Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Experimental Design, Growth Conditions, and Sampling
2.2. Total Nitrogen and Mineral Content Analysis
2.3. SPAD Index, Carbon Dioxide Assimilation Rate, and Chlorophyll Fluorescence Determination
2.4. Untargeted Metabolomics
2.5. Statistics and Chemometric Interpretation of Metabolites
3. Results
3.1. Biometric Parameters
3.2. Total Nitrogen and Mineral Accumulation
3.3. Physiological and Biochemical Parameters
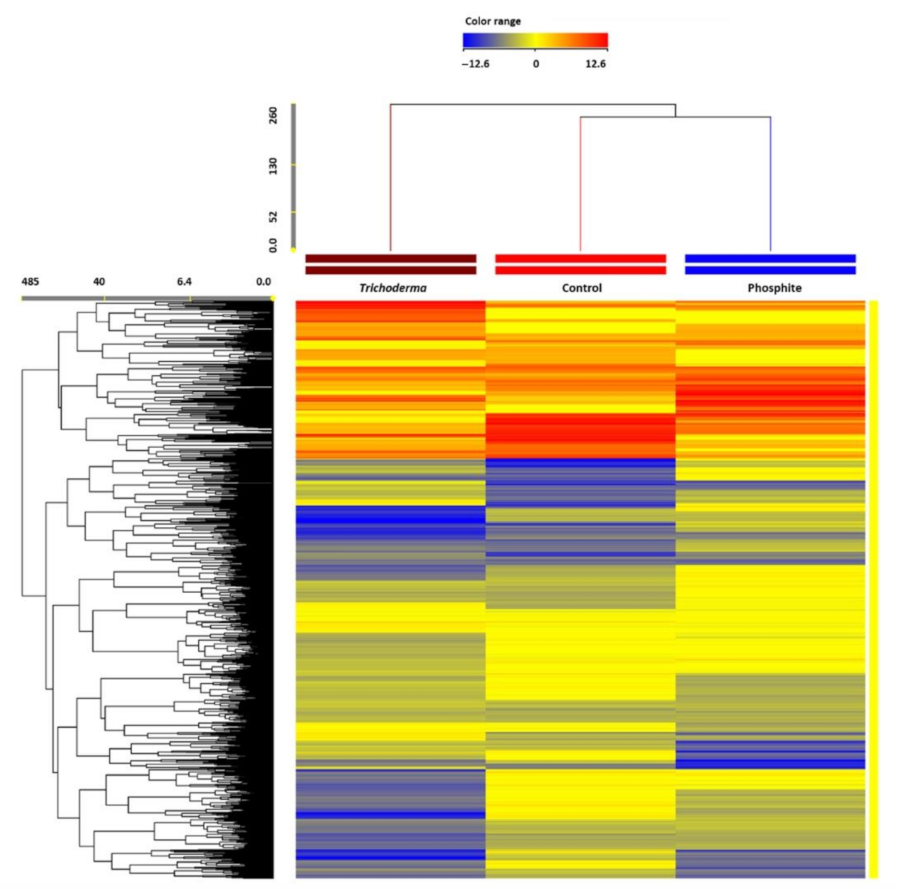
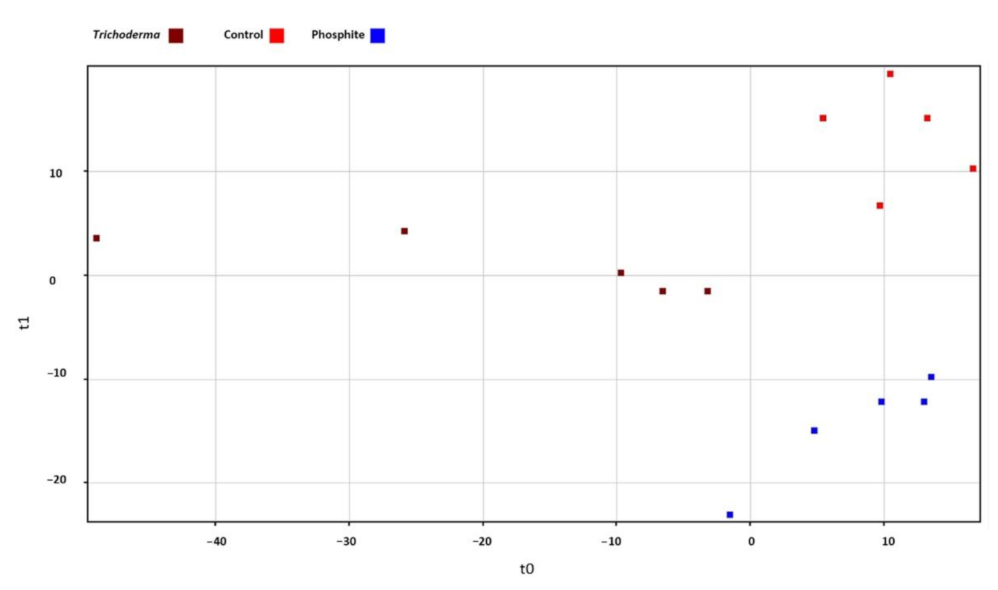
