Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Crop Materials and Experimental Design
2.2. Experimental Measurements
2.2.1. Photosynthetic Characteristics
2.2.2. Relative electrolyte conductivity
2.2.3. Relative Water Content
2.2.4. Proline Content
2.2.5. Indicators of Oxidative Damage
2.2.6. Antioxidant Enzyme Activity
2.3. Statistical Analysis
3. Results
3.1. Effects of SA on the Growth and Biomass of the Maize Seedlings
3.2. Effect of SA Treatment on the Cell Membrane Permeability and MDA Content
3.3. Effects of SA Treatment on the Relative Water Content and Proline Content of Maize Seedlings
3.4. Effects of SA Treatment on Maize Seedling Photosynthesis
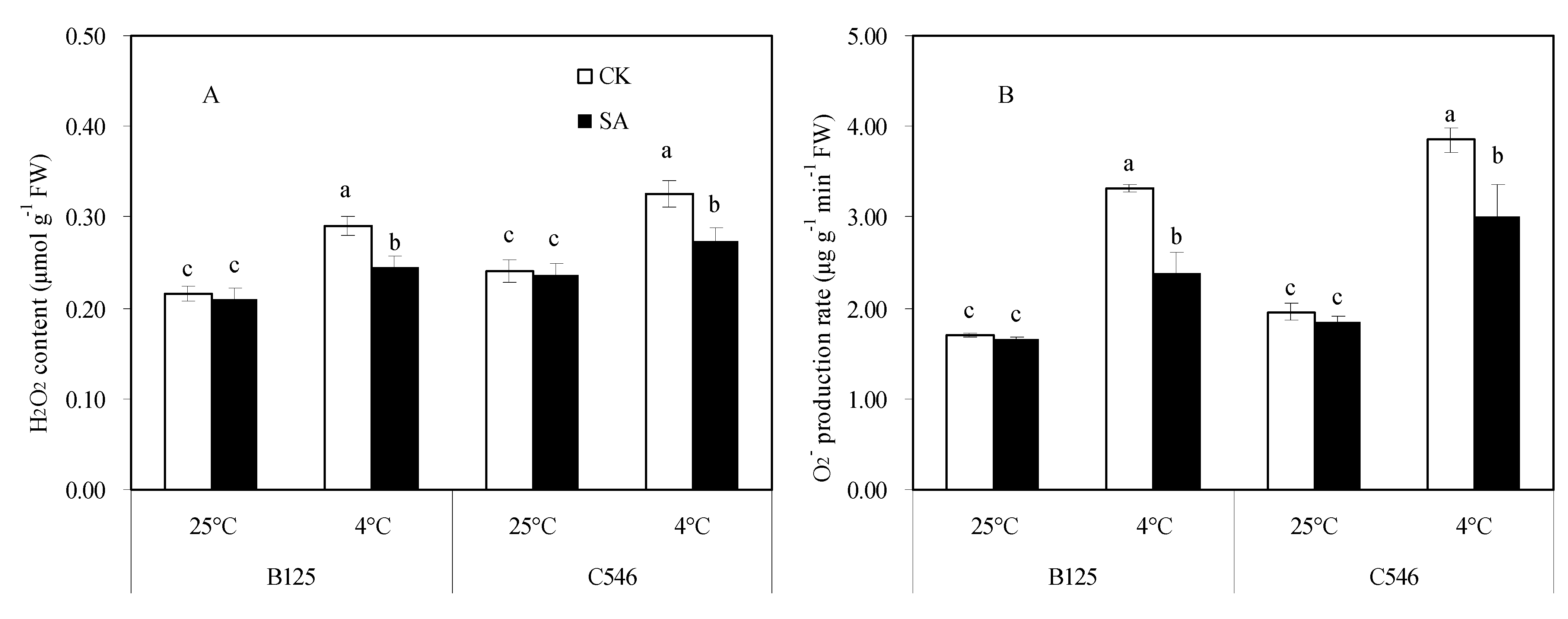
3.5. Effects of SA Treatment on the ROS Content of Maize Seedlings
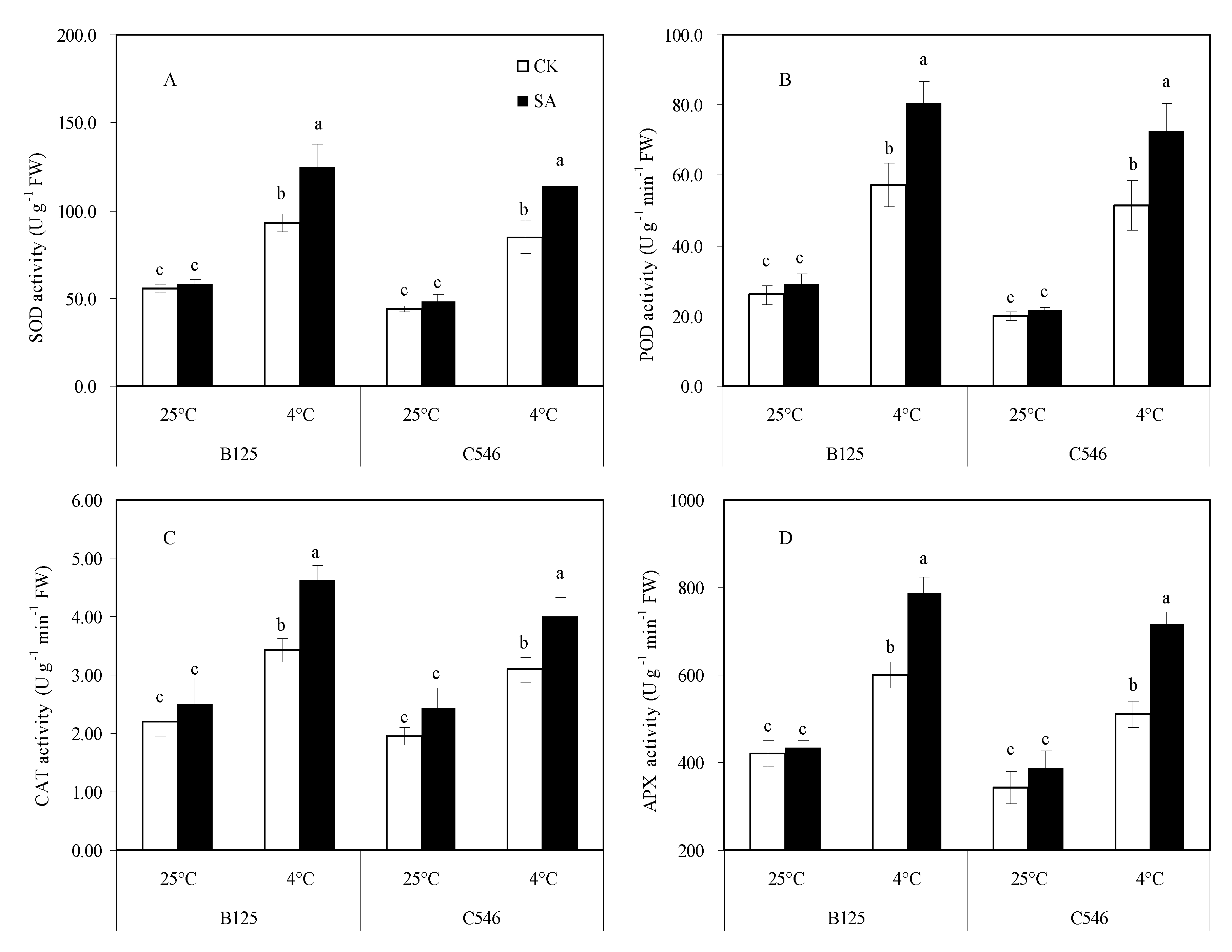
3.6. Effects of SA Treatment on Antioxidant Enzyme System
4. Discussion
4.1. Growth and Physiological Changes in Maize Seedlings under Chilling Stress
4.2. Effects of SA on Growth and Physiological Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Wu, P.; Zhang, W.; Yang, Z.; Liu, H.; Ahammed, G.J.; Cui, J. Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci. Hortic. Amst. 2020, 261, 108953. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. Forest Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Tian, L.X.; Li, J.; Bi, W.S.; Zuo, S.Y.; Li, L.J.; Li, W.L.; Sun, L. Effects of waterlogging stress at different growth stages on the photosynthetic characteristics and grain yield of spring maize (Zea mays L.) under field conditions. Agr. Water Manag. 2019, 218, 250–258. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K.H.M. Salt stress in maize: Effects, resistance mechanisms and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef] [Green Version]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in ecophysiology, osmolytes, and secondary metabolites of the medicinal plants of mentha piperita and catharanthus roseus subjected to drought and heat stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Sowinski, P.; Rudzinska-Langwald, A.; Adamczyk, J.; Kubica, I.; Fronk, J. Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J. Plant Physiol. 2005, 162, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Majlath, I.; Pal, M.; Gondor, O.K.; Rudnoy, S.; Olah, C.; Vankova, R.; Kalapos, B.; Janda, T. Janus-faced nature of light in the cold acclimation processes of maize. Front. Plant Sci. 2018, 9, 850. [Google Scholar] [CrossRef] [Green Version]
