The Dynamics of Selenium Uptake by Maize (Zea mays L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil
2.2. Plant Material and Experimental Design
2.3. Estimation of Se Content in Plants
2.4. Relative Rate of Dry Mass Accumulation and Relative Rate of Selenium Uptake by Maize
- YieldTn—plant dry mass [g pot−1] harvested at Tn;
- Tn—phase of plant growth/harvest;
- ∆dTn−1—number of days since previous harvest phase.
- SeUTn—Se uptake [µg pot−1] by plants harvested at Tn;
- Tn—phase of plant growth/harvest;
- ∆dTn−1—number of days since previous plant harvest phase.
- YieldTn—plant dry mass [g pot−1] harvested at Tn;
- Tn—phase of plant growth/harvest;
- SeCTn—Se content [µg kg−1] in plants harvested at Tn.
2.5. Statistical Analysis
3. Results and Discussion
3.1. Plant Yield
3.2. Selenium in Plants
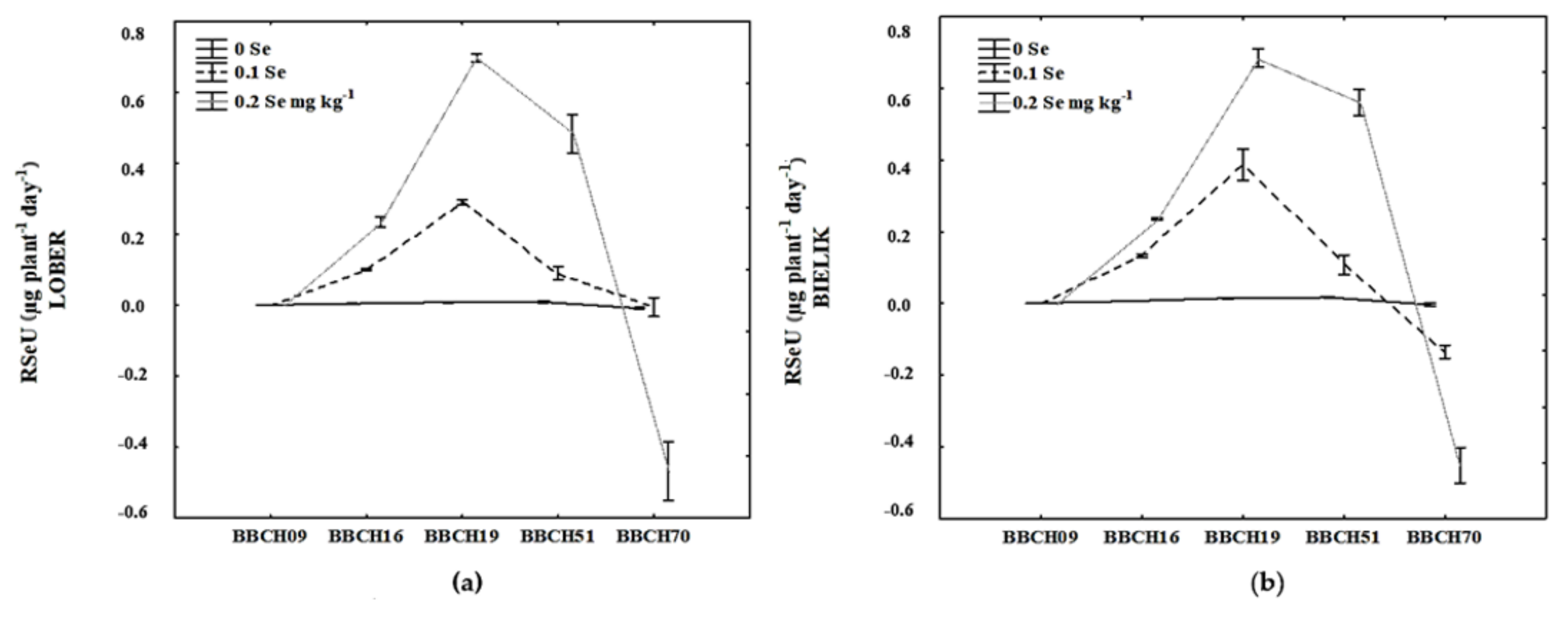
3.3. Rate of SE Uptake (RSeU)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ebrahimi, N.; Hartikainen, H.; Simojoki, A.; Hajiboland, R.; Seppanen, M. Dynamics of dry matter and selenium accumulation in oilseed rape (Brassica napus L.) in response to organic and inorganic selenium treatments. Agric. Food Sci. 2015, 24, 104–117. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Mao, H.; Zhao, H.; Huang, D. Increasing Se concentration in maize grain with soil- or foliar-applied selenite on the Loess Plateau in China. Field Crop. Res. 2013, 150, 83–90. [Google Scholar] [CrossRef]
- White, J.P. Selenium metabolism in plants. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Żarczyński, P.; Sobiech, P.; Snarska, A.; Stopyra, A.; Wieteska, M.; Płaczek, A. The effect of micronutrient deficiencies on the health status of transition cows. J. Elem. 2017, 22, 1223–1234. [Google Scholar] [CrossRef]
- Płaczek, A.; Stępień, P.; Żarczyński, P.; Patorczyk-Pytlik, B. Methods for enrichment of animal diets with selenium. J. Elem. 2019, 24, 1159–1172. [Google Scholar] [CrossRef]
- Kruzhel, B.; Bąkowska, M.; Vovk, S.; Nowakowska, E.; Sergei, P. Selenium in the diet of ruminants. Acta Sci. Pol. Zootech. 2014, 13, 5–16. [Google Scholar]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in food chain and animal nutrition: Lessons from nature-review. Asian-Aust. J. Anim. Sci. 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- Suchý, P.; Straková, E.; Herzig, I. Selenium in poultry nutrition: A review. Czech J. Anim. Sci. 2014, 59, 495–503. [Google Scholar] [CrossRef]
- Klusonova, I.; Skladanka, J.; Hodulikova, L.; Skarpa, P.; Adam, V. The influence of foliar application of selenium on content of glutathione in the forage of perennial ryegrass (Lolium perenne L.). In Mendel Net, Proceedings of the International Ph.D. Students Conference, Brno, Czech Republic, 11–12 November 2015; Polák, O., Cerkal, R., Březinová Belcredi, N., Eds.; Mendel University: Brno, Czech Republic, 2015; pp. 131–136. Available online: https://mnet.mendelu.cz/mendelnet2015/mnet_2015_full.pdf (accessed on 10 March 2021).
