |
| Harvest Index | 2019 | 10.11–50.82 | 20.78 ± 6.69 | 29.50 |
| 2020 | 10.11–54.56 | 23.18 ± 7.12 | 30.73 |
| Traits | Year | DF | DM | NTP | NLP | PH | NSPKL | PL | SPKL | LL | LW | BM | YP | HI | TSWT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 2019 | 0.52 ** | |||||||||||||
| DM | 2020 | 0.86 ** | |||||||||||||
| NTP | 2019 | −0.23 ** | −0.23 ** | ||||||||||||
| NTP | 2020 | 0.07 | 0.09 | ||||||||||||
| NLP | 2019 | 0.07 | −0.01 | 0.30 ** | |||||||||||
| NLP | 2020 | 0.11 | 0.09 | 0.20 * | |||||||||||
| PH | 2019 | −0.36 ** | −0.39 ** | 0.50 ** | 0.12 * | ||||||||||
| PH | 2020 | −0.011 | −0.13 | 0.25 * | 0.09 | ||||||||||
| NSPKL | 2019 | −0.014 * | 0.05 | 0.20 ** | 0.11 * | 0.21 ** | |||||||||
| NSPKL | 2020 | 0.05 | 0.07 | 0.20 * | −0.06 | −0.01 | |||||||||
| PL | 2019 | 0.02 | −0.03 | 0.07 | 0.04 | 0.05 | 0.03 | ||||||||
| PL | 2020 | −0.08 | −0.10 | 0.03 | −0.07 | 0.107 | 0.06 | ||||||||
| SPKL | 2019 | −0.01 | 0.07 | 0.22 ** | 0.05 | 0.34 ** | 0.19 ** | 0.35 ** | |||||||
| SPKL | 2020 | −0.02 | −0.03 | 0.15 | −0.05 | 0.21 * | 0.08 | 0.22 * | |||||||
| LL | 2019 | −0.13 * | −0.03 | 0.46 ** | 0.20 ** | 0.46 ** | 0.32 ** | −0.01 | 0.20 ** | ||||||
| LL | 2020 | 0.02 | −0.06 | 0.09 | 0.05 | 0.06 | 0.10 | 0.18 | 0.12 | ||||||
| LW | 2019 | 0.15 * | 0.20 ** | 0.16 ** | 0.12 * | 0.06 | 0.15 ** | −0.02 | 0.09 | 0.28 ** | |||||
| LW | 2020 | −0.16 | −0.09 | 0.29 ** | 0.15 | 0.26 ** | 0.09 | 0.18 | 0.24 * | 0.011 | |||||
| BM | 2019 | 0.07 | 0.08 | 0.09 | 0.05 | 0.04 | −0.04 | 0.07 | 0.04 | 0.11 * | −0.02 | ||||
| BM | 2020 | −0.01 | −0.03 | 0.38 ** | 0.15 | 0.52 ** | 0.13 | 0.15 | 0.19 | 0.39 ** | 0.27 ** | ||||
| YP | 2019 | 0.01 | 0.03 | 0.21 ** | 0.14 * | 0.20 ** | 0.12 * | 0.01 | 0.10 * | 0.21 ** | 0.06 | 0.28 ** | |||
| YP | 2020 | −0.09 | −0.17 | 0.20 * | 0.24 * | 0.01 | −0.16 | −0.06 | 0.11 | 0.09 | 0.24 * | 0.15 | |||
| HI | 2019 | −0.06 | −0.04 | 0.12 * | 0.08 | 0.15 ** | 0.012 * | −0.07 | 0.03 | 0.10 | 0.09 | −0.58 ** | 0.57 ** | ||
| HI | 2020 | −0.06 | −0.11 | −0.15 | 0.02 | −0.40 ** | −0.21 * | −0.13 | −0.09 | −0.21 * | −0.04 | −0.64 ** | 0.62 ** | ||
| TSWT | 2019 | −0.04 | −0.17 ** | 0.06 | 0.04 | 0.11 * | −0.07 | −0.03 | −0.03 | 0.01 | −0.23 ** | 0.17 ** | 0.53 ** | 0.28 ** | |
| TSWT | 2020 | −0.08 | −0.06 | −0.01 | 0.05 | −0.09 | −0.20 * | 0.06 | −0.06 | 0.13 | −0.03 | −0.06 | 0.16 | 0.16 |
| PCA 2019 | PCA 2020 | |||||||
|---|---|---|---|---|---|---|---|---|
| Traits | PC1 | PC2 | PC3 | PC4 | PC1 | PC2 | PC3 | PC4 |
| DF | 0.23 | 0.33 | 0.40 | −0.09 | −0.05 | 0.52 | 0.39 | 0.04 |
