Phylogenetic Analyses of Rhizobia Isolated from Nodules of Lupinus angustifolius in Northern Tunisia Reveal Devosia sp. as a New Microsymbiont of Lupin Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Sites, Isolation of Bacterial Strains and Culture Conditions
2.2. Nodulation and Cross-Inoculation Test
2.3. DNA Isolation, PCR Amplifications and Sequencing
2.4. Phenotypic Characterization
2.5. Phylogenetic Analyses
3. Results
3.1. Isolation and Symbiotic Efficiency of L. angustifolius-Nodulating Bacteria from Northern Tunisia
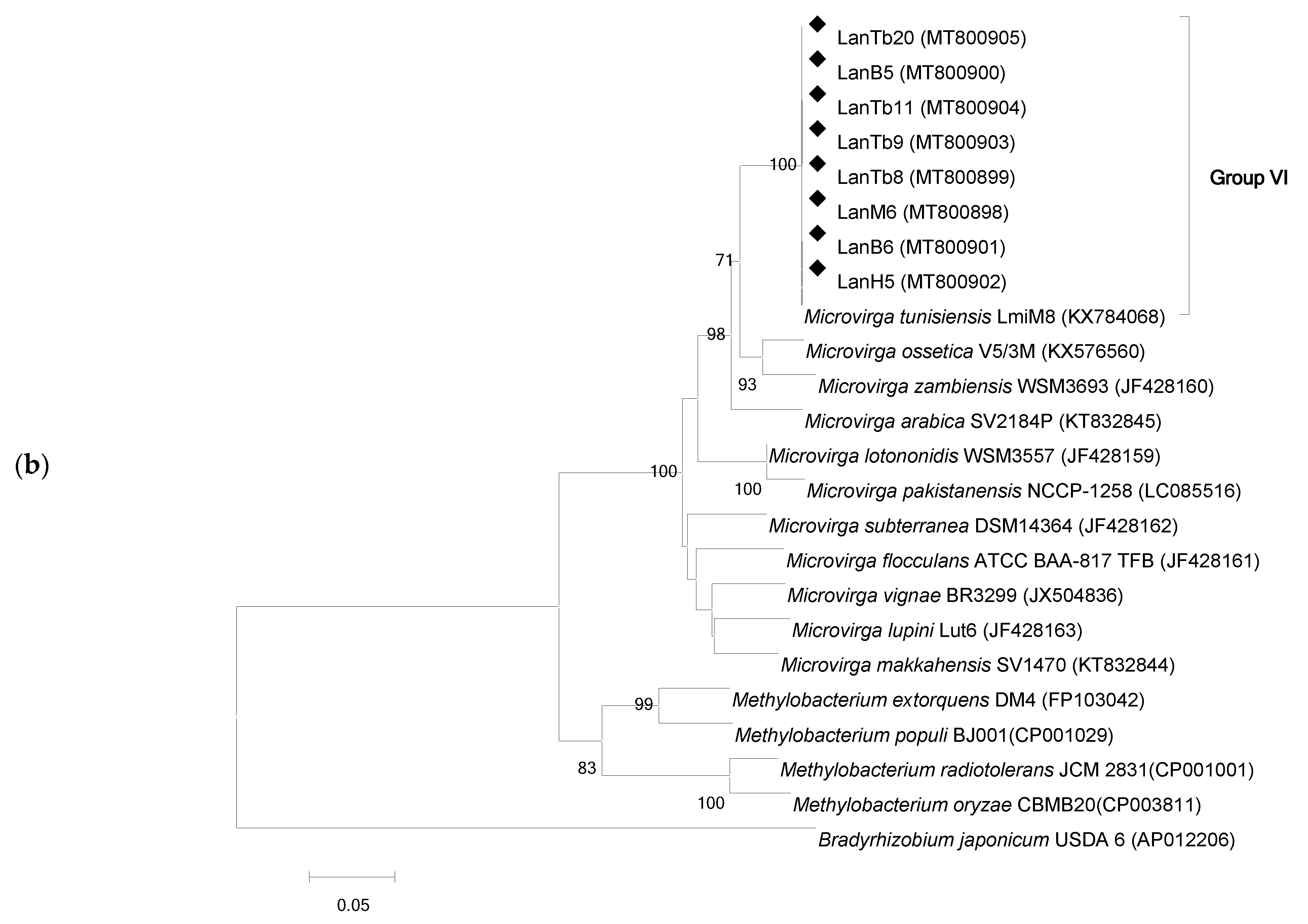
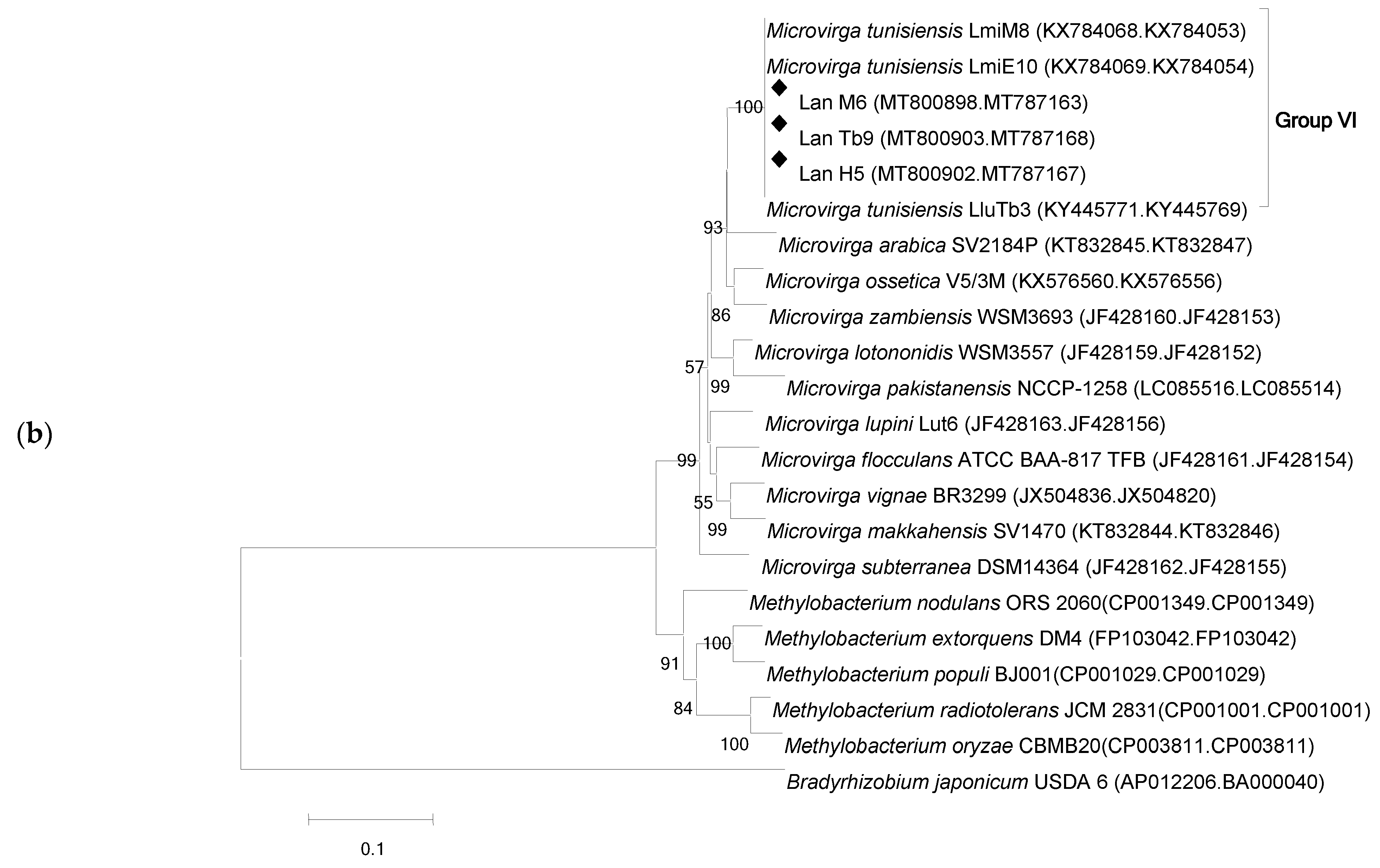
3.2. Phylogenetic Analysis Based on recA and gyrB Genes
3.3. 16S rRNA Gene Phylogeny
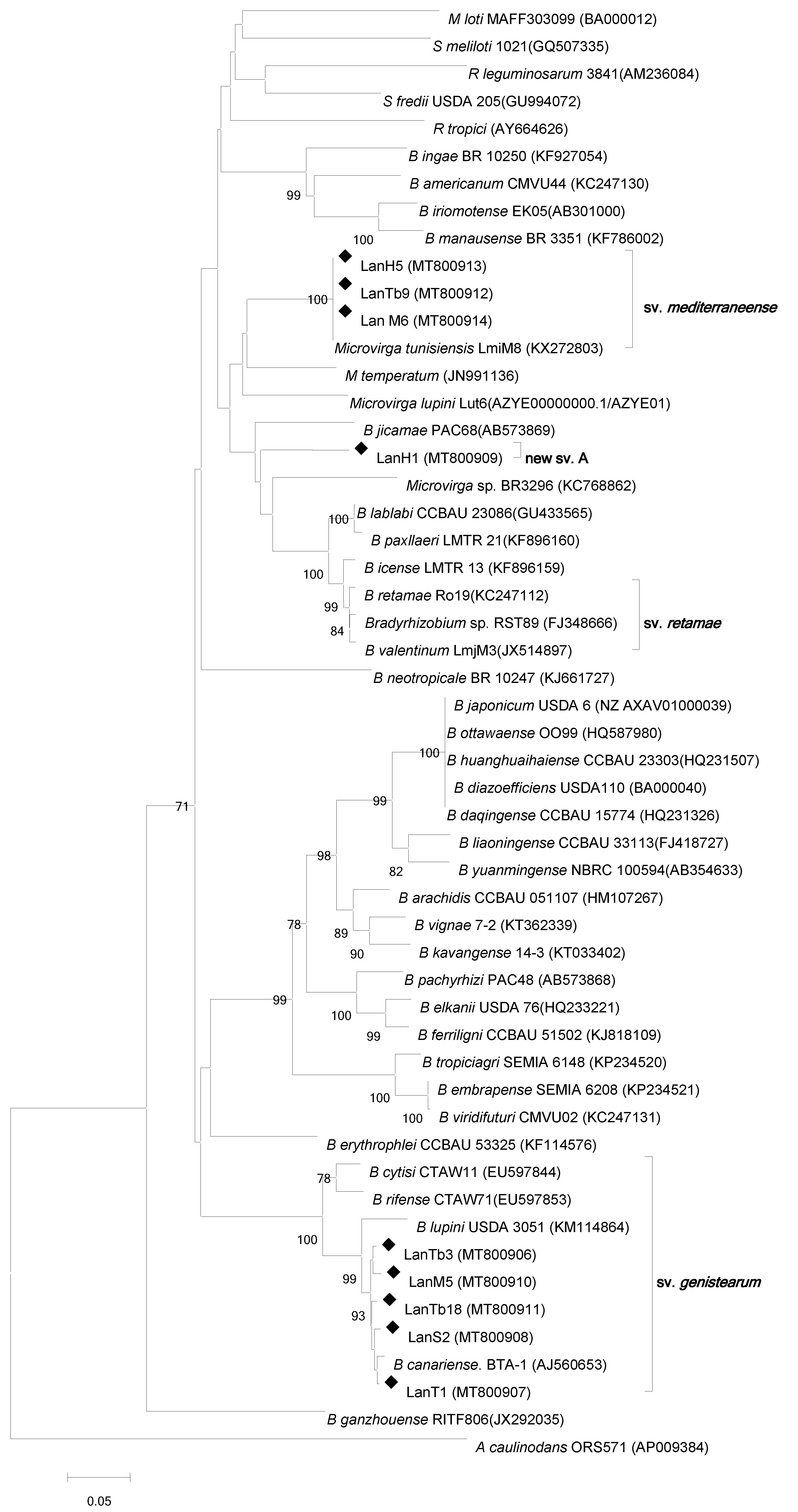
3.4. NodC Gene Phylogeny
3.5. Host Range of Rhizobial Strains Isolated from L. angustifolius Nodules
3.6. Phenotypic Analyses of Narrow-Leaf Lupin-Nodulating Strains
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Allen, E.K.; Allen, O.N. The Leguminosae: A Source Book of Characteristics, Uses and Nodulation; University of Wisconsin Press: Madison, WI, USA, 1981; p. 812. [Google Scholar]
- Cardoso, D.; Pennington, R.T.; de Queiroz, L.P.; Boatwright, J.S.; Van Wyk, B.E.; Wojciechowski, M.F.; Lavin, M. Reconstructing the deep-branching relationships of the papilionoid legumes. S. Afr. J. Bot. 2013, 89, 58–79. [Google Scholar] [CrossRef]
- Ainouché, A.K.; Bayer, R.J. Phylogenetic relationships in Lupinus (Fabaceae: Papilionoideae) based on internal transcribed spacer sequences (ITS) of nuclear ribosomal DNA. Am. J. Bot. 1999, 86, 590–607. [Google Scholar] [CrossRef]
- Drummond, C.S.; Eastwood, R.J.; Miotto, S.T.S.; Hughes, C.E. Multiple continental radiations and correlates of diversification in Lupinus (Leguminosae): Testing for key innovation within complete taxón sampling. Syst. Biol. 2012, 61, 443–460. [Google Scholar] [CrossRef]
- Cristofolini, G.; Chiapella, L.F. Origin and diversification of Genisteae (Fabaceae): A serosystematic purview. Webbia 1984, 38, 105–122. [Google Scholar] [CrossRef]
- Cristofolini, G. The biodiversity of the “Leguminosae-Genisteae” and its genesis. Lagascalia 1997, 19, 121–128. [Google Scholar]
- Mahé, F.; Pascual, H.; Coriton, O.; Huteau, V.; Navarro Perris, A.; Misset, M.T.; Aınouche, A. New data and phylogenetic placement of the enigmatic Old World lupin: Lupinus mariae-josephae H. Pascual. Genet. Res. Crop Evol. 2011, 58, 101–114. [Google Scholar] [CrossRef]
- Naganowska, B.; Wolko, B.S.; Liwinska, E.; Kaczmarek, Z.; Schifino-Wittmann, M.T. 2C DNA variation and relationships among New World species of the genus Lupinus (Fabaceae). Plant Syst. Evol. 2006, 256, 147–157. [Google Scholar] [CrossRef]
