Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
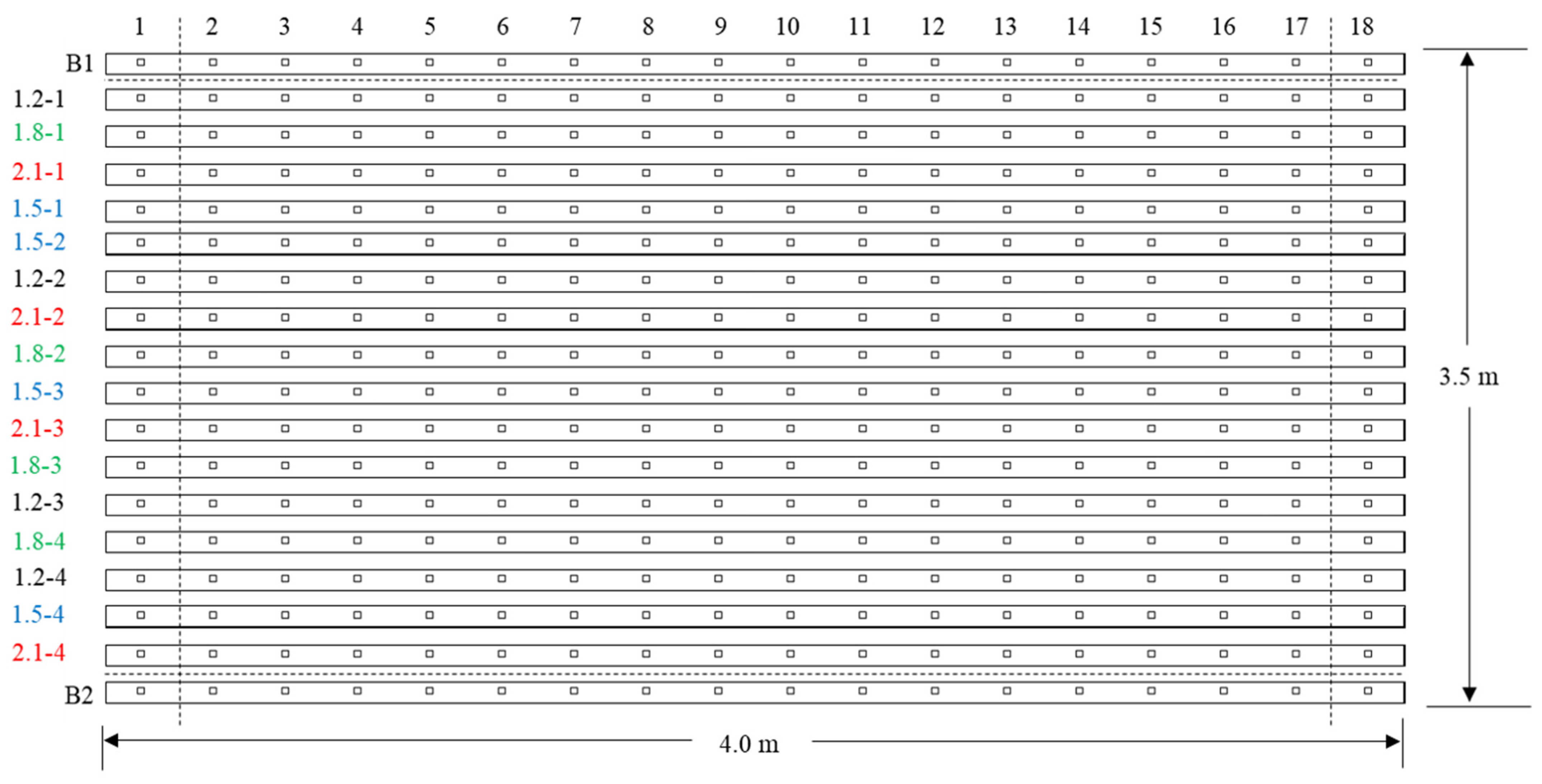
2.2. Treatments and Experimental Design
2.3. Measurement of Gas Exchange Properties and Light Response Curve
2.4. Measurement of Leaf Area and Relative Chlorophyll Content (SPAD)
2.5. Harvesting and Yield Measurements
2.6. Measurement of Tissue Nutrient Analysis
2.7. Measurement of Total Glucosinolates
2.8. Measurement of Total Anthocyanins
2.9. Measurement of Chlorophyll and Carotenoids
2.10. Statistical Analysis
3. Results
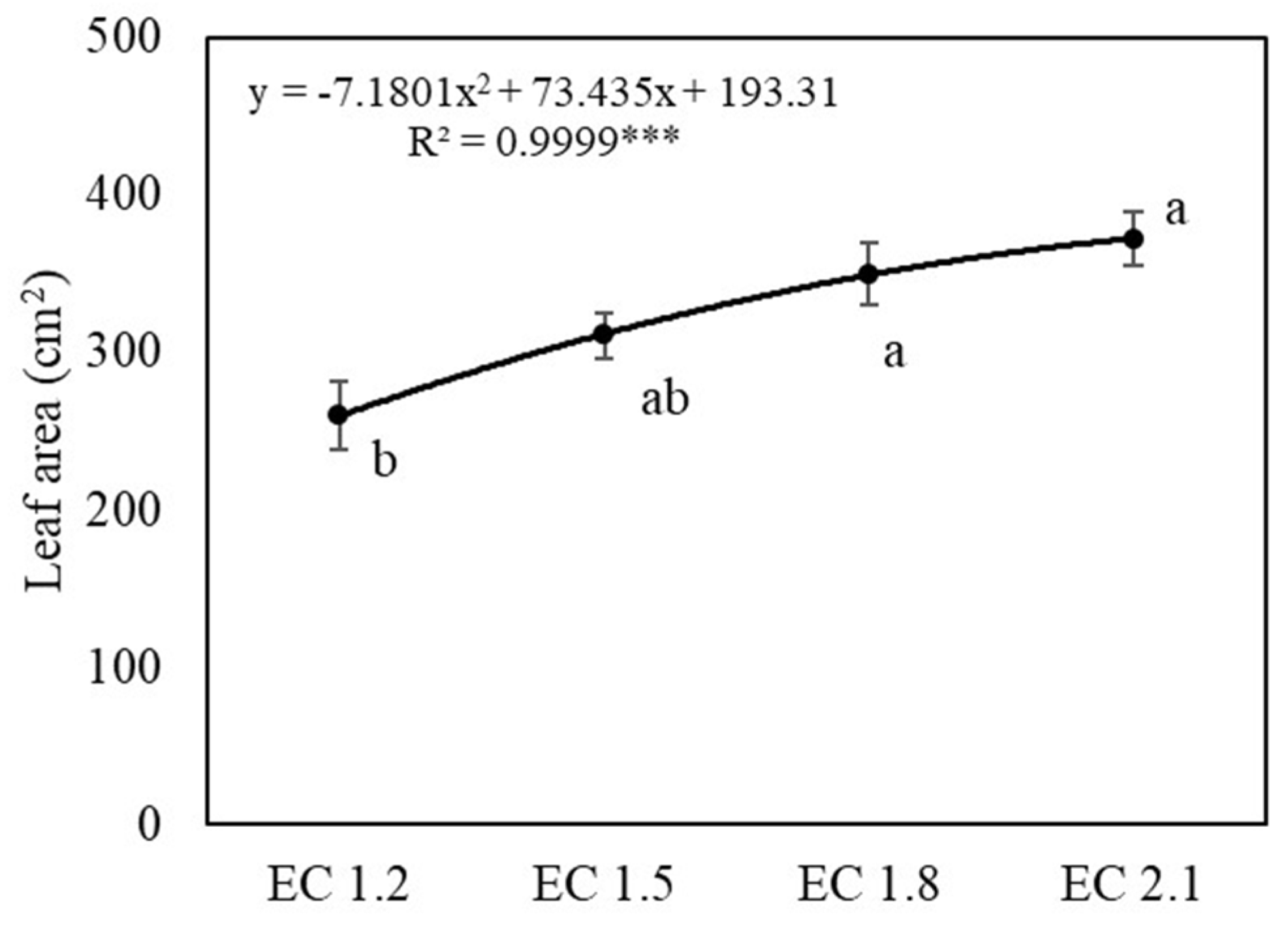
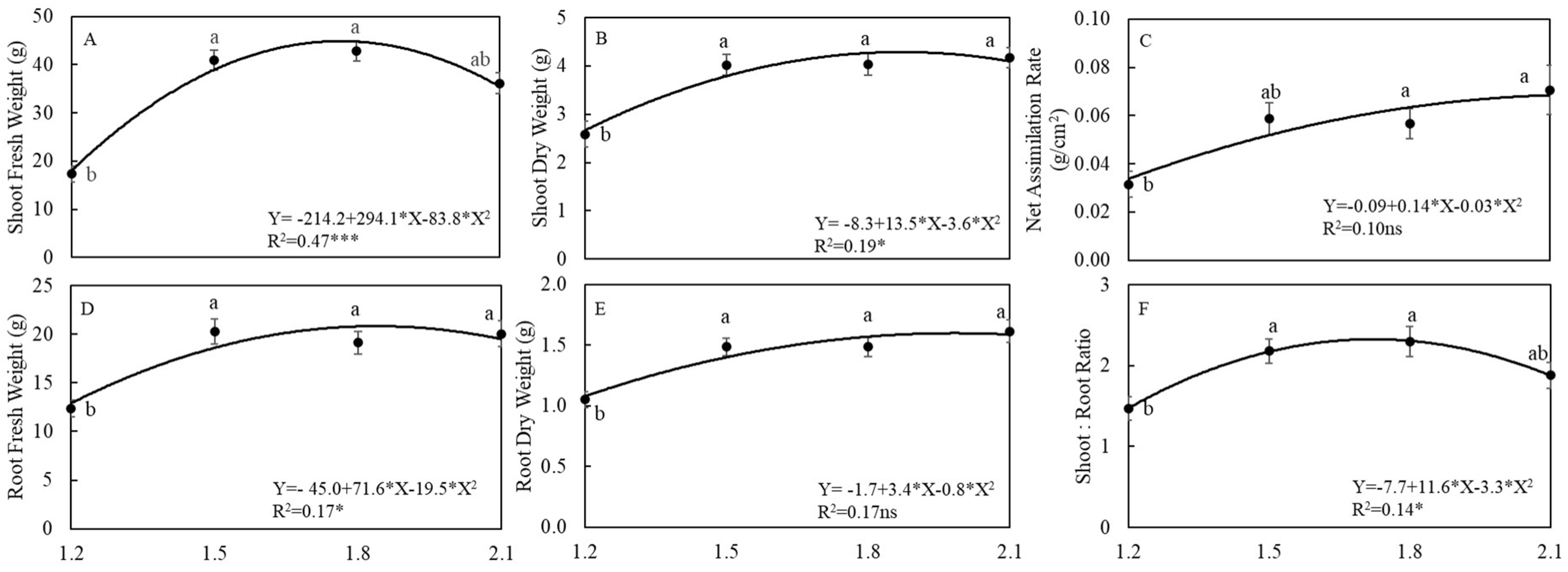
3.1. Plant Growth and Yields
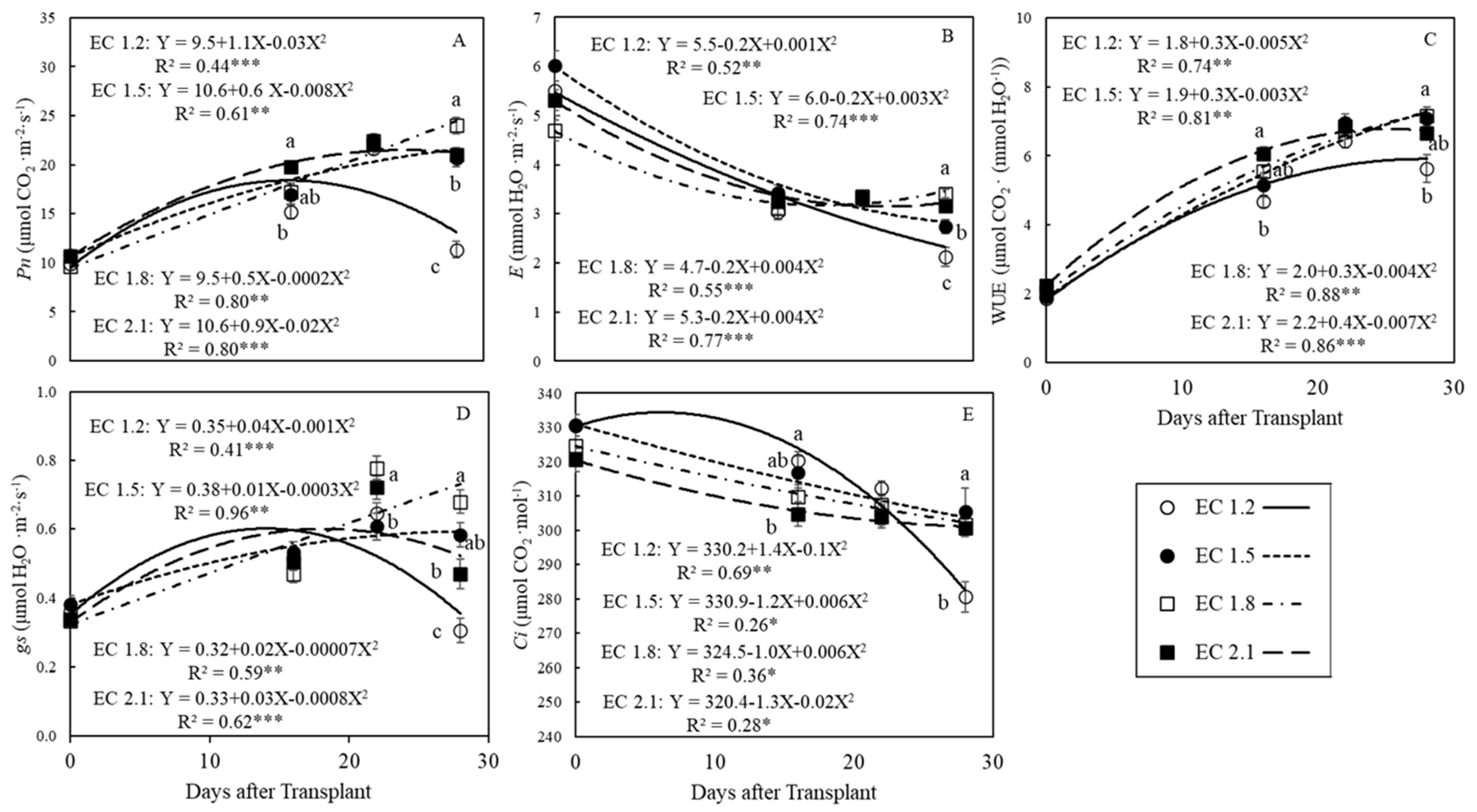
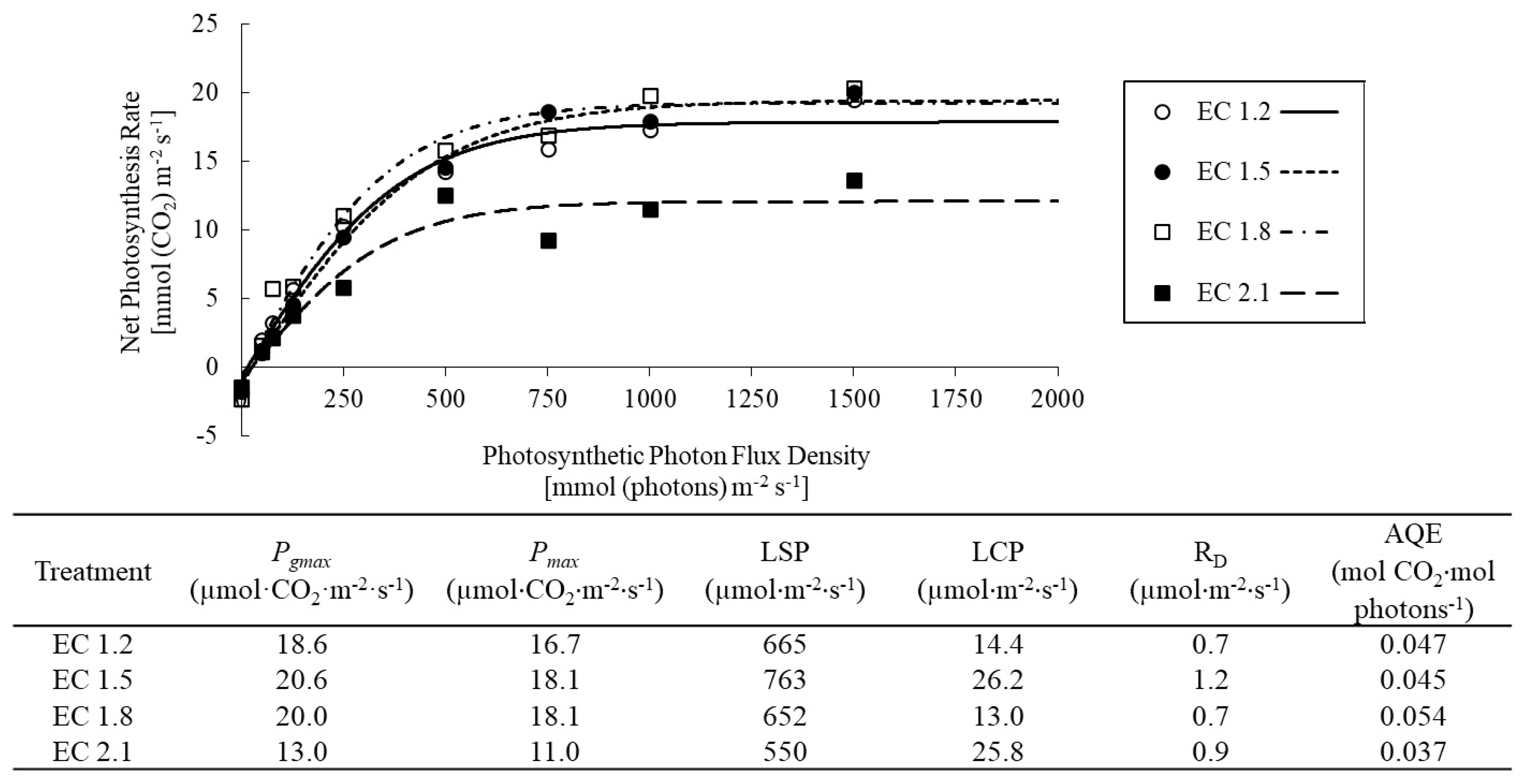
3.2. Leaf Gas Exchange Indices
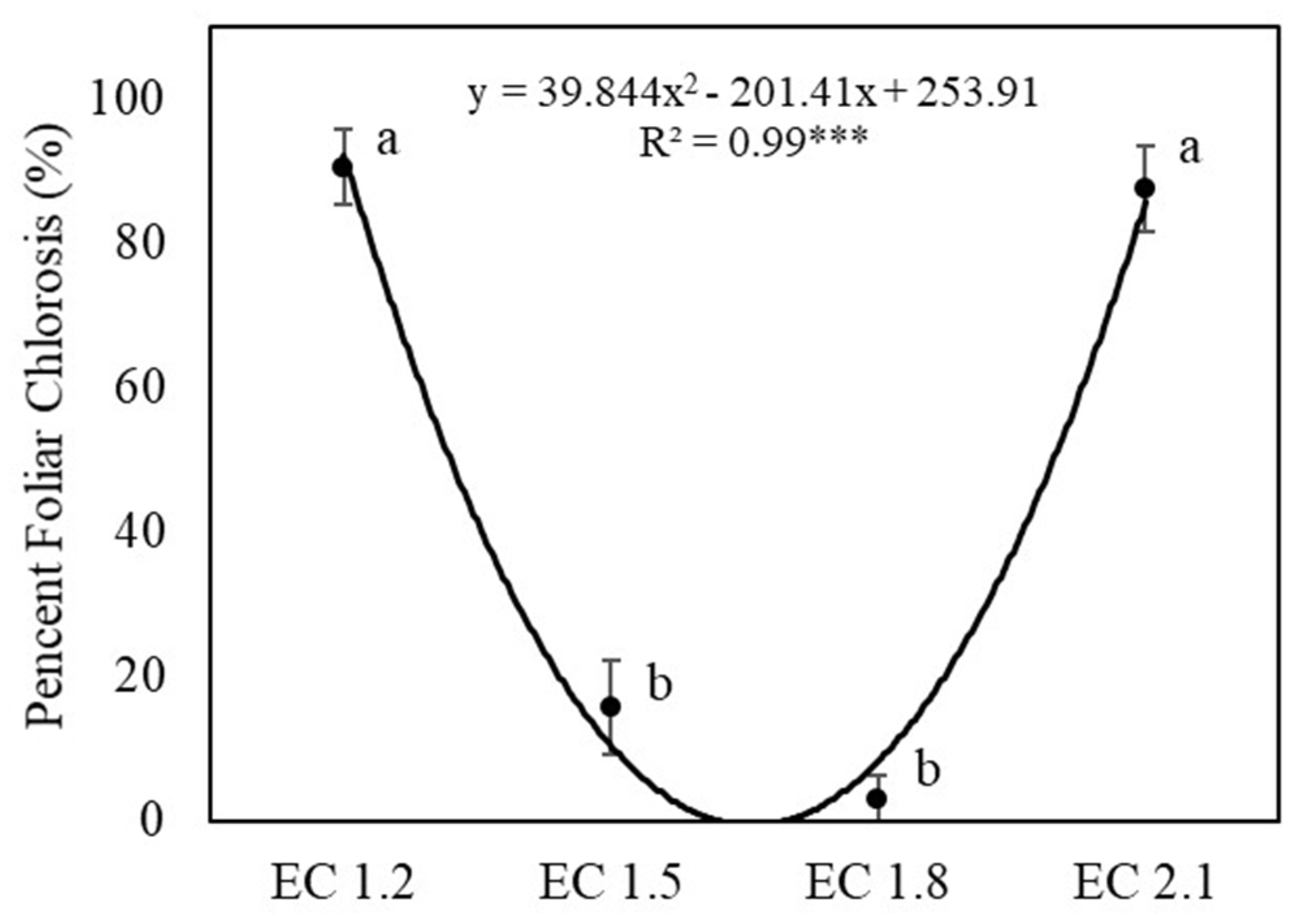
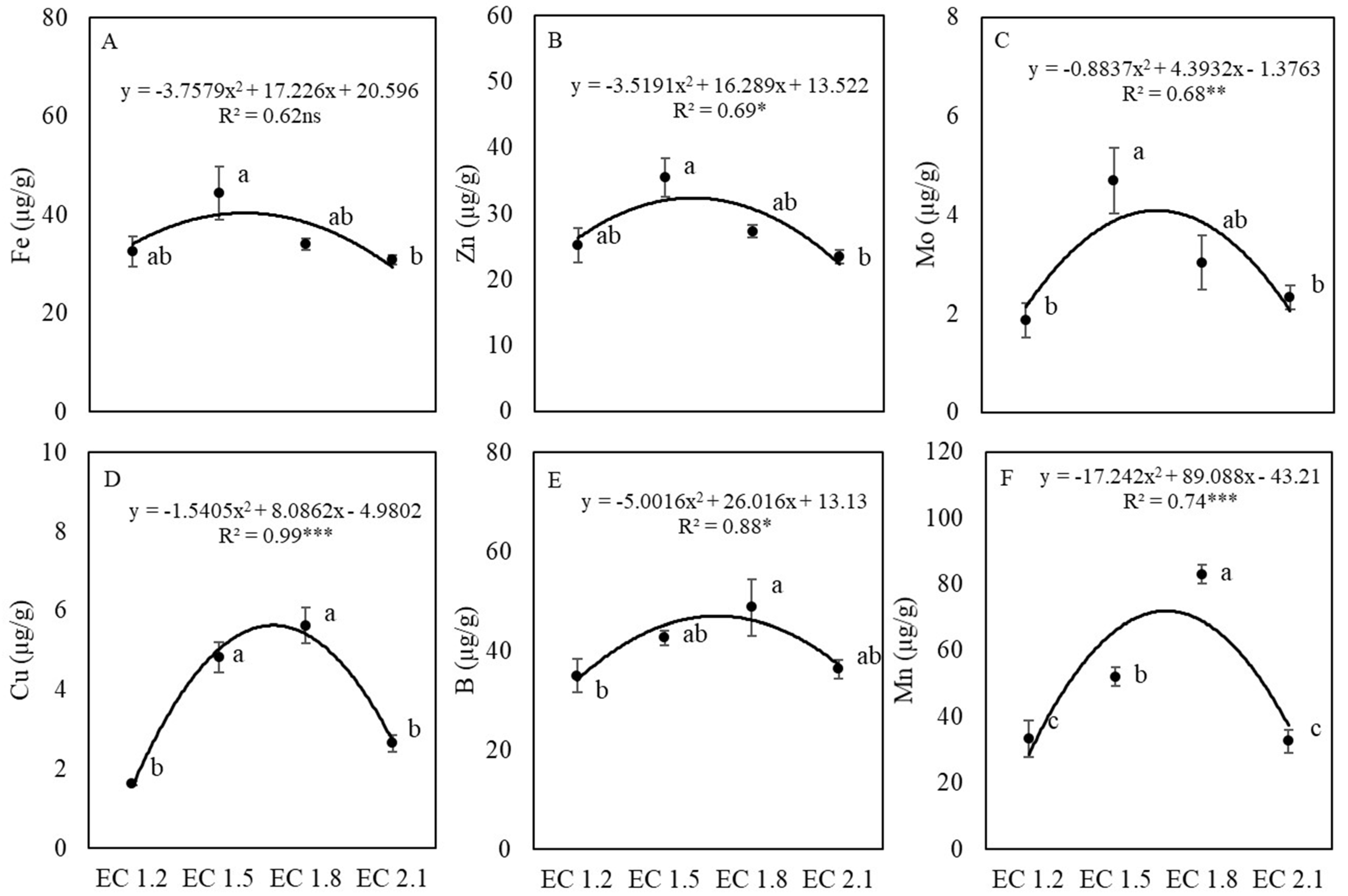
3.3. Leaf Nutrient and Nitrate Contents
3.4. Leaf Phytochemical Content
4. Discussion
4.1. EC Affected Growth and Yield of Arugula
4.2. EC Affected Leaf Nutrient Concentrations of Arugula
4.3. EC Affected Leaf Gas Exchange Indices in Arugula
4.4. EC Affected Plant Phytochemical Content
4.5. EC Affected Leaf Nitrate Content and Potential Influence on Human Health
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hall, M.K.D.; Jobling, J.J.; Rogers, G.S. Some perspectives on rocket as a vegetable crop: A review. Veg. Crops Res. Bull. 2012, 76, 21–41. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Ferrante, A. Nitrates and Glucosinolates as strong determinants of the nutritional quality in rocket leafy salads. Nutrients 2014, 6, 1519–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonasia, A.; Lazzizera, C.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant. Sci. 2017, 8, 300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambo, P.; Lunari, G.; Gianquinto, G.; Pimpini, F. Primi risultati di coltivazione dello spinacio (Spinacia Oleracea L.) in floating system. Italus Hortus 2001, 8, 64–69. [Google Scholar]
