Organic Oat Response to Variety, Seeding Rate, and Nutrient Source and Rate
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
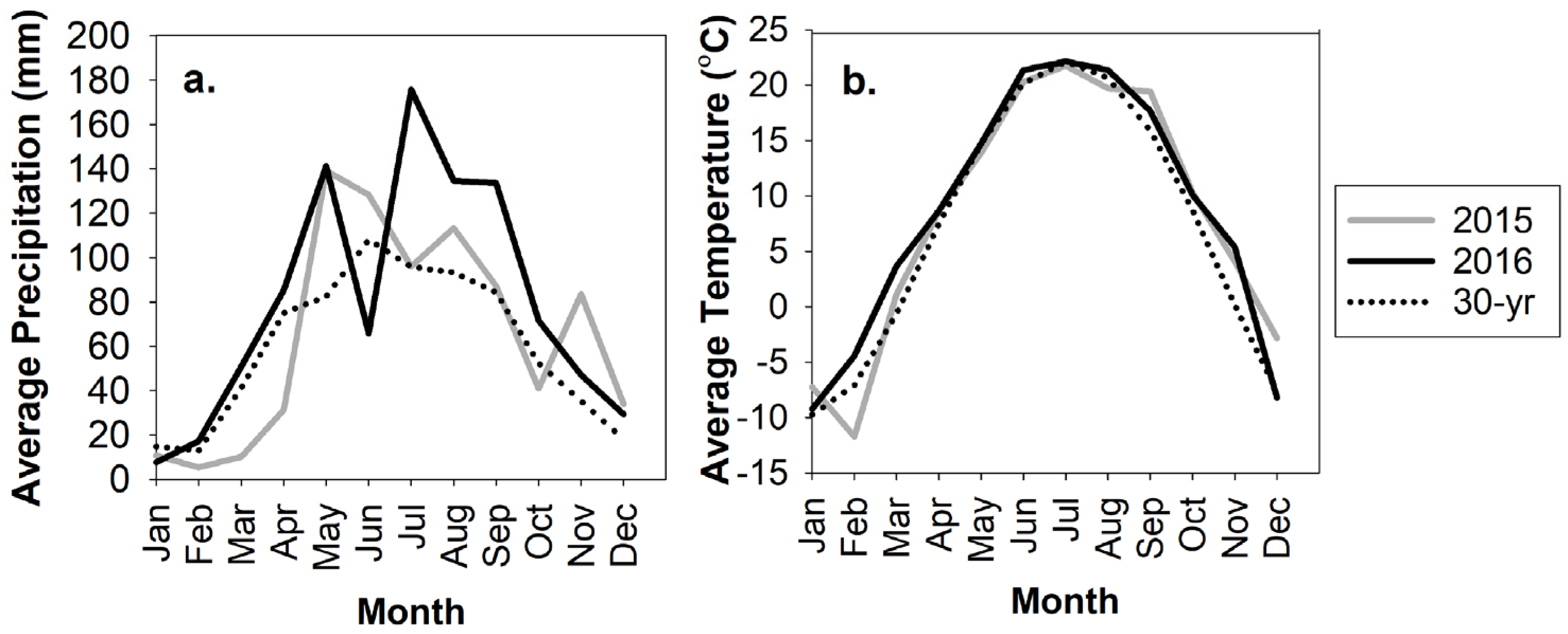
3.1. Weather
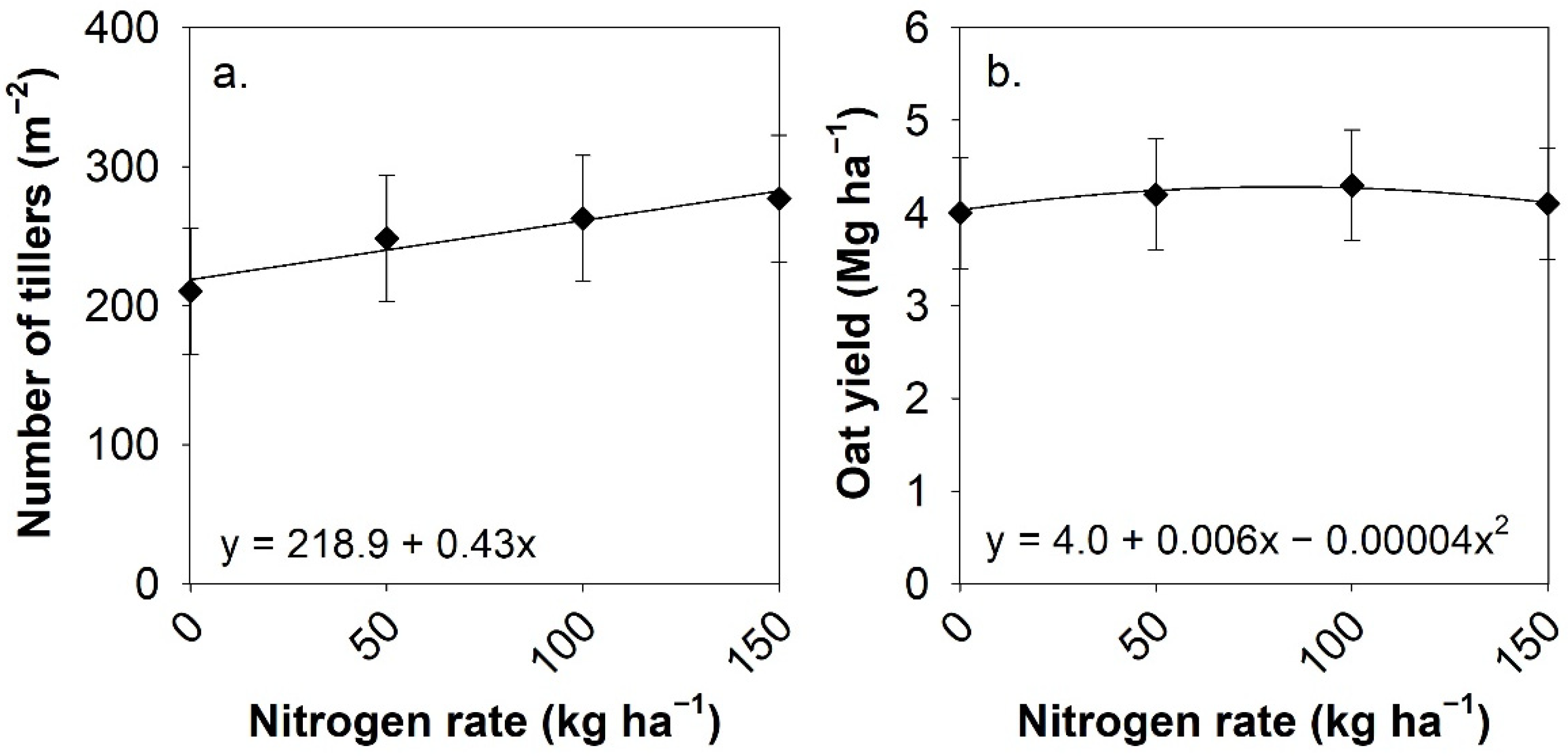
3.2. Plant Population and Tillers per Plant
3.3. Grain Yield
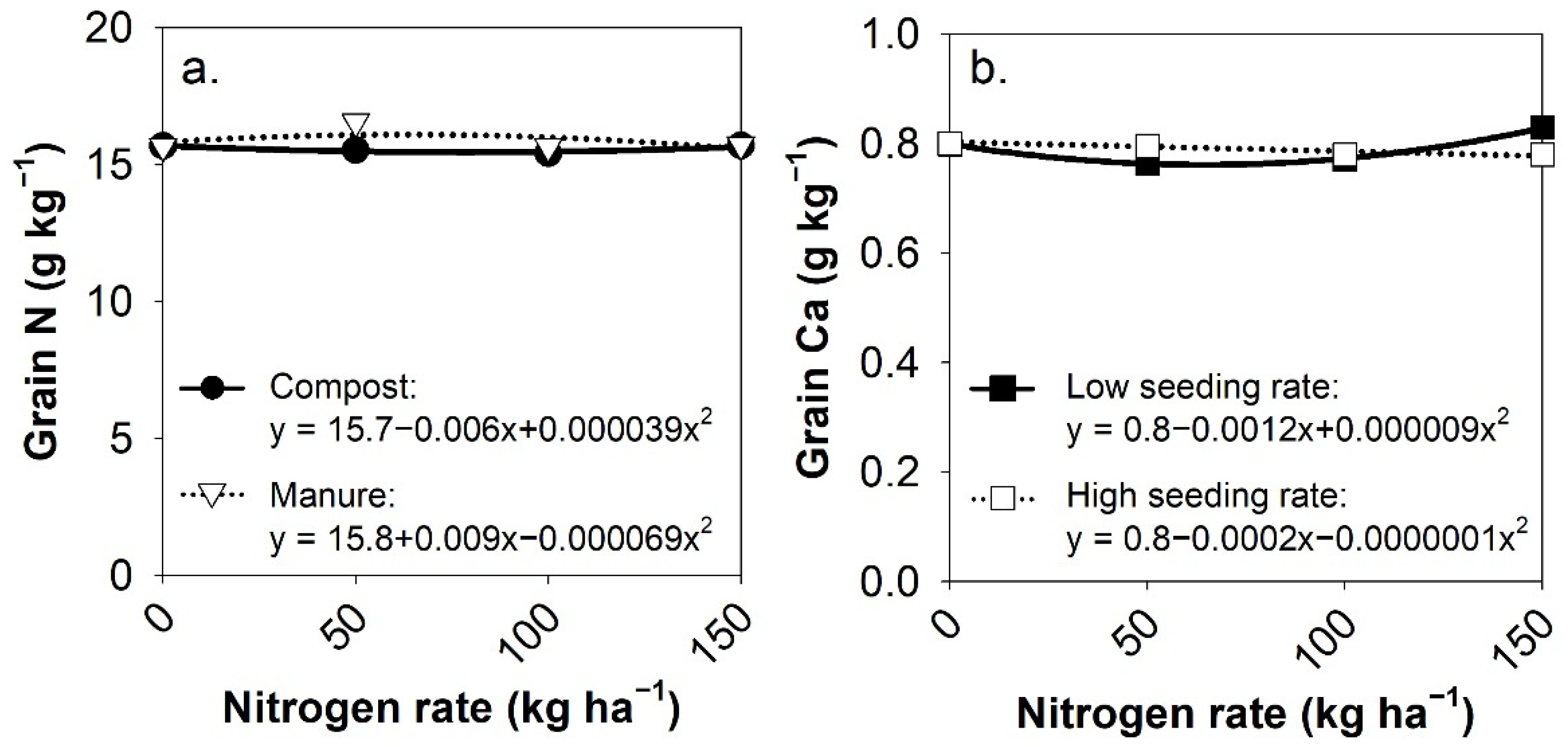
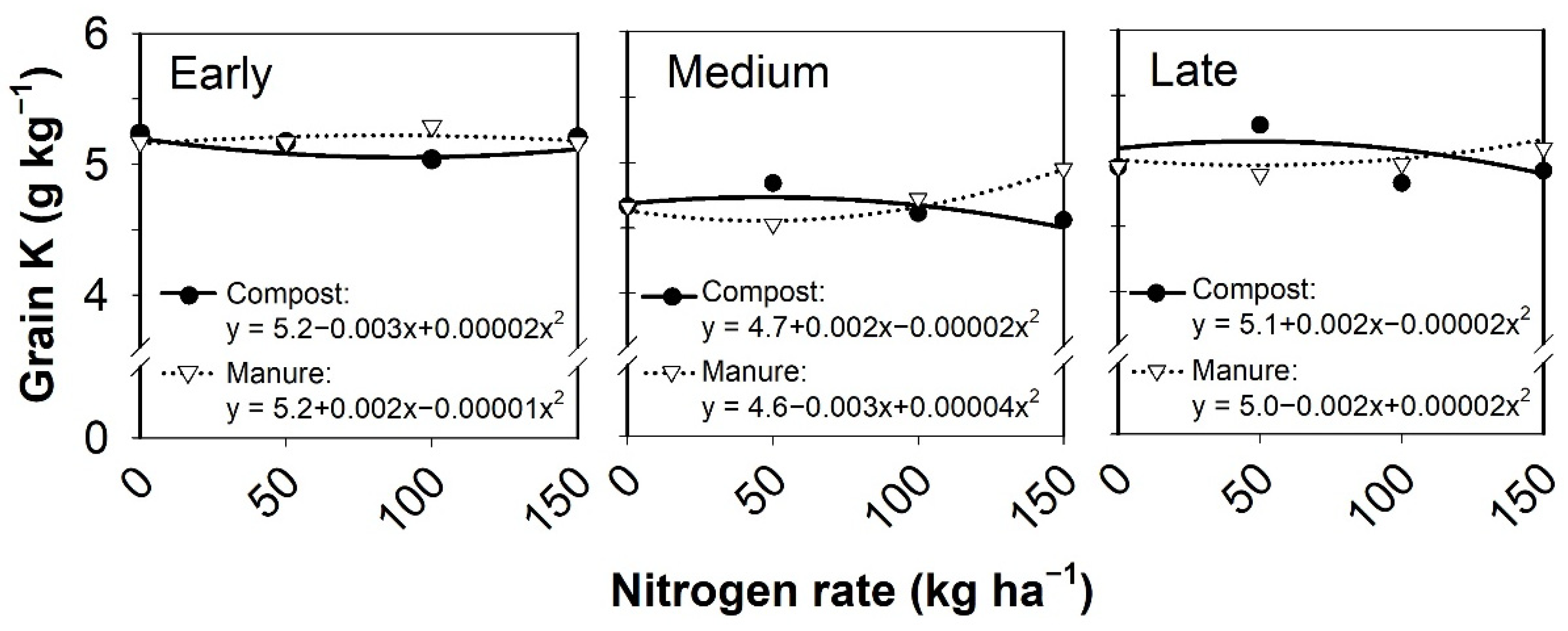
3.4. Grain Nutrient Content
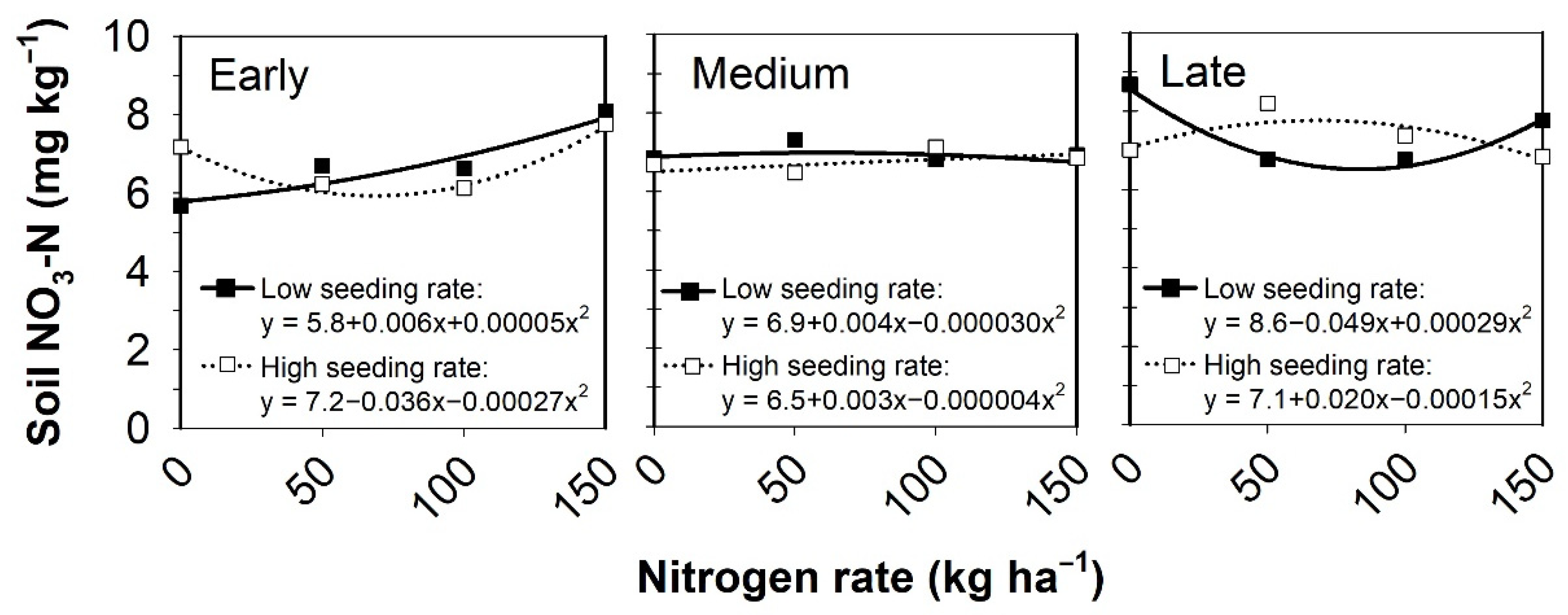
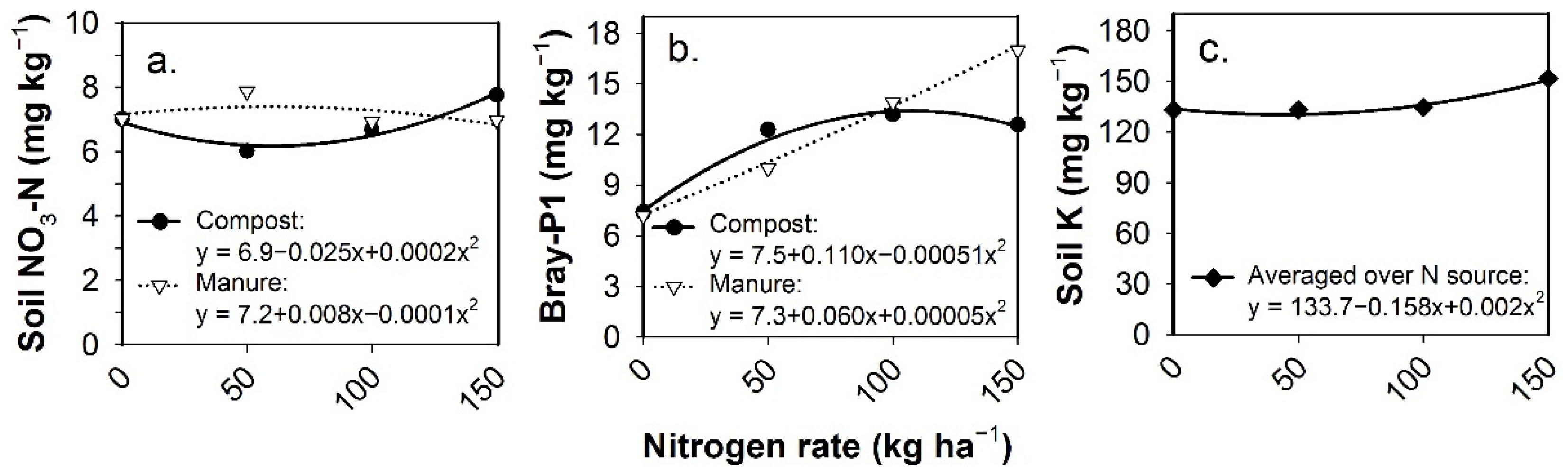
3.5. Post-Harvest Residual Soil Nutrients