- Sprent, J.I. Nodulation in Legumes; Royal Botanic Gardens, Kew: London, UK, 2001; p. 146. [Google Scholar]
- Coba de la Peña, T.; Pueyo, J.J. Legumes in the reclamation of marginal soils, from cultivar and inoculant selection to transgenic approaches. Agron. Sustain. Dev. 2012, 32, 65–91. [Google Scholar] [CrossRef]
- Pueyo, J.J.; Quiñones, M.A.; Coba de la Peña, T.; Fedorova, E.E.; Lucas, M.M. Nitrogen and phosphorus interplay in lupin root nodules and cluster roots. Front. Plant Sci. 2021, 12, 644218. [Google Scholar] [CrossRef]
- Falkengren-Grerup, U.; Schottelndreier, M. Vascular plants as indicators of nitrogen enrichment in soils. Plant Ecol. 2004, 172, 51–62. [Google Scholar] [CrossRef]
- Bahmanyar, M.A.; Ranjbar, G.A. The role of potassium in improving growth índices and increasing amount of grain nutrient elements of wheat cultivars. J. Appl. Sci. 2008, 8, 1280–1285. [Google Scholar] [CrossRef][Green Version]
- Leporatti, M.L.; Ghedira, K. Comparative analysis of medicinal plants used in traditional medicine in Italy and Tunisia. J. Ethnobiol. Ethnomed. 2009, 5, 31. [Google Scholar] [CrossRef]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Editorial: Protein crops: Food and feed for the future. Front. Plant Sci. 2017, 8, 105. [Google Scholar] [CrossRef][Green Version]
- Andrews, M.; Andrews, M.E. Specificity in legume-rhizobia symbioses. Int. J. Mol. Sci. 2017, 18, 705. [Google Scholar] [CrossRef] [PubMed]
- Jarabo-Lorenzo, A.; Perez-Galdona, R.; Donate-Correa, J.; Rivas, R.; Velazquez, E.; Hernandez, M.; Temprano, F.; Martinez-Molina, E.; Ruiz-Argueso, T.; Leon-Barrios, M. Genetic diversity of bradyrhizobial populations from diverse geographic origins that nodulate Lupinus spp. and Ornithopus spp. Syst. Appl. Microbiol. 2003, 26, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Stepkowski, T.; Zak, M.; Moulin, L.; Kroliczak, J.; Golinska, B.; Narozna, D.; Safronova, V.I.; Madrzak, C.J. Bradyrhizobium canariense and Bradyrhizobium japonicum are the two dominant rhizobium species in root nodules of lupin and serradella plants growing in Europe. Syst. Appl. Microbiol. 2011, 34, 368–375. [Google Scholar] [CrossRef]
- Velázquez, E.; Valverde, A.; Rivas, R.; Gomis, V.; Peix, A.; Gantois, I.; Igual, J.M.; Leon-Barrios, M.; Willems, A.; Mateos, P.F.; et al. Strains nodulating Lupinus albus on different continents belong to several new chromosomal and symbiotic lineages within Bradyrhizobium. Antonie Leeuwenhoek 2010, 97, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Mellal, H.; Yacine, B.; Boukaous, L.; Khouni, S.; Benguedouar, A.; Castellano-Hinojosa, A.; Bedmar, E.J. Phylogenetic diversity of Bradyrhizobium strains isolated from root nodules of Lupinus angustifolius grown wild in the North East of Algeria. Syst. Appl. Microbiol. 2019, 42, 397–402. [Google Scholar] [CrossRef]
