Grain Quality and Starch Physicochemical Properties of Chalky Rice Mutant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Sample Preparation
2.3. Scanning Electron Microscopy
2.4. Analysis of Rice Grain Quality
2.5. Measurement of Thermal Properties
2.6. Starch Fine Structure Measurement
2.7. Starch Crystalline Structure Analysis
2.8. Statistical Analysis
3. Results and Discussion
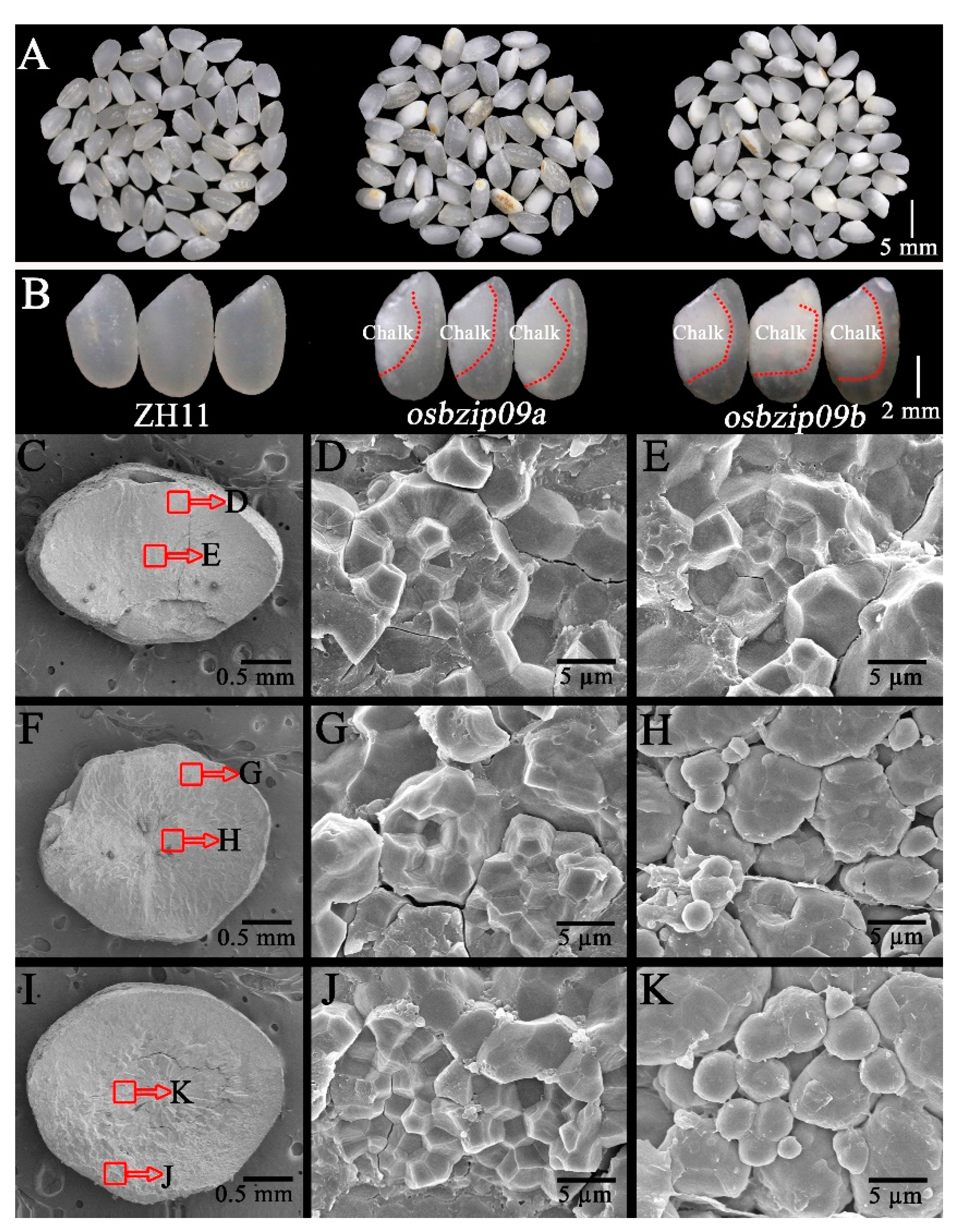
3.1. Analysis of Grain Quality Profiles
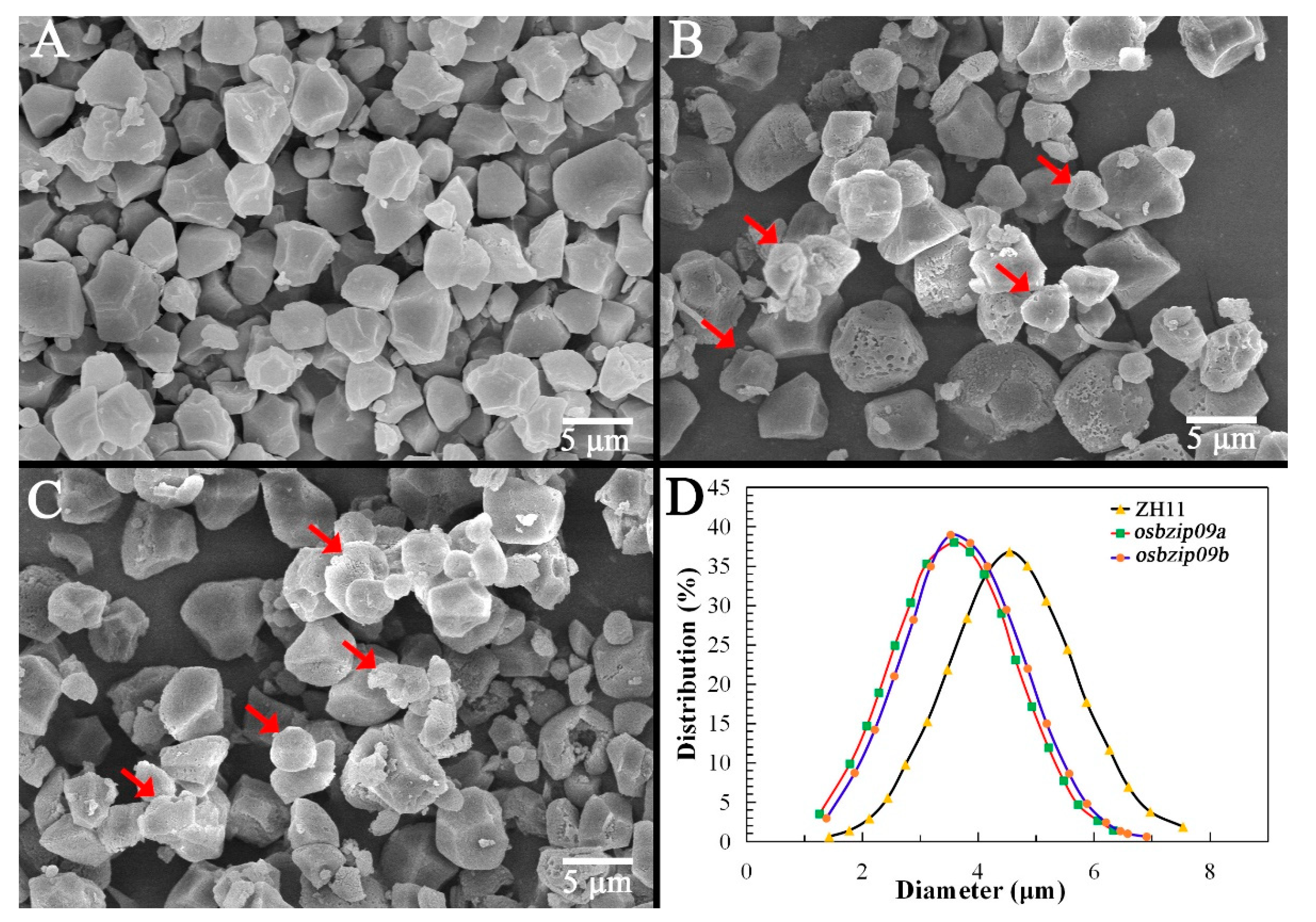
3.2. Analysis of Starch Granular Structure
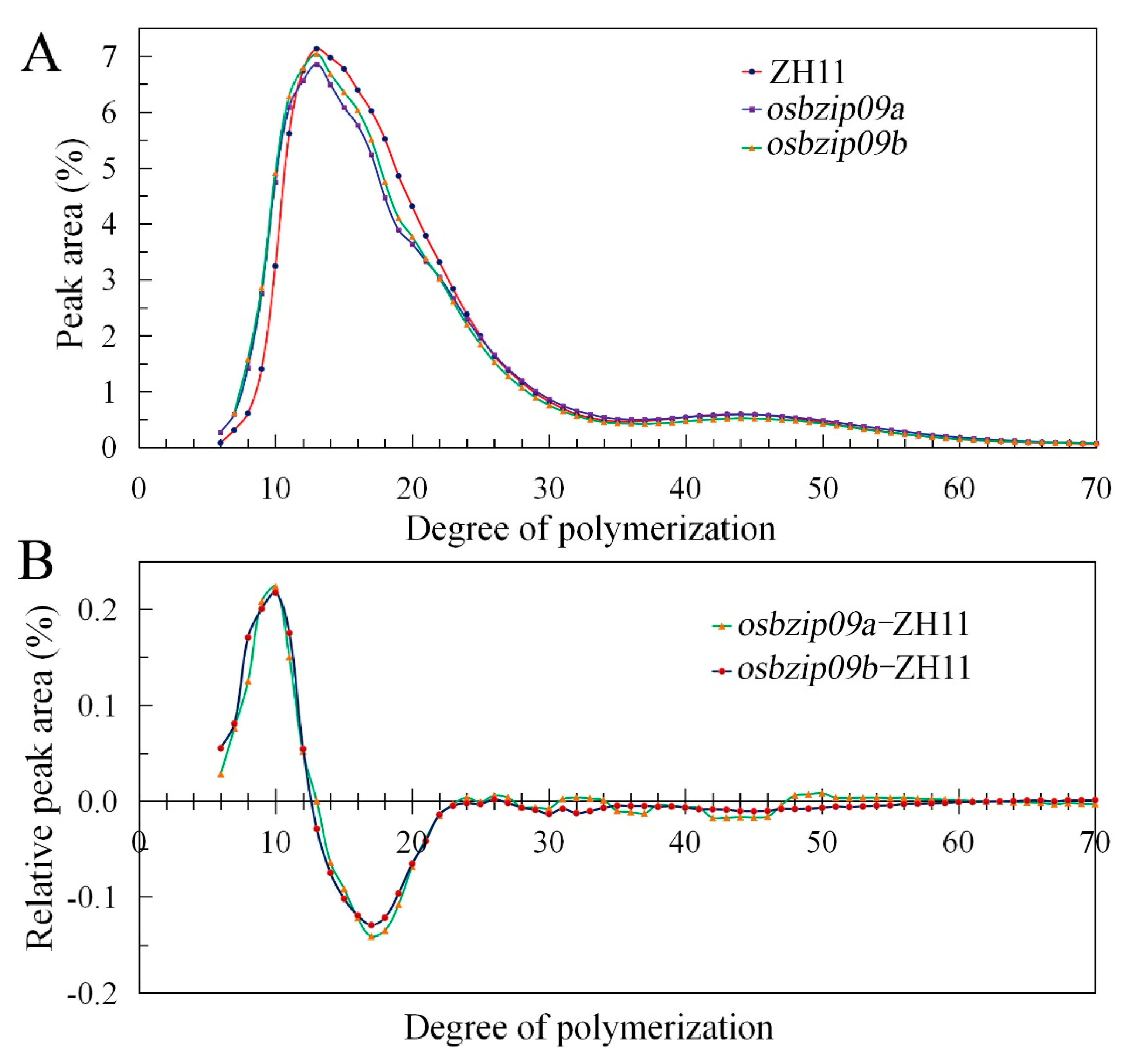
3.3. Starch Fine Structure Analysis
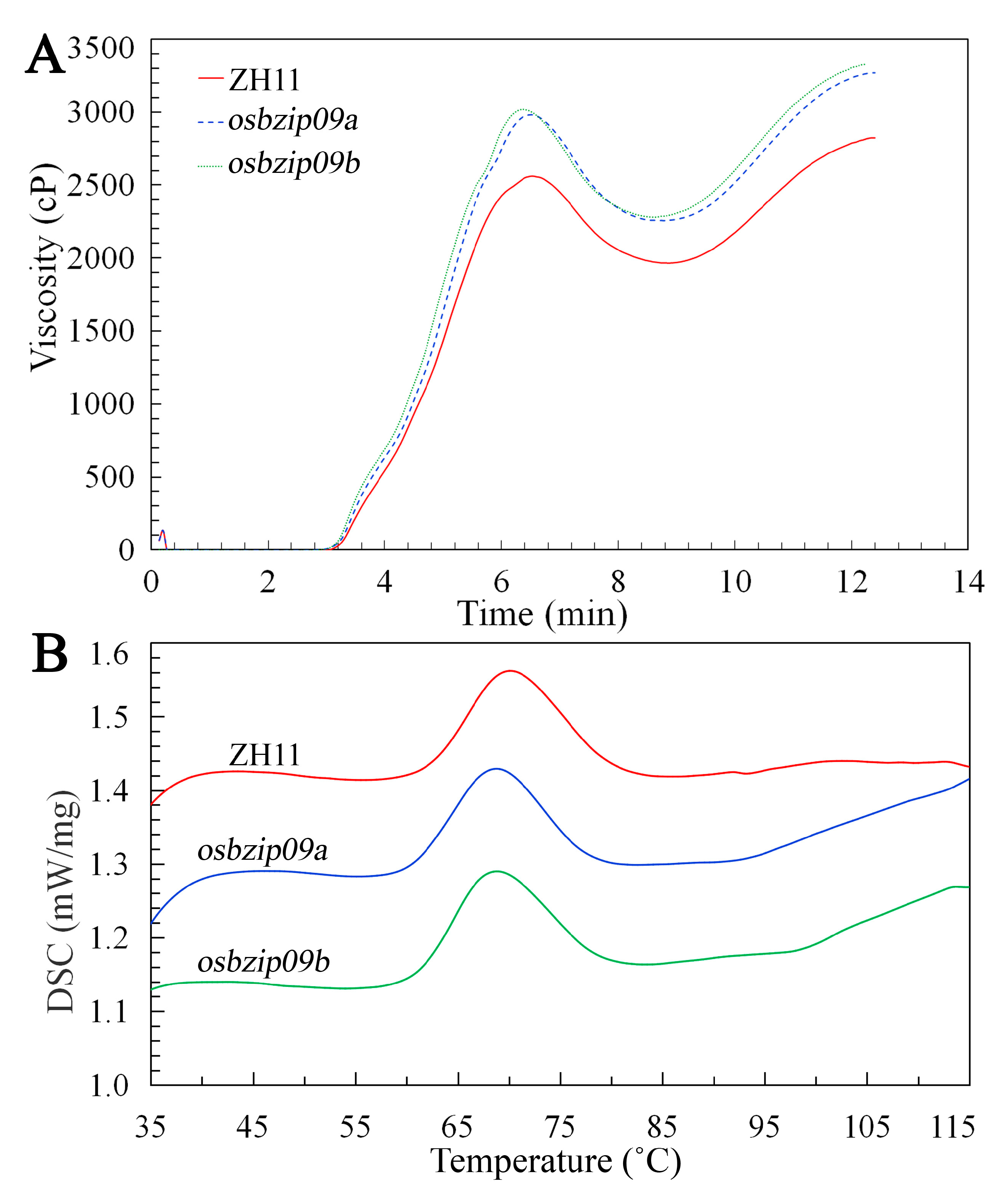
3.4. Comparison of Rice Starch Pasting Properties
3.5. Thermal Properties of Starch
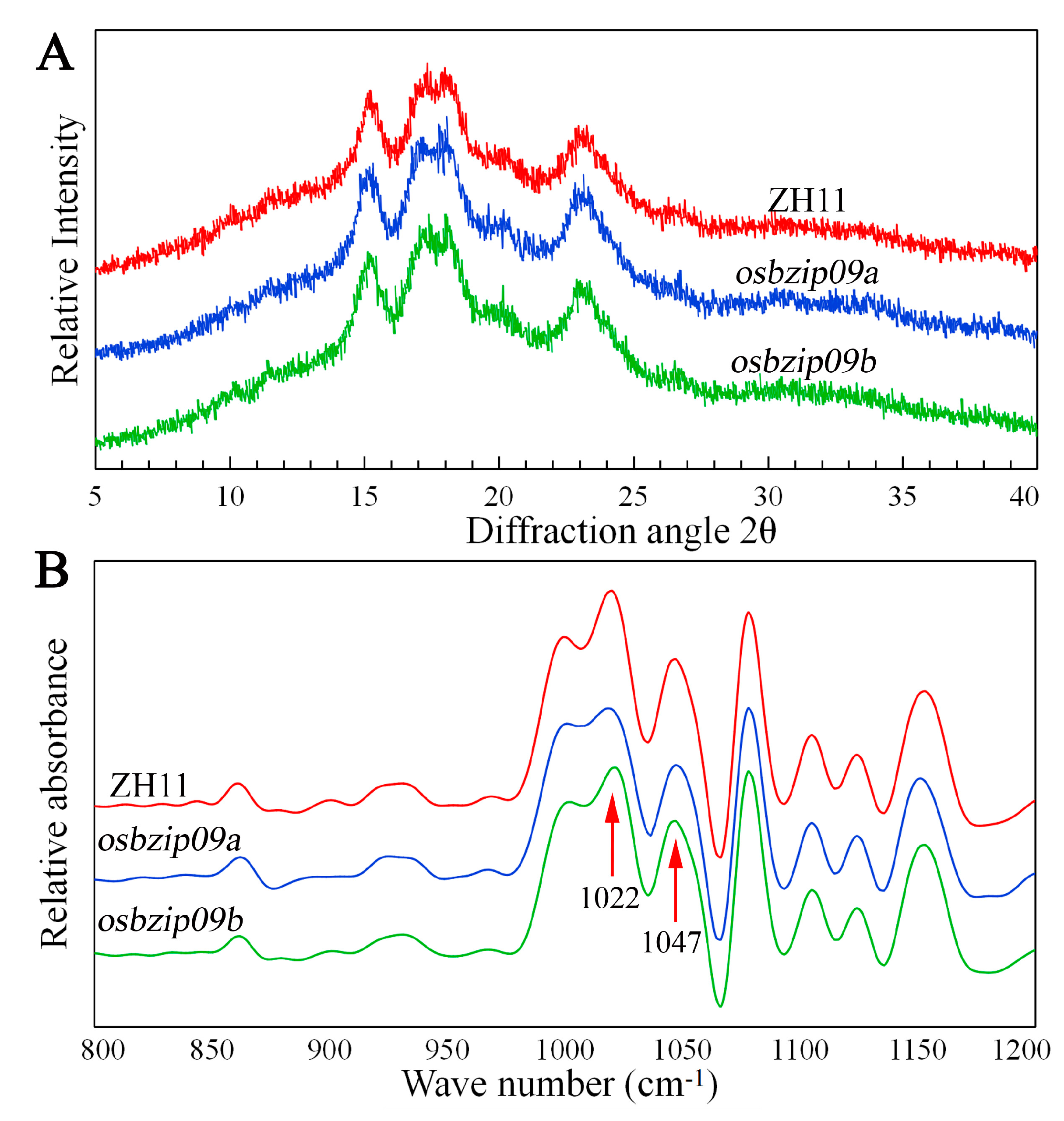
3.6. Starch Crystalline Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fitzgerald, M.A.; McCouch, S.R.; Hall, R.D. Not just a grain of rice: The quest for quality. Trends Plant Sci. 2009, 14, 133–1339. [Google Scholar] [CrossRef] [PubMed]
- Custodio, M.C.; Cuevas, R.P.; Ynion, J.; Laborte, A.G.; Velasco, M.L.; Demont, M. Rice quality: How is it defined by consumers, industry, food scientists, and geneticists? Trends Food Sci. Technol. 2019, 92, 122–137. [Google Scholar] [CrossRef]
- Li, H.Y.; Gilbert, R.G. Starch molecular structure: The basis for an improved understanding of cooked rice texture. Carbohydr. Polym. 2018, 195, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Butardo, V.M., Jr.; Sreenivasulu, N.; Juliano, B.O. Improving rice grain quality: State-of-the-art and future prospects. Methods Mol. Biol. 2019, 1892, 19–55. [Google Scholar]