- De Freitas, E.M.; Giovanelli, L.B.; Delazari, F.T.; dos Santos, M.L.; Pereira, S.B.; da Silva, D.J.H. Arugula Production as a function of irrigation depths and potassium fertilization. Rev. Bras. Eng. Agríc. Ambient. 2017, 21, 197–202. [Google Scholar] [CrossRef]
- Traka, M.H. Health benefits of glucosinolates. Adv. Bot. Res. 2016, 80, 247–279. [Google Scholar] [CrossRef]
- Wilson, D.W.; Nash, P.; Buttar, H.S.; Griffiths, K.; Singh, R.; De Meester, F.; Horiuchi, R.; Takahashi, T. The role of food antioxidants, benefits of functional foods, and influence of feeding habits on the health of the older person: An overview. Antioxidants 2017, 6, 81. [Google Scholar] [CrossRef] [Green Version]
- Correia, C.C.S.A.; da Cunha, F.F.; Mantovani, E.C.; da Silva, D.J.H.; Dias, S.H.B.; de Sousa Ferreira, T. Irrigation of arugula cultivars in the region of Zona da Mata Mineira. Semin. Cienc. Agrar. 2019, 40, 1101–1114. [Google Scholar] [CrossRef] [Green Version]
- Nicola, S.; Pignata, G.; Casale, M. Advanced soilless growing systems for standard, safe and premium leafy vegetable production. In Proceedings of the VI ISHS Balkan Symposium on Vegetables and Potatoes, Zagreb, Croatia, 29 September 2014; p. 11. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Son, J.E.; Kim, H.J.; Ahn, T.I. Hydroponic systems. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 213–221. [Google Scholar] [CrossRef]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1998, 78, 5–38. [Google Scholar] [CrossRef]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables? J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Francois, L.E.; Maas, E.V. Crop response and management on saltaffected soils. In Handbook of Plant and Crop Stress: Books in Soils, Plants, and the Environment; Marcel Dekker Inc.: New York, NY, USA, 1994. [Google Scholar]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food. Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and Quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2014, 172, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Bell, L.; Methven, L.; Signore, A.; Oruna-Concha, M.J.; Wagstaff, C. Analysis of seven salad rocket (Eruca sativa) accessions: The relationships between sensory attributes and volatile and non-volatile compounds. Food Chem. 2016, 218, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; Schrijver, R.D.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, S219–S265. [Google Scholar] [CrossRef]
- Goharrizi, K.J.; Riahi-Madvar, A.; Rezaee, F.; Pakzad, R.; Bonyad, F.J.; Ahsaei, M.G. Effect of salinity stress on enzymes’ activity, ions concentration, oxidative stress parameters, biochemical traits, content of sulforaphane, and CYP79F1 gene expression level in Lepidium draba plant. J. Plant Growth Regul. 2019, 39, 1075–1094. [Google Scholar] [CrossRef]
- Lidder, S.; Webb, A.J. Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate-nitrite-nitric oxide pathway. Br. J. Clin. Pharmacol. 2012, 75, 677–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sørensen, J.N.; Johansen, A.S.; Kaack, K. Marketable and nutritional quality of leeks as affected by water and nitrogen supply and plant age at harvest. J. Sci. Food Agric. 1995, 68, 367–373. [Google Scholar] [CrossRef]
- Van Eysinga, J.P.N.L.R.; van der Meijs, M.Q. Effect of nitrogen nutrition and global radiation on yield and nitrate content of lettuce grown under glass. Commun. Soil Sci. Plant Anal. 2008, 16, 1293–1300. [Google Scholar] [CrossRef]
- Ferrante, A.; Incrocci, L.; Maggini, R.; Tognoni, F.; Serra, G. Preharvest and postharvest strategies for reducing nitrate content in rocket (Eruca sativa). In Proceedings of the XXVI International Horticultural Congress: Issues and Advances in Postharvest Horticulture, Toronto, ON, Canada, 11 August 2002; Volume 628, pp. 153–159. [Google Scholar] [CrossRef]
- Santamaria, P.; Elia, A.; Serio, F. Effect of solution nitrogen concentration on yield, leaf element content, and water and nitrogen use efficiency of three hydroponically-grown rocket salad genotypes. J. Plant Nutr. 2006, 25, 245–258. [Google Scholar] [CrossRef]