- Granada, C.E.; Beneduzi, A.; Lisboa, B.B.; Turchetto-Zolet, A.C.; Vargas, L.K.; Passaglia, L.M. Multilocus sequence analysis reveals taxonomic differences among Bradyrhizobium sp. symbionts of Lupinus albescens plants growing in arenized and non-arenized areas. Syst. Appl. Microbiol. 2015, 38, 323–329. [Google Scholar] [CrossRef]
- Durán, D.; Rey, L.; Sánchez-Cañizares, C.; Navarro, A.; Imperial, J.; Ruiz-Argüeso, T. Genetic diversity of indigenous rhizobial symbionts of the Lupinus mariae-josephae endemism from alkaline-limed soils within its area of distribution in Eastern Spain. Syst. Appl. Microbiol. 2012, 36, 128–136. [Google Scholar] [CrossRef]
- Trujillo, M.E.; Willems, A.; Abril, A.; Planchuelo, A.M.; Rivas, R.; Ludena, D.; Mateos, P.F.; Martinez-Molina, E.; Velázquez, E. Nodulation of Lupinus albus by strains of Ochrobactrum lupini sp. nov. Appl. Environ. Microbiol. 2004, 71, 1318–1327. [Google Scholar] [CrossRef]
- González-Sama, A.; Lucas, M.M.; De Felipe, M.R.; Pueyo, J.J. An unusual infection mechanism and nodule morphogenesis in white lupin (Lupinus albus). New Phytol. 2004, 163, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.; Velázquez, E.; Fernández-Santos, F.; Vizcaíno, N.; Rivas, R.; Mateos, P.F.; Martínez-Molina, E.; Igual, J.M.; Willems, A. Phyllobacterium trifolii sp. nov., nodulating Trifolium and Lupinus in Spanish soils. Int. J. Syst. Evol. Microbiol. 2005, 55, 1985–1989. [Google Scholar] [CrossRef]
- El-Idrissi, M.M.; Aujjar, N.; Belabed, A.; Dessaux, Y.; Filali-Maltouf, A. Characterization of rhizobia isolated from carob tree (Ceratonia siliqua). J. Appl. Bacteriol. 1996, 80, 165–173. [Google Scholar] [CrossRef]
- Ardley, J.K.; Parker, M.A.; De Meyer, S.E.; Trengove, R.D.; O’Hara, G.W.; Reeve, W.G.; Yates, R.J.; Dilworth, M.J.; Willems, A.; Howieson, J.G. Microvirga lupini sp. nov., Microvirga lotononidis sp. nov. and Microvirga zambiensis sp. nov are alphaproteobacterial root-nodule bacteria that specifically nodulate and fix nitrogen with geographically and taxonomically separate legume hosts. Int. J. Syst. Evol. Microbiol. 2012, 62, 2579–2588. [Google Scholar] [PubMed]
- Msaddak, A.; Rejili, M.; Duran, D.; Rey, L.; Mars, M.; Palacios, J.; Ruiz-Argueso, T.; Imperial, J. Microvirga tunisiensis sp. nov., a root nodule symbiotic bacterium isolated from Lupinus micranthus and L. luteus grown in Northern Tunisia. Syst. Appl. Microbiol. 2019, 42, 6. [Google Scholar] [CrossRef]