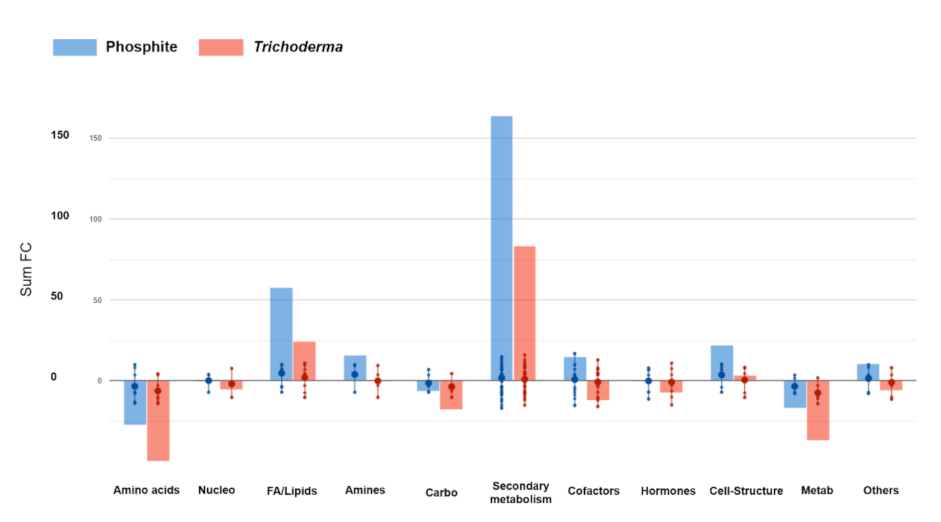
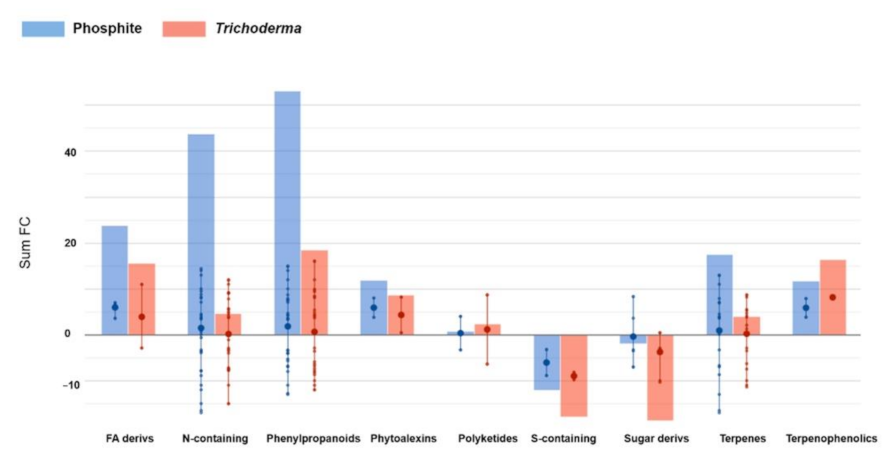
3.4. Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shennan, S.; Downey, S.S.; Timpson, A.; Edinborough, K.; Colledge, S.; Kerig, T.; Manning, K.; Thomas, M.G. Regional population collapse followed initial agriculture booms in mid-Holocene Europe. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Commission European. Ecosystem Goods and Services. Available online: https://ec.europa.eu/environment/nature/info/pubs/docs/ecosystem.pdf (accessed on 27 February 2021).