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McBride, W.D.; Greene, C. The Profitability of Organic Soybean Production. Renew. Agric. Food Syst. 2009, 24, 276–284. [Google Scholar] [CrossRef] [Green Version]
- Weisberger, D.A.; Wiedenhoeft, M.H.; Smith, M.A.; Liebman, M.Z. Balancing Objectives in an Organic Oat Rotation Year: Implications of Planting Date and Crop Density. Agron. J. 2019, 111, 816–825. [Google Scholar] [CrossRef]
- Teasdale, J.R.; Mangum, R.W.; Radhakrishnan, J.; Cavigelli, M.A. Weed Seedbank Dynamics in Three Organic Farming Crop Rotations. Agron. J. 2004, 96, 1429–1435. [Google Scholar] [CrossRef]
- Nair, A.; Delate, K. Composting, crop rotation, and cover crop practices in organic vegetable production. In Organic Farming for Sustainable Agriculture; Nandwani, D., Ed.; Springer International Publishing: Basel, Switzerland, 2016; pp. 231–257. ISBN 978-3-319-26803-3. [Google Scholar]
- Delate, K.; Cambardella, C.A. Agroecosystem Performance during Transition to Certified Organic Grain Production. Agron. J. 2004, 96, 1288. [Google Scholar] [CrossRef]
- Delbridge, T.A.; Coulter, J.A.; King, R.P.; Sheaffer, C.C.; Wyse, D.L. Economic Performance of Long-Term Organic and Conventional Cropping Systems in Minnesota. Agron. J. 2011, 103, 1372–1382. [Google Scholar] [CrossRef]
- USDA-NASS Certified Organic Survey 2016 Summary; USDA-National Agricultural Statistics Service: Washington, DC, USA, 2017; Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/zg64tk92g/70795b52w/4m90dz33q/OrganicProduction-09-20-2017_correction.pdf (accessed on 22 September 2020).
