Overcompensation Can Be an Ideal Breeding Target
Abstract
1. Introduction
2. The Overcompensation Controversy: Does Defoliation Result in Dependable Yield Increase?
3. Yield Response to Defoliation Varies Greatly among Genotypes
4. Genotype, Environment, as Well as Method and Timing of Defoliation, Are All Critical for Positive Maize Yield Response
5. Positive Yield Responses to Defoliation Typically Occur in Drought Stress Environment
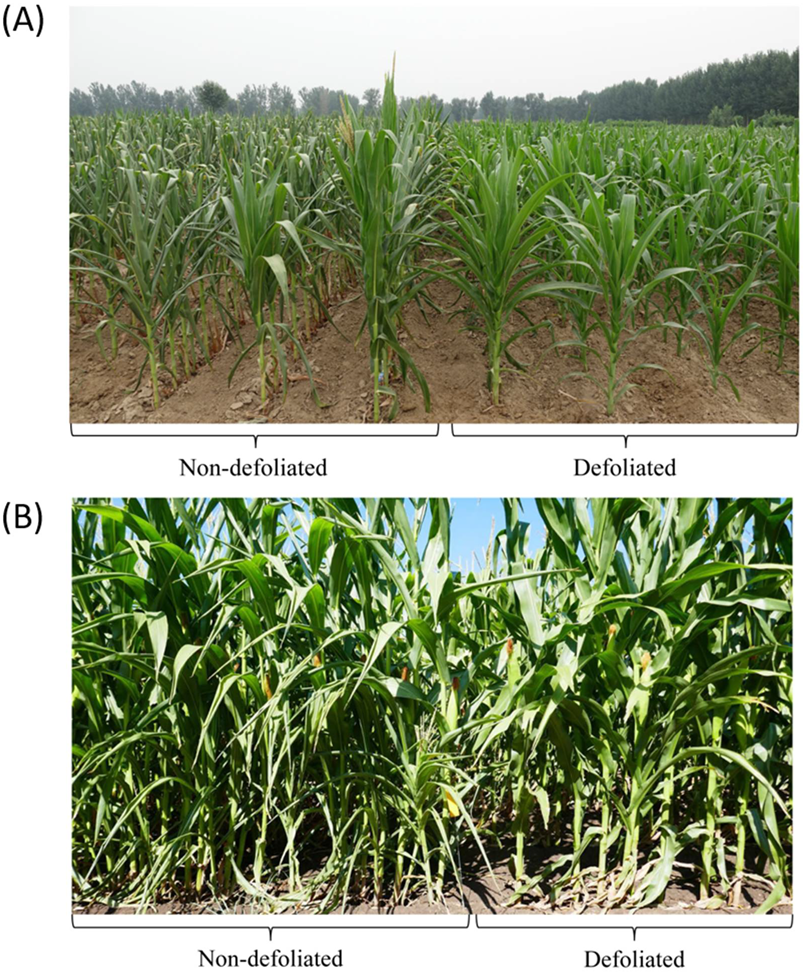
6. Changes in Plant Morphology and Yield Components in Maize Crops Responded Positively to Defoliation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global Food Demand and the Sustainable Intensification of Agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A. Selectable Traits to Increase Crop Photosynthesis and Yield of Grain Crops. J. Exp. Bot. 2000, 51 (Suppl. S1), 447–458. [Google Scholar] [CrossRef] [PubMed]
- Maschinski, J.; Whitham, T. The Continuum of Plant Responses to Herbivory: The Influence of Plant Association, Nutrient Availability, and Timing. Am. Nat. 1989, 134, 1–9. [Google Scholar] [CrossRef]
- Nilsson, P.; Tuomi, J.; Astrom, M. Even Repeated Grazing May Select for Overcompensation. Ecology 1996, 77, 1942–1946. [Google Scholar] [CrossRef]
- Lennartsson, T.; Tuomi, J.; Nilsson, P. Evidence for an Evolutionary History of Overcompensation in the Grassland Biennial Gentianella Campestris (Gentianaceae). Am. Nat. 1997, 149, 1147–1155. [Google Scholar] [CrossRef]
- Mauricio, R.; Rausher, M.D.; Burdick, D.S. Variation in the Defense Strategies of Plants: Are Resistance and Tolerance Mutually Exclusive? Ecology 1997, 78, 1301–1311. [Google Scholar] [CrossRef]
- Weinig, C.; Stinchcombe, J.R.; Schmitt, J. QTL Architecture of Resistance and Tolerance Traits in Arabidopsis Thaliana in Natural Environments. Mol. Ecol. 2003, 12, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Siddappaji, M.H.; Scholes, D.R.; Bohn, M.; Paige, K.N. Overcompensation in Response to Herbivory in Arabidopsis Thaliana: The Role of Glucose-6-Phosphate Dehydrogenase and the Oxidative Pentose-Phosphate Pathway. Genetics 2013, 195, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Scholes, D.R.; Paige, K.N. Plasticity in Ploidy Underlies Plant Fitness Compensation to Herbivore Damage. Mol. Ecol. 2014, 23, 4862–4870. [Google Scholar] [CrossRef] [PubMed]
- Poveda, K.; Jiménez, M.I.G.; Kessler, A. The Enemy as Ally: Herbivore-Induced Increase in Crop Yield. Ecol. Appl. 2010, 20, 1787–1793. [Google Scholar] [CrossRef]
- Stieha, C.; Poveda, K. Tolerance Responses to Herbivory: Implications for Future Management Strategies in Potato. Ann. Appl. Biol. 2015, 166, 208–217. [Google Scholar] [CrossRef]