- Salas-Zapata, W.A.; Ortiz-Muñoz, S.M. Analysis of meanings of the concept of sustainability. Sustain. Dev. 2019, 27, 153–161. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Li, W. Analyses of urban ecosystem based on information entropy. Ecol. Modell. 2006, 197, 1–12. [Google Scholar] [CrossRef]
- FAO. How to Feed the World in 2050. Insights from an Expert Meet; Food and Agriculture Organization of the United Nations: Rome, Italy, 2009; pp. 1–35. [Google Scholar]
- Talukder, B.; Blay-Palmer, A.; van Loon, G.W.; Hipel, K.W. Towards complexity of agricultural sustainability assessment: Main issues and concerns. Environ. Sustain. Indic. 2020, 6, 100038. [Google Scholar] [CrossRef]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic Responses of Maize Shoots and Roots Elicited by Combinatorial Seed Treatments with Microbial and Non-microbial Biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, S.L.; Comite, E.; El-nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of trichoderma harzianum, 6-pentyl-α-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Cuciniello, A.; Cenvinzo, V.; Bonini, P.; Colla, G.; Rouphael, Y. Yield and nutritional quality of Vesuvian piennolo tomato PDO as affected by farming system and biostimulant application. Agronomy 2019, 9, 505. [Google Scholar] [CrossRef]
- Colla, G.; Cardarelli, M.; Bonini, P.; Rouphael, Y. Foliar applications of protein hydrolysate, plant and seaweed extracts increase yield but differentially modulate fruit quality of greenhouse tomato. HortScience 2017, 52, 1214–1220. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Nardi, S. Transcriptome-wide identification of differentially expressed genes in Solanum lycopersicon L. In response to an Alfalfa-protein hydrolysate using microarrays. Front. Plant Sci. 2017, 8, 1159. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational research on trichoderma: From ’Omics to the field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L. Trichoderma: A Multi-Purpose Tool for Integrated Pest Management. In Principles of Plant-Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing Berlin: Cham, Switzerland, 2015; pp. 345–353. [Google Scholar]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Pelagio-Flores, R.; Esparza-Reynoso, S.; Garnica-Vergara, A.; López-Bucio, J.; Herrera-Estrella, A. Trichoderma-induced acidification is an early trigger for changes in Arabidopsis root growth and determines fungal phytostimulation. Front. Plant Sci. 2017, 8, 822. [Google Scholar] [CrossRef]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Harman, G.E. Overview of mechanisms and uses of Trichoderma spp. Phytopathology 2006, 96, 190–194. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotechnol. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef]
- Wu, L.; Gao, X.; Xia, F.; Joshi, J.; Borza, T.; Wang-Pruski, G. Biostimulant and fungicidal effects of phosphite assessed by GC-TOF-MS analysis of potato leaf metabolome. Physiol. Mol. Plant Pathol. 2019, 106, 49–56. [Google Scholar] [CrossRef]
- Groves, E.; Howard, K.; Hardy, G.; Burgess, T. Role of salicylic acid in phosphite-induced protection against Oomycetes; a Phytophthora cinnamomic—Lupinus augustifolius model system. Eur. J. Plant Pathol. 2015, 141, 559–569. [Google Scholar] [CrossRef]
- Aćimović, S.G.; Zeng, Q.; McGhee, G.C.; Sundin, G.W.; Wise, J.C. Control of fire blight (Erwinia amylovora) on apple trees with trunk-injected plant resistance inducers and antibiotics and assessment of induction of pathogenesis-related protein genes. Front. Plant Sci. 2015, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Moor, U.; Põldma, P.; Tõnutare, T.; Karp, K.; Starast, M.; Vool, E. Effect of phosphite fertilization on growth, yield and fruit composition of strawberries. Sci. Hortic. 2009, 119, 264–269. [Google Scholar] [CrossRef]
- Glinicki, R.; Jadczuk-tobjasz, E. The Effect of Plant Stimulant / Fertilizer “Resistim” on Growth and Development of Strawberry Plants. J. Fruit Ornam. Plant Res. 2010, 18, 111–124. [Google Scholar]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium phosphite increases tolerance to UV-B in potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Villate, A.; San Nicolas, M.; Gallastegi, M.; Aulas, P.A.; Olivares, M.; Usobiaga, A.; Etxebarria, N.; Aizpurua-Olaizola, O. Review: Metabolomics as a prediction tool for plants performance under environmental stress. Plant Sci. 2021, 303, 110789. [Google Scholar] [CrossRef] [PubMed]
- FreshPlaza. Presente e Futuro Dello Zucchino in Italia. Available online: https://www.freshplaza.it/article/9202994/presente-e-futuro-dello-zucchino-in-italia/ (accessed on 10 April 2021).