- Chaves, M.M.; Osorio, J.; Pereira, J.S. Water use efficiency and photosynthesis. In Water Use Efficiency in Plant Biology; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Jassby, A.D.; Platt, T. Mathematical Formulation of the relationship between photosynthesis and light for phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Lobo, F.D.A.; de Barros, M.P.; Dalmagro, H.J.; Dalmolin, A.C.; Pereira, W.E.; de Souza, E.C.; Vourlitis, G.L.; Rodríguez Ortiz, C.E. Fitting Net photosynthetic light-response curves with Microsoft Excel—A critical look at the models. Photosynthetica 2013, 51, 445–456. [Google Scholar] [CrossRef]
- Jones, J.B., Sr.; Wolf, B.; Mills, H.A. Microwave digestion using CEM microwave digestion system. In Plant Analysis Handbook; Micro-Macro Publishing: Athens, GA, USA, 1991. [Google Scholar]
- Gavlak, R.; Horneck, D.; Miller, R.O. Soil, Plant and Water Reference Methods for the Western Region, 3rd ed.; WCC-103 Publication: Fort Collins, CO, USA, 2005. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Protein (Crude) in Animal Feed. Combustion Method (Dumas Method). Official Methods of Analysis of the Association of Official Analytical Chemists; VELP Scientifica: Usmate Velate, Italy, 1990. [Google Scholar]
- Mawlong, I.; Sujith Kumar, M.S.; Gurung, B.; Singh, K.H.; Singh, D. A simple spectrophotometric method for estimating total glucosinolates in mustard de-oiled cake. Int. J. Food Prop. 2017, 20, 3274–3281. [Google Scholar] [CrossRef]
- Sukwattanasinit, T.; Burana-Osot, J.; Sotanaphun, U. Spectrophotometric method for quantitative determination of total anthocyanins and quality characteristics of roselle (Hibiscus sabdariffa). Planta Med. 2007, 73, 1517–1522. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–383. [Google Scholar]