- Rautio, P.; Huhta, A.P.; Piippo, S.; Tuomi, J.; Juenger, T.; Saari, M.; Aspi, J. Overcompensation and Adaptive Plasticity of Apical Dominance in Erysimum Strictum (Brassicaceae) in Response to Simulated Browsing and Resource Availability. Oikos 2005, 111, 179–191. [Google Scholar] [CrossRef]
- Paige, K.N.; Whitham, T.G. Flexible Life History Traits: Shifts by Scarlet Gilia in Response to Pollinator Abundance. Ecology 1987, 68, 1691–1695. [Google Scholar] [CrossRef]
- Paige, K.N. Overcompensation in Response to Mammalian Herbivory: From Mutualistic to Antagonistic Interactions. Ecology 1992, 73, 2076–2085. [Google Scholar] [CrossRef]
- Paige, K.N. Regrowth Following Ungulate Herbivory in Ipomopsis Aggregata: Geographic Evidence for Overcompensation. Oecologia 1999, 118, 316–323. [Google Scholar] [CrossRef]
- Anderson, L.L.; Paige, K.N. Multiple Herbivores and Coevolutionary Interactions in an Ipomopsis Hybrid Swarm. Evol. Ecol. 2003, 17, 139–156. [Google Scholar] [CrossRef]
- Agrawal, A.A. Induced Responses to Herbivory and Increased Plant Performance. Science 1998, 279, 1201–1202. [Google Scholar] [CrossRef]
- Agrawal, A.A.; Strauss, S.Y.; Stout, M.J. Costs of Induced Responses and Tolerance to Herbivory in Male and Female Fitness Components of Wild Radish. Evolution 1999, 53, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Poveda, K.; Díaz, M.; Ramirez, A. Can Overcompensation Increase Crop Production? Ecology 2018, 99, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Bergelson, J.; Crawley, M.J. Herbivory and Ipomopsis Aggregate: The Disadvantages of Being Eaten. Am. Nat. 1992, 139, 870–882. [Google Scholar] [CrossRef]
- Belsky, A.J.; Carson, W.P.; Jensen, C.L.; Fox, G.A. Overcompensation by Plants: Herbivore Optimization or Red Herring? Evol. Ecol. 1993, 7, 109–121. [Google Scholar] [CrossRef]
- Echarte, L.; Andrade, F.H.; Sadras, V.O.; Abbate, P. Kernel Weight and Its Response to Source Manipulations during Grain Filling in Argentinean Maize Hybrids Released in Different Decades. Field Crops Res. 2006, 96, 307–312. [Google Scholar] [CrossRef]
- Hanway, J.J. Defoliation Effect on Different Corn (Zea Mays, L.) Hybrids as Influenced by Plant Population and Stage of Development. Agron. J. 1969, 61, 534–538. [Google Scholar] [CrossRef]
- Hicks, D.R.; Crookston, R.K. Defoliation Boosts Corn Yield. Crop. Soil 1976, 29, 12–13. [Google Scholar]
- Crookston, R.K.; Hicks, D.R. Early Defoliation Affects Corn Grain Yields 1. Crop. Sci. 1977, 18, 485–489. [Google Scholar] [CrossRef]
- Hicks, D.R.; Nelson, W.W.; Ford, J.H. Defoliation Effects on Corn Hybrids Adapted to the Northern Corn Belt. Agron. J. 1977, 69, 387–390. [Google Scholar] [CrossRef]