- ISTAT, Italian National Institute of Statistics. Coltivazioni: Ortive. Available online: http://dati.istat.it/Index.aspx?QueryId=33703 (accessed on 10 April 2021).
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Agronomy Monograph 9; Black, C.A., Evans, D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Kitajima, M.; Butler, W.L. Excitation spectra for Photosystem I and Photosystem II in chloroplasts and the spectral characteristics of the distribution of quanta between the two photosystems. BBA Bioenerg. 1975, 408, 297–305. [Google Scholar] [CrossRef]
- Miras-Moreno, B.; Corrado, G.; Zhang, L.; Senizza, B.; Righetti, L.; Bruni, R.; El-Nakhel, C.; Sifola, M.I.; Pannico, A.; De Pascale, S.; et al. The metabolic reprogramming induced by sub-optimal nutritional and light inputs in soilless cultivated green and red butterhead lettuce. Int. J. Mol. Sci. 2020, 21, 6381. [Google Scholar] [CrossRef]
- Salek, R.M.; Neumann, S.; Schober, D.; Hummel, J.; Billiau, K.; Kopka, J.; Correa, E.; Reijmers, T.; Rosato, A.; Tenori, L.; et al. COordination of Standards in MetabOlomicS (COSMOS): Facilitating integrated metabolomics data access. Metabolomics 2015, 11, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Chiaiese, P.; Colla, G.; De Pascale, S.; Rouphael, Y. Metabolic insights into the anion-anion antagonism in sweet basil: Effects of different nitrate/chloride ratios in the nutrient solution. Int. J. Mol. Sci. 2020, 21, 2482. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Dreher, K.; Karp, P.D. The challenge of constructing, classifying, and representing metabolic pathways. FEMS Microbiol. Lett. 2013, 345, 85–93. [Google Scholar] [CrossRef]
- Verma, M.; Brar, S.K.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Antagonistic fungi, Trichoderma spp.: Panoply of biological control. Biochem. Eng. J. 2007, 37, 1–20. [Google Scholar] [CrossRef]
- Rossall, S.; Qing, C.; Paneri, M.; Bennett, M.; Swarup, R. A ‘growing’ role for phosphites in promoting plant growth and development. In Proceedings of the II World Congress on the Use of Biostimulants in Agriculture, Florence, Italy, 16–19 November 2015; pp. 61–68. [Google Scholar]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Woo, S.L.; Ruocco, M.; Vinale, F.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Lanzuise, S.; Manganiello, G.; Lorito, M. Trichoderma-based Products and their Widespread Use in Agriculture. Open Mycol. J. 2014, 8, 71–126. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma strains and metabolites enhances soybean productivity and nutrient content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradices and Trichoderma atroviride acts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef]
- Salwan, R.; Rialch, N.; Sharma, V. Bioactive volatile metabolites of Trichoderma: An overview. Second. Metab. Plant Growth Promot. Rhizomicroorg. Discov. Appl. 2019, 87–111. [Google Scholar] [CrossRef]
- Altomare, C.; Norvell, W.A.; Björkman, T.; Harman, G.E. Solubilization of phosphates and micronutrients by the plant-growth- promoting and biocontrol fungus Trichoderma harzianum Rifai 1295-22. Appl. Environ. Microbiol. 1999, 65, 2926–2933. [Google Scholar] [CrossRef]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Phosphite as an inductor of adaptive responses to stress and stimulator of better plant performance. In Biotic and Abiotic Stress Tolerance in Plants; Vats, S., Ed.; Springer: Singapore, 2018; pp. 203–238. ISBN 9789811090295. [Google Scholar]
- Stewart, A.; Hill, R. Applications of Trichoderma in Plant Growth Promotion. In Biotechnology and Biology of Trichoderma; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. ISBN 9780444595768. [Google Scholar]
