Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Soil Sampling and Treatment
2.3. Monitoring of the Physicochemical Properties of the Soil
2.4. Biochemical Analyses
2.5. Microbiological Analyses
2.6. Statistical Analysis
2.7. Molecular Docking Study
3. Results
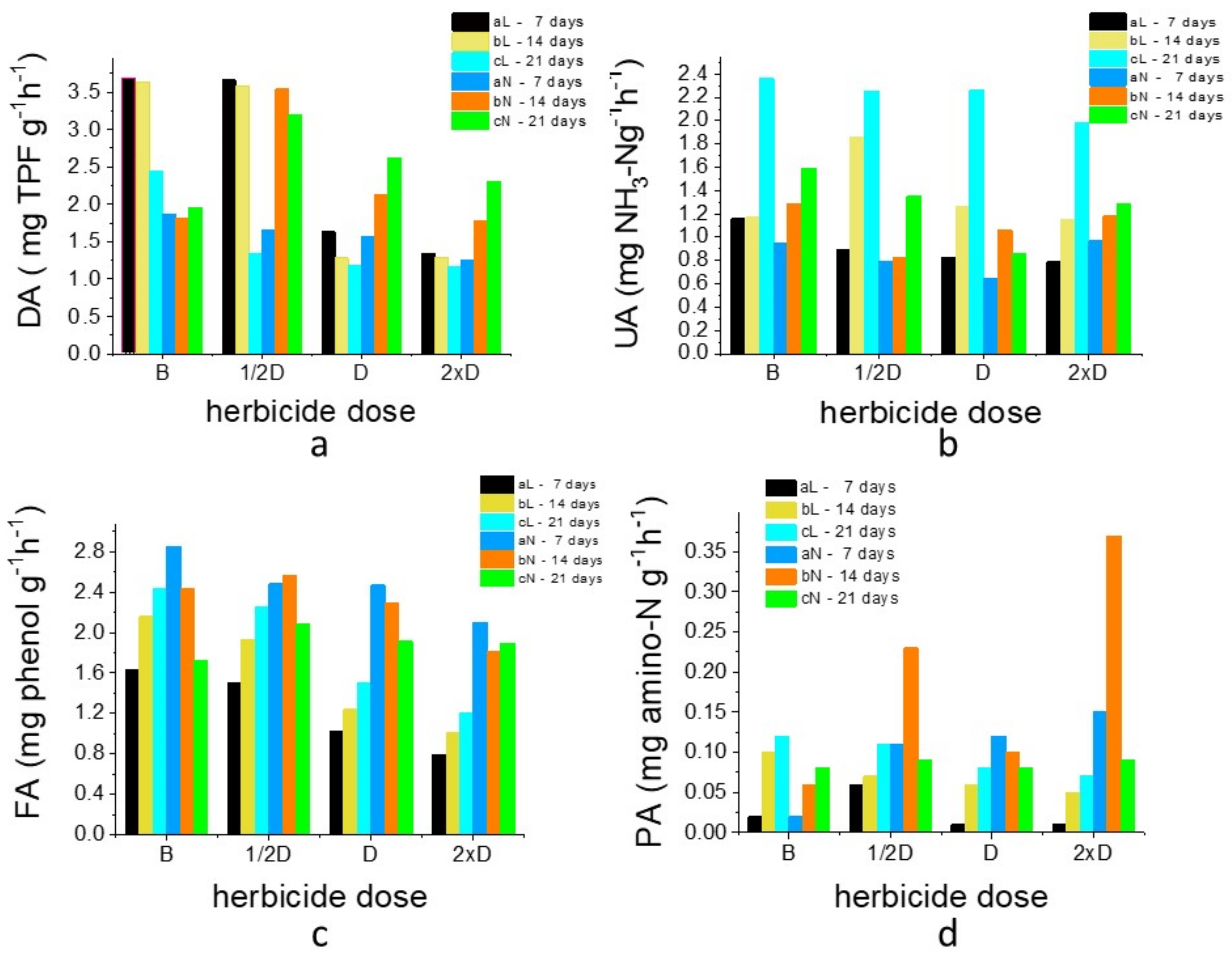
3.1. Assessment of the Activities of Some Enzymes Found in Soil
3.2. Correlation between the Activities of Enzymes Found in Soil for all the Experimental Variants
3.3. Correlation between the Activities of Enzymes Found in Soil and Physicochemical Properties of the Soil
3.4. Microbiological Analyses
3.5. Correlation between the Population of the Soil Microorganism and Applied Doses of Oxyfluorfen
3.6. Correlation between the Population of the Soil Microorganism and Physicochemical Properties of the Soil
3.7. Molecular Docking Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Utobo, E.B.; Tewari, L. Soil enzymes as bioindicators of soil ecosystem status. Appl. Ecol. Environ. Res. 2015, 13, 147–169. [Google Scholar]
- Cao, P.; Hall, E.; Zhang, E. Soil sampling sensor system on a mobile robot. In Proceedings of the SPIE Intelligent Robots and Computer Vision XXI: Algorithms, Techniques and Active Vision, Providence, RI, USA, 27–31 October 2003; Volume 5267, pp. 304–310. [Google Scholar]
- Zaid, A.M.; Mayouf, M.; Farouj, Y.S. The effects of post-emergence herbicides on soil microflora and nitrogen fixing bacteria in Pea field. Int. J. Chem. Environ. Biol. Sci. 2014, 2, 40–42. [Google Scholar]
- Sapundjieva, K.; Kalinova, S.; Kartalska, Y.; Naydenov, M. Influence of pendimetalin herbicide upon soil microflora. Pochvoznanie Agrokhim. Ekol. 2008, 42, 49–55. [Google Scholar]
- Gupta, P.K. Pesticide exposure—Indian scene. Toxicology 2004, 198, 83–90. [Google Scholar] [CrossRef]
- Crouzet, O.; Batisson, I.; Besse-Hoggan, P.; Bonnemoy, F.; Bardot, C.; Poly, F.; Bohatier, J.; Mallet, C. Response of soil microbial communities to the herbicide mesotrione: A dose-effect microcosm approach. Soil Biol. Biochem. 2010, 42, 193–202. [Google Scholar] [CrossRef]
- Romero, M.C.; Urrutia, M.I.; Reinoso, H.E.; Kiernan, M.M. Benzo[a]pyrene degradation by soil filamentous fungi. J. Yeast Fungal Res. 2010, 1, 25–29. [Google Scholar]
- Garbisu, C.; Alkorta, I.; Epelde, L. Assessment of soil quality using microbial properties and attributes of ecological relevance. Appl. Soil Ecol. 2011, 49, 1–4. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Popescu, R.; Bordean, D.M.; Vladoiu, D.L.; Mituletu, M.; Ostafe, V. The effect of chlorsulfurone and MCPB-Na on the enzymatic activity of microorganisms. J. Serb. Chem. Soc. 2014, 79, 1075–1084. [Google Scholar] [CrossRef]
- Filimon, M.N.; Popescu, R.; Verdes, D.; Dumitrescu, G.; Voia, O.S.; Ahmadi, M.; Dronca, D. The effects of difenoconazole treatment on microorganism from soil. Rev. Chim. 2018, 69, 1129–1133. [Google Scholar] [CrossRef]
