Evaluation of the Effectiveness of Spirotetramat on the Diaspine Scale Parlatoria pergandii in Citrus Orchards
Abstract
:1. Introduction
2. Materials and Methods
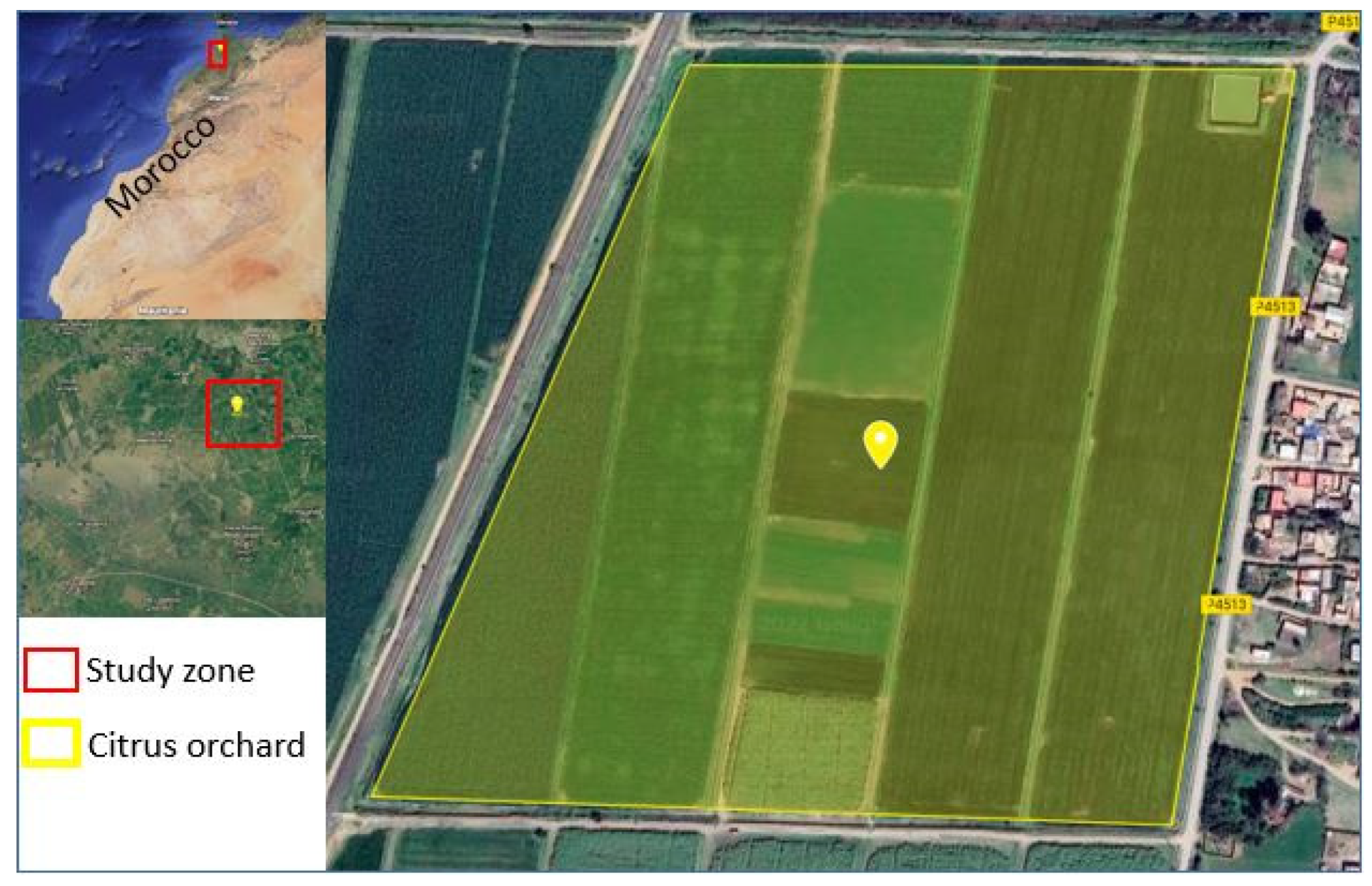
2.1. Study Area
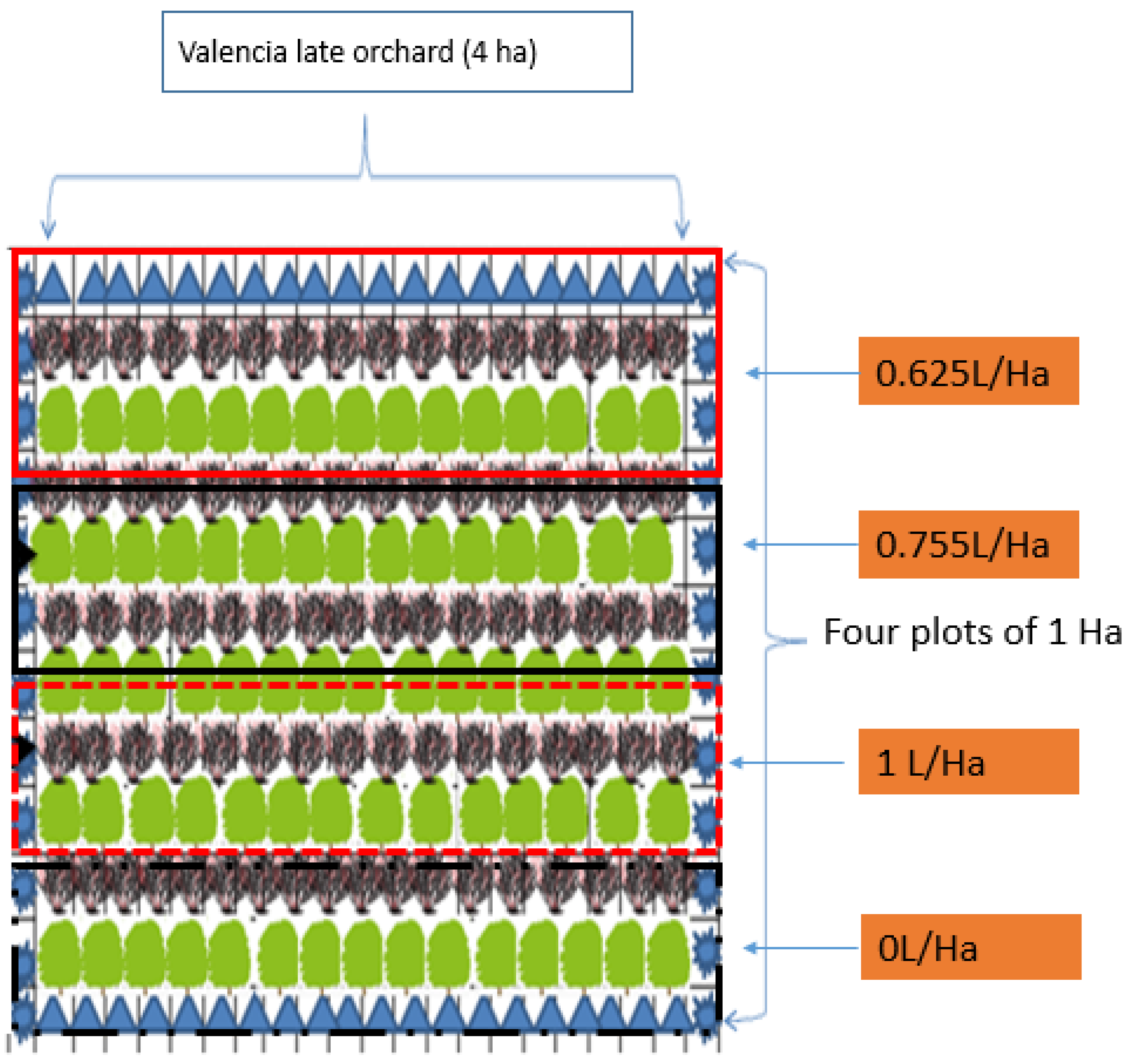
2.2. Sampling Design
2.3. Statistics
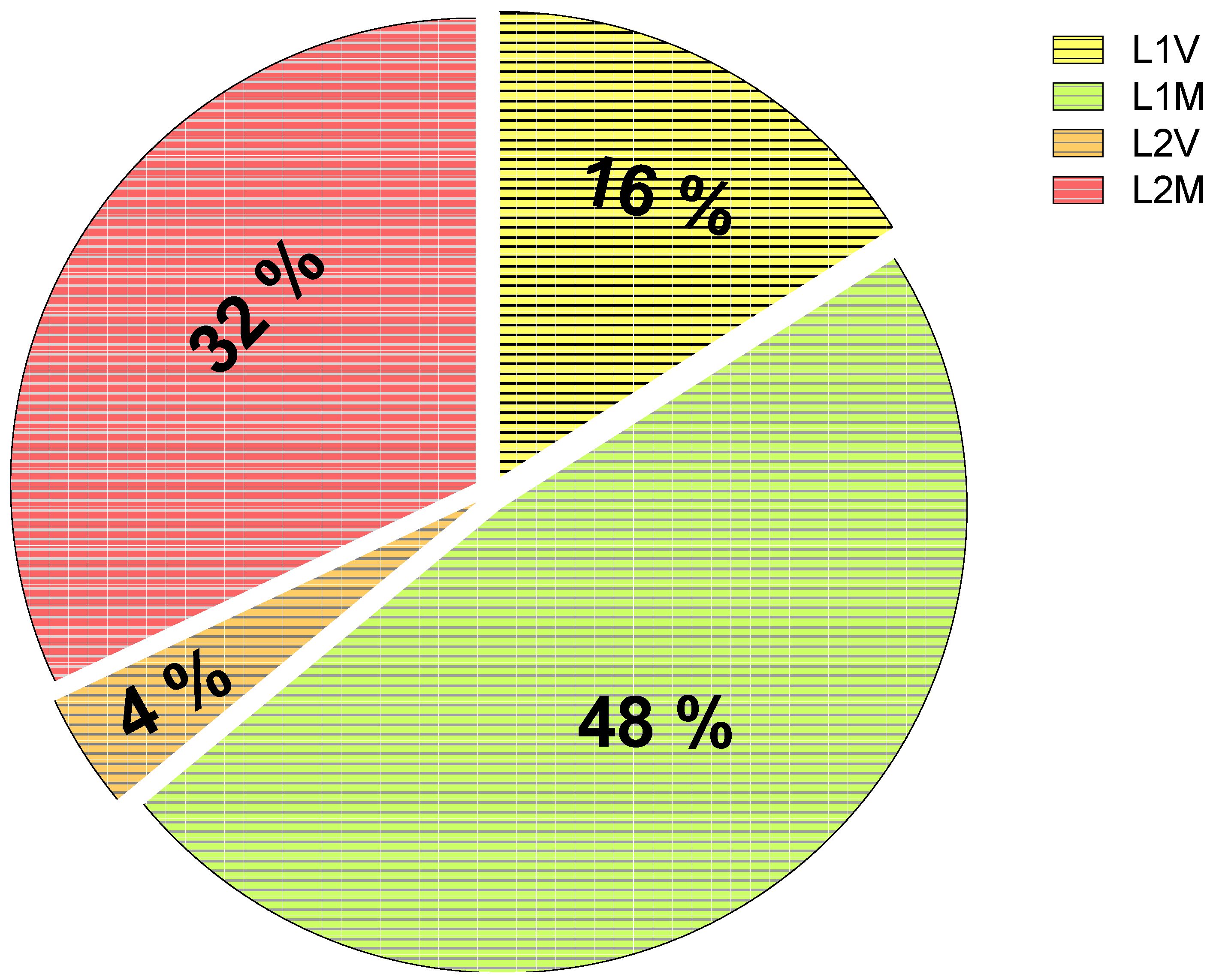
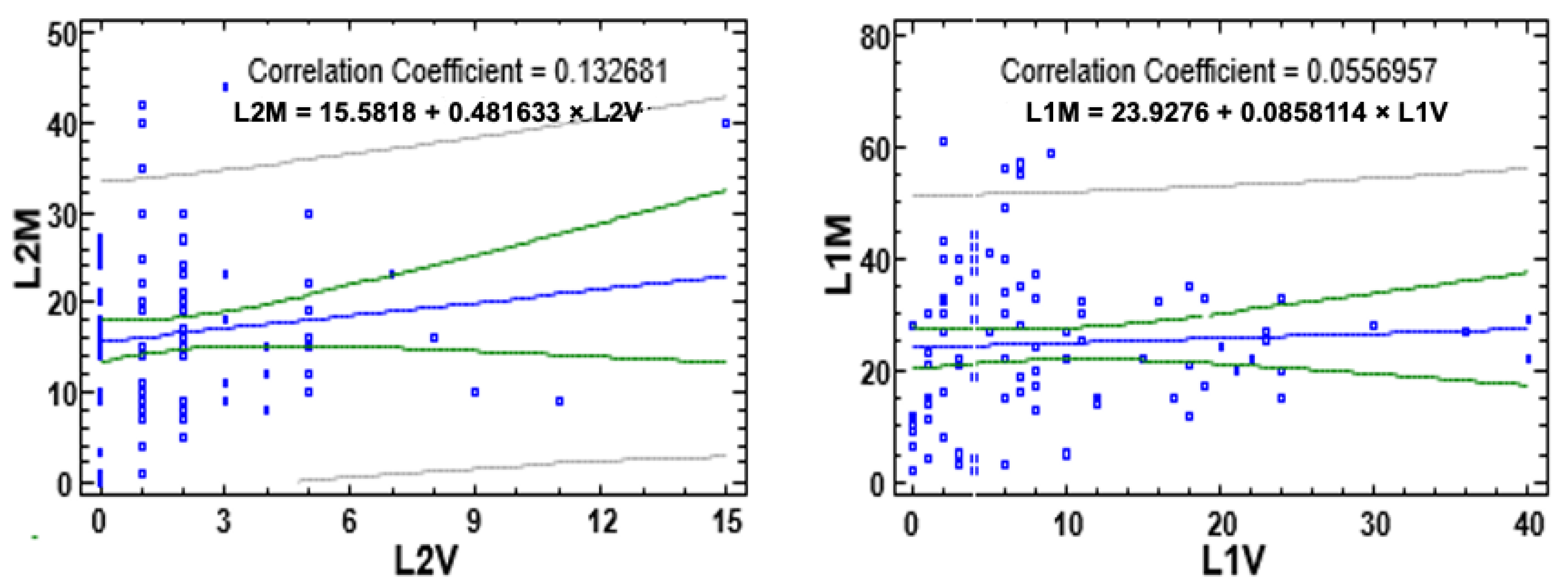
3. Results
3.1. Impact on Larvae
3.2. Impact on Females
4. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dambier, D.; Benyahia, H.; Pensabene-Bellavia, G.; Kaçar, Y.A.; Froelicher, Y.; Belfalah, Z.; Lhou, B.; Handaji, N.; Printz, B.; Morillon, R. Somatic Hybridization for Citrus Rootstock Breeding: An Effective Tool to Solve Some Important Issues of the Mediterranean Citrus Industry. Plant Cell Rep. 2011, 30, 883–900. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.D. Citron cultivation, production and uses in the Mediterranean region. In Medicinal and Aromatic Plants of the Middle-East; Springer: Berlin/Heidelberg, Germany, 2014; pp. 199–214. [Google Scholar]
- El-Otmani, M.; Coggins, C.W.; Duymovic, A. Citrus Cultivars and Production in Morocco. HortScience 1990, 25, 1343–1346. [Google Scholar] [CrossRef]
- Oubelkacem, A.; Scardigno, A.; Choukr-Allah, R. Treated Wastewater Reuse on Citrus in Morocco: Assessing the Economic Feasibility of Irrigation and Nutrient Management Strategies. Integr. Environ. Assess. Manag. 2020, 16, 898–909. [Google Scholar] [CrossRef]
- Porat, R.; Weiss, B.; Cohen, L.; Daus, A.; Aharoni, N. Reduction of Postharvest Rind Disorders in Citrus Fruit by Modified Atmosphere Packaging. Postharvest Biol. Technol. 2004, 33, 35–43. [Google Scholar] [CrossRef]
- Prusky, D. Reduction of the Incidence of Postharvest Quality Losses, and Future Prospects. Food Secur. 2011, 3, 463–474. [Google Scholar] [CrossRef]