- MCIA Oats; Minnesota Crop Improvement Association: Saint Paul, MN, USA, 2020; Available online: http://docs.mncia.org/public/website/UMN-Oat-Brochure-2020.pdf (accessed on 23 June 2021).
- Delate, K.; Duffy, M.; Chase, C.; Holste, A.; Friedrich, H.; Wantate, N. An Economic Comparison of Organic and Conventional Grain Crops in a Long-Term Agroecological Research (LTAR) Site in Iowa. Am. J. Alternative Agric. 2003, 18, 59–69. [Google Scholar] [CrossRef]
- Coleman, P. Guide for Organic Crop Producers; USDA-Agricultural Marketing Service—National Organic Program: Washington, DC, USA, 2012; Available online: https://www.ams.usda.gov/sites/default/files/media/CropProducersGuide.pdf (accessed on 23 June 2021).
- Mason, H.; Navabi, A.; Frick, B.; O’Donovan, J.; Spaner, D. Cultivar and Seeding Rate Effects on the Competitive Ability of Spring Cereals Grown under Organic Production in Northern Canada. Agron. J. 2007, 99, 1199–1207. [Google Scholar] [CrossRef]
- Olsen, J.M.; Griepentrog, H.-W.; Nielsen, J.; Weiner, J. How Important Are Crop Spatial Pattern and Density for Weed Suppression by Spring Wheat? Weed Sci. 2012, 60, 501–509. [Google Scholar] [CrossRef]
- Benaragama, D.; Shirtliffe, S.J. Integrating Cultural and Mechanical Methods for Additive Weed Control in Organic Systems. Agron. J. 2013, 105, 1728–1734. [Google Scholar] [CrossRef]
- Ma, B.L.; Biswas, D.K.; Zhou, Q.P.; Ren, C.Z. Comparisons among Cultivars of Wheat, Hulled and Hulless Oats: Effects of N Fertilization on Growth and Yield. Can. J. Plant Sci. 2012, 92, 1213–1222. [Google Scholar] [CrossRef]
- Ma, B.L.; Zheng, Z.; Pageau, D.; Vera, C.; Fregeau-Reid, J.; Xue, A.; Yan, W. Nitrogen and Phosphorus Uptake, Yield and Agronomic Traits of Oat Cultivars as Affected by Fertilizer N Rates under Diverse Environments. Nutr. Cycl. Agroecosyst. 2017, 108, 245–265. [Google Scholar] [CrossRef]
- Mulder, E.G. Effect of Mineral Nutrition on Lodging of Cereals. Plant Soil 1954, 5, 246–306. [Google Scholar] [CrossRef]
- USDA Organic production and handling requirements—§205.203. In Electronic Code of Federal Regulations (eCFR); Part 205—National Organic Program; United Stated Department of Agriculture: Washington, DC, USA, 2020; Available online: https://www.ecfr.gov (accessed on 21 June 2021).
- Ahmed, S.; Mickelson, S.; Pederson, C.; Baker, J.; Kanwar, R.; Lorimor, J.; Webber, D. Swine Manure Rate, Timing, and Application Method Effects on Post-Harvest Soil Nutrients, Crop Yield, and Water Quality Implications in a Corn-Soybean Rotation. Trans. ASABE 2013, 56, 395–408. [Google Scholar] [CrossRef]
- Eghball, B.; Power, J.F. Phosphorus- and Nitrogen-Based Manure and Compost Applications: Corn Production and Soil Phosphorus. Soil Sci. Soc. Am. J. 1999, 63, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Fuerst, E.P.; Koenig, R.T.; Kugler, J.; Painter, K.; Stannard, M.; Goldberg, J. Organic Alfalfa Management Guide; Washington State University Extension: Pullman, WA, USA, 2009; Available online: https://www.nrcs.usda.gov/Internet/FSE_PLANTMATERIALS/publications/wapmcpu10176.pdf (accessed on 23 June 2021).
- Yost, M.A.; Coulter, J.A. Managing the Rotation from Alfalfa to Corn. 2018. Available online: https://extension.umn.edu/corn-cropping-systems/managing-rotation-alfalfa-corn (accessed on 6 April 2021).
- Jungers, J.M.; Kaiser, D.E.; Lamb, J.F.S.; Lamb, J.A.; Noland, R.L.; Samac, D.A.; Wells, M.S.; Sheaffer, C.C. Potassium Fertilization Affects Alfalfa Forage Yield, Nutritive Value, Root Traits, and Persistence. Agron. J. 2019, 111, 2843–2852. [Google Scholar] [CrossRef]
- Schmitt, M.A.; Shaeffer, C.C.; Randall, G.W. Preplant Manure and Commercial P and K Fertilizer Effects on Alfalfa Production. J. Prod. Agric. 1993, 6, 385–390. [Google Scholar] [CrossRef]
- Wilson, M. Guidelines for Manure Application Rates. University of Minnesota Extension. 2020. Available online: https://extension.umn.edu/manure-land-application/manure-application-rates (accessed on 21 June 2021).