- Saeki, M.; Toyota, K. Effect of bensulfuron-methyl (a sulfonyurea herbicide) on the soil bacterial community of a paddy soil microcosm. Biol. Fertil. Soils 2004, 40, 110–118. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Dumontet, S.; Mazzatura, A.; Pasquale, V. Toxic effects of four sulphonylureas herbicides on soil microbial biomass. J. Environ. Sci. Health Part B 2012, 47, 653–659. [Google Scholar] [CrossRef]
- Da Silva, G.S.; Melo, C.A.D.; Fialho, C.M.T.; Santos, L.D.T.; Costa, M.D.; da Silva, A.A. Impact of sulfentrazone, isoxaflutole and oxyfluorfen on the microorganisms of two forest soils, Bragantia. Campinas 2014, 73, 292–299. [Google Scholar]
- Zhang, Q.; Zhu, L.; Wang, J.; Xie, H.; Wang, J.; Wang, F.; Sun, F. Effects of fomesafen on soil enzyme activity, microbial population, and bacterial community composition. Environ. Monit. Assess. 2014, 186, 2801–2812. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, S.; Zheng, D.; Feng, S. Effects of cadmium, zinc and lead on soil enzyme activities. J. Environ. Sci. 2006, 18, 1135–1141. [Google Scholar] [CrossRef]
- Guo, H.; Chen, G.F.; Lu, Z.P.; Zhao, H.; Yang, H. Alteration of microbial properties and community structure in soils exposed to napropamide. J. Environ. Sci. 2009, 21, 494–502. [Google Scholar] [CrossRef]
- Rasool, N.; Reshi, Z.A. Effect of the fungicide Mancozeb at different application rates on enzyme activities in a silt loam soil of the Kashmir Himalaya, India. Trop. Ecol. 2010, 51, 199–205. [Google Scholar]
- Xiong, D.; Gao, Z.; Fu, B.; Sun, H.; Tian, S.; Xiao, Y.; Qin, Z. Effect of pyrimorph on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2013, 56, 44–48. [Google Scholar] [CrossRef]
- Rasool, N.; Reshi, Z.A.; Shah, M.A. Effect of butachlor (G) on soil enzyme activity. Eur. J. Soil Biol. 2014, 61, 94–100. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Roseb, M.T.; Rose, T.J.; Zwieten, L. Effect of glyphosate and a commercial formulation on soil functionality assessed by substrate induced respiration and enzyme activity. Eur. J. Soil Biol. 2018, 85, 64–72. [Google Scholar] [CrossRef]
- Alister, C.A.; Gomez, P.A.; Rojas, S.; Kogan, M. Pendimethalin and oxyfluorfen degradation under two irrigation conditions over four years’ application. J. Environ. Sci. Health Part B 2009, 44, 337–343. [Google Scholar] [CrossRef]
- Filimon, M.N.; Voia, S.O.; Vlădoiu, D.L.; Isvoran, A.; Ostafe, V. Temperature dependent effect of difenoconazole on enzymatic activity from the soil. J. Serb. Chem. Soc. 2015, 80, 1127–1137. [Google Scholar] [CrossRef]
- Das, A.C.; Debnath, A.; Mukherjee, D. Effect of the herbicides oxadiazon and oxyfluorfen on phosphates solubilizing microorganisms and their persistence in rice fields. Chemosphere 2003, 53, 217–221. [Google Scholar] [CrossRef]