- Tena, A.; Garcia-Marí, F. Current Situation of Citrus Pests and Diseases in the Mediterranean Basin. IOBC Bull. 2011, 62, 365–368. [Google Scholar]
- Gutierrez, A.P.; Ponti, L. Prospective Analysis of the Geographic Distribution and Relative Abundance of Asian Citrus Psyllid (Hemiptera: Liviidae) and Citrus Greening Disease in North America and the Mediterranean Basin. Fla. Entomol. 2013, 96, 1375–1391. [Google Scholar] [CrossRef]
- Ali, N. Communautés de Nématodes Phytoparasites Associés à L’olivier: Réponse Aux Forçages Anthropiques et Environnementaux. Ph.D. Thesis, Montpellier SupAgro, Montpellier, France, 2015. [Google Scholar]
- Mazih, A. Current Situation of Citrus Pests and the Control Methods in Use in Morocco. IOBC WPRS Bull. 2008, 38, 10–16. [Google Scholar]
- Mazih, A. Status of Citrus IPM in the Southern Mediterranean Basin Morocco, North Africa. Acta Hortic. 2015, 1065, 1097–1103. [Google Scholar] [CrossRef]
- Elekcioğlu, N.Z.; Ölçülü, M. Determination of Parasitoid and Predator Species of Chaff Scale [Parlatoria Pergandii Comstock (Hemiptera: Diaspididae)] in Citrus Orchards in Eastern Mediterranean Region of Turkey. Bitki Koruma Bülteni 2018, 58, 131–139. [Google Scholar]
- Jendoubi, H.; Suma, P.; Russo, A. Response of the Parlatoria Citrus Scale Insects (Hemiptera, Coccoidea) to Coloured Sticky Traps. In Proceedings of the VII International Scientific Agriculture Symposium, “Agrosym 2016”, Jahorina, Bosnia and Herzegovina, 6–9 October 2016; pp. 1529–1535. [Google Scholar]
- Jaouad, M.; Moinina, A.; Ezrari, S.; Lahlali, R. Key Pests and Diseases of Citrus Trees with Emphasis on Root Rot Diseases: An Overview. Moroc. J. Agric. Sci. 2020, 1, 149–160. [Google Scholar]