- Marshall, H.G.; Kolb, F.L.; Roth, G.W. Effects of Nitrogen Fertilizer Rate, Seeding Rate, and Row Spacing on Semidwarf and Conventional Height Spring Oat. Crop Sci. 1987, 27, 572–575. [Google Scholar] [CrossRef]
- May, W.E.; Mohr, R.M.; Lafond, G.P.; Johnston, A.M.; Stevenson, F.C. Effect of Nitrogen, Seeding Date and Cultivar on Oat Quality and Yield in the Eastern Canadian Prairies. Can. J. Plant Sci. 2004, 84, 1025–1036. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture—Natural Resources Conservation Service Web Soil Survey. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/survey/?cid=nrcseprd1464818 (accessed on 4 July 2021).
- Recommended Chemical Soil Test Procedures for the North Central Region; Brown, J.R. (Ed.) Missouri Agricultural Experiment Station SB 1001: Columbia, MO, USA, 1998; Available online: https://www.canr.msu.edu/uploads/234/68557/rec_chem_soil_test_proce55c.pdf (accessed on 23 June 2021).
- Murphy, J.; Riley, J.P. A Modified Single Solution Method for the Determination of Phosphate in Natural Waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Eghball, B. Nitrogen Mineralization from Field-Applied Beef Cattle Feedlot Manure or Compost. Soil Sci. Soc. Am. J. 2000, 64, 2024–2030. [Google Scholar] [CrossRef]
- Peters, J.; Combs, S.; Hoskins, B.; Jarman, J.; Kovar, J.; Watson, M.; Wolf, A.; Wolf, N. Recommended Methods of Manure Analysis; Peters, J., Ed.; University of Wisconsin-Extension: Madison, WI, USA, 2003; Available online: https://cdn.shopify.com/s/files/1/0145/8808/4272/files/A3769.pdf (accessed on 23 June 2021).
- Gavlak, R.; Horneck, D.A.; Miller, R.O. Soil, Plant, and Water Reference Methods for the Western Region, 3rd ed.; Western Rural Development Cente: Corvallis, OR, USA, 2005; 207p. [Google Scholar]
- Doane, T.A.; Horwáth, W.R. Spectrophotometric Determination of Nitrate with a Single Reagent. Anal. Lett. 2003, 36, 2713–2722. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 14.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2016; Available online: https://documentation.sas.com/doc/en/statcdc/14.2/statug/titlepage.htm (accessed on 23 June 2021).
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute: Cary, NC, USA, 2007; ISBN 978-1-59994-078-6. [Google Scholar]
- Kim, T.K. T Test as a Parametric Statistic. Korean J. Anesth. 2015, 68, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Sorrells, M.E.; Simmons, S.R. Influence of environment on the development and adaptation of oat. In Oat Science and Technology; American Society of Agronomy, Inc. and Crop Science Society of America, Inc.: Madison, WI, USA, 1992; pp. 115–163. ISBN 978-0-89118-225-2. [Google Scholar]
- Wiersma, J.; Ransom, J.; Hofman, V. Seeding Rate for Small Grains. 2018. Available online: https://extension.umn.edu/planting-small-grains/seeding-rate-small-grains (accessed on 2 January 2021).
- Ahmad, A.H.; Wahid, A.; Khalidg, F.; Fiaz, N.; Zamir, M.S.I. Impact of Organic and Inorganic Sources of Nitrogen and Phosphorus Fertilizers on Growth, Yield and Quality of Forage Oat (Avena sativa L.). Cercet. Agron. Mold. 2011, 44, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Bilal, M.; Ayub, M.; Tariq, M.; Tahir, M.; Nadeem, M.A. Dry Matter Yield and Forage Quality Traits of Oat (Avena Sativa L.) under Integrative Use of Microbial and Synthetic Source of Nitrogen. J. Saudi Soc. Agric. Sci. 2017, 16, 236–241. [Google Scholar] [CrossRef] [Green Version]
- Reeves, D.L.; Sraon, H.S. How an oat plant develops. In Research Bulletins of the South Dakota Agricultural Experiment Station (1887–2011); South Dakota Agricultural Experiment Station, South Dakota State University: Brookings, SD, USA, 1976; p. 15. [Google Scholar]
- Wiersma, J.; Dill-Macky, R.; Rines, H.; Tiede, T.; Smith, K. 2016 Oat Field Crop Trials Results; Minnesota Agricultural Experiment Station: Saint Paul, MN, USA, 2016; Available online: https://www.maes.umn.edu/sites/maes.umn.edu/files/oat_2016_final-update.pdf (accessed on 23 June 2021).
- Entz, M.H.; Guilford, R.; Gulden, R. Crop Yield and Soil Nutrient Status on 14 Organic Farms in the Eastern Portion of the Northern Great Plains. Can. J. Plant Sci. 2001, 81, 351–354. [Google Scholar] [CrossRef]
- Langemeier, M.; O’Donnell, M. Conventional and Organic Enterprise Net Returns. In Farmdoc Daily 2020. Available online: https://farmdocdaily.illinois.edu/2020/09/conventional-and-organic-enterprise-net-returns.html (accessed on 1 April 2021).
- Caffe-Treml, M.; Guiterrez, L.; Smith, K.P.; Kleinjan, J. 2017–2018 Multi-State Organic Oat Variety Trial Results; South Dakota State University Extension: Brookings, SD, USA, 2019; Available online: https://extension.sdstate.edu/sites/default/files/2019-05/P-00107.pdf (accessed on 23 June 2021).