- Pose-Juan, E.; Sanchez-Martin, M.J.; Andrades, M.S.; Rodriguez-Cruz, M.S.; Herrero-Hernandez, E. Pesticide residues in vineyard soils from Spain: Spatial and temporal distributions. Sci. Total Environ. 2015, 514, 351–358. [Google Scholar] [CrossRef]
- Sokolova, T.V.; Gulidova, V.A. Change of the biological activity of soils under the effect of herbicides. Zashchita Karantin Rast. 2010, 8, 46–47. [Google Scholar]
- Ghosh, R.K.; Jana, P.K.; Nongmaithem, D.; Pal, D.; Bera, S.; Mallick, S.; Barman, S.K.; Kole, R.K. Prospects of botanical herbicides in system of crop intensification in the Gangetic Inceptisols of India. In Proceedings of the 6th International Workshop on Software Clones, Hangzhou, China, 17–22 June 2012; pp. 116–117. [Google Scholar]
- Mougi, A. The roles of amensalistic and commensalistic interactions in large ecological network stability. Sci. Rep. 2016, 6, 29929. [Google Scholar] [CrossRef]
- Vlǎdoiu, D.L.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Effects of herbicides and fungicides on the soil chitinolytic activity. A molecular docking approach. Ecol. Chem. Eng. S 2015, 22, 439–450. [Google Scholar] [CrossRef][Green Version]
- Vlǎdoiu, D.L.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Computational analysis of difenoconazole interaction with soil chitinases. J. Phys. Conf. Ser. 2015, 574, 012012. [Google Scholar] [CrossRef]
- Vlădoiu, D.L.; Filimon, M.N.; Ostafe, V.; Isvoran, A. Assessment of pesticides interactions with Bacillus pasteurii urease. A computational study. Rom. J. Phys. 2015, 60, 583–592. [Google Scholar]
- Isvoran, A. Computational study concerning the effect of some pesticides on the Proteus mirabilis catalase activity. AIP Conf. Proc. 2016, 1722, 130001. [Google Scholar]
- Filimon, M.N.; Roman, D.L.; Caraba, I.V.; Isvoran, A. Assessment of the effect of application of the herbicide S-metolachlor on the activity of some enzymes found in soil. Agriculture 2021, 11, 469. [Google Scholar] [CrossRef]
- Huma, T.; Maryam, A.; Qamar, T.U. Molecular modeling and docking of wheat hydroquinone glucosyl transferase by using hydroquinone, phenyl phosphorodiamate and n-(n butyl) phosphorothiocictriamide as inhibitors. Bioinformation 2014, 10, 124–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, K.D.; Labala, R.K.; Devi, T.B.; Singh, N.I.; Chanu, H.D.; Sougrakpam, S.; Nameirakpam, B.S.; Sahoo, D.; Rajashekar, Y. Biochemical efficacy, molecular docking and inhibitory effect of 2,3-dimethylmaleic anhydride on insect acetylcholinesterase. Sci. Rep. 2017, 7, 12483. [Google Scholar] [CrossRef] [PubMed]
- EPA-738-F02–013. Oxyfluorfen RED Facts. 2002. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/oxyfluorfen_red.pdf (accessed on 26 August 2021).