- Tounsi-Hammami, S.; Le Roux, C.; Dhane-Fitouri, S.; De Lajudie, P.; Duponnois, R.; Ben Jeddi, F. Genetic diversity of rhizobia associated with root nodules of white lupin (Lupinus albus L.) in Tunisian calcareous soils. Syst. Appl. Microbiol. 2019, 42, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Sakane, T.; Yokota, A. Transfer of “Pseudomonas riboflavin” (Foster 1944), a gram-negative, motile rod with long-chain 3-hydroxy fatty acids, to Devosia riboflavin gen. nov., sp. nov., nom. rev. Int. J. Syst. Bacteriol. 1996, 46, 16–22. [Google Scholar] [CrossRef][Green Version]
- Hoque, M.S.; Broadhurst, L.M.; Thrall, P.H. Genetic characterization of root-nodule bacteria associated with Acacia salicina and A. stenophylla (Mimosaceae) across south-eastern Australia. Int. J. Syst. Evol. Microbiol. 2011, 61, 299–309. [Google Scholar] [CrossRef]
- Bautista, V.V.; Monsalud, R.G.; Yokota, A. Devosia yakushimensis sp nov., isolated from root nodules of Pueraria lobata (Willd.) Ohwi. Int. J. Syst. Evol. Microbiol 2010, 60, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Rivas, R.; Velazquez, E.; Willems, A.; Vizcaino, N.; Subba-Rao, N.S.; Mateos, P.F.; Gillis, M.; Dazzo, F.B.; Martinez-Molina, E. A new species of Devosia that forms a unique nitrogen-fixing root-nodule symbiosis with the aquatic legume Neptunia natans (L.f.) Druce. Appl. Environ. Microbiol. 2002, 68, 5217–5222. [Google Scholar] [CrossRef]
- Rejili, M.; Msaddak, A.; Filali, I.; Benabderrahim, M.A.; Mars, M.; Marın, M. New chromosomal lineages within Microvirga and Bradyrhizobium genera nodulate Lupinus angustifolius growing on different Tunisian soils. FEMS Microbiol. Ecol. 2019, 95, fiz118. [Google Scholar] [CrossRef]
- Vincent, J.M. A Manual for the Practical Study of Root Nodule Bacteria; International Biological Programme, Number 15; Blackwell Scientific: Oxford, UK, 1970. [Google Scholar]
- Msaddak, A.; Durán, D.; Rejili, M.; Mars, M.; Ruiz-Argüeso, T.; Imperial, J.; Palacios, J.; Rey, L. Diverse bacteria affiliated with the genera Microvirga, Phyllobacterium and Bradyrhizobium nodulate Lupinus micranthus growing in soils of Northern Tunisia. Appl. Environ. Microbiol. 2017, 83, 6. [Google Scholar] [CrossRef] [PubMed]
- Baele, M.; Baele, P.; Vaneechoutte, M.; Storms, V.; Butaye, P.; Devriese, L.A.; Verschraegen, G.; Gillis, M.; Haesebrouck, F. Application of tRNA intergenic spacer PCR for identification of Enterococcus species. J. Clin. Microbiol. 2000, 38, 4201–4207. [Google Scholar] [CrossRef]