- Longchamp, M.; Castrec-Rouelle, M.; Biron, P.; Bariac, T. Variations in the accumulation, localization and rate of metabolization of selenium in mature Zea mays plants supplied with selenite or selenate. Food Chem. 2015, 182, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Chilimba, A.D.C.; Young, S.D.; Black, C.R.; Meacham, M.C.; Lammel, J.; Broadley, M.R. Agronomic biofortification of maize with selenium (Se) in Malawi. Field Crops Res. 2012, 125, 118–128. [Google Scholar] [CrossRef]
- Masanza, B.; Maida, J.H.A.; Chilimba, A.D.C.; Lowole, M.W.; Nalivata, P.C. Liming and selenium application impact on plant available selenium in selected soils of Malawi. J. Soil Sci. Environ. Manage 2016, 7, 115–122. [Google Scholar] [CrossRef][Green Version]
- El-Ramady, H.; Abdalla, N.; Taha, H.S.; Alshaal, T.; El-Henawy, A.; Faizy, S.E.-D.A.; Shams, M.S.; Youssef, S.M.; Shalaby, T.; Bayoumi, Y.; et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Al-Tameemi, H.J.H.; AL-Amiri, N.J.; Hassan, M.J. Effect of Selenium Fertilization on Dry Weight, Concentration, and Sele-nium uptake in Shoot of Maize (Zea mays L.) Growing in Iraqi Calcareous Soils. Merit Res. J. Agric. Sci. Soil Sci. 2018, 6, 001–006. [Google Scholar] [CrossRef]
- Płaczek, A.; Patorczyk-Pytlik, B. Changes in the content of selenium in aerial parts of maize varieties, depending on the growing period and soil texture. J. Elem. 2020, 25, 787–800. [Google Scholar] [CrossRef]
- Riedell, W.E. Mineral-nutrient synergism and dilution responses to nitrogen fertilizer infield-grown maize. J. Plant Nutr. Soil Sci. 2010, 173, 869–874. [Google Scholar] [CrossRef]
- McDowell, L.R.; Valle, G.; Cristaldi, L.; Davis, P.A.; Rosendo, O.; Willinson, N.S. Selenium availability and methods of selenium supplementation for grossing ruminants. In Proceedings of the 13th Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 11–12 January 2002; pp. 86–102. [Google Scholar]
- Xing, K.; Zhou, S.; Wu, X.; Zhu, Y.; Kong, J.; Shao, T.; Tao, X. Concentrations and characteristics of selenium in soil samples from Dashan Region, a selenium-enriched area in China. J. Soil Sci. Plant Nutr. 2015, 61, 889–897. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. BBCH. Monograph. Quedlinburg 2018, 28–31. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development of 23 January 2003 “On the Methodology of Analytical Proceedings in the Scope of Determining the Content of Nutrients and Feed Additives in Feed Materials, Prefixes and Compound Feeds” (J. of Laws 66, 614), In Polish. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20030660614 (accessed on 1 May 2021).