- Yen, J.; Sheu, W.; Wang, Y. Dissipation of the herbicide oxyfluorfen in subtropical soils and its potential to contaminate groundwater. Ecotoxicol. Environ. Saf. 2003, 54, 151–156. [Google Scholar] [CrossRef]
- USEPA. Reregistration Eligibility Decisions (REDs) Database on Oxyfluorfen (42874–0303). USEPA 738-R-02–014. October 2002. Available online: http://www.epa.gov/pesticides/reregistration/status.htm (accessed on 24 January 2021).
- Frank, R.; Clegg, B.S.; Ritcey, G. Disappearance of oxyfluorfen (Goal) from onions and organic soils. Bull. Environ. Contam. Toxicol. 1991, 46, 485–491. [Google Scholar] [CrossRef]
- Weed Science Society of America (WSSA). Herbicide Handbook, 8th ed.; Weed Science Society of America: Lawrence, KS, USA, 2002; pp. 357–360. [Google Scholar]
- Tale, K.S.; Ingole, S. A review on role of physico-chemical properties in soil quality. Chem. Rev. Lett. 2015, 4, 57–66. [Google Scholar]
- Horrocks, R.D.; Valentine, J.F. Chapter 11. Soil fertility and forage production. In Harvested Forages; Academic Press: Cambridge, MA, USA, 1999; pp. 187–224. [Google Scholar]
- Bordean, D.M.; Borozan, A.B.; Cojocariu, L.; Moigradean, D.; Cojocariu, A.; Dragos, N.; Pirvulescu, L.; Alda, S.; Horablaga, M. Seasonal variation in nutrient content of some leafy vegetables from Banat County, Romania. Rev. Agric. Rural Dev. 2013, 2, 170–174. [Google Scholar]
- Baethgen, W.E.; Alley, M.M. A manual colorimetric procedure for measuring ammonium nitrogen in soil and plant Kjeldahl digests. Commun. Soil. Sci. Plant Anal. 1989, 20, 961–969. [Google Scholar] [CrossRef]
- Amponsah, D.; Etsey, G.; Nagai, H. Determination of amount of phosphate and sulphate in soil samples from university of Cape Coast Farm. Int. J. Sci. Technol. Res. 2014, 3, 211–215. [Google Scholar]
- Uwah, E.I.; Abah, J.; Ndahi, N.P.; Ogugbuaja, V.O. Concentration levels of nitrate and nitrite in soils and some leafy vegetables obtained in Maiduguri, Nigeria. J. Appl. Sci. Environ. Sanit. 2009, 4, 233–244. [Google Scholar]
- Schinner, F.; Öhlinger, R.; Kandeler, E.; Margesin, R. Methods in Soil Biology; Springer: Berlin/Heidelberg, Germany, 1996; p. 241. [Google Scholar]
- Alef, K.; Nannipieri, P. Methods in Applied Soil Microbiology and Biochemistry; Academic Press: London, UK, 1995; p. 316. [Google Scholar]
- Krámer, M.; Erdei, G. Primenenie metoda opredeleniia aktivnosti fosfatazî v agrohimiceskih issledovaniiah. Pocivovedenie 1959, 9, 99–102. [Google Scholar]
- Filimon, M.N.; Nica, V.D.; Ostafe, V.; Bordean, D.M.; Borozan, A.B.; Vlad, D.C.; Popescu, R. Use of enzymatic tools for biomonitoring inorganic pollution in aquatic sediments: A case study (Bor, Serbia). Chem. Cent. J. 2013, 7, 59. [Google Scholar] [CrossRef][Green Version]
- Dragan-Bularda, M. Microbiologie Generala-Lucrari Practice; Editura Universitatii Babes-Bolyai: Cluj-Napoca, Romania, 2000; pp. 189–191. (In Romanian) [Google Scholar]
- Filimon, M.N.; Voia, S.O.; Popescu, R.; Dumitrescu, G.; Petculescu Ciochina, L.; Mituletu, M.; Vlad, D.C. The effect of some insecticides on soil microorganisms based on enzymatic and bacteriological analyses. Rom. Biotechnol. Lett. 2015, 20, 10439–10447. [Google Scholar]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Rumsey, D.J. How to interpret a correlation coefficient R. In Statistics for Dummies, 2nd ed.; Wiley: Hoboken, NJ, USA, 2003; p. 286. ISBN 978-0470537039. [Google Scholar]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Shoichet, B.K. ZINC—A free database of commercially available compounds for virtual screening. J. Chem. Inf. Model. 2005, 45, 177–182. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011, 39, W270–W277. [Google Scholar] [CrossRef]