- Arenstein, Z.; Ausher, R.; Beicht, W.; Blair, B.D.; Blazquez, C.H.; Dinoor, A.; Dishon, I.; Edelbaum, G.; Elkana, J.; Eshel, J. Advisory Work in Crop Pest and Disease Management; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Michael, G.; Ong’amo, G.O.; Nderitu, J.; Watson, G.W.; Kinuthia, W. Diversity of Scale Insects (Hemiptera: Coccomorpha) Attacking Citrus Trees in Machakos, Makueni, Kilifi and Kwale Counties, Kenya. J. Agric. Sci. Pract. 2021, 6, 79–85. [Google Scholar]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide Exposure, Safety Issues, and Risk Assessment Indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.-Y.; Black, C. Taiwanese Farm Workers’ Pesticide Knowledge, Attitudes, Behaviors and Clothing Practices. Int. J. Environ. Health Res. 2015, 25, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Costa-Comelles, J.; Alonso, A.; Rodriguez, J.M. Efficacy of Some Citrus Pesticides on the Diaspidid Scales Lepidosaphes Beckii (Newman) and Parlatoria Pergandii Comstock (Homoptera, Diaspididae) and Impact on Nontarget Organisms. Bol. Sanid. Veg. Plagas Esp. 1994, 20, 357–369. [Google Scholar]
- Berni, I.; Menouni, A.; El, I.G.; Duca, R.-C.; Kestemont, M.-P.; Godderis, L.; El, S.J. Understanding Farmers’ Safety Behavior Regarding Pesticide Use in Morocco. Sustain. Prod. Consum. 2021, 25, 471–483. [Google Scholar] [CrossRef]
- El Bakouri, H.; Aassiri, A.; Morillo, J.; Usero, J.; Khaddor, M.; Ouassini, A. Pesticides and Lipids Occurrence in Tangier Agricultural Soil (Northern Morocco). Appl. Geochem. 2008, 23, 3487–3497. [Google Scholar] [CrossRef]
- Abbassi, M. Notes Bio-Écologiques Sur Parlatoria Pergandei COMSTOCK (Homopt. Coccidae) Au Maroc. Fruits 1975, 30, 179–184. [Google Scholar]
- García-Marí, F.; Rodrigo, E. Life Cycle of the Diaspidids Aonidiella Aurantii, Lepidosaphes Beckii and Parlatoria Pergandii in an Orange Grove in Valencia (Spain). IOBC WPRS Bull. 1995, 18, 118. [Google Scholar]
- Jebbour, Y.; Abbassi, M. Recrudescence Du Pou Violet Parlatoria Pergandii (Comstock). Homoptera Diaspi-Didae, Sur Agrumes Au Maroc: Biologie et Écologie de l’espèce. Al Awamia 2006, 3, 118–119. [Google Scholar]