- Radl, V.; Simões-Araújo, J.L.; Leite, J.; Passos, S.R.; Martins, L.M.V.; Xavier, G.R.; Rumjanek, N.G.; Baldani, J.I.; Zilli, J.E. Microvirga vignae sp. nov., a root nodule symbiotic bacterium isolated from cowpea grown in semi-arid Brazil. Int. J. Syst. Evol. Microbiol. 2014, 64, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Guerrouj, K.; Ruíz-Díez, B.; Chahboune, R.; Ramírez-Bahena, M.H.; Abdelmoumen, H.; Quinones, M.A.; ElIdrissi, M.M.; Velázquez, E.; Fernández-Pascual, M.; Bedmar, E.J.; et al. Definition of a novel symbiovar (sv. retamae) within Bradyrhizobium retamae sp. nov., nodulating Retama sphaerocarpa and Retama monosperma. Syst. Appl. Microbiol. 2013, 36, 218–223. [Google Scholar] [PubMed]
- Msaddak, A.; Rejili, M.; Duran, D.; Rey, L.; Palacios, J.M.; Imperial, J.; Ruiz-Argueso, T.; Mars, M. Definition of two new symbiovars, sv. lupini and sv. mediterranense, within the genera Bradyrhizobium and Phyllobacterium efficiently nodulating Lupinus micranthus in Tunisia. Syst. Appl. Microbiol. 2018, 41, 487–493. [Google Scholar] [CrossRef]
- Chamber, M.A.; Iruthayathas, E.E. Nodulation and nitrogen fixation by fast- and slow-growing rhizobia strains of soybean on several temperate and tropical legumes. Plant Soil 1988, 112, 239–245. [Google Scholar] [CrossRef]
- El-Akhal, M.R.; Rincón, A.; Arenal, F.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Genetic diversity and symbiotic efficiency of rhizobial isolates obtained from nodules of Arachis hypogaea in Northwestern Morocco. Soil Biol. Biochem. 2008, 40, 2911–2914. [Google Scholar] [CrossRef]
- Vinuesa, P.; León-Barrios, M.; Silva, C.; Willems, A.; Jarabo-Lorenzo, A.; Pérez-Galdona, R.; Werner, D.; Martínez-Romero, E. Bradyrhizobium canariense sp. nov., an acid-tolerant endosymbiont that nodulates endemic genistoid legumes (Papilionoideae: Genisteae) from the Canary Islands, along with Bradyrhizobium japonicum bv. genistearum, Bradyrhizobium genospecies alpha and Bradyrhizobium genospecies beta. Int. J. Syst. Evol. Microbiol 2005, 55, 569–575. [Google Scholar]
- Subba-Rao, N.S.; Mateos, P.F.; Baker, D.; Pankratz, H.S.; Palma, J.; Dazzo, F.B.; Sprent, J.I. The unique root-nodule symbiosis between Rhizobium and the aquatic legume, Neptunia natans (L. f.) Druce. Planta 1995, 196, 311–320. [Google Scholar] [CrossRef]
- Miller, M.S.; Pepper, I.L. Survival of a fast-growing strain of lupin rhizobia in Sonoran Desert soils. Soil Biol. Biochem. 1988, 20, 323–327. [Google Scholar] [CrossRef]
- Pudelko, K.; Madrzak, J. Populations of Rhizobium and Bradyrhizobium nodulating Lupinus in Poland. In Abstracts of Biological Fixation for Ecology and Sustainable Agriculture, 2nd ed.; European Nitrogen Conference and NATO Advanced Research Workshop: Poznan, Poland, 1996; p. 156, Scientific Publishers OWN. [Google Scholar]
- Andam, C.P.; Parker, M.A. A novel Alpha-Proteobacterial root nodule symbiont associated with Lupinus texensis. Appl. Environ. Microbiol. 2007, 73, 5687–5691. [Google Scholar] [CrossRef]
