The Survival and Parasitism Rate of Tamarixia radiata (Hymenoptera: Eulophidae) on Its Host Exposed to Beauveriabassiana (Ascomycota: Hypocreales)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insects Culture
2.2. Fungal Strain and Conidia Suspension Preparation
2.3. Diaphorina Citri First Parasitized by Tamarixia radiata and Afterward Treated with Beauveria bassiana Suspension
2.4. Diaphorina Citri First Sprayed with Beauveria bassiana and Afterward Exposed to Tamarixia radiata
2.5. Statistical Analysis
3. Results
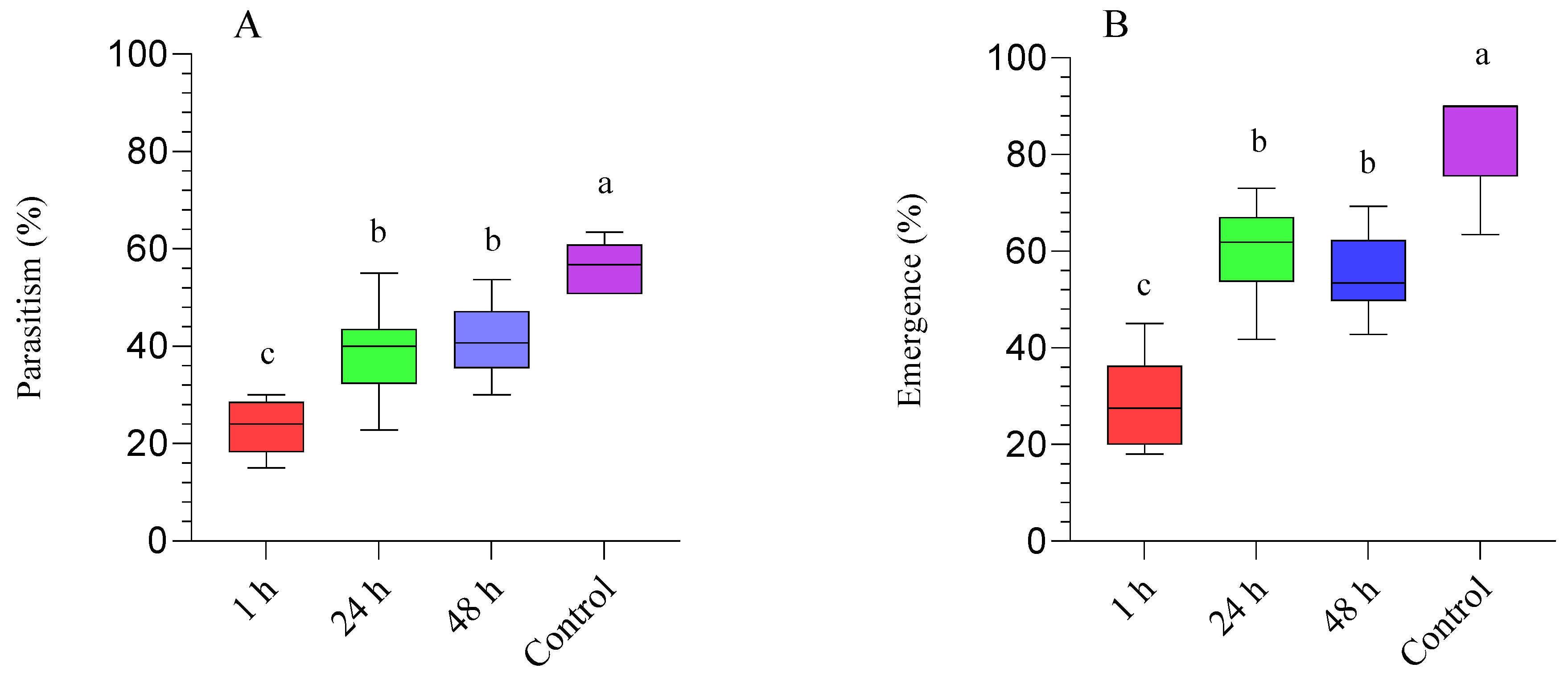
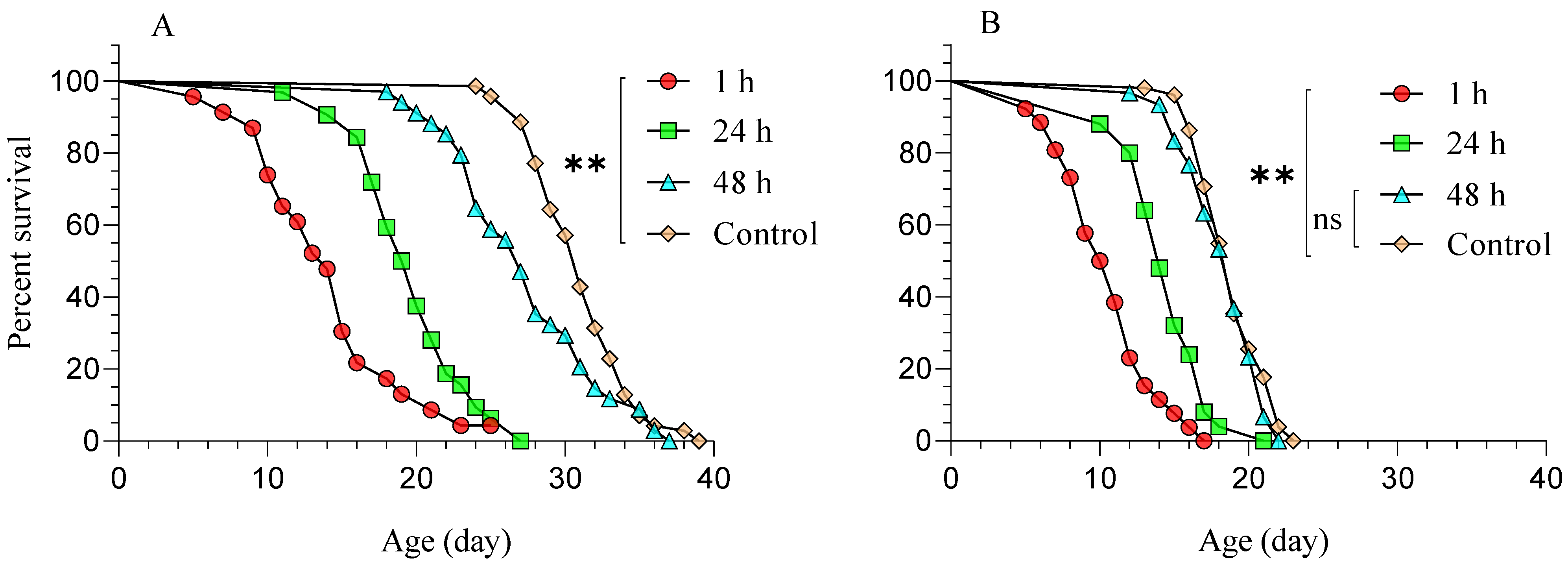
3.1. Diaphorina Citri Parasitized by Tamarixia radiata and Subsequently Treated with Beauveria bassiana (BB-12)
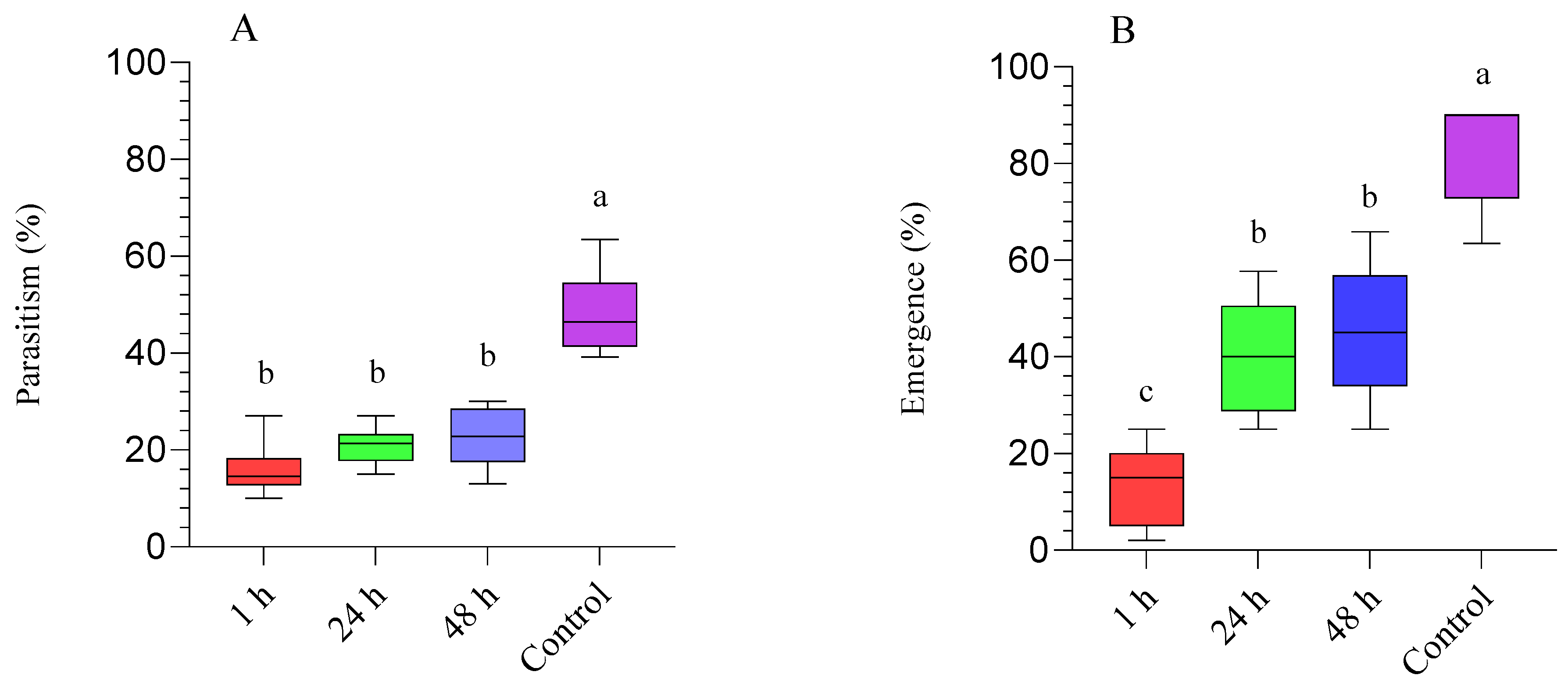
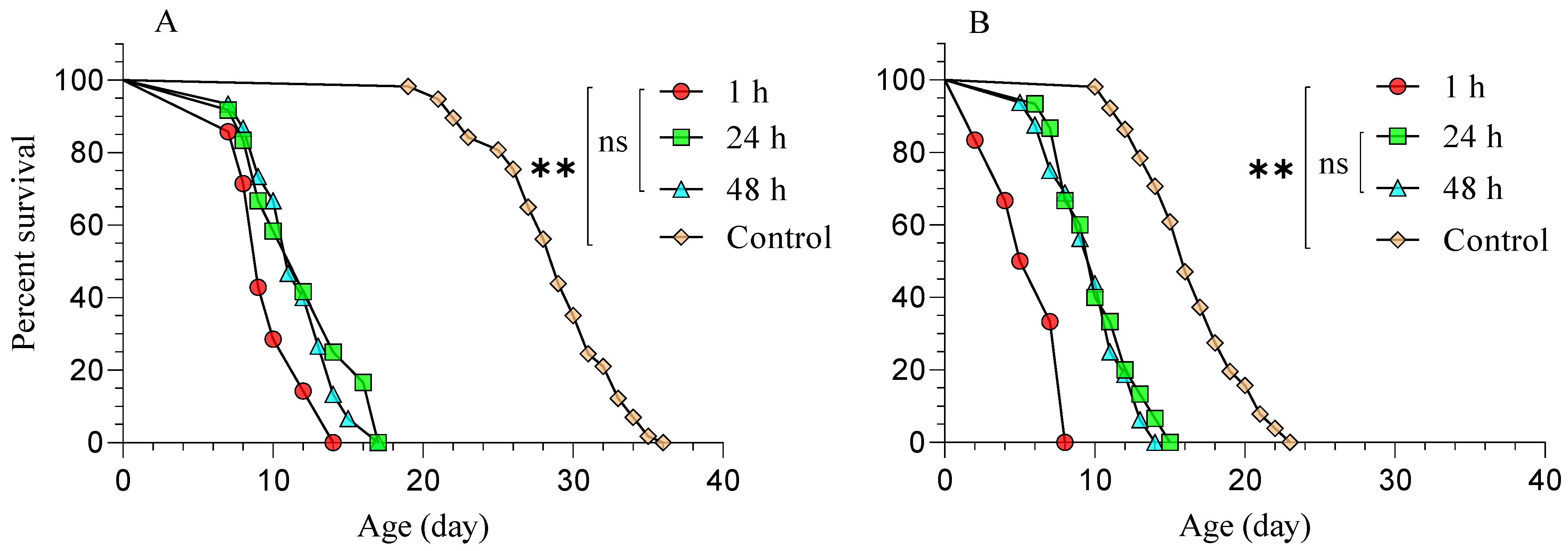
3.2. Diaphorina Citri Treated with Beauveria bassiana (BB-12) and Parasitized by Tamarixia radiata
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Rogers, M.E.; Hall, D.G.; Stansly, P.A. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J. Econ. Entomol. 2009, 102, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.M. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- Grafton-Cardwell, E.E.; Stelinski, L.L.; Stansly, P.A. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu. Rev. Entomol. 2013, 58, 413–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal suppression of Asian citrus psyllid Diaphorina citri (Hemiptera: Liviidae) vector of huanglongbing pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar] [CrossRef] [Green Version]
- Gharalari, A.; Nansen, C.; Lawson, D.; Gilley, J.; Munyaneza, J.; Vaughn, K. Knockdown mortality, repellency, and residual effects of insecticides for control of adult Bactericera cockerelli (Hemiptera: Psyllidae). J. Econ. Entomol. 2009, 102, 1032–1038. [Google Scholar] [CrossRef]
- Naeem, A.; Freed, S.; Jin, F.L.; Akmal, M.; Mehmood, M.J.C.P. Monitoring of insecticide resistance in Diaphorina citri Kuwayama (Hemiptera: Psyllidae) from citrus groves of Punjab, Pakistan. Crop Prot. 2016, 86, 62–68. [Google Scholar] [CrossRef]