- Rodrigo, M.E.; Garcia-Mari, F.; Rodriguez-Reina, J.M.; Olmeda, T. Colonization of Growing Fruit by the Armored Scales Lepidosaphes Beckii, Parlatoria Pergandii and Aonidiella Aurantii (Hom., Diaspididae). J. Appl. Entomol. 2004, 128, 569–575. [Google Scholar] [CrossRef]
- Rosen, D.; De Bach, P. Species of Aphytis of the World (Hymenoptera: Aphelinidae); Series Entomologica; Junk, W., Ed.; The Hague: Boston, MA, USA; London, UK, 1979; Volume 17. [Google Scholar]
- Rodrigo, E.; Troncho, P.; García-Marí, F. Parasitoeds (Hym.: Aphelinidae) of Three Scale Insects (Hom.: Diaspididae) in a Citrus Grove in Valencia, Spain. Entomophaga 1996, 41, 77–94. [Google Scholar] [CrossRef]
- Planes, L.; Catalán, J.; Tena, A.; Porcuna, J.L.; Jacas, J.A.; Izquierdo, J.; Urbaneja, A. Lethal and Sublethal Effects of Spirotetramat on the Mealybug Destroyer, Cryptolaemus Montrouzieri. J. Pest Sci. 2013, 86, 321–327. [Google Scholar] [CrossRef]
- Kühnhold, J.; Klueken, A.M.; De Maeyer, L.; Van Waetermeulen, X.; Brück, E.; Elbert, A. Movento®, an Innovative Solution for Sucking Insect Pest Control in Agriculture: Field Performance in Fruits and Vegetables. Bayer Crop. J. 2008, 61, 279–306. [Google Scholar]
- Mohapatra, S.; Deepa, M.; Jagadish, G.K. An Efficient Analytical Method for Analysis of Spirotetramat and Its Metabolite Spirotetramat-Enol by HPLC. Bull. Environ. Contam. Toxicol. 2012, 88, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Hassouni, T.; Belghyti, D. Distribution of Gastrointestinal Helminths in Chicken Farms in the Gharb Region—Morocco. Parasitol. Res. 2006, 99, 181–183. [Google Scholar] [CrossRef]
- Benyahia, H.; Talha, A.; Fadli, A.; Chetto, O.; Omari, F.E.; Beniken, L. Performance of ‘Valencia Late’Sweet Orange (Citrus Sinensis) on Different Rootstocks in the Gharb Region (Northwestern Morocco). Annu. Res. Rev. Biol. 2017, 20, 1–11. [Google Scholar] [CrossRef]