- Barrera, L.L.; Trujillo, M.E.; Goodfellow, M.; Garcia, F.J.; Hernandez-Lucas, I.; Davila, G.; van Berkum, P.; Martinez-Romero, E. Biodiversity of bradyrhizobia nodulating Lupinus spp. Int. J. Syst. Bacteriol. 1997, 47, 1086–1091. [Google Scholar] [CrossRef]
- Fernández-Pascual, M.; Pueyo, J.J.; de Felipe, M.R.; Golvano, M.P.; Lucas, M.M. Singular features of the Bradyrhizobium Lupinus symbiosis. Dyn. Soil Dyn. Plant. 2007, 1, 1–16. [Google Scholar]
- Ryan-Salter, T.P.; Black, A.D.; Andrews, M.; Moot, D.J. Identification and effectiveness of rhizobial strains that nodulate Lupinus polyphyllus. Proc N. Z. Grassl. Assoc. 2014, 76, 61–66. [Google Scholar] [CrossRef]
- Ueda, T.; Suga, Y.; Yahiro, N.; Matsuguchi, T. Phylogeny of SymPlasmids of rhizobia by PCR-based sequencing of a nodC segment. J. Bacteriol. 1995, 177, 468–472. [Google Scholar] [CrossRef][Green Version]
- Laguerre, G.; Nour, S.M.; Macheret, V.; Sanjuan, J.J.; Drouin, P.; Amarger, N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 2001, 147, 981–993. [Google Scholar] [CrossRef]
- Rincón, A.; Arenal, F.; González, I.; Manrique, E.; Lucas, M.M.; Pueyo, J.J. Diversity of rhizobial bacteria isolated from nodules of the gypsophyte Ononis tridentata L. growing in Spanish soils. Microb. Ecol. 2008, 56, 223–233. [Google Scholar]
- Cordero, I.; Ruiz-Díez, B.; de la Peña, T.C.; Balaguer, L.; Lucas, M.M.; Rincón, A.; Pueyo, J.J. Rhizobial diversity, symbiotic effectiveness and structure of nodules of Vachellia macracantha. Soil Biol. Biochem. 2016, 96, 39–54. [Google Scholar] [CrossRef]
- Rogel, M.A.; Ormeno-Orrillo, E.; Martinez Romero, E. Symbiovars in rhizobia reflect bacterial adaptation to legumes. Syst. Appl. Microbiol. 2011, 34, 96–104. [Google Scholar] [CrossRef]
- Sánchez-Canizares, C.; Rey, L.; Durán, D.; Temprano, F.; Sánchez-Jiménez, P.; Navarro, A.; Polajnar, M.; Imperial, J.; Ruiz-Argüeso, T. Endosymbiotic bacteria nodulating a new endemic lupine Lupinus mariae-josephi from alkaline soils in Eastern Spain represent a new lineage within the Bradyrhizobium genus. Syst. Appl. Microbiol. 2011, 34, 207–215. [Google Scholar] [CrossRef]
- Barcellos, F.G.; Menna, P.; da Silva Batista, J.S.; Hungria, M. Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inoculant strain to indigenous diazotrophs Sinorhizobium (Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian Savannah soil. Appl. Environ. Microbiol. 2007, 73, 2635–2643. [Google Scholar] [CrossRef]
- Ruiz-Díez, B.; Fajardo, S.; Puertas-Mejía, M.A.; de Felipe, R.; Fernández-Pascual, M. Stress tolerance, genetic analysis and symbiotic properties of root-nodulating bacteria isolated from Mediterranean leguminous shrubs in Central Spain. Arch. Microbiol. 2009, 191, 35–46. [Google Scholar] [CrossRef]