- Patindol, J.; Wang, Y. J. Fine structures and physicochemical properties of starches from chalky and translucent rice kernels. J. Agric. Food Chem. 2003, 51, 2777–2784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, L.; Zhang, J.; Cai, X.L.; Liu, Q.Q.; Wei, C.X. Relationships between transparency, amylose content, starch cavity, and moisture of brown rice kernels. J. Cereal Sci. 2019, 90, 102854. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Chen, S.J.; Ren, X.Y.; Lu, Y.; Liu, D.R.; Cai, X.L.; Li, Q.F.; Gao, J.P.; Liu, Q.Q. Molecular Structure and physicochemical properties of starches from rice with different amylose contents resulting from modification of OsGBSSI activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef]
- Yamakawa, H.; Hirose, T.; Kuroda, M.; Yamaguchi, T. Comprehensive expression profiling of rice grain filling-related genes under high temperature using DNA microarray. Plant Physiol. 2007, 144, 258–277. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.L.; Guo, T.; Wan, X.Y.; Wang, H.Y.; Zhu, M.Z.; Li, A.L.; Su, N.; Shen, Y.Y.; Mao, B.G.; Zhai, H.Q.; et al. Transcriptome analysis of grain-filling caryopses reveals involvement of multiple regulatory pathways in chalky grain formation in rice. BMC Genom. 2010, 11, 730. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, T.; Yamakawa, H.; Nakata, M.; Kuroda, M.; Hakata, M. Suppression of phospholipase D genes improves chalky grain production by high temperature during the grain-filling stage in rice. Biosci. Biotechnol. Biochem. 2019, 83, 1102–1110. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch-composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Jeon, J.S.; Ryoo, N.; Hahn, T.R.; Walia, H.; Nakamura, Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Biochem. 2010, 48, 383–392. [Google Scholar] [CrossRef]