- Mansouri, I.; Squalli, W.; El Agy, A.; El-Hassani, A.; El Ghadraoui, L.; Dakki, M. Comparison of Nesting Features and Breeding Success of Turtle Dove Streptopelia Turtur between Orchards and Riparian Habitats. Int. J. Zool. 2021, 2021, 5566398. [Google Scholar] [CrossRef]
- Cankaya, S. A Comparative Study of Some Estimation Methods for Parameters and Effects of Outliers in Simple Regression Model for Research on Small Ruminants. Trop. Anim. Health Prod. 2009, 41, 35–41. [Google Scholar] [CrossRef]
- Abdel-Aty, M.; Abdalla, M.F. Modeling Drivers’ Diversion from Normal Routes under ATIS Using Generalized Estimating Equations and Binomial Probit Link Function. Transportation 2004, 31, 327–348. [Google Scholar] [CrossRef]
- Berlinger, M.J.; Gol’berg, A.M. The Effect of the Fruit Sepals on the Citrus Mealybug Population and on Its Parasite. Entomol. Exp. Appl. 1978, 24, 238–243. [Google Scholar] [CrossRef]
- Franco, J.C.; Suma, P.; Da Silva, E.B.; Blumberg, D.; Mendel, Z. Management Strategies of Mealybug Pests of Citrus in Mediterranean Countries. Phytoparasitica 2004, 32, 507–522. [Google Scholar] [CrossRef]
- Carrière, Y. Haplodiploidy, Sex, and the Evolution of Pesticide Resistance. J. Econ. Entomol. 2003, 96, 1626–1640. [Google Scholar] [CrossRef]
- Kedia, A.; Prakash, B.; Mishra, P.K.; Singh, P.; Dubey, N.K. Botanicals as Eco Friendly Biorational Alternatives of Synthetic Pesticides against Callosobruchus Spp. (Coleoptera: Bruchidae)-A Review. J. Food Sci. Technol. 2015, 52, 1239–1257. [Google Scholar] [CrossRef]
- Amirahmadi, M.; Shoeibi, S.; Abdollahi, M.; Rastegar, H.; Khosrokhavar, R.; Hamedani, M.P. Monitoring of Some Pesticides Residue in Consumed Tea in Tehran Market. Iran. J. Environ. Health Sci. Eng. 2013, 10, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, U.; Sandhu, K.S. Effect of Handling and Processing on Pesticide Residues in Food-A Review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbaneja, A.; Grout, T.G.; Gravena, S.; Wu, F.; Cen, Y.; Stansly, P.A. Citrus pests in a global world. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–348. [Google Scholar]
- Belkhiri, D.; Biche, M.; Mehaoua, M.S. Effet de Spirotetram Ate Surl’ Evolution des Larves et des Adultes de Parlatoria Blanchardi du Palmier Dattier en Algerie Parlatoria Blanchardi of the Date Palm in Algeria. Courr. Savoir 2018, 25, 157–161. [Google Scholar]
- Stanley, J.; Preetha, G. Pesticide Toxicity to Non-Target Organisms: Exposure, Toxicity and Risk Assessment Methodologies; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-94-017-7752-0. [Google Scholar]
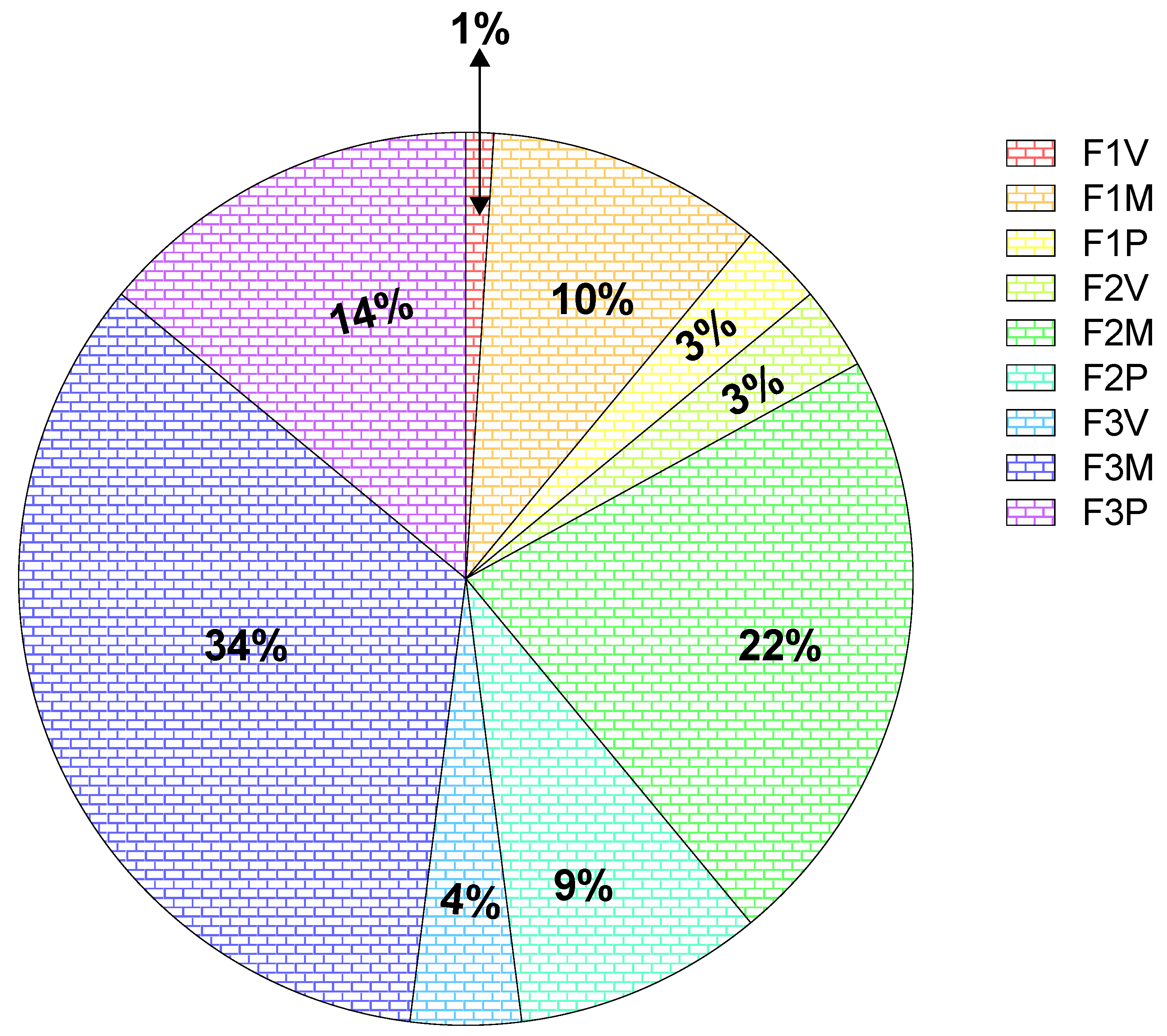
| Compared Parameters | t | df | p-Value |
|---|---|---|---|
| L1V-L1M | −10.223 | 98 | 0.000 |
| L2V-L2M | −16.183 | 98 | 0.000 |
| L1V-L2V | 7.948 | 98 | 0.000 |
| L1M-L2M | 5.263 | 98 | 0.000 |
| L1V | L1M | L2V | L2M | |
|---|---|---|---|---|
| T0 | 16.08 a ± 2.32 | 17.70 b ± 1.73 | 3.95 a ± 0.53 | 11.75 b ± 1.19 |
| T1 | 5.04 b ± 0.68 | 26.08 ab ± 2.89 | 0.91 b ± 0.17 | 14.95 ab ± 1.19 |
| T2 | 6.12 b ± 1.46 | 29.37 a ± 2.94 | 0.79 b ± 0.15 | 17.33 a ± 2.17 |
| T3 | 6.92 b ± 1.30 | 25.37 ab ± 2.68 | 2.22 b ± 0.61 | 21.48 a ± 1.82 |
| Sum of Squares | df | Mean Square | F | p-Value | ||
|---|---|---|---|---|---|---|
| F1V | Between Groups | 61.365 | 3 | 20.455 | 5.476 | 0.002 |
| Within Groups | 343.625 | 92 | 3.735 | |||
| Total | 404.990 | 95 | ||||
| F2V | Between Groups | 158.531 | 3 | 52.844 | 4.687 | 0.004 |
| Within Groups | 1037.208 | 92 | 11.274 | |||
| Total | 1195.740 | 95 | ||||
| F3V | Between Groups | 125.531 | 3 | 41.844 | 1.974 | 0.123 |
| Within Groups | 1950.208 | 92 | 21.198 | |||
| Total | 2075.740 | 95 | ||||