| DM | 0.20 | 0.43 | 0.38 | 0.01 | −0.05 | 0.54 | 0.37 | 0.02 |
| NT/P | −0.43 | 0.10 | −0.13 | −0.03 | 0.32 | 0.01 | 0.31 | −0.20 |
| NL/P | −0.20 | 0.16 | 0.12 | −0.03 | 0.10 | −0.04 | 0.43 | 0.14 |
| PH | −0.45 | −0.04 | −0.23 | −0.01 | 0.41 | −0.06 | −0.07 | 0.14 |
| NSPKL | −0.25 | 0.21 | 0.00 | 0.20 | 0.25 | −0.08 | 0.03 | −0.30 |
| PL | −0.07 | 0.19 | −0.10 | −0.17 | 0.22 | −0.10 | −0.13 | 0.02 |
| SPK L | −0.23 | 0.28 | −0.07 | −0.05 | 0.31 | −0.21 | 0.17 | −0.29 |
| LL | −0.39 | 0.25 | −0.02 | 0.03 | 0.26 | −0.01 | 0.07 | 0.34 |
| LW | −0.12 | 0.41 | 0.14 | 0.25 | 0.17 | 0.19 | −0.10 | −0.47 |
| BM | −0.03 | 0.20 | −0.05 | −0.71 | 0.02 | −0.42 | 0.49 | −0.08 |
| Y/P | −0.31 | −0.13 | 0.50 | −0.25 | 0.51 | 0.04 | 0.04 | 0.20 |
| HI | −0.24 | −0.28 | 0.46 | 0.40 | −0.39 | −0.34 | 0.33 | −0.21 |
| 1000 Seed weight | −0.17 | −0.38 | 0.32 | −0.37 | −0.06 | −0.21 | 0.14 | 0.56 |
| % of Variance | 20.66 | 13.14 | 12.55 | 11.25 | 19.83 | 15.39 | 12.55 | 8.66 |
| Cum. % of Variance | 20.66 | 33.80 | 46.35 | 57.60 | 19.83 | 35.22 | 47.77 | 56.43 |
| Traits ± SD Cluster | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | Cluster 5 | Cluster 6 | Cluster 7 | Cluster 8 | Cluster 9 |
|---|---|---|---|---|---|---|---|---|---|
| DF | 153.7 ± 4.3 | 151.7 ± 3.6 | 150.9 ± 3.3 | 152.3 ± 4.2 | 151.1 ± 3.6 | 150.9 ± 3.7 | 153 ± 5.2 | 158.7 ± 5.8 | 165 ± 2 |
| DM | 182.7 ± 4.6 | 181.3 ± 3.7 | 180.9 ± 3.9 | 182.8 ± 5.4 | 181.3 ± 4 | 180.5 ± 3.5 | 189.4 ± 3.4 | 188.3 ± 5.6 | 181 ± 2 |
| NT/P | 6.6 ± 1.4 | 7.9 ± 1.5 | 8.02 ± 1.5 | 7.2 ± 1.5 | 7.6 ± 1.3 | 7.8 ± 1.5 | 6.5 ± 1.3 | 5.9 ± 1.2 | 8.2 ± 0.2 |
| NL/P | 6.2 ± 1.1 | 6.6 ± 1.3 | 6.5 ± 1.1 | 6.5 ± 1.2 | 6.5 ± 1.2 | 6.6 ± 1.3 | 6 ± 1.01 | 6.3 ± 1.1 | 7.8 ± 0.2 |
| PH | 96.7 ± 10.1 | 110.8 ± 9.3 | 115.7 ± 12.1 | 104.5 ± 15.8 | 109.7 ± 10.6 | 116.5 ± 12 | 83.2 ± 5.8 | 74.2 ± 7.6 | 95.8 ± 0.2 |
| NSPKL | 7.8 ± 1.6 | 8.5 ± 1.7 | 8.8 ± 1.6 | 8.6 ± 1.6 | 8.7 ± 1.6 | 8.8 ± 1.6 | 9.6 ± 2 | 8.2 ± 1.5 | 7 ± 0.2 |
| PL | 17.5 ± 2.4 | 18.1 ± 2.1 | 18.2 ± 2.2 | 18.2 ± 2.2 | 18.1 ± 2.1 | 18 ± 1 | 16.6 ± 2.5 | 18.1 ± 1.5 | 16.8 ± 0.2 |
| SPK L | 19.3 ± 2.1 | 20.1 ± 2.01 | 20.5 ± 1.9 | 19.9 ± 2.2 | 20 ± 2 | 20.3 ± 2 | 20.3 ± 1.7 | 18.9 ± 2 | 18 ± 0.2 |
| LL | 31.4 ± 6.5 | 34.4 ± 5.2 | 35.03 ± 1.8 | 32.6 ± 5.78 | 34 ± 5.2 | 34.3 ± 4.9 | 32 ± 5.9 | 27.1 ± 4.6 | 29.4 ± 0.2 |
| LW | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.26 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 28.3 | 1.9 ± 0.2 | 1.8 ± 0.23 | 1.6 ± 1.2 |
| BM | 92.7 ± 12.6 | 122.5 ± 9.5 | 116 ± 8.3 | 117.5 ± 7.7 | 119.5 ± 8.5 | 91.5 ± 23.8 | 61.1 ± 4.7 | 83 ± 10 | 78 ± 2 |