- Louarn, G.; Chenu, K.; Fournier, C.; Andrieu, B.; Giauffret, C. Relative contributions of light interception and radiation use efficiency to the reduction of maize productivity under cold temperatures. Funct. Plant Biol. 2008, 35, 885–899. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Hoglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances, and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Wise, R.R.; Naylor, A.W. Chilling-enhanced photooxidation: The peroxidative destruction of lipids during chilling injury to photosynthesis and ultrastructure. Plant Physiol. 1987, 83, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Klessig, D.F.; Choi, H.W.; Dempsey, D.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Gobbato, E.; Kracher, B.; Qiu, J.; Bautor, J.; Parker, J.E. A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol. 2017, 213, 1802–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, C.; Tsai, C.J.; Unsicker, S.B.; Xue, L.; Reichelt, M.; Gershenzon, J.; Hammerbacher, A. Salicylic acid activates poplar defense against the biotrophic rust fungus Melampsora larici-populina via increased biosynthesis of catechin and proanthocyanidins. New Phytol. 2019, 221, 960–975. [Google Scholar] [CrossRef] [Green Version]
- Kosová, K.; Prášil, I.T.; Vítámvás, P.; Dobrev, P.; Motyka, V.; Floková, K.; Novák, O.; Turečková, V.; Rolčik, J.; Pešek, B.; et al. Complex phytohormone responses during the cold acclimation of two wheat cultivars differing in cold tolerance, winter Samanta and spring Sandra. J. Plant Physiol. 2012, 169, 567–576. [Google Scholar] [CrossRef]
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2017, 255, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, J.; Peng, Y.; Huang, B. Metabolic pathways regulated by abscisic acid, salicylic acid and gamma-aminobutyric acid in association with improved drought tolerance in creeping bentgrass (Agrostis stolonifera). Physiol. Plant. 2017, 159, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Yu, Q.; Ma, Y.; Gu, W.; Yang, D. Physiological changes associated with enhanced cold resistance during maize (Zea mays) germination and seedling growth in response to exogenous calcium. Crop Pasture Sci. 2020, 71, 529. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photo peroxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Elstner, E.F.; Heupel, A. Inhibition of nitrite formation from hydroxylammoniumchloride: A simple assay for superoxide dismutase. Anal. Biochem. 1976, 70, 616–620. [Google Scholar] [CrossRef]