- Allen, J.R.; McKee, G.W.; McGahen, J.H. Leaf Number and Maturity in Hybrid Corn 1. Agron. J. 1973, 65, 233–235. [Google Scholar] [CrossRef]
- Lauer, J.G.; Roth, G.W.; Bertram, M. Impact of Defoliation on Corn Forage Yield. Agron. J. 2004, 96, 1459–1463. [Google Scholar] [CrossRef]
- Roth, G.W.; Lauer, J.G. Impact of Defoliation on Corn Forage Quality. Agron. J. 2008, 100, 651–657. [Google Scholar] [CrossRef]
- Adee, E.A.; Paul, L.E.; Nafziger, E.D.; Bollero, G.A. Yield Loss of Corn Hybrids to Incremental Defoliation. Crop. Manag. 2005, 4, 1–9. [Google Scholar] [CrossRef]
- Dungan, G.H. Losses to the Corn Crop Caused by Leaf Injury. Plant. Physiol. 1934, 9, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Dungan, G.H. Relation of Blade Injury to the Yielding Ability of Corn Plants. J. Am. Soc. Agron. 1930, 22, 164–170. [Google Scholar] [CrossRef]
- Hume, A.N.; Franzke, C. The Effect of Certain Injuries to Leaves of Corn Plant upon Weights of Grain Produced. Agron. J. 1929, 21, 1156–1164. [Google Scholar] [CrossRef]
- Culpepper, C.W.; Magoon, C.A. Effects of Defoliation and Root Pruning on the Chemical Composition of Sweet Corn Kernels. J. Agric. Res. 1930, 40, 575–583. [Google Scholar]
- Andrade, F.H.; Aguirrezabal, L.A.N.; Rizzali, R.H. Growth and Performance Compared. In Basis for Management of Maize, Sunflower and Soybeans; Andrade, F.H., Sadras, V.O., Eds.; INTA-FCA (UNMdP): Balcarce, Argentina, 2000. [Google Scholar]
- Jenkins, M.T. Influence of Climate and Weather on Growth of Corn. In Yearbook of Agriculture: Climate and Man; USDA and USDA Forest Serv.: Washington, DC, USA, 1941. [Google Scholar]
- Egharevba, P.N.; Horrosks, R.D.; Zuber, M.S. Dry Matter Accumulation in Maize in Response to Defoliation. Agron. J. 1976, 68, 40–43. [Google Scholar] [CrossRef]
- Tilahun, A. Quantitative and Physiological Traits in Maize (Zea Mays) Associated with Different Levels of Moisture, Plant Density and Leaf Defoliation in Ethiopia. In Proceedings of the First National Maize Workshop of Ethiopia, Addis Abeba, Ethiopia, 5–7 May 1992; pp. 74–80. [Google Scholar]
- Eldredge, J.C. The Effect of Injury in Imitation of Hail Damage on the Development of the Corn Plant. Iowa Agric. Home Econ. Exp. Stn. Ames. 1935, 16, 1. [Google Scholar]
- Cloninger, F.D.; Zuber, M.S.; Horrocks, R.D. Synchronization of Flowering in Corn (Zea Mays, L.) by Clipping Young Plants. Agron. J. 1974, 66, 270–272. [Google Scholar] [CrossRef]
- Pang, X.G.; Shi, Y.; Kan, Y.W.; Liu, X.; Gao, Q.H.; Zhang, G.; Yuan, W.G. Study on the Effect of Defoliation at Different Leaf Stage on Maize Yield. Xiandainongyekeji 2016, 20, 45. (In Chinese) [Google Scholar]