- Grosdidier, A.; Zoete, V.; Michielin, O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins 2007, 67, 1010–1025. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Application; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Baćmaga, M.; Boros, E.; Kucharski, J.; Wyszkowska, J. Enzymatic activity in soil contaminated with the Aurora 40 WG herbicide. Environ. Prot. Eng. 2012, 38, 91–102. [Google Scholar]
- Busse, M.D.; Ratcliff, A.W.; Shestak, C.J. Glyphosate toxicity and the effects of long term vegetation control on soil microbial communities. Soil Biol. Biochem. 2001, 33, 1777–1789. [Google Scholar] [CrossRef]
- Gomez, E.; Ferreras, L.; Lovotti, L.; Fernandez, E. Impact of glyphosate application on microbial biomass and metabolic activity in a Vertic Argiudoll from Argentina. Eur. J. Soil Biol. 2009, 45, 163–167. [Google Scholar] [CrossRef]
- Gianfreda, L.; Rao, M.A. The influence of pesticides on soil enzymes. In Soil Enzymology Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 22, pp. 293–312. [Google Scholar]
- Bhattarai, A.; Bhattarai, B.; Pandey, S. Variation of soil microbial population in different soil horizons. J. Microbiol. Exp. 2015, 2, 00044. [Google Scholar] [CrossRef]
- Nannipieri, P.; Kandeler, E.; Ruggiero, P. Enzyme activities and microbiological and biochemical processes in soil. In Enzymes in the Environment; Burns, R.G., Dick, R., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 1–33. [Google Scholar]
- Araújo, A.S.F.; Monteiro, R.T.R.; Abarkeli, R.B. Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 2003, 52, 799–804. [Google Scholar] [CrossRef]
- Zabaloy, M.C.; Gómez, M.A. Microbial respiration in soils of the Argentine Pampas after metsulfuron-methyl, 2,4-D and glyphosate treatments. Commun. Soil Sci. Plant Anal. 2008, 39, 370–385. [Google Scholar] [CrossRef]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology, Soil Biology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 22. [Google Scholar]
- Lupwayi, N.Z.; Hanson, K.G.; Harker, K.N.; Clayton, G.W.; Blackshaw, R.E.; O’Donovan, J.T.; Johnson, E.N.; Gan, Y.; Irvine, R.B.; Monreal, M.A. Soil microbial biomass, functional diversity and enzyme activity in glyphosate-resistant wheat–canola rotations under low-disturbance direct seeding and conventional tillage. Soil Biol. Biochem. 2007, 39, 1418–1427. [Google Scholar] [CrossRef]
- Kucharski, J.; Wyszkawska, J. Biological properties of soil contaminated with the herbicide Apyros 75 WG. J. Elem. 2008, 13, 357–371. [Google Scholar]
- Baruah, M.; Mishra, R.R. Effect of herbicides butachlor, 2,4-d and oxyfluorfen on enzyme activities and CO2 evolution in submerged paddy field soil. Plant. Soil 1986, 96, 287–291. [Google Scholar] [CrossRef]
- Ramesh, A.; Joshi, O.P.; Billore, S.D. Effect of herbicides on soil dehydrogenase and urease activity in soybean (Glycine max). Indian J. Agric. Sci. 2000, 70, 218–219. [Google Scholar]
- Muñoz-Leoz, B.; Garbisu, C.; Charcosset, J.Y.; Sánchez-Pérez, J.M.; Antigüedad, I.; Romera, E.R. Non-target effects of three formulated pesticides on microbially-mediated processes in a clay-loam soil. Sci. Total Environ. 2013, 449, 345–354. [Google Scholar] [CrossRef]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican + mesosulfuron-methyl + iodosulfuron-methyl-sodium. Environ. Sci. Pollut. Res. 2015, 22, 643–656. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Herrera-Cervera, J.A.; Lluch, C.; Tejera, N.A. Trehalose metabolism in root nodules of the model legume Lotus japonicus in response to salt stress. Physiol. Plant 2006, 128, 701–709. [Google Scholar] [CrossRef]