- Zhu, J.H.; Yu, W.W.; Zhang, C.Q.; Zhu, Y.J.; Xu, J.L.; Li, E.P.; Gilbert, R.G.; Liu, Q.Q. New insights into amylose and amylopectin biosynthesis in rice endosperm. Carbohydr. Polym. 2020, 230, 115656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F. Relationships between amylopectin internal molecular structure and physicochemical properties of starch. Trends Food Sci. Tech. 2018, 78, 234–242. [Google Scholar] [CrossRef]
- Li, C.; Wu, A.; Yu, W.W.; Hu, Y.M.; Li, E.P.; Zhang, C.Q.; Liu, Q.Q. Parameterizing starch chain-length distributions for structure-property relations. Carbohydr. Polym. 2020, 241, 116390. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.X.; Qian, Q.; Liu, Q.Q.; Yan, M.X.; Liu, X.F.; Yan, C.J.; Liu, G.F.; Gao, Z.Y.; Tang, S.Z.; Zeng, D.L.; et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. Proc. Natl. Acad. Sci. USA 2009, 106, 21760–21765. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Xia, D.; He, Y.Q. Rice grain quality—Traditional traits for high quality rice and health-plus substances. Mol. Breed. 2020, 40, 1–17. [Google Scholar] [CrossRef]
- Pandey, M.K.; Rani, N.S.; Madhav, M.S.; Sundaram, R.M.; Varaprasad, G.S.; Sivaranjani, A.K.; Bohra, A.; Kumar, G.R.; Kumar, A. Different isoforms of starch-synthesizing enzymes controlling amylose and amylopectin content in rice (Oryza sativa L.). Biotechnol. Adv. 2012, 30, 1697–1706. [Google Scholar] [CrossRef] [Green Version]
- Toyosawa, Y.; Kawagoe, Y.; Matsushima, R.; Crofts, N.; Ogawa, M.; Fukuda, M.; Kumamaru, T.; Okazaki, Y.; Kusano, M.; Saito, K.; et al. Deficiency of starch synthase IIIa and IVb alters starch granule morphology from polyhedral to spherical in rice endosperm. Plant Physiol. 2016, 170, 1255–1270. [Google Scholar] [CrossRef] [Green Version]