- Tiwari, S.; Stelinski, L.L.; Rogers, M.E. Biochemical basis of organophosphate and carbamate resistance in Asian citrus psyllid. J. Econ. Entomol. 2012, 105, 540–548. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef] [Green Version]
- Patt, J.M.; Chow, A.; Meikle, W.G.; Gracia, C.; Jackson, M.A.; Flores, D.; Sétamou, M.; Dunlap, C.A.; Avery, P.B.; Hunter, W.B. Efficacy of an autodisseminator of an entomopathogenic fungus, Isaria fumosorosea, to suppress Asian citrus psyllid, Diaphorina citri, under greenhouse conditions. Biol. Control 2015, 88, 37–45. [Google Scholar] [CrossRef]
- Shah, P.; Pell, J. Entomopathogenic fungi as biological control agents. Appl. Microbiol. Biotechnol. 2003, 61, 413–423. [Google Scholar] [CrossRef]
- Ibarra-Cortés, K.H.; González-Hernández, H.; Guzmán-Franco, A.W. Susceptibility of nymphs and adults of Diaphorina citri to the entomophathogenic fungus Hirsutella citriformis. Biocontrol Sci. Technol. 2017, 27, 433–438. [Google Scholar] [CrossRef]
- Keppanan, R.; Krutmuang, P.; Sivaperumal, S.; Hussain, M.; Bamisile, B.S.; Aguila, L.C.R.; Dash, C.K.; Wang, L. Synthesis of mycotoxin protein IF8 by the entomopathogenic fungus Isaria fumosorosea and its toxic effect against adult Diaphorina citri. Int. J. Biol. Macromol. 2018, 125, 1203–1211. [Google Scholar] [CrossRef]
- Hoy, M.A.; Nguyen, R.; Jeyaprakash, A. Classical biological control of Asian citrus psylla. Citrus Ind. 2001, 81, 48–50. [Google Scholar]
- Avery, P.B.; Wekesa, V.W.; Hunter, W.B.; Hall, D.G.; McKenzie, C.L.; Osborne, L.S.; Powell, C.A.; Rogers, M.E. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci. Technol. 2011, 21, 1065–1078. [Google Scholar] [CrossRef]
- Meyer, J.M.; Hoy, M.A.; Boucias, D.G. Isolation and characterization of an Isaria fumosorosea isolate infecting the Asian citrus psyllid in Florida. J. Invertebr. Pathol. 2008, 99, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Lezama-Gutiérrez, R.; Molina-Ochoa, J.; Chávez-Flores, O.; Ángel-Sahagún, C.A.; Skoda, S.R.; Reyes-Martínez, G.; Barba-Reynoso, M.; Rebolledo-Domínguez, O.; Ruíz-Aguilar, G.M.; Foster, J.E. Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae) in Persian lime under field conditions. Int. J. Trop. Insect Sci. 2012, 32, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.M.; Hoy, M.A.; Boucias, D.G. Morphological and molecular characterization of a Hirsutella species infecting the Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), in Florida. J. Invertebr. Pathol. 2007, 95, 101–109. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G.; Meyer, J.M.; Kriss, A.B.; Gottwald, T.R.; Boucias, D.G. Observations on the entomopathogenic fungus Hirsutella citriformis attacking adult Diaphorina citri (Hemiptera: Psyllidae) in a managed citrus grove. BioControl 2012, 57, 663–675. [Google Scholar] [CrossRef]
- Alemán, J.; Baños, H.; Ravelo, J. Diaphorina citri y la enfermedad huanglongbing: Una combinación destructiva para la producción citrícola. Rev. Prot. Veg. 2007, 22, 154–165. [Google Scholar]
- Portalanza, D.E.; Sanchez, L.; Plúas, M.; Felix, I.; Costa, V.A.; Dias-Pini, N.d.S.; Ferreira-Stafanous, S.; Gómez-Torres, M.L. First records of parasitoids attacking the Asian citrus psyllid in Ecuador. Rev. Bras. Entomol. 2017, 61, 107–110. [Google Scholar] [CrossRef]
- Ramos Aguila, L.C.; Hussain, M.; Huang, W.; Lei, L.; Bamisile, B.S.; Wang, F.; Chi, H.; Wang, L. Temperature-Dependent Demography and Population Projection of Tamarixia radiata (Hymenoptera: Eulophidea) reared on Diaphorina citri (Hemiptera: Liviidae). J. Econ. Entomol. 2020, 113, 55–63. [Google Scholar] [CrossRef]
- Aubert, B.; Quilici, S. Biological control of the African and Asian citrus psyllids (Homoptera: Psylloidea), through eulophid and encyrtid parasites (Hymenoptera: Chalcidoidea) in Reunion Island. Int. Organ. Citrus Virol. Conf. Proc. (1957–2010) 1984, 9, 1957–2010. [Google Scholar]
- Étienne, J.; Quilici, S.; Marival, D.; Franck, A. Biological control of Diaphorina citri (Hemiptera: Psyllidae) in Guadeloupe by imported Tamarixia radiata (hymenoptera: Eulophidae). Fruits 2001, 56, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G.; Hentz, M.G.; Adair, R.C., Jr. Population ecology and phenology of Diaphorina citri (Hemiptera: Psyllidae) in two Florida citrus groves. Environ. Entomol. 2008, 37, 914–924. [Google Scholar] [CrossRef] [Green Version]