- Marschner, H. Marschner’s Mineral. Nutrition of Higher Plants; Academic Press: London, UK, 2002; ISBN 0123849063. [Google Scholar]
- Rouphael, Y.; Carillo, P.; Colla, G.; Fiorentino, N.; Sabatino, L.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cirillo, V.; Shabani, E.; et al. Appraisal of combined applications of trichoderma virens and a biopolymer-based biostimulant on lettuce agronomical, physiological, and qualitative properties under variable n regimes. Agronomy 2020, 10, 196. [Google Scholar] [CrossRef]
- Hernández, I.; Munné-Bosch, S. Linking phosphorus availability with photo-oxidative stress in plants. J. Exp. Bot. 2015, 66, 2889–2900. [Google Scholar] [CrossRef]
- Harman, G.E.; Doni, F.; Khadka, R.B.; Uphoff, N. Endophytic strains of Trichoderma increase plants’ photosynthetic capability. J. Appl. Microbiol. 2021, 130, 529–546. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Bernardo, L.; Kane, D.; Trevisan, M.; Lucini, L. Zinc excess triggered polyamines accumulation in lettuce root metabolome, as compared to osmotic stress under high salinity. Front. Plant Sci. 2016, 7, 842. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, L.; Carletti, P.; Badeck, F.W.; Rizza, F.; Morcia, C.; Ghizzoni, R.; Rouphael, Y.; Colla, G.; Terzi, V.; Lucini, L. Metabolomic responses triggered by arbuscular mycorrhiza enhance tolerance to water stress in wheat cultivars. Plant Physiol. Biochem. 2019, 137, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Baffi, C.; Colla, G. A vegetal biopolymer-based biostimulant promoted root growth in melon while triggering brassinosteroids and stress-related compounds. Front. Plant Sci. 2018, 9, 472. [Google Scholar] [CrossRef]
- Nejat, N.; Mantri, N. Plant immune system: Crosstalk between responses to biotic and abiotic stresses the missing link in understanding plant defence. Curr. Issues Mol. Biol. 2017, 23, O2. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Gomes, G.L.G.C.; Velini, E.D.; Carbonari, C.A. Fosfito de potássio não protege plantas de milho contra os efeitos fitotóxicos do glyphosate1. Pesqui. Agropecuária Trop. 2015, 45, 291–296. [Google Scholar] [CrossRef]
- Batista, P.F.; Müller, C.; Merchant, A.; Fuentes, D.; Silva-Filho, R. de O.; da Silva, F.B.; Costa, A.C. Biochemical and physiological impacts of zinc sulphate, potassium phosphite and hydrogen sulphide in mitigating stress conditions in soybean. Physiol. Plant 2020, 168, 456–472. [Google Scholar] [CrossRef] [PubMed]
- Kosmacz, M.; Sokołowska, E.M.; Bouzaa, S.; Skirycz, A. Towards a functional understanding of the plant metabolome. Curr. Opin. Plant Biol. 2020, 55, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Grubb, C.D.; Abel, S. Glucosinolate metabolism and its control. Trends Plant Sci. 2006, 11, 89–100. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Glucosinolates and the clubroot disease: Defense compounds or auxin precursors? Phytochem. Rev. 2009, 8, 135–148. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of glucosinolate biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Y.; Xu, L.; Dong, J.; Zhu, X.; Ying, J.; Wang, Q.; Fan, L.; Li, C.; Liu, L. Methyl jasmonate, salicylic acid and abscisic acid enhance the accumulation of glucosinolates and sulforaphane in radish (Raphanus sativus L.) taproot. Sci. Hortic. 2019, 250, 159–167. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early signaling events induced by elicitors of plant defenses. Mol. Plant Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Salazar-Iribe, A.; De-la-Peña, C. Auxins, the hidden player in chloroplast development. Plant Cell Rep. 2020, 39, 1595–1608. [Google Scholar] [CrossRef]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.N.; Geng, C.C.; Li, D.D.; Xu, S.W.; Mao, D.D.; Umbreen, S.; Loake, G.J.; Cui, B.M. Nitrate reductase-mediated nitric oxide regulates the leaf shape in arabidopsis by mediating the homeostasis of reactive oxygen species. Int. J. Mol. Sci. 2019, 20, 2235. [Google Scholar] [CrossRef] [PubMed]