| YP | 20.7 ± 5.6 | 21.2 ± 5.4 | 22.1 ± 5.7 | 21.1 ± 5.6 | 21.7 ± 5.7 | 20.6 ± 5.1 | 17.9 ± 2.6 | 19.5 ± 4.6 | 38.2 ± 0.2 |
| HI | 22.3 ± 4.8 | 17.3 ± 4.1 | 19 ± 4.5 | 17.9 ± 4.1 | 18.1 ± 4.1 | 23.7 ± 7.6 | 29.4 ± 5 | 23.5 ± 4.6 | 48.9 ± 0.7 |
| TSWT | 25.3 ± 5.2 | 25 ± 4.8 | 26.1 ± 5.1 | 24.8 ± 5.1 | 25.7 ± 5 | 24.6 ± 4.8 | 21.6 ± 3 | 23.8 ± 4.1 | 35 ± 0.2 |
| Traits | CL1 | CL2 | CL3 | CL4 | CL5 |
|---|---|---|---|---|---|
| DF | 150.6 ± 4.9 | 150.7 ± 4.6 | 150.7 ± 4 | 150 ± 3.8 | 151.1 ± 5.1 |
| DM | 180.2 ± 4.7 | 180 ± 4.52 | 180 ± 5.1 | 179.3 ± 4.6 | 179.7 ± 5.4 |
| NTP | 7 ± 1.3 | 7.44 ± 1.4 | 6.6 ± 1.5 | 6.4 ± 1.1 | 6.8 ± 1.4 |
| NLP | 8 ± 1.7 | 8.48 ± 2.5 | 7.6 ± 2.1 | 7.2 ± 2 | 7.8 ± 1.6 |
| PH | 110.8 ± 11.9 | 126.19 ± 144.27 | 87.9 ± 8.9 | 96.2 ± 20.9 | 83 ± 5.2 |
| NSPKL | 9.3 ± 1.9 | 8.97 ± 2 | 8.7 ± 1.5 | 8.1 ± 1 | 9 ± 2.1 |
| PL | 18.9 ± 2.6 | 20.9 ± 3.6 | 18.2 ± 2.6 | 18 ± 3.2 | 19.4 ± 2.3 |
| SPKL | 20.8 ± 13 | 23.83 ± 2.8 | 21.6 ± 3.5 | 20.3 ± 4 | 20.9 ± 4 |
| LL | 35.9 ± 6.2 | 35.37 ± 7.2 | 30.4 ± 6.6 | 30.2 ± 6.6 | 35.9 ± 7.1 |
| LW | 1.7 ± 0.2 | 1.9 ± 3.1 | 1.6 ± .3.6 | 1.5 ± .4 | 1.6 ± .2.4 |
| BM | 123.6 ± 12.9 | 125.9 ± 11.8 | 79.4 ± .9 | 76.5 ± 7.4 | 115.2 ± 12.4 |
| YP | 23.3 ± 0.3 | 26.3 ± 6.24 | 25.3 ± 6.5 | 25 ± 6.5 | 25.8 ± 4.3 |
| HI | 19.1 ± 4.8 | 20.9 ± 4.4 | 32. ± 8.1 | 33.2 ± 10.1 | 22.4 ± 3.4 |
| TSWT | 24.9 ± 4.4 | 24.7 ± 3.8 | 25.2. ± 4.4 | 26.6 ± 2.9 | 25.2 ± 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ihsan, M.; Nazir, N.; Ghafoor, A.; Khalil, A.A.K.; Zahoor, M.; Nisar, M.; Khames, A.; Ullah, R.; Shah, A.B. Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis. Agronomy 2021, 11, 1713. https://doi.org/10.3390/agronomy11091713
Ihsan M, Nazir N, Ghafoor A, Khalil AAK, Zahoor M, Nisar M, Khames A, Ullah R, Shah AB. Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis. Agronomy. 2021; 11(9):1713. https://doi.org/10.3390/agronomy11091713
Chicago/Turabian StyleIhsan, Mohammad, Nausheen Nazir, Abdul Ghafoor, Atif Ali Khan Khalil, Muhammad Zahoor, Mohammad Nisar, Ahmed Khames, Riaz Ullah, and Abdul Bari Shah. 2021. "Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis" Agronomy 11, no. 9: 1713. https://doi.org/10.3390/agronomy11091713
APA StyleIhsan, M., Nazir, N., Ghafoor, A., Khalil, A. A. K., Zahoor, M., Nisar, M., Khames, A., Ullah, R., & Shah, A. B. (2021). Genetic Diversity in Local and Exotic Avena sativa L. (Oat) Germplasm Using Multivariate Analysis. Agronomy, 11(9), 1713. https://doi.org/10.3390/agronomy11091713