- Hoddle, M.S. Foreign exploration for natural enemies of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae), in the Punjab of Pakistan for use in a classical biological control program in California USA. Pak. Entomol. 2012, 34, 1–5. [Google Scholar]
- Michaud, J. Natural mortality of Asian citrus psyllid (Homoptera: Psyllidae) in central Florida. Biol. Control 2004, 29, 260–269. [Google Scholar] [CrossRef]
- Tamayo-Mejía, F.; Tamez-Guerra, P.; Guzmán-Franco, A.W.; Gomez-Flores, R. Developmental stage affects survival of the ectoparasitoid Tamarixia triozae exposed to the fungus Beauveria bassiana. Biol. Control 2016, 93, 30–36. [Google Scholar] [CrossRef]
- Roy, H.E.; Pell, J.K. Interactions between entomopathogenic fungi and other natural enemies: Implications for biological control. Biocontrol Sci. Technol. 2000, 10, 737–752. [Google Scholar] [CrossRef]
- Hochberg, M.E. Intra-host interactions between a braconid endoparasitoid, Apanteles glomeratus, and a baculovirus for larvae of Pieris brassicae. J. Anim. Ecol. 1991, 60, 51–63. [Google Scholar] [CrossRef]
- Brobyn, P.J.; Clark, S.J.; Wilding, N. The effect of fungus infection of Metopolophium dirhodum [Hom.: Aphididae] on the oviposition behaviour of the aphid parasitoid Aphidius rhopalosiphi [Hym.: Aphidiidae]. Entomophaga 1988, 33, 333–338. [Google Scholar] [CrossRef]
- Baverstock, J.; Roy, H.E.; Pell, J.K. Entomopathogenic fungi and insect behaviour: From unsuspecting hosts to targeted vectors. In The Ecology of Fungal Entomopathogens; Springer: Berlin/Heidelberg, Germany, 2009; pp. 89–102. [Google Scholar]