- Lu, N.; Bernardo, E.L.; Tippayadarapanich, C.; Takagaki, M.; Kagawa, N.; Yamori, W. Growth and accumulation of secondary metabolites in perilla as affected by photosynthetic photon flux density and electrical conductivity of the nutrient solution. Front. Plant Sci. 2017, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-J.; Chiami, K.; Ishii, G. Effect of ammonium: Nitrate nutrient ratio on nitrate and glucosinolate contents of hydroponically-G. J. Soil Sci. Plant Nutr. 2010, 52, 387–393. [Google Scholar] [CrossRef]
- Calori, A.H.; Factor, T.L.; Lima Júnior, S.; Moraes, L.A.; Barbosa, P.J.; Tivelli, S.W.; Purquerio, L.F. Electrical conductivity and plant spacing on baby leaf table beet and lettuce production. Hortic. Bras. 2014, 32, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. Var. acephala in floating raft culture: Nutrient concentration and growing season effects on lettuce. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Sago, Y.; Shigemura, A. Quantitative nutrient management reduces nitrate accumulation in hydroponic butterhead lettuces grown under artificial lighting. HortScience 2018, 53, 963–967. [Google Scholar] [CrossRef]
- Samarakoon, U.C.; Fyffe, C.; Bale, J.; Ling, P.; Basnagala, S.; Donley, N.; Altland, J. Effect of Electrical conductivity on the productivity and nutrient uptake of Lactuca sativa L. grown using nutrient film technique (NFT). In Proceedings of the International Symposium on Growing Media, Soilless Cultivation, and Compost Utilization in Horticulture, Portland, OR, USA, 20–25 August 2019; pp. 137–144. [Google Scholar] [CrossRef]
- Samarakoon, U.; Palmer, J.; Ling, P.; Altland, J. Effects of electrical conductivity, pH, and foliar application of calcium chloride on yield and tipburn of Lactuca sativa grown using the nutrient–film technique. HortScience 2020, 55, 1265–1271. [Google Scholar] [CrossRef]