- Hawrylak-Nowak, B. Changes in Anthocyanin Content as Indicator of Maize Sensitivity to Selenium. J. Plant Nutr. 2008, 31, 1232–1242. [Google Scholar] [CrossRef]
- Naseem, M.; Anwar-ul-Haq, M.; Wang, X.; Farooq, N.; Awais, M.; Sattar, H.; Malik, A.H.; Mustafa, A.; Ahmad, J.; El-Esawi, M.A. Influence of Selenium on Growth, Physiology, and Antioxidant Responses in Maize Varies in a Dose-Dependent Manner. J. Food Qual. 2021, 9. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Devi, D.D.; Shanker, A.K.; Sheeba, J.A.; Bangarusamy, U. Impact of selenium spray on monocarpic senescence of soybean (Glycine Max L.). J. Food Agric. Environ. 2004, 2, 44–47. [Google Scholar]
- Shafiq, S.; Adeel, M.; Raza, H.; Iqbal, R.; Ahmad, Z.; Naeem, M.; Sheraz, M.; Ahmed, U.; Azmi, U.R. Effects of Foliar Application of Selenium in Maize (Zea Mays L.) under Cadmium Toxicity. Biol. Forum 2019, 11, 61–71. [Google Scholar]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singh, D.; Singh, K. Effect of selenium application on arsenic uptake in rice (Oryza sativa L.). Environ. Monit. Assess 2017, 189, 430. [Google Scholar] [CrossRef]
- Kaur, M.; Sharma, S. Influence of selenite and selenate on growth, leaf physiology and antioxidant defense system in wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 5700–5710. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Hu, C.; Zhao, X.; Guo, Z. Effect of sulphate on selenium uptake and translocation in rape (Brassica napus L.) supplied with selenate or selenite. Plant Soil 2016, 399, 295–304. [Google Scholar] [CrossRef]
- Cartes, P.; Gianfreda, L.; Mora, M. Uptake of Selenium and its Antioxidant Activity in Ryegrass When Applied as Selenate and Selenite Forms. Plant Soil 2005, 276, 359–367. [Google Scholar] [CrossRef]
- Thavarajah, D.; Thavarajah, P.; Vial, E.; Gebhardt, M.; Lacher, C.; Kumar, S.; Combs, G.F. Will selenium increase lentil (Lens culinaris Medik) yield and seed quality? Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef]
- Boldrin, P.F.; Faquin, V.; Ramos, S.J.; Boldrin, K.V.F.; Ávila, F.W.; Guimarães Guilherme, L.R. Soil and foliar application of selenium in rice biofortification. J. Food Compos. Anal. 2013, 31, 238–244. [Google Scholar] [CrossRef]
- Sharma, S.; Bansal, A.; Dhillon, S.K.; Dhillon, K.S. Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Soil 2010, 329, 339–348. [Google Scholar] [CrossRef]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Mombo, S.; Schreck, E.; Dumat, C.; Laplanche, C.; Pierart, A.; Longchamp, M.; Besson, P.; Castrec-Rouelle, M. Bioaccessibility of selenium after human ingestion in relation to its chemical species and compartmentalization in maize. Environ. Geochem. Health 2016, 38, 869–883. [Google Scholar] [CrossRef]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) uptake and dynamic changes of Se content in soil–plant systems. Environ Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef] [PubMed]
- Hefnawy, A.E.G.; Tórtora-Pérez, J.L. The importance of selenium and the effects of its deficiency in animal health. Small Rumin. Res. 2010, 89, 185–192. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Yang, F.; Chen, L.; Hu, Q.; Pan, G. Effect of the application of selenium on selenium content of soybean and its products. Biol Trace Elem Res. 2003, 93, 249–256. [Google Scholar] [CrossRef]