- Bamisile, B.S.; Senyo Akutse, K.; Dash, C.K.; Qasim, M.; Ramos Aguila, L.C.; Ashraf, H.J.; Huang, W.; Hussain, M.; Chen, S.; Wang, L. Effects of seedling age on colonization patterns of Citrus limon plants by endophytic Beauveria bassiana and Metarhizium anisopliae and their influence on seedlings growth. J. Fungi 2020, 6, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Qasim, M.; Ramos Aguila, L.C.; Wang, F.; Keppanan, R.; Wang, L. Endophytic Beauveria bassiana in foliar-treated Citrus limon plants acting as a growth suppressor to three successive generations of Diaphorina citri Kuwayama (Hemiptera: Liviidae). Insects 2019, 10, 176. [Google Scholar] [CrossRef] [Green Version]
- Dash, C.K.; Bamisile, B.S.; Keppanan, R.; Qasim, M.; Lin, Y.; Islam, S.U.; Hussain, M.; Wang, L. Endophytic entomopathogenic fungi enhance the growth of Phaseolus vulgaris L.(Fabaceae) and negatively affect the development and reproduction of Tetranychus urticae Koch (Acari: Tetranychidae). Microb. Pathog. 2018, 125, 385–392. [Google Scholar] [CrossRef]
- Humber, R.A. Fungi: Identification. In Manual of Techniques in Insect Pathology; Elsevier: Amsterdam, The Netherlands, 1997; pp. 153–185. [Google Scholar]
- Humber, R.A. Identification of entomopathogenic fungi. Man. Tech. Invertebr. Pathol. 2012, 2, 151–187. [Google Scholar]
- Martins, I.; Silva, R.; Alencar, J.; Silva, K.; Cividanes, F.; Duarte, R.; Agostini, L.; Polanczyk, R. Interactions between the entomopathogenic fungi Beauveria bassiana (Ascomycota: Hypocreales) and the aphid parasitoid Diaeretiella rapae (Hymenoptera: Braconidae) on Myzus persicae (Hemiptera: Aphididae). J. Econ. Entomol. 2014, 107, 933–938. [Google Scholar] [CrossRef]
- Rashki, M.; Kharazi-Pakdel, A.; Allahyari, H.; Van Alphen, J. Interactions among the entomopathogenic fungus, Beauveria bassiana (Ascomycota: Hypocreales), the parasitoid, Aphidius matricariae (Hymenoptera: Braconidae), and its host, Myzus persicae (Homoptera: Aphididae). Biol. Control 2009, 50, 324–328. [Google Scholar] [CrossRef]
- Lord, J.C. Response of the wasp Cephalonomia tarsalis (Hymenoptera: Bethylidae) to Beauveria bassiana (Hyphomycetes: Moniliales) as free conidia or infection in its host, the sawtoothed grain beetle, Oryzaephilus surinamensis (Coleoptera: Silvanidae). Biol. Control 2001, 21, 300–304. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.; Qureshi, J.; Stansly, P.; Stelinski, L.J.J. Behavioral response of Tamarixia radiata (Waterston)(Hymenoptera: Eulophidae) to volatiles emanating from Diaphorina citri Kuwayama (Hemiptera: Psyllidae) and citrus. J. Insect Behav. 2010, 23, 447–458. [Google Scholar] [CrossRef]