| F1M | Between Groups | 1766.365 | 3 | 588.788 | 8.181 | 0.000 |
| Within Groups | 6621.375 | 92 | 71.971 | |||
| Total | 8387.740 | 95 | ||||
| F2M | Between Groups | 3655.031 | 3 | 1218.344 | 6.511 | 0.000 |
| Within Groups | 17,213.958 | 92 | 187.108 | |||
| Total | 20,868.990 | 95 | ||||
| F3M | Between Groups | 11,303.865 | 3 | 3767.955 | 13.589 | 0.000 |
| Within Groups | 25,509.292 | 92 | 277.275 | |||
| Total | 36,813.156 | 95 | ||||
| F1P | Between Groups | 618.698 | 3 | 206.233 | 19.971 | 0.000 |
| Within Groups | 950.042 | 92 | 10.327 | |||
| Total | 1568.740 | 95 | ||||
| F2P | Between Groups | 78.781 | 3 | 26.260 | 0.382 | 0.767 |
| Within Groups | 6332.208 | 92 | 68.828 | |||
| Total | 6410.990 | 95 | ||||
| F3P | Between Groups | 279.750 | 3 | 93.250 | 0.516 | 0.672 |
| Within Groups | 16,634.750 | 92 | 180.813 | |||
| Total | 16,914.500 | 95 | ||||
| F1V | F1M | F1P | F2V | F2M | F2P | F3V | F3M | F3P | |
|---|---|---|---|---|---|---|---|---|---|
| T0 | 2.83 a ± 0.41 | 9.75 b ± 0.94 | 6.20 a ± 0.79 | 6.20 a ± 0.92 | 21.16 b ± 2.27 | 23.91 a ± 1.51 | 12.08 a ± 0.98 | 42.41 b ± 2.28 | 40.25 a ± 1.87 |
| T1 | 1.95 ab ± 0.59 | 18.83 a ± 2.32 | 4.75 ab ± 0.93 | 3.66 b ± 0.77 | 37.16 a ± 2.21 | 14.5 b ± 0.99 | 4.5 b ± 0.50 | 52.25 ab ± 3.20 | 20.45 b ± 1.22 |
| T2 | 1.16 ab ± 0.20 | 18.16 a ± 1.82 | 3.87 ab ± 0.87 | 2.58 b ± 0.31 | 40.20 a ± 3.49 | 8.79 c ± 0.69 | 4.58 b ± 0.53 | 57.25 a ± 5.07 | 13.45 c ± 1.23 |
| T3 | 0.66 b ± 0.15 | 17.14 a ± 1.67 | 2.90 b ± 0.43 | 2.47 b ± 0.30 | 32.95 a ± 2.23 | 8.95 c ± 1.02 | 5.57 b ± 0.59 | 54.04 ab ± 4.41 | 11.57 c ± 1.11 |
| Estimate | Standard Error | Wald Test | p-Value | ||
|---|---|---|---|---|---|
| Survival | Intercept | −1.073 | 0.530 | 4.101 | 0.043 |
| T0 | −0.118 | 0.038 | 9.523 | 0.002 | |
| T1 | 0.063 | 0.028 | 4.991 | 0.055 | |
| T2 | −0.001 | 0.002 | 0.525 | 0.539 | |
| T3 | −0.136 | 0.563 | 0.561 | 0.056 | |