- Seppänen, M.M.; Ebrahimi, N.; Kontturi, J.; Hartikainen, H.; Lopez Heras, I.; Cámara, C.; Madrid, Y. Dynamics of selenium uptake and metabolism of organic selenium species in the leaves and seeds of Brassica napus L. Agric. Food Sci. 2018, 27, 38–49. [Google Scholar] [CrossRef]
- Jiang, Y.; Zeng, Z.H.; Bu, Y.; Ren, C.Z.; Li, J.Z.; Han, J.J.; Tao, C.; Zhang, K.; Wang, X.X.; Lu, G.X.; et al. Effects of selenium fertilizer on grain yield, Se uptake and distribution in common buckwheat (Fagopyrum esculentum Moench). Plant Soil Environ. 2015, 61, 371–377. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Ann. Rev. Plant Physiol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Hell, R., Mendel, R.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 17, pp. 225–241. [Google Scholar] [CrossRef]
- Winkel, L.H.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium cycling across soil-plant-atmosphere interfaces: A critical review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]




| Yield (g pot−1) | RMG (g plant−1 day−1) | Se Content (µg kg−1) | Se uptake (µg pot−1) | RSeU (µg plant−1 day−1) | |
|---|---|---|---|---|---|
| Variety (A) | |||||
| LOBER | 77.9 (7.45–156) | 0.24 (0.00–0.52) | 835 (10.0–2670) | 45.3 (0.45–179) | 0.09 (–0.61–0.72) |
| BIELIK | 71.0 (8.97–140) | 0.22 (0.00–0.43) | 992 (28.0–2774) | 50.2 (0.68–186) | 0.10 (–0.52–0.72) |
| Se dose (mg kg−1) (B) | |||||
| 0 | 73.3 (7.45–152) | 0.28 (0.06–0.46) | 41.5 (10.0–82.0) | 2.17 (0.45–5.40) | 0.01 (–0.01–0.02) |
| 0.1 | 74.7 (9.07–156) | 0.29 (0.08–0.51) | 862 (324–1696) | 41.5 (10.9–67.3) | 0.12 (–0.16–0.43) |
| 0.2 | 75.4 (9.62–154) | 0.28 (0.08–0.52) | 1838 (662–2774) | 99.6 (25.06–187) | 0.25 (–0.61–0.72) |
| BBCH (C) | |||||
| BBCH09 | - | 0.00 | - | - | 0.00 |
| BBCH16 | 9.43 (7.45–11.1) | 0.08 (0.06–0.10) | 1382 (55.0–2774) | 13.5 (0.45–29.7) | 0.12 (0.00–0.26) |
| BBCH19 | 37.7 (33.3–41.0) | 0.31 (0.27–0.35) | 1166 (30.0–2548) | 44.7 (1.00–92.8) | 0.35 (0.01–0.72) |
| BBCH51 | 106 (97.9–120) | 0.44 (0.38–0.52) | 722 (20.0–1811) | 77.6 (2.13–186) | 0.21 (0.00–0.60) |
| BBCH70 | 144 (131–156) | 0.30 (0.23–0.37) | 385 (10.0–919) | 55.1 (1.52–121) | −0.18 (–0.61–0.05) |
| A | * LSD0.05 0.83 | * LSD0.05 0.01 | * LSD0.05 28.36 | * LSD0.05 2.25 | ns |
| B | * LSD0.05 1.01 | ns | * LSD0.05 34.74 | * LSD0.05 2.75 | * LSD0.05 0.02 |
| C | * LSD0.05 1.17 | * LSD0.05 0.01 | * LSD0.05 40.11 | * LSD0.05 3.18 | * LSD0.05 0.03 |
| A × B × C | * LSD0.05 2.87 | * LSD0.0 50.02 | * LSD0.05 98.25 | * LSD0.05 7.78 | * LSD0.05 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płaczek, A.; Patorczyk-Pytlik, B. The Dynamics of Selenium Uptake by Maize (Zea mays L.). Agronomy 2021, 11, 1305. https://doi.org/10.3390/agronomy11071305
Płaczek A, Patorczyk-Pytlik B. The Dynamics of Selenium Uptake by Maize (Zea mays L.). Agronomy. 2021; 11(7):1305. https://doi.org/10.3390/agronomy11071305
Chicago/Turabian StylePłaczek, Aldona, and Barbara Patorczyk-Pytlik. 2021. "The Dynamics of Selenium Uptake by Maize (Zea mays L.)" Agronomy 11, no. 7: 1305. https://doi.org/10.3390/agronomy11071305
APA StylePłaczek, A., & Patorczyk-Pytlik, B. (2021). The Dynamics of Selenium Uptake by Maize (Zea mays L.). Agronomy, 11(7), 1305. https://doi.org/10.3390/agronomy11071305