- Brinkman, M.A.; Rho, Y.D. Response of Three Oat Cultivars to N Fertilizer. Crop Sci. 1984, 24, 973–977. [Google Scholar] [CrossRef]
- Hamill, M.L. The Effect of Cultivar, Seeding Date, Seeding Rate and Nitrogen Fertility on Oat (Avena sativa L.) Yield and Milling Quality. Master’s Thesis, University of Manitoba, Winnipeg, MB, Canada, 2002. [Google Scholar]
- Lafond, G.P.; May, W.E.; Holzapfel, C.B. Row Spacing and Nitrogen Fertilizer Effect on No-Till Oat Production. Agron. J. 2013, 105, 1–10. [Google Scholar] [CrossRef] [Green Version]
- May, W.E.; Brandt, S.; Hutt-Taylor, K. Response of Oat Grain Yield and Quality to Nitrogen Fertilizer and Fungicides. Agron. J. 2020, 112, 1021–1034. [Google Scholar] [CrossRef] [Green Version]
- Pecio, A.; Bichonski, A. Nitrogen Fertilization and Fungicide Application as Elements of Oat Production. Pol. J. Environ. Stud. 2010, 19, 1297–1305. [Google Scholar]
- Buckley, K.E.; Mohr, R.M.; Therrien, M.C. Yield and Quality of Oat in Response to Varying Rates of Swine Slurry. Can. J. Plant Sci. 2010, 90, 645–653. [Google Scholar] [CrossRef]
- Yost, M.A.; Coulter, J.A.; Russelle, M.P.; Sheaffer, C.C.; Kaiser, D.E. Alfalfa Nitrogen Credit to First-Year Corn: Potassium, Regrowth, and Tillage Timing Effects. Agron. J. 2012, 104, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Grain Millers, Inc. The Growth and Development of Oats: A Production Guide; Grain Millers, Inc.: Eden Prairie, MN, USA, 2017; Available online: http://allamakeeswcd.org/wp-content/uploads/2017/10/Oat-Production-Guide.pdf (accessed on 26 March 2021).
- Ciolek, A.; Makarska, E.; Wesolowski, M.; Cierpiala, R. Content of Selected Nutrients in Wheat, Barley, and Oat Grain from Organic and Conventional Farming. J. Elem. 2012, 17, 181–189. [Google Scholar] [CrossRef]
- Malhi, S.S.; Johnston, A.M.; Schoenau, J.J.; Wang, Z.L.; Vera, C.L. Seasonal Biomass Accumulation and Nutrient Uptake of Wheat, Barley and Oat on a Black Chernozem Soil in Saskatchewan. Can. J. Plant Sci. 2006, 86, 1005–1014. [Google Scholar] [CrossRef]
- Kaiser, D. Oat Fertilizer Recommendations. 2018. Available online: https://extension.umn.edu/crop-specific-needs/oat-fertilizer-recommendations (accessed on 15 January 2021).
- Nelson, N.O.; Janke, R.R. Phosphorus Sources and Management in Organic Production Systems. HortTechnology 2007, 17, 442–454. [Google Scholar] [CrossRef] [Green Version]
- Nesme, T.; Colomb, B.; Hinsinger, P.; Watson, C.A. Soil Phosphorus Management in Organic Cropping Systems: From Current Practices to Avenues for a More Efficient Use of P Resources. In Organic Farming, Prototype for Sustainable Agricultures: Prototype for Sustainable Agricultures; Bellon, S., Penvern, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 23–45. ISBN 978-94-007-7927-3. [Google Scholar]
- Ochsner, T.E.; Schumacher, T.W.; Venterea, R.T.; Feyereisen, G.W.; Baker, J.M. Soil Water Dynamics and Nitrate Leaching under Corn–Soybean Rotation, Continuous Corn, and Kura Clover. Vadose Zone J. 2018, 17, 170028. [Google Scholar] [CrossRef] [Green Version]
- Pfarr, C.J.; Wilson, M.L.; Coulter, J.A.; Fernández, F.G. Liquid Swine Manure as a Sidedressed Nitrogen Source for Corn. Agron. J. 2020, 112, 5206–5221. [Google Scholar] [CrossRef]
- Eghball, B.; Wienhold, B.J.; Gilley, J.E.; Eigenberg, R.A. Mineralization of Manure Nutrients. J. Water Conserv. 2002, 57, 470–472. [Google Scholar]
- Larney, F.J.; Sullivan, D.M.; Buckley, K.E.; Eghball, B. The Role of Composting in Recycling Manure Nutrients. Can. J. Soil Sci. 2006, 86, 597–611. [Google Scholar] [CrossRef]
- Pagliari, P.H.; Laboski, C.A.M. Dairy Manure Treatment Effects on Manure Phosphorus Fractionation and Changes in Soil Test Phosphorus. Biol. Fertil. Soils 2013, 49, 987–999. [Google Scholar] [CrossRef]
- Pagliari, P.H. Variety and solubility of phosphorus forms in animal manure and their effects on soil test phosphorus. In Applied Manure and Nutrient Chemistry for Sustainable Agriculture and Environment; Springer: Dordrecht, The Netherlands, 2014; pp. 141–161. [Google Scholar]
- Pagliari, P.H.; Laboski, C.A.M. Effects of Manure Inorganic and Enzymatically Hydrolyzable Phosphorus on Soil Test Phosphorus. Soil Sci. Soc. Am. J. 2014, 78, 1301. [Google Scholar] [CrossRef]
- Duncan, E.W.; King, K.W.; Williams, M.R.; LaBarge, G.; Pease, L.A.; Smith, D.R.; Fausey, N.R. Linking Soil Phosphorus to Dissolved Phosphorus Losses in the Midwest. Agric. Environ. Lett. 2017, 2, 170004. [Google Scholar] [CrossRef]
- Maguire, R.O.; Sims, J.T. Soil Testing to Predict Phosphorus Leaching. J. Environ. Qual. 2002, 31, 1601–1609. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.; Daniel, T.C.; Sims, J.T.; Pote, D.H. Determining Environmentally Sound Soil Phosphorus Levels. J. Soil Water Conserv. 1996, 51, 160–166. [Google Scholar]
- Elser, J.; Bennett, E. A Broken Biogeochemical Cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Rosen, C.J. Potassium for Crop Production. 2018. Available online: https://extension.umn.edu/phosphorus-and-potassium/potassium-crop-production (accessed on 6 April 2021).