- Neocleous, D.; Savvas, D. The effects of phosphorus supply limitation on photosynthesis, biomass production, nutritional quality, and mineral nutrition in lettuce grown in a recirculating nutrient solution. Sci. Hortic. 2019, 252, 379–387. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; Zhao, H.; Guo, D.; He, L.; Liu, F.; Zhou, Q.; Nandwani, D.; Hui, D.; Yu, J. Electrical conductivity of nutrient solution influenced photosynthesis, quality, and antioxidant enzyme activity of pakchoi (Brassica campestris L. Ssp. Chinensis) in a hydroponic system. PLoS ONE 2018, 13, e0202090. [Google Scholar] [CrossRef]
- Ortiz-Hernández, Y.D.; Martínez-Gutiérrez, G.A.; Urrestarazu, M.; Vasquez-Vasquez, L.; Escamirosa-Tinoco, C. Productivity under shade and different nutrient solution of hydroponic watercress (Nasturtium officinale R. BR.). J. Plant Nutr. 2015, 38, 1495–1504. [Google Scholar] [CrossRef]
- Bakhshandeh, E. Quantification of the effect of environmental factors on seed germination and seedling growth of Eruca (Eruca sativa) using mathematical models. J. Plant Growth Regul. 2019, 39, 190–204. [Google Scholar] [CrossRef]
- Tabatabaie, S.J.; Nazari, J. Influence of nutrient concentrations and NaCl salinity on the growth, photosynthesis, and essential oil content of peppermint and memon verbena. Turk. J. Agric. For. 2007, 31, 245–253. [Google Scholar]
- James, E.C.; van Iersel, M.W. Fertilizer concentration affects growth and flowering of subirrigated petunias and begonias. HortScience 2001, 36, 40–44. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.; Pill, W. Cultural practices to speed the growth of microgreen arugula (Roquette; Eruca vesicaria subsp. sativa). J. Hortic. Sci. Biotechnol. 2015, 85, 171–176. [Google Scholar] [CrossRef]
- Campos Júnior, J.E.; Santos Júnior, J.A.; Silva, Ê.F.D.F.; Martins, J.B.; Rolim, M.M. Consumption, efficiency and water content of arugula under different management of brackish nutritional solutions. Eng. Agríc. 2018, 38, 885–892. [Google Scholar] [CrossRef]
- Mattson, N.; Merrill, T. Symptoms of Common Nutrient Deficiencies in Hydroponic Arugula. eGRO Research Updates. 2017. Available online: http://e-gro.org/pdf/2017-2.pdf (accessed on 28 June 2021).