- Fu, F.F.; Xue, H.W. Coexpression analysis identifies Rice Starch Regulator1, a rice AP2/EREBP family transcription factor, as a novel rice starch biosynthesis regulator. Plant Physiol. 2010, 154, 927–938. [Google Scholar] [CrossRef]
- Wang, J.C.; Xu, H.; Zhu, Y.; Liu, Q.Q.; Cai, X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013, 64, 3453–3466. [Google Scholar] [CrossRef]
- Bello, B.K.; Hou, Y.; Zhao, J.; Jiao, G.; Wu, Y.; Li, Z.; Wang, Y.; Tong, X.; Wang, W.; Yuan, W.; et al. NF-YB1-YC12-bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.). Plant Biotechnol. J. 2019, 17, 1222–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Chen, Z.C.; Zhang, Q.; Meng, S.S.; Wei, C.X. The NAC Transcription factors OsNAC20 and OsNAC26 regulate starch and storage protein synthesis. Plant Physiol. 2020, 184, 1775–1791. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.C.; Wang, C.X.; Lu, C.Y.; Wang, J.D.; Zhou, Y.; Xiong, M.; Zhang, C.Q.; Liu, Q.Q.; Li, Q.F. Genome-wide identification and expression analysis of OsbZIP09 target genes in rice reveal its mechanism of controlling seed germination. Int. J. Mol. Sci. 2021, 22, 1661. [Google Scholar] [CrossRef]
- Zhu, L.J.; Liu, Q.Q.; Sang Y., J.; Gu, M.H.; Shi, Y.C. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 2010, 120, 94–100. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Zhou, L.H.; Zhu, Z.B.; Lu, H.W.; Zhou, X.Z.; Qian, Y.T.; Li, Q.F.; Lu, Y.; Gu, M.H.; Liu, Q.Q. Characterization of grain quality and starch fine structure of two japonica rice (Oryza sativa) cultivars with good sensory properties at different temperatures during the filling stage. J. Agric. Food Chem. 2016, 64, 4048–4057. [Google Scholar] [CrossRef]