- El-Akhal, M.R.; Rincon, A.; El Mourabit, N.; Pueyo, J.J.; Barrijal, S. Phenotypic and genotypic characterizations of rhizobia isolated from root nodules of peanut (Arachis hypogaea L.) grown in Moroccan soils. J. Basic Microbiol. 2009, 49, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Howieson, J.G.; Fillery, I.R.P.; Legocki, A.B.; Sikorski, M.M.; Stepkowski, T.; Minchin, F.R.; Dilworth, M.J. Nodulation, nitrogen fixation and nitrogen balance. In Lupins as Crop Plants: Biology, Production and Utilization; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Wallingford, UK, 1998; pp. 149–180. [Google Scholar]
- Kulkarni, S.; Nautiyal, C.S. Characterization of high tolerant rhizobia isolated from Prosopis juliflora grown in alkaline soil. J. Gen. Appl. Microbiol. 1999, 45, 213–220. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El-Akhal, M.R.; Rincon, A.; Coba de la Pena, T.; Lucas, M.M.; El Mourabit, N.; Barrijal, S.; Pueyo, J.J. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2013, 15, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Gault, R.R.; Corbin, E.J.; Boundy, K.A.; Brockwell, J. Nodulation studies on legumes exotic to Australia: Lupinus and Ornithopus spp. Aust. J. Exp. Agric. 1986, 26, 37–48. [Google Scholar] [CrossRef]
- Kozhemyakov, A.P.; Cheremisov, B.M.; Kurlovich, B.S.; Tikhonovich, I.A.; Kartuzova, L.T.; Tchetkova, S.A.; Emeljanenko, T.A. Evaluation of the biological nitrogen fixing ability of lupin (Lupinus L.). Plant Genet. Resour. Newsl. 2000, 123, 66–77. [Google Scholar]



| Site | Number of Isolates | Isolate Code | Isolate Code Number | Soil pH | Environment | Bioclimatic Stage | Soil Texture |
|---|---|---|---|---|---|---|---|
| Takelsa | 5 | LanT | 1, 3, 5, 6, 7 | 7.5 | Area with orange trees | semi-arid superior | sandy |
| Mraissa | 5 | LanM | 4, 5, 6, 7, 9 | 8.0 | Area planted with cereals | semi-arid superior | sandy |
| Borj Hfaiedh | 2 | LanB | 5, 6 | 8.5 | Area with olive trees | semi-arid superior | sandy |
| Hammamet | 3 | LanH | 1, 2, 5 | 9.0 | Semiurban area, next to the sea | semi-arid superior | sandy |
| Sejnane | 2 | LanS | 2, 5 | 7.5 | Semiurban area | humid | clay |
| Tabarka | 15 | LanTb | 3, 4, 5, 6, 8, 9, 11, 12, 14, 15, 16, 17, 18, 19, 20 | 6.5 | Area planted with cereals | humid | sandy |
| Host Plant | ||||||||
|---|---|---|---|---|---|---|---|---|
| L. angustifolius | L. luteus | L. micranthus | L. mariae-josephae | Retama sphaerocarpa | Vigna unguiculata | Glycine max | ||
| Strain | PG | |||||||
| LanT1 | I | + | + | + | +W | +W | +W | - |
| LanM5 | I | + | + | + | - | +W | +W | - |
| LanTb3 | I | + | + | + | - | +W | +W | - |
| LanTb18 | I | + | + | + | +W | +W | +W | - |