- Crookston, R.K.; Hicks, D.R. Effect of Early Defoliation on Maize Growth and Yield: An Eleven-Year Perspective. Crop. Sci. 1988, 28, 371–373. [Google Scholar] [CrossRef]
- Vasilas, B.L.; Seif, R.D. Defoliation Effects on Two Corn Inbreds and Their Single-Cross Hybrid. Agron. J. 1985, 77, 816–820. [Google Scholar] [CrossRef]
- Shanahan, J.F.; Nielsen, D.C. Influence of Growth Retardants (Anti-Gibberelins) on Corn Vegetative Growth, Water Use, and Grain Yield under Different Levels of Water Stress. Agron. J. 1987, 79, 103–109. [Google Scholar] [CrossRef]
- Shapiro, C.A.; Peterson, T.A.; Flowerday, A.D. Yield Loss Due to Simulated Hail Damage on Corn: A Comparison of Actual and Predicted Values. Agron. J. 1986, 78, 585–589. [Google Scholar] [CrossRef]
- Ren, Q.Y. Experiments on Seedling in Maize. Com. Agri. Sci. Tech. 1980, 4, 9. (In Chinese) [Google Scholar]
- Bao, J.; Yang, C. Studies on Seedling Defoliation in Maize. Shannxi Agric. Sci. 1986, 4, 11–12. (In Chinese) [Google Scholar]
- Xie, P.J.; Gao, K.; Jie, G.Y.; Wang, Z.W. Application of Defoliation Technology in Spring Maize in Taoyuan. Pri. Agric. Tech. Ext. 2018, 1, 9. (In Chinese) [Google Scholar]
- Zhang, F.L.; Su, B.J.; Zong, Y.H.; Li, L.Q.; Li, L. Effect of Defoliation on Yield and Other Agronomic Traits in Maize. Bull. Agric. Sci. Technol. 2016, 11, 129–132. (In Chinese) [Google Scholar]
- Zhang, C.Y.; Su, B.J.; Zong, Y.H.; Liu, Y.B.; Li, L.; Zhang, F.L. Preliminary Results of Defoliation on Maize Yield in Hei-Long-Jiang Provice. Res. Rep. 2016, 1005-269012-0091-03, 91–93. (In Chinese) [Google Scholar]
- Li, L.; Zhang, G.P.; Su, B.J.; Zong, Y.H.; Zhang, F.L. Studies on the Influence of Seedling Cutting to Plant Morphology and Yield in Maize. Exp. Stud. 2018, 4, 91–95, (In Chinese with English abstract). [Google Scholar]
- Li, Y.P.; Liu, Y.L.; Sun, S.; Wang, T.; Zhai, W.B. Effects of Defoliation on Maize Growth and Yield. Agric. Jilin 2019, 4, 48. [Google Scholar]
- Feng, G.; Wang, X.J.; Wang, X.F.; Yu, B.; Zhao, C.H.; Tong, S.H.; Cao, Z.B. Effect of Maize Cut Seedling on Plant Main Characteristics and Yield. Liaoning Agric. Sci. 2018, 6, 73–75. [Google Scholar]
- Dungan, G.H.; Gausman, H.W. Clipping Corn Plant to Delay Their Development. Agron. J. 1951, 43, 90–93. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Marasini, M.; Zong, Y.; Su, B.J.; Li, L.; Zhang, F.L. Studies on the Effects of Seedling Cut on Growth and Development of Spring Maize. Highlights Sci. Pap. Online 2018, 11, 212–216. Available online: https://max.book118.com/html/2019/0311/8035002054002012.shtm (accessed on 21 August 2019). (In Chinese with English abstract).