- Cai, J.W.; Man, J.M.; Huang, J.; Liu, Q.Q.; Wei, W.X.; Wei, C.X. Relationship between structure and functional properties of normal rice starches with different amylose contents. Carbohydr. Polym. 2015, 125, 35–44. [Google Scholar] [CrossRef]
- Su, Y.; Rao, Y.C.; Hu, S.K.; Yang, Y.L.; Gao, Z.Y.; Zhang, G.H.; Liu, J.; Hu, J.; Yan, M.X.; Dong, G.J.; et al. Map-based cloning proves qGC-6, a major QTL for gel consistency of japonica/indica cross, responds by Waxy in rice (Oryza sativa L.). Theor. Appl. Genet. 2011, 123, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.M.; Zhong, L.J.; Wang, F.; Zhang, G.P. Differences in cooking and eating properties between chalky and translucent parts in rice grains. Food Chem. 2005, 90, 39–46. [Google Scholar] [CrossRef]
- Zhu, A.K.; Zhang, Y.X.; Zhang, Z.H.; Wang, B.F.; Xue, P.; Cao, Y.R.; Chen, Y.Y.; Li, Z.H.; Liu, Q.N.; Cheng, S.H.; et al. Genetic dissection of qPCG1 for a quantitative trait locus for percentage of chalky grain in rice (Oryza sativa L.). Front Plant Sci. 2018, 9, 1173. [Google Scholar] [CrossRef]
- Xu, H.; Li, X.F.; Zhang, H.; Wang, L.C.; Zhu, Z.G.; Gao, J.P.; Li, C.S.; Zhu, Y. High temperature inhibits the accumulation of storage materials by inducing alternative splicing of OsbZIP58 during filling stage in rice. Plant Cell Environ. 2020, 43, 1879–1896. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhu, C.C.; Zhou, Y.; Xiong, M.; Wang, J.D.; Bai, H.; Lu, C.Y.; Zhang, C.Q.; Liu, Q.Q.; Li, Q.F. OsbZIP09, a unique osbzip transcription factor of rice, promotes rather than suppresses seed germination by attenuating abscisic acid pathway. Rice Sci. 2021, 28, 358–367. [Google Scholar]