- Chien, C.; Chu, Y.; Ku, S. Parasitic strategy, morphology and life history of Tamarixia radiata (Hymenoptera; Eulophidae). Chin. J. Entomol. 1991, 11, 264–281. [Google Scholar]
- Chow, A.; Dunlap, C.A.; Jackson, M.A.; Flores, D.; Patt, J.M.; Sétamou, M. Oviposition behavior and survival of Tamarixia radiata (Hymenoptera: Eulophidae), an ectoparasitoid of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Liviidae), on hosts exposed to an entomopathogenic fungus, Isaria fumosorosea (Hypocreales: Cordycipitaceae), under laboratory conditions. J. Econ. Entomol. 2016, 109, 1995–2005. [Google Scholar] [PubMed]
- van Lenteren, J.C.; Fransen, J.J. Survival of the parasitoid Encarsia formosa after treatment of parasitized greenhouse whitefly larvae with fungal spores of Aschersonia aleyrodis. Entomol. Exp. Appl. 1994, 71, 235–243. [Google Scholar] [CrossRef]
- Mesquita, A.L.; Lacey, L.A. Interactions among the entomopathogenic fungus, Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes), the parasitoid, Aphelinus asychis (Hymenoptera: Aphelinidae), and their aphid host. Biol. Control 2001, 22, 51–59. [Google Scholar] [CrossRef]
- Ibarra-Cortés, K.H.; González-Hernández, H.; Guzmán-Franco, A.W.; Ortega-Arenas, L.D.; Villanueva-Jiménez, J.A.; Robles-Bermúdez, A. Interactions between entomopathogenic fungi and Tamarixia radiata (Hymenoptera: Eulophidae) in Diaphorina citri (Hemiptera: Liviidae) populations under laboratory conditions. J. Pest Sci. 2018, 91, 373–384. [Google Scholar] [CrossRef]
- El-Sufty, R.; Führer, E. Interrelationships between Cydia pomonella L. (Lep., Tortricidae), Ascogaster quadridentatus Wesm. (Hym., Braconidae) and the fungus Beauveria bassiana (Bals.) Vuill. Z. Angew. Entomol. 1985, 99, 504–511. [Google Scholar] [CrossRef]