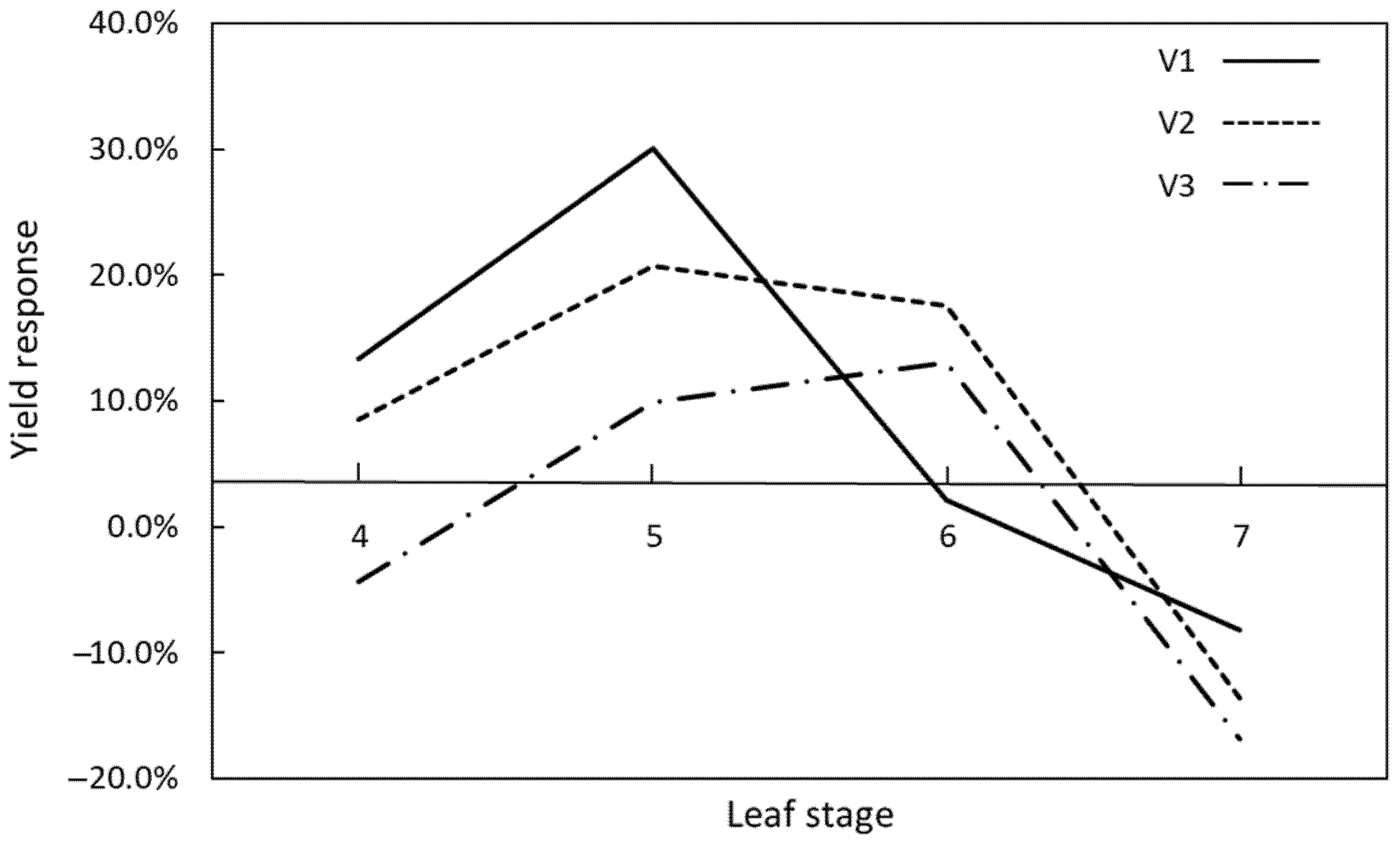


| Year | Location | Hybrid | Kernel Yield (kg/ha) | Yield Change Following Defoliation | p Value | |
|---|---|---|---|---|---|---|
| Control | Defoliated | |||||
| 2016 | Langfang | Jinrui88 | 2441.3 | 3930.0 | 61.0% | p < 0.05 |
| (China) | Cangyu76 | 7222.5 | 8756.3 | 21.2% | p < 0.05 | |
| Wuke2 | 10,796.3 | 11,010.0 | 2.0% | p > 0.05 | ||
| Yufeng303 | 12,853.3 | 12,115.0 | −5.7% | p < 0.05 | ||
| Shiyu9 | 4507.5 | 4125.0 | −9.3% | p < 0.05 | ||
| Xianyu335 | 9967.5 | 8343.0 | −16.3% | p < 0.05 | ||
| Jufeng558 | 9761.3 | 7646.3 | −21.7% | p < 0.05 | ||
| Gatton | PAC727IT | 5125.2 | 5510.8 | 7.5% | p < 0.05 | |
| (Australia) | PAC606IT | 5853.4 | 5874.2 | 0.4% | p > 0.05 | |
| 2017 | Tangshan | Jinsai29 | 6784.5 | 7701.0 | 13.5% | p < 0.05 |
| (China) | Huanong887 | 10,692.7 | 11,596.0 | 8.4% | p < 0.05 | |
| Jinghai5 | 6829.5 | 5667.0 | −20.5% | p < 0.05 | ||
| Zhengdan958 | 7959.5 | 6344.0 | −25.5% | p < 0.05 | ||
| Year | Location (Latitude/Longitude) | Genotype | Yield (kg/ha) | Difference | Reference | |
|---|---|---|---|---|---|---|
| Non-Defoliation | Defoliation | |||||
| 2014 | Langfang, Hebei (39.520° N/116.680° E) | Chengyu13 | 7731.8 | 8499.3 | 9.9% | [41] |
| Yuhe988 | 7419.3 | 8954.0 | 20.7% | |||
| Heyu187 | 7299.9 | 9495.0 | 30.1% | |||
| 2016 | Tongliao, Neimenggu (43.517° N/121.967° E) | Chunyu968 | 11,444.3 | 13,350.8 | 16.7% | [49] |
| Langfang, Hebei (39.520° N/116.680° E) | Nongda372 | 6982.5 | 7638.8 | 9.4% | ||
| Linfen, Shanxi (36.359° N/111.558° E) | Huaneng887 | 7130.3 | 9582.8 | 34.4% | ||