- Heinrich, A.; Falen, J.; Stone, A. High Soil Test Phosphorus and Potassium Levels on a Long-Term Organic Farm: Trends, Causes, and Solutions. 2020. Available online: https://eorganic.org/node/20735 (accessed on 23 June 2021).
| Year | 0–15 cm | |||
|---|---|---|---|---|
| pH | Organic Matter g kg−1 | Bray P-1 | K | |
| mg kg−1 | ||||
| 2015 | 7.3 | 49 | 10 | 108 |
| 2016 | 7.3 | 53 | 7 | 230 |
| Year | Nutrient Source | Nutrient Concentration | Target N Application Rate kg N ha−1 | Actual Application Rates | |||||
|---|---|---|---|---|---|---|---|---|---|
| TN | P | K | Rate Mg ha−1 | TN | P | K | |||
| kg Mg−1 | kg ha−1 | ||||||||
| 2015 | Compost | 8.0 | 4.4 | 5.5 | 50 | 6.3 | 50.2 | 27.6 | 34.5 |
| 100 | 12.6 | 100.4 | 55.2 | 69.0 | |||||
| 150 | 18.8 | 150.6 | 82.9 | 103.6 | |||||
| Manure | 9.4 | 2.6 | 7.1 | 50 | 5.4 | 50.6 | 14.0 | 38.2 | |
| 100 | 10.8 | 101.1 | 28.0 | 76.4 | |||||
| 150 | 16.1 | 151.7 | 42.0 | 114.6 | |||||
| 2016 | Compost | 7.4 | 5.0 | 4.8 | 50 | 6.7 | 49.8 | 33.5 | 32.3 |
| 100 | 13.5 | 99.5 | 67.0 | 64.6 | |||||
| 150 | 20.2 | 149.3 | 100.5 | 96.8 | |||||
| Manure | 8.3 | 5.3 | 6.9 | 50 | 6.1 | 50.2 | 32.1 | 41.8 | |
| 100 | 12.1 | 100.5 | 64.2 | 83.5 | |||||
| 150 | 18.2 | 150.7 | 96.2 | 125.3 | |||||
| Variety 1 | Maturity Group | Plant Population 2 | Yield |
|---|---|---|---|
| Plants m−2 | Mg ha−1 | ||
| Tack/Saber | Early | 261.1 ± 25.6 b | 4.0 ± 0.6 b |
| Shelby | Medium | 356.8 ± 25.6 a | 3.8 ± 0.6 c |
| Deon | Late | 261.6 ± 25.6 b | 4.8 ± 0.6 a |
| Variety 1 | Maturity Group | Grain Nutrient Content 2 | |||||
|---|---|---|---|---|---|---|---|
| N | P | K 3 | Ca | Mg | S | ||
| g kg−1 | |||||||
| Tack/Saber | Early | 16.0 ± 0.4 a | 3.9 ± 0.2 a | 5.2 ± 1.7 a | 0.77 ± 0.01 b | 1.4 ± 0.2 a | 1.5 ± 0.1 b |
| Shelby | Medium | 15.2 ± 0.4 b | 3.8 ± 0.2 a | 4.7 ± 1.7 b | 0.95 ± 0.01 a | 1.3 ± 0.2 c | 1.5 ± 0.1 b |
| Deon | Late | 15.9 ± 0.4 a | 3.8 ± 0.2 a | 5.0 ± 1.7 ab | 0.65 ± 0.01 c | 1.4 ± 0.2 b | 1.6 ± 0.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilson, M.L.; Evans, E.E.; Klossner, L.; Pagliari, P.H. Organic Oat Response to Variety, Seeding Rate, and Nutrient Source and Rate. Agronomy 2021, 11, 1418. https://doi.org/10.3390/agronomy11071418
Wilson ML, Evans EE, Klossner L, Pagliari PH. Organic Oat Response to Variety, Seeding Rate, and Nutrient Source and Rate. Agronomy. 2021; 11(7):1418. https://doi.org/10.3390/agronomy11071418
Chicago/Turabian StyleWilson, Melissa L., Emily E. Evans, Lee Klossner, and Paulo H. Pagliari. 2021. "Organic Oat Response to Variety, Seeding Rate, and Nutrient Source and Rate" Agronomy 11, no. 7: 1418. https://doi.org/10.3390/agronomy11071418
APA StyleWilson, M. L., Evans, E. E., Klossner, L., & Pagliari, P. H. (2021). Organic Oat Response to Variety, Seeding Rate, and Nutrient Source and Rate. Agronomy, 11(7), 1418. https://doi.org/10.3390/agronomy11071418