- Jana, S.; Choudhuri, M.A. Glycolate metabolism of three submersed aquatic angiosperms during ageing. Aquat. Bot. 1982, 12, 345–354. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Hernández, J.A.; Jiménez, A.; Mullineaux, P.; Sevilla, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defenses. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Sohn, Y.G.; Lee, B.H.; Kang, K.Y.; Lee, J.J. Effects of NaCl stress on germination, antioxidant responses, and proline content in two rice cultivars. J. Plant Biol. 2005, 48, 201–208. [Google Scholar] [CrossRef]
- Kang, H.M.; Saltveit, M.E. Activity of enzymatic antioxidant defense systems in chilled and heat shocked cucumber seedling radicles. Physiol. Plant. 2001, 113, 548–556. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Mauch, F. The role of abscisic acid in plant pathogen interactions. Curr. Opin. Plant Biol. 2005, 8, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Ploschuk, E.L.; Bado, L.A.; Salinas, M.; Wassner, D.F.; Windauer, L.B.; Insausti, P. Photosynthesis and fluorescence responses of Jatropha curcas to chilling and freezing stress during early vegetative stages. Environ. Exp. Bot. 2014, 102, 18–26. [Google Scholar] [CrossRef]
- Aroca, R.; Tognoni, F.; Irigoyen, J.J.; Sánchez-Díaz, M.; Pardossi, A. Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol. Biochem. 2001, 39, 1067–1073. [Google Scholar] [CrossRef]
- Rymen, B.; Fiorani, F.; Kartal, F.; Vandepoele, K.; Inzé, D.; Beemster, G.T.S. Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes 1[W][OA]. Plant Physiol. 2007, 143, 1429–1438. [Google Scholar] [CrossRef] [Green Version]
- Stone, P.; Sorensen, I.; Jamieson, I. Effect of soil temperature on phenology, canopy development, biomass and yield of maize in a cool-temperate climate. Field Crop. Res. 1999, 63, 169–178. [Google Scholar] [CrossRef]
- Sun, Y.; He, Y.; Irfan, A.R.; Liu, X.; Yu, Q.; Zhang, Q.; Yang, D. Exogenous Brassinolide Enhances the Growth and Cold Resistance of Maize (Zea mays L.) Seedlings under Chilling Stress. Agronomy 2020, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, N.; Erika, H.; Christian, K. Critically low soil temperatures for root growth and root morphology in three alpine plant species. Alp. Bot. 2016, 126, 11–21. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Li, M.; Lin, L.; Zhang, Y.; Sui, N. ZmMYB31, a R2R3-MYB transcription factor in maize, positively regulates the expression of CBF genes and enhances resistance to chilling and oxidative stress. Mol. Biol. Rep. 2019, 46, 3937–3944. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, E.W.; Fridovich, I.S. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J. Biol. Chem. 1975, 250, 8812–8817. [Google Scholar] [CrossRef]