| 2016 | Yichun, Heilongjiang (47.727° N/128.841° E) | Demeiya1 | 7740.0 | 9075.0 | 17.2% | [50] |
| Harbin, Heilongjiang (45.803° N/126.535° E) | Nongyu7 | 4887.0 | 5467.5 | 11.9% | ||
| Qiqihar, Heilongjiang (47.354° N/123.918° E) | Jida935 | 4434.0 | 4990.5 | 12.6% | ||
| 2017 | Taoyuan, Hunan (28.902° N/111.488° E) | Qiyu7 | 9704.0 | 9975.0 | 2.8% | [48] |
| 2016 | Langfang, Hebei (39.520° N/116.680° E) | Heyu187 | 7290.0 | 9500.0 | 30.3% | [51] |
| 2017 | Zhengdan958 | 6090.0 | 7180.0 | 17.9% | ||
| 2018 | Fengcheng, Liaoning (40.452° N/124.066° E) | Liangyu99 | 10,139.2 | 11,336.5 | 11.8% | [53] |
| Hongshuo899 | 7716.4 | 10,284.8 | 33.3% | |||
| 2019 | Jilin, Jilin (43.501° N/126.330° E) | Xianyu335 | 11,512.6 | 12,990.9 | 12.8% | [52] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Z.; Powell, J.J.; Ye, X.; Liu, X.; Yuan, Z.; Liu, C. Overcompensation Can Be an Ideal Breeding Target. Agronomy 2021, 11, 1376. https://doi.org/10.3390/agronomy11071376
Zheng Z, Powell JJ, Ye X, Liu X, Yuan Z, Liu C. Overcompensation Can Be an Ideal Breeding Target. Agronomy. 2021; 11(7):1376. https://doi.org/10.3390/agronomy11071376
Chicago/Turabian StyleZheng, Zhi, Jonathan J. Powell, Xueling Ye, Xueqiang Liu, Zhongwei Yuan, and Chunji Liu. 2021. "Overcompensation Can Be an Ideal Breeding Target" Agronomy 11, no. 7: 1376. https://doi.org/10.3390/agronomy11071376
APA StyleZheng, Z., Powell, J. J., Ye, X., Liu, X., Yuan, Z., & Liu, C. (2021). Overcompensation Can Be an Ideal Breeding Target. Agronomy, 11(7), 1376. https://doi.org/10.3390/agronomy11071376