| Treatment | n° Nymphs Exposed | Pre-Adult Duration | Sex Ratio | |
|---|---|---|---|---|
| Female | Male | |||
| 1 h | 20 | 10.35 ± 0.11a | 9.15 ± 0.10a | 0.48 ± 0.06a |
| 24 h | 20 | 10.85 ± 0.25a | 9.45 ± 0.15a | 0.56 ± 0.04a |
| 48 h | 20 | 10.90 ± 0.10a | 9.60 ± 0.16a | 0.53 ± 0.04a |
| Control | 20 | 10.20 ± 0.16a | 9.40 ± 0.12a | 0.58 ± 0.01a |
| Treatment | n° Nymphs Exposed | Pre-Adult Duration | Sex Ratio | |
|---|---|---|---|---|
| Female | Male | |||
| 1 h | 20 | 10.35 ± 0.16a | 9.25 ± 0.13a | 0.28 ± 0.13a |
| 24 h | 20 | 10.80 ± 0.20a | 9.30 ± 0.13a | 0.31 ± 0.10a |
| 48 h | 20 | 10.50 ± 0.19a | 9.40 ± 0.12a | 0.41 ± 0.11a |
| Control | 20 | 10.40 ± 0.16a | 9.45 ± 0.11a | 0.51 ± 0.02a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos Aguila, L.C.; Akutse, K.S.; Ashraf, H.J.; Bamisile, B.S.; Lin, J.; Dai, J.; Wang, H.; Wang, L. The Survival and Parasitism Rate of Tamarixia radiata (Hymenoptera: Eulophidae) on Its Host Exposed to Beauveriabassiana (Ascomycota: Hypocreales). Agronomy 2021, 11, 1496. https://doi.org/10.3390/agronomy11081496
Ramos Aguila LC, Akutse KS, Ashraf HJ, Bamisile BS, Lin J, Dai J, Wang H, Wang L. The Survival and Parasitism Rate of Tamarixia radiata (Hymenoptera: Eulophidae) on Its Host Exposed to Beauveriabassiana (Ascomycota: Hypocreales). Agronomy. 2021; 11(8):1496. https://doi.org/10.3390/agronomy11081496
Chicago/Turabian StyleRamos Aguila, Luis Carlos, Komivi Senyo Akutse, Hafiza Javaria Ashraf, Bamisope Steve Bamisile, Jingyi Lin, Jiawang Dai, Huiting Wang, and Liande Wang. 2021. "The Survival and Parasitism Rate of Tamarixia radiata (Hymenoptera: Eulophidae) on Its Host Exposed to Beauveriabassiana (Ascomycota: Hypocreales)" Agronomy 11, no. 8: 1496. https://doi.org/10.3390/agronomy11081496
APA StyleRamos Aguila, L. C., Akutse, K. S., Ashraf, H. J., Bamisile, B. S., Lin, J., Dai, J., Wang, H., & Wang, L. (2021). The Survival and Parasitism Rate of Tamarixia radiata (Hymenoptera: Eulophidae) on Its Host Exposed to Beauveriabassiana (Ascomycota: Hypocreales). Agronomy, 11(8), 1496. https://doi.org/10.3390/agronomy11081496