| Scale | 1.000 | 0.000 | |||
| Mortalities | Intercept | −1.046 | 0.480 | 5.212 | 0.065 |
| T0 | −0.161 | 0.073 | 8.431 | 0.042 | |
| T1 | 0.058 | 0.033 | 9.047 | 0.002 | |
| T2 | −0.051 | 0.012 | 10.321 | 0.001 | |
| T3 | −0.456 | 0.451 | 0.621 | <0.001 | |
| Scale | 1.000 | 0.000 | |||
| Parasitoid Survival | Intercept | −1.064 | 0.690 | 5.654 | 0.007 |
| T0 | −0.543 | 0.053 | 10.632 | 0.001 | |
| T1 | 0.454 | 0.032 | 7.431 | 0.05 | |
| T2 | −0.034 | 0.0453 | 0.565 | 0.789 | |
| T3 | −0.136 | 0.563 | 0.674 | 0.796 | |
| Scale | 1.000 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assouguem, A.; Kara, M.; Mansouri, I.; Imtara, H.; AlZain, M.N.; Mechchate, H.; Conte, R.; Squalli, W.; Farah, A.; Lazraq, A. Evaluation of the Effectiveness of Spirotetramat on the Diaspine Scale Parlatoria pergandii in Citrus Orchards. Agronomy 2021, 11, 1562. https://doi.org/10.3390/agronomy11081562
Assouguem A, Kara M, Mansouri I, Imtara H, AlZain MN, Mechchate H, Conte R, Squalli W, Farah A, Lazraq A. Evaluation of the Effectiveness of Spirotetramat on the Diaspine Scale Parlatoria pergandii in Citrus Orchards. Agronomy. 2021; 11(8):1562. https://doi.org/10.3390/agronomy11081562
Chicago/Turabian StyleAssouguem, Amine, Mohammed Kara, Ismail Mansouri, Hamada Imtara, Mashail N. AlZain, Hamza Mechchate, Raffaele Conte, Wafae Squalli, Abdellah Farah, and Abderrahim Lazraq. 2021. "Evaluation of the Effectiveness of Spirotetramat on the Diaspine Scale Parlatoria pergandii in Citrus Orchards" Agronomy 11, no. 8: 1562. https://doi.org/10.3390/agronomy11081562
APA StyleAssouguem, A., Kara, M., Mansouri, I., Imtara, H., AlZain, M. N., Mechchate, H., Conte, R., Squalli, W., Farah, A., & Lazraq, A. (2021). Evaluation of the Effectiveness of Spirotetramat on the Diaspine Scale Parlatoria pergandii in Citrus Orchards. Agronomy, 11(8), 1562. https://doi.org/10.3390/agronomy11081562