- Gong, H.; Zhu, X.; Chen, K.; Wang, S.; Zhang, C. Silicon alleviates oxidative damage of wheat plants in pots under drought. Plant Sci. 2005, 169, 313–321. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, M.; Gong, X.; Liu, C.; Hong, M.; Wang, L.; Hong, F. Influence of lanthanides on the antioxidative defense system in maize seedlings under cold stress. Biol. Trace Elem. Res. 2011, 142, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Bhusal, N.; Bhusal, S.J.; Yoon, T. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Aniszewski, T.; Drozdov, S.N.; Kholoptseva, E.S.; Kurets, V.K.; Obshatko, L.A.; Popov, E.G.; Talanov, A.V. Effects of light and temperature parameters on net photosynthetic carbon dioxide fixation by whole plants of five lupin species (Lupinus albus L., Lupinus angustifoliu L., Lupinus luteus L., Lupinus mutabilis Sweet. Lupinus polyphyllus Lindl.). Acta Agric. Scand. Sect. B-Soil Plant Sci. 2001, 51, 17–27. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J.C. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Jiang, B.C.; Shi, Y.T.; Peng, Y.; Jia, Y.X.; Yan, Y.; Dong, X.J.; Hui, L.; Dong, J.; Li, J.; Gong, Z.Z.; et al. Cold-Induced CBF–PIF3 Interaction Enhances Freezing Tolerance by Stabilizing the phyB Thermosensor in Arabidopsis. Mol. Plant. 2020, 13, 894–906. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.G. Osmotic adjustment and plant adaptation to environmental changes related to drought and salinity. Environ. Rev. 2010, 18, 309–319. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, J.; Chang, Q.; Gu, C.; Song, A.; Chen, S.; Dong, B.; Chen, F. Cold acclimation induces freezing tolerance via antioxidative enzymes, proline metabolism and gene expression changes in two chrysanthemum species. Mol. Biol. Rep. 2014, 41, 815–822. [Google Scholar] [CrossRef]
- Ahmad, I.; Basra, S.M.A.; Wahid, A. Exogenous application of ascorbic acid, salicylic acid and hydrogen peroxide improve the productivity of hybrid maize at low temperature stress. Int. J. Agric. Biol. 2014, 16, 1560–8530. [Google Scholar]
- Bari, R.; Jones, J.D. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.B.; Tian, L.; Tian, R.R.; Pan, Q.H.; Zhan, J.C.; Wen, P.F.; Chen, J.Y.; Zhang, P.; Wang, W.; Huang, W.D. Involvement of phospholipase D in the low temperature acclimation-induced thermo tolerance in grape berry. Plant Physiol. Biochem. 2009, 47, 504–510. [Google Scholar] [CrossRef]