- Walters, K.J.; Currey, C.J. Effects of nutrient solution concentration and daily light integral on growth and nutrient concentration of several basil species in hydroponic production. HortScience 2018, 53, 1319–1325. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Alkhaled, B.; Livieratos, I.; Stamatakis, A.; Ntatsi, G.; Savvas, D. Impact of nitrogen source and supply level on growth, yield and nutritional value of two contrasting ecotypes of Cichorium spinosum L. grown hydroponically: Effect of NH4/total-N ratios on stamnagathi growth. J. Sci. Food Agric. 2017, 98, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.W.; Fisher, P.R. Quantifying the acidic and basic effects of vegetable and herb species in peat-based substrate and hydroponics. HortScience 2019, 54, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Cavins, T.J.; Whipker, B.E.; Fonteno, W.C. Timing of PourThru affects pH, electrical conductivity, and leachate volume. Commun. Soil Sci. Plant Anal. 2005, 36, 1573–1581. [Google Scholar] [CrossRef]
- Ralph, P.J. Photosynthetic responses of Halophila ovalis (R. Br.) hook. f. to osmotic stress. J. Exp. Mar. Biol. Ecol. 1998, 227, 203–220. [Google Scholar] [CrossRef]
- Ruiz-Lozano, J.M.; Aroca, R. Host response to osmotic stresses: Stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants. In Arbuscular Mycorrhizas: Physiology and Function; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2010; pp. 239–256. [Google Scholar]
- Çalişkan, B.; Çalişkan, A.C. Potassium nutrition in plants and its interactions with other nutrients in hydroponic culture. In Potassium—Improvement of Quality in Fruits and Vegetables through Hydroponic Nutrient Management; InTechOpen: London, UK, 2019; Volume 9, pp. 9–22. [Google Scholar]
- Bugbee, B. Nutrient Management in Recirculating Hydroponic Culture; International Society for Horticultural Science: Palmerston North, New Zealand, 2003; Volume 648, pp. 99–112. [Google Scholar]
- Albornoz, F.; Lieth, J.H. Over fertilization limits lettuce productivity because of osmotic stress. Chil. J. Agric. Res. 2015, 75, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Eraslan, F.; Inal, A.; Savasturk, O.; Gunes, A. Changes in antioxidative system and membrane damage of lettuce in response to salinity and boron toxicity. Sci. Hortic. 2007, 114, 5–10. [Google Scholar] [CrossRef]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on metabolism: New knowledge gained from multi-level analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef]
- Maathuis, F.J. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Niinemets, Ü. Photosynthesis and resource distribution through plant canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Yamori, W.; Kondo, E.; Sugiura, D.; Terashima, I.; Suzuki, Y.; Makino, A. Enhanced leaf photosynthesis as a target to increase grain yield: Insights from transgenic rice lines with variable rieske fes protein content in the cytochrome b 6 / f complex. Plant Cell Environ. 2016, 39, 80–87. [Google Scholar] [CrossRef]
- Chen, R.Y.; Song, S.W.; Song, C.Z.; Liu, H.C.; Sun, G.W.; Huang, D.F. Effect of the nitrogen nutrient on pigments of flower stalk in Chinese kale. Appl. Mech. Mater. 2011, 142, 37–41. [Google Scholar] [CrossRef]
- Eckardt, N.A. A New chlorophyll degradation pathway. Plant Cell 2009, 21, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hörtensteiner, S.; Kräutler, B. Chlorophyll breakdown in higher plants. Biochim. Biophys. Acta 2010, 1807, 977–988. [Google Scholar] [CrossRef] [Green Version]
- Delgado, R.; Martín, P.; del Álamo, M.; González, M.-R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Kataoka, I.; Kubo, Y.; Sugiura, A.; Tomana, T. Changes in L-phenylalanine ammonia-lyase activity and anthocyanin synthesis during berry ripening of three grape cultivars. J. Jpn. Soc. Hortic. Sci. 1983, 52, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Keller, M.; Harzdina, G. Interaction of nitrogen availability during bloom and light intensity during veraison. II. Effects on anthocyanin and phenolic development during grape ripening. Am. J. Enol. Vitic. 1998, 49, 341–349. [Google Scholar]
- Gershenzon, J. (Ed.) Changes in the levels of plant secondary metabolites under water and nutrient stress. In Phytochemical Adaptations to Stress; Recent Advances in Phytochemistry; Springer Science & Business Media, LLC: Boston, MA, USA, 1984; Volume 18, pp. 273–320. [Google Scholar] [CrossRef]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.C.; Martínez-Sánchez, A.; Selma, M.V.; Tudela, J.A.; Baixauli, C.; Gil, M.I. Influence of nutrient solutions in an open-field soilless system on the quality characteristics and shelf life of fresh-cut red and green lettuces (Lactuca sativa L.) in different seasons: Influence of nutrient solutions on soilless-grown fresh-cut Lactuca sativa L. J. Sci. Food Agric. 2012, 93, 415–421. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E.; Tognoni, F.; Serra, G.; Ferrante, A.; Piagessi, A. Use of biostimulants for reducing nutrient solution concentration in floating system. In Proceedings of the III International Symposium on Models for Plant Growth, Environmental Control and Farm Management in Protected Cultivation, Wageningen, The Netherlands, 29 October 2006; pp. 477–484. [Google Scholar] [CrossRef]