- Peppino Margutti, M.; Reyna, M.; Meringer, M.V.; Racagni, G.E.; Villasuso, A.L. Lipid signalling mediated by PLD/PA modulates proline and H2O2 levels in barley seedlings exposed to short- and long-term chilling stress. Plant Physiol. Biochem. 2017, 113, 149–160. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexindependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungus Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Rey-Burusco, M.F.; Daleo, G.R.; Feldman, M.L. Identification of potassium phosphite responsive miRNAs and their targets in potato. PLoS ONE 2019, 14, e0222346. [Google Scholar] [CrossRef]
| Treatment | Fresh Biomass Production | Dry Biomass Production | Root Dry Weight |
|---|---|---|---|
| g plant−1 | g plant−1 | g plant−1 | |
| Control | 340.1 ± 10.17 b | 43.3 ± 1.24 b | 2.6 ± 0.36 b |
| Phosphite | 364.5 ± 13.31 ab | 43.7 ± 1.60 b | 4.2 ± 0.96 ab |
| Trichoderma | 390.9 ± 24.92 a | 47.2 ± 1.75 a | 5.6 ± 1.22 a |
| Significance | * | * | * |
| Treatment | Total N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| g kg−1 dw | g kg−1 dw | g kg−1 dw | g kg−1 dw | g kg−1 dw | g kg−1 dw | |
| Control | 23.9 ± 2.89 | 2.6 ± 0.24 b | 24.1 ± 4.22 | 14.0 ± 4.32 | 3.7 ± 0.25 b | 1.9 ± 0.38 |
| Phosphite | 21.4 ± 1.24 | 3.1 ± 0.11 ab | 23.9 ± 2.20 | 15.3 ± 0.77 | 3.9 ± 0.22 ab | 2.2 ± 0.42 |
| Trichoderma | 22.7 ± 2.75 | 3.6 ± 0.34 a | 24.3 ± 2.50 | 16.1 ± 0.29 | 4.5 ± 0.31 a | 2.5 ± 0.38 |
| Significance | ns | * | ns | ns | * | ns |
| Treatment | SPAD Index | Pn | Fv/Fm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 16 | 23 | 30 | 16 | 23 | 30 | 16 | 23 | 30 | |
| DAT | DAT | DAT | |||||||
| Control | 58.0 ± 1.22 | 51.1 ± 0.72 b | 49.3 ± 0.84 b | 9.3 ± 2.01 | 11.1 ± 1.23 b | 8.0 ± 0.68 b | 0.78 ± 0.02 | 0.75 ± 0.02 | 0.75 ± 0.01 b |
| Phosphite | 55.7 ± 2.58 | 55.0 ± 0.87 a | 50.3 ± 2.28 ab | 10.2 ± 2.52 | 12.7 ± 1.15 ab | 9.5 ± 0.78 b | 0.78 ± 0.03 | 0.75 ± 0.02 | 0.75 ± 0.01 b |
| Trichoderma | 60.0 ± 0.89 | 55.3 ± 0.78 a | 54.0 ± 1.14 a | 10.3 ± 1.03 | 15.4 ± 2.28 a | 12.3 ± 1.45 a | 0.80 ± 0.01 | 0.78 ± 0.02 | 0.78 ± 0.01 a |
| Significance | ns | ** | * | ns | ** | *** | ns | ns | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formisano, L.; Miras-Moreno, B.; Ciriello, M.; El-Nakhel, C.; Corrado, G.; Lucini, L.; Colla, G.; Rouphael, Y. Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants. Agronomy 2021, 11, 1205. https://doi.org/10.3390/agronomy11061205
Formisano L, Miras-Moreno B, Ciriello M, El-Nakhel C, Corrado G, Lucini L, Colla G, Rouphael Y. Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants. Agronomy. 2021; 11(6):1205. https://doi.org/10.3390/agronomy11061205
Chicago/Turabian StyleFormisano, Luigi, Begoña Miras-Moreno, Michele Ciriello, Christophe El-Nakhel, Giandomenico Corrado, Luigi Lucini, Giuseppe Colla, and Youssef Rouphael. 2021. "Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants" Agronomy 11, no. 6: 1205. https://doi.org/10.3390/agronomy11061205
APA StyleFormisano, L., Miras-Moreno, B., Ciriello, M., El-Nakhel, C., Corrado, G., Lucini, L., Colla, G., & Rouphael, Y. (2021). Trichoderma and Phosphite Elicited Distinctive Secondary Metabolite Signatures in Zucchini Squash Plants. Agronomy, 11(6), 1205. https://doi.org/10.3390/agronomy11061205