- Hanashiro, I.; Abe, J.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high performance anion exchange chromatography. Carbohyd. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Nishi, A.; Nakamura, Y.; Tanaka, N.; Satoh, H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001, 127, 459–472. [Google Scholar] [CrossRef]
- Fujita, N.; Yoshida, M.; Asakura, N.; Ohdan, T.; Miyao, A.; Hirochika, H.; Nakamura, Y. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006, 140, 1070–1084. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Francisco, P.B., Jr.; Hosaka, Y.; Sato, A.; Sawada, T.; Kubo, A.; Fujita, N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005, 58, 213–227. [Google Scholar] [CrossRef] [PubMed]
- López-González, C.; Juárez-Colunga, S.; Morales-Elías, N.C.; Tiessen, A. Exploring regulatory networks in plants: Transcription factors of starch metabolism. Peer J. 2019, 7, e6841. [Google Scholar] [CrossRef]
- Cai, Y.; Xie, D.L.; Wang, Z.Y.; Hong, M.M. Interaction of rice bZIP protein REB with the 5′-upstream region of both rice sbe1 gene and waxy gene. Chin. Sci. Bull. 2002, 47, 310–314. [Google Scholar] [CrossRef]
- Xu, Y.J.; Ying, Y.N.; Ouyang, S.H.; Duan, X.L.; Sun, H.; Jiang, S.K.; Sun, S.C.; Bao, J.S. Factors affecting sensory quality of cooked japonica rice. Rice Sci. 2018, 25, 330–339. [Google Scholar]
- Noosuk, P.; Hill, S.E.; Pradipasena, P.; Mitchell, J.R. Structure-viscosity relationships for Thai rice starches. Starch/Starke 2003, 55, 337–344. [Google Scholar] [CrossRef]
- Li, H.Y.; Lei, N.Y.; Yan, S.; Yang, J.Y.; Yu, T.; Wen, Y.Y.; Wang, J.; Sun, B.G. The importance of amylopectin molecular size in determining the viscoelasticity of rice starch gels. Carbohydr. Polym. 