| LanS2 | II | + | + | + | - | +W | +W | - |
| LanTb17 | III | + | - | - | - | - | - | - |
| LanH1 | IV | + | + | + | + | + | - | - |
| LanTb5 | V | + | + | + | - | +W | - | - |
| LanM6 | VI | + | + | + | +W | +W | - | - |
| LanH5 | VI | + | + | + | +W | +W | - | - |
| LanTb9 | VI | + | + | + | +W | +W | - | - |
| Strain | PG | GT (h) | T Range (°C) | pH Range | Salt Tolerance (NaCl %) | Antibiotics Resistance | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.5 | 1 | 2 | Amp | Gen | Tet | Spe | Kan | Str | Chl | |||||
| LanT1 | I | 10 | 20–28 | 4–11 | + | - | - | - | + | - | + | - | - | - | + |
| LanM5 | I | 9 | 20–28 | 4–11 | + | - | - | - | + | - | + | - | - | - | + |
| LanTb3 | I | 9.5 | 20–28 | 4–11 | + | - | - | - | + | - | + | - | - | - | + |
| LanTb18 | I | 8.7 | 20–28 | 4–11 | + | - | - | - | + | - | + | - | - | - | + |
| LanS2 | II | 9.3 | 20–37 | 4–11 | + | +/- | - | - | - | - | -/+ | - | - | - | -/+ |
| LanTb17 | III | 8 | 20–37 | 4–12 | + | +/- | - | - | + | - | + | -/+ | + | - | + |
| LanH1 | IV | 18 | 20–37 | 4–11 | + | +/- | - | - | - | - | -/+ | - | - | - | -/+ |
| LanTb5 | V | 4 | 20–28 | 4–11 | + | + | +/- | - | - | - | - | + | + | + | - |
| LanM6 | VI | 4.6 | 20–37 | 4–12 | + | + | +/- | - | - | - | - | - | - | - | - |
| LanH5 | VI | 4.6 | 20–37 | 4–12 | + | + | +/- | - | - | - | - | - | - | - | - |
| LanTb9 | VI | 4.6 | 20–37 | 4–12 | + | + | +/- | - | - | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Msaddak, A.; Rey, L.; Imperial, J.; Palacios, J.M.; Mars, M.; Pueyo, J.J. Phylogenetic Analyses of Rhizobia Isolated from Nodules of Lupinus angustifolius in Northern Tunisia Reveal Devosia sp. as a New Microsymbiont of Lupin Species. Agronomy 2021, 11, 1510. https://doi.org/10.3390/agronomy11081510
Msaddak A, Rey L, Imperial J, Palacios JM, Mars M, Pueyo JJ. Phylogenetic Analyses of Rhizobia Isolated from Nodules of Lupinus angustifolius in Northern Tunisia Reveal Devosia sp. as a New Microsymbiont of Lupin Species. Agronomy. 2021; 11(8):1510. https://doi.org/10.3390/agronomy11081510
Chicago/Turabian StyleMsaddak, Abdelhakim, Luis Rey, Juan Imperial, José Manuel Palacios, Mohamed Mars, and José J. Pueyo. 2021. "Phylogenetic Analyses of Rhizobia Isolated from Nodules of Lupinus angustifolius in Northern Tunisia Reveal Devosia sp. as a New Microsymbiont of Lupin Species" Agronomy 11, no. 8: 1510. https://doi.org/10.3390/agronomy11081510
APA StyleMsaddak, A., Rey, L., Imperial, J., Palacios, J. M., Mars, M., & Pueyo, J. J. (2021). Phylogenetic Analyses of Rhizobia Isolated from Nodules of Lupinus angustifolius in Northern Tunisia Reveal Devosia sp. as a New Microsymbiont of Lupin Species. Agronomy, 11(8), 1510. https://doi.org/10.3390/agronomy11081510