- Alberici, A.; Quattrini, E.; Penati, M.; Martinetti, L.; Gallina, P.M.; Ferrante, A.; Schiavi, M. Effect of the reduction of nutrient solution concentration on leafy vegetables quality grown in floating system. In Proceedings of the International Symposium on High Technology for Greenhouse System Management: Greensys2007, Naples, Italy, 4 October 2007; Volume 801, pp. 1167–1176. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The antioxidants changes in ornamental flowers during development and senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooke, D.; Steward, W.P.; Gescher, A.J.; Marczylo, T. Anthocyans from fruits and vegetables—Does bright colour signal cancer chemopreventive activity? Eur. J. Cancer 2005, 41, 1931–1940. [Google Scholar] [CrossRef]
- Padilla, G.; Cartea, M.E.; Velasco, P.; de Haro, A.; Ordás, A. Variation of glucosinolates in vegetable crops of Brassica rapa. Phytochemistry 2006, 68, 536–545. [Google Scholar] [CrossRef]
- Schonhof, I.; Krumbein, A.; Brückner, B. Genotypic effects on glucosinolates and sensory properties of broccoli and cauliflower. Nahr. Food 2004, 48, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant. Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Pereira, V.A., Jr.; de Arruda, I.N.Q.; Stefani, R. Active chitosan/PVA films with anthocyanins from Brassica Oleraceae (red cabbage) as time-temperature indicators for application in intelligent food packaging. Food Hydrocoll. 2014, 43, 180–188. [Google Scholar] [CrossRef]
- Abdel Mohsen, M.A.; Hassan, A.A.M.; El-Sewedy, S.M.; Aboul-Azm, T.; Magagnotti, C.; Fanelli, R.; Airoldi, L. Biomonitoring of N-nitroso compounds, nitrite and nitrate in the urine of Egyptian bladder cancer patients with or without Schistosoma haematobium infection. Int. J. Cancer 1999, 82, 789–794. [Google Scholar] [CrossRef]
- Mensinga, T.T.; Speijers, G.J.A.; Meulenbelt, J. Health implications of exposure to environmental nitrogenous compounds. Toxicol. Rev. 2003, 22, 41–51. [Google Scholar] [CrossRef]
- Parks, S.E.; Huett, D.O.; Campbell, L.C.; Spohr, L.J. Nitrate and nitrite in Australian leafy vegetables. Aust. J. Agric. Res. 2008, 59, 632–638. [Google Scholar] [CrossRef]
- Colla, G.; Kim, H.-J.; Kyriacou, M.C.; Rouphael, Y. Nitrate in fruits and vegetables. Sci. Hortic. 2018, 237, 221–238. [Google Scholar] [CrossRef]
- Guadagnin, S.G.; Rath, S.; Reyes, F.G.R. Evaluation of the nitrate content in leaf vegetables produced through different agricultural systems. Food Addit. Contam. 2005, 22, 1203–1208. [Google Scholar] [CrossRef]
- Milkowski, A.; Garg, H.K.; Coughlin, J.R.; Bryan, N.S. Nutritional epidemiology in the context of nitric oxide biology: A risk–benefit evaluation for dietary nitrite and nitrate. Nitric Oxide 2009, 22, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Weitzberg, E.; Lundberg, J.O. Novel aspects of dietary nitrate and human health. Annu. Rev. Nutr. 2013, 33, 129–159. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.; Brandt, P.A. Nitrate food additives series. In WHO Food Additives Series; International Programme on Chemical Safety (IPCS): Geneva, Switzerland, 2003. [Google Scholar]
- European Commission. Regulation (EU) No 1258/2011 of 2 december 2011 amending regulation (EC) No. 1881/2006 as regards maximum levels for nitrates in foodstuffs. J. Eur. Union L. 2011, 320, 15–17. [Google Scholar]




| Treatments | Pre-EC | Post-EC | Pre-pH | Post-pH |
|---|---|---|---|---|
| EC 1.2 | 1.24 ± 0.07 d | 1.25 ± 0.01 d | 7.20 ± 0.08 a | 5.80 ± 0.01 a |
| EC 1.5 | 1.49 ± 0.04 c | 1.53 ± 0.02 c | 7.08 ± 0.07 a | 5.81 ± 0.01 a |
| EC 1.8 | 1.82 ± 0.05 b | 1.81 ± 0.01 b | 6.98 ± 0.09 a | 5.80 ± 0.01 a |
| EC 2.1 | 2.10 ± 0.04 a | 2.11 ± 0.01 a | 6.98 ± 0.09 a | 5.81 ± 0.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution. Agronomy 2021, 11, 1340. https://doi.org/10.3390/agronomy11071340
Yang T, Samarakoon U, Altland J, Ling P. Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution. Agronomy. 2021; 11(7):1340. https://doi.org/10.3390/agronomy11071340
Chicago/Turabian StyleYang, Teng, Uttara Samarakoon, James Altland, and Peter Ling. 2021. "Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution" Agronomy 11, no. 7: 1340. https://doi.org/10.3390/agronomy11071340
APA StyleYang, T., Samarakoon, U., Altland, J., & Ling, P. (2021). Photosynthesis, Biomass Production, Nutritional Quality, and Flavor-Related Phytochemical Properties of Hydroponic-Grown Arugula (Eruca sativa Mill.) ‘Standard’ under Different Electrical Conductivities of Nutrient Solution. Agronomy, 11(7), 1340. https://doi.org/10.3390/agronomy11071340