- Shi, Q.; Bao, Z.; Zhu, Z.; Ying, Q.; Qian, Q. Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of cucumis sativa L. Plant Growth Regul. 2006, 48, 127–135. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Qin, G.; Cui, H.; Wang, Q. Salicylic acid analogues with biological activity may induce chilling tolerance of maize (Zea mays) seeds. Botany 2012, 90, 845–855. [Google Scholar] [CrossRef]
- Malan, C.; Greyling, M.M.; Gressel, J. Correlation between Cu Zn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990, 69, 157–166. [Google Scholar] [CrossRef]
- Bandurska, H.; Ski, A.S. The effect of salicylic acid on barley response to water deficit. Acta Physiol. Plant 2005, 27, 379–386. [Google Scholar] [CrossRef]
- Mateo, A.; Funck, D.; Mu Hlenbock, P.; Kular, B.; Mullineaux, P.M.; Karpinski, A.S. Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J. Exp. Bot. 2006, 57, 1795–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Inbred Lines | Temperature (°C) | SA (mg/L) | Plant Height (cm) | Root Length (cm) | Seedling DW (mg) | Root DW (mg) |
|---|---|---|---|---|---|---|
| B125 | 25 | 0 | 22.4 ± 0.5 a | 22.6 ± 1.6 ab | 130.4 ± 5.4 b | 61.5 ± 1.4 b |
| 50 | 23.8 ± 0.7 a | 25.0 ± 1.2 a | 152.7 ± 4.1 a | 71.0 ± 1.9 a | ||
| 4 | 0 | 20.5 ± 0.6 b | 19.5 ± 0.7 c | 103.7 ± 3.6 c | 46.3 ± 1.8 c | |
| 50 | 22.3 ± 0.4 a | 22.1 ± 1.4 b | 126.3 ± 3.9 b | 60.8 ± 4.5 b | ||
| C546 | 25 | 0 | 25.0 ± 0.5 ab | 24.1 ± 0.7 ab | 138.6 ± 5.2 b | 84.8 ± 2.8 b |
| 50 | 27.6 ± 0.8 a | 26.7 ± 1.2 a | 166.8 ± 7.9 a | 98.4 ± 6.3 a | ||
| 4 | 0 | 19.7 ± 0.8 c | 20.7 ± 0.3 c | 108.5 ± 3.4 c | 61.3 ± 1.6 c | |
| 50 | 23.5 ± 0.7 b | 23.4 ± 0.5 b | 137.4 ± 4.1 b | 84.2 ± 3.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, D.; Wang, Q.; Song, X.; Wang, Y.; Yang, X.; Qin, D.; Xie, T.; Yang, D. Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics. Agronomy 2021, 11, 1341. https://doi.org/10.3390/agronomy11071341
Zhang Q, Li D, Wang Q, Song X, Wang Y, Yang X, Qin D, Xie T, Yang D. Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics. Agronomy. 2021; 11(7):1341. https://doi.org/10.3390/agronomy11071341
Chicago/Turabian StyleZhang, Qian, Dongmei Li, Qi Wang, Xiangyu Song, Yingbo Wang, Xilang Yang, Dongling Qin, Tenglong Xie, and Deguang Yang. 2021. "Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics" Agronomy 11, no. 7: 1341. https://doi.org/10.3390/agronomy11071341
APA StyleZhang, Q., Li, D., Wang, Q., Song, X., Wang, Y., Yang, X., Qin, D., Xie, T., & Yang, D. (2021). Exogenous Salicylic Acid Improves Chilling Tolerance in Maize Seedlings by Improving Plant Growth and Physiological Characteristics. Agronomy, 11(7), 1341. https://doi.org/10.3390/agronomy11071341