- Nannipieri, P.; Sequi, P.; Fusi, P. Humus and enzyme activity. In Humic Substances in Terrestrial Ecosystems; Piccolo, A., Ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 293–328. [Google Scholar]
- Maddela, N.R.; Venkateswarlu, K. Impact of acephate and buprofezin on soil proteases. In Insecticides-Soil Microbiota Interactions; Springer: Berlin/Heidelberg, Germany, 2018; pp. 57–64. [Google Scholar]
- Perucci, P.; Scarponi, L. Effects of the herbicide imazethapyr on soil microbial biomass and various soil enzyme activities. Biol. Fertil. Soils 1994, 17, 237–240. [Google Scholar] [CrossRef]
- Wainwright, M.J. A review of the effect of pesticides on microbial activity in soils. Eur. J. Soil Sci. 2006, 29, 287–298. [Google Scholar] [CrossRef]
- El Hussein, A.A.; Mohamed, A.T.; El Siddig, M.A.; Sherif, A.M.; Osman, A.G. Effects of oxyfluorfen herbicide on microorganisms in loam and silt loam soils. Res. J. Environ. Sci. 2012, 6, 134–145. [Google Scholar] [CrossRef][Green Version]
- Bera, S.; Ghosh, R.K. Microflora population and physico-chemical properties of soil of potato as influenced by oxyfluorfen 23.5% EC. Univ. J. Agric. Res. 2014, 2, 135–140. [Google Scholar] [CrossRef]
- Liang, B.; Lu, P.; Li, H.; Li, R.; Li, S.; Huang, X. Biodegradation of fomesafen by strain Lysinibacillus sp. ZB-1 isolated from soil. Chemosphere 2009, 77, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Q.; Zhang, J.; Zhang, J.; Huang, X.; Lu, P.; Li, S.-P. Microbial degradation of fomesafen by a newly isolated strain Pseudomonas zeshuii BY-1 and the biochemical degradation pathway. J. Agric. Food Chem. 2012, 60, 7104–7110. [Google Scholar] [CrossRef] [PubMed]




| Oxyfluorfen Dose | Dose Abbreviation | Lot I—Soil Preserved in Laboratory Conditions with Controlled Values of Physiochemical Parameters | Lot II—Soil Preserved in Field Condition with Variable Physicochemical Parameters | ||||
|---|---|---|---|---|---|---|---|
| untreated soil | B | aL measurements are made after 7 days of incubation | bL measurements are made after 14 days of incubation | cL measurements are made after 21 days of incubation | aN measurements are made after 7 days of incubation | bN measurements are made after 14 days of incubation | cN measurements are made after 21 days of incubation |
| 1 g/kg of soil | ½ D | ||||||
| 2 g/kg of soil | D | ||||||
| 4 g/kg of soil | 2× D | ||||||
| Enzyme | ∆G (kcal/mol) |
|---|---|
| dehydrogenase from Clostridium beijerinckii | −7.578 |
| phosphatase from Bacillus subtilis | −8.006 |
| urease from Bacillus pasteurii | It does not bind to the active site of the enzyme. |
| protease from Serratia sp. E-15 and Serratiamarcescens | |
| proteinase from Streptomyces griseus | −7.226 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimon, M.N.; Roman, D.L.; Bordean, D.M.; Isvoran, A. Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms. Agronomy 2021, 11, 1702. https://doi.org/10.3390/agronomy11091702
Filimon MN, Roman DL, Bordean DM, Isvoran A. Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms. Agronomy. 2021; 11(9):1702. https://doi.org/10.3390/agronomy11091702
Chicago/Turabian StyleFilimon, Marioara Nicoleta, Diana Larisa Roman, Despina Maria Bordean, and Adriana Isvoran. 2021. "Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms" Agronomy 11, no. 9: 1702. https://doi.org/10.3390/agronomy11091702
APA StyleFilimon, M. N., Roman, D. L., Bordean, D. M., & Isvoran, A. (2021). Impact of the Herbicide Oxyfluorfen on the Activities of Some Enzymes Found in Soil and on the Populations of Soil Microorganisms. Agronomy, 11(9), 1702. https://doi.org/10.3390/agronomy11091702