2019, 212, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.X.; Jobling, S.A.; Millar, A.; Morell, M.K.; Li, Z.Y. Allelic effects on starch structure and properties of six starch biosynthetic genes in a rice recombinant inbred line population. Rice 2015, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.Q.; Zhou, J.H.; Chen, S.J.; Fan, X.L.; Li, Q.F.; Lu, Y.; Wang, M.; Yu, H.X.; Yi, C.D.; Tang, S.Z.; et al. Wxlv, the ancestral allele of rice waxy gene. Mol. Plant 2019, 12, 1157–1166. [Google Scholar] [CrossRef] [Green Version]
- Li, H.Y.; Wen, Y.Y.; Wang, J.; Sun, B.G. The molecular structures of leached starch during rice cooking are controlled by thermodynamic effects, rather than kinetic effects. Food Hydrocolloid. 2017, 73, 295–299. [Google Scholar] [CrossRef]
- Vandeputte, G.E.; Vermeylen, R.; Geeroms, J.; Delcour, J.A. Rice starches. I. Structural aspects provide insight into crystallinity characteristics and gelatinization behaviour of granular starch. J. Cereal Sci. 2003, 38, 43–52. [Google Scholar] [CrossRef]
- Blazek, J.M.; Gilbert, E.P. Application of small-angle X-ray and neutron scattering techniques to the characterisation of starch structure: A review. Carbohydr. Polym. 2011, 85, 281–293. [Google Scholar] [CrossRef]
- Gidley, M.J.; Bulpin, P.V. Crystallisation of malto-oligosaccharides as models of the crystalline forms of starch: Minimum chain-length requirement for the formation of double helices. Carbohydr. Polym. 1987, 161, 291–300. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Tan, I.; Flanagan, B.M.; Halley, P.J.; Whittaker, A.K.; Gidley, M.J. A method for estimating the nature and relative proportions of amorphous, single, and double-helical components in starch granules by (13)C CP/MAS NMR. Biomacromolecules 2007, 8, 885–891. [Google Scholar] [CrossRef]
| Lines | CR (%) | CD (%) | AAC (%) | GC (%) | PC (%) | TSC (%) |
|---|---|---|---|---|---|---|
| ZH11 | 18.86 ± 3.14 b | 31.24 ± 4.53 b | 15.01 ± 0.22 b | 85.31 ± 6.13 a | 7.02 ± 0.31 a | 87.18 ± 0.74 a |
| osbizip09a | 68.63 ± 5.67 a | 69.58 ± 8.11 a | 17.64 ± 0.36 a | 72.26 ± 5.75 b | 7.36 ± 0.24 a | 86.12 ± 0.81 a |
| osbizip09b | 65.24 ± 6.21 a | 71.15 ± 6.05 a | 17.28 ± 0.47 a | 74.19 ± 4.72 b | 7.41 ± 0.16 a | 86.43 ± 0.69 a |
| Lines | To (°C) | Tp (°C) | Tc (°C) | ΔH (J G−1) | RC (%) | 1047/1022 cm−1 |
|---|---|---|---|---|---|---|
| ZH11 | 62.01 ± 0.13 a | 70.45 ± 0.49 a | 80.45 ± 0.49 a | 10.21 ± 0.27 a | 28.16 ± 0.24 a | 0.84 ± 0.02 a |
| osbizip09a | 60.30 ± 0.28 b | 68.40 ± 0.43 b | 77.45 ± 0.35 b | 8.31 ± 0.25 b | 26.54 ± 0.34 b | 0.78 ± 0.01 b |
| osbizip09b | 60.88 ± 0.39 b | 68.90 ± 0.28 b | 78.20 ± 0.42 b | 8.32 ± 0.31 b | 26.72 ± 0.21 b | 0.77 ± 0.02 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-X.; Zhu, C.-C.; Lu, C.-Y.; Yang, Y.; Li, Q.-F.; Liu, Q.-Q.; Zhang, C.-Q. Grain Quality and Starch Physicochemical Properties of Chalky Rice Mutant. Agronomy 2021, 11, 1575. https://doi.org/10.3390/agronomy11081575
Wang C-X, Zhu C-C, Lu C-Y, Yang Y, Li Q-F, Liu Q-Q, Zhang C-Q. Grain Quality and Starch Physicochemical Properties of Chalky Rice Mutant. Agronomy. 2021; 11(8):1575. https://doi.org/10.3390/agronomy11081575
Chicago/Turabian StyleWang, Chu-Xin, Cheng-Chao Zhu, Chen-Ya Lu, Yong Yang, Qian-Feng Li, Qiao-Quan Liu, and Chang-Quan Zhang. 2021. "Grain Quality and Starch Physicochemical Properties of Chalky Rice Mutant" Agronomy 11, no. 8: 1575. https://doi.org/10.3390/agronomy11081575
APA StyleWang, C.-X., Zhu, C.-C., Lu, C.-Y., Yang, Y., Li, Q.-F., Liu, Q.-Q., & Zhang, C.-Q. (2021). Grain Quality and Starch Physicochemical Properties of Chalky Rice Mutant. Agronomy, 11(8), 1575. https://doi.org/10.3390/agronomy11081575





