Carbon Mineralization Dynamics of Organic Materials and Their Usage in the Restoration of Degraded Tropical Tea-Growing Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site and Soil Characteristics
2.2. Preparation of Organic Amendments and Their Characteristics
2.3. Soil Incubation
2.4. Mineralization Kinetics
2.5. Microbial Biomass Carbon and Nitrogen
2.6. Soil Enzyme Activities
2.7. Statistical Analysis
3. Results
3.1. Carbon Mineralization Dynamics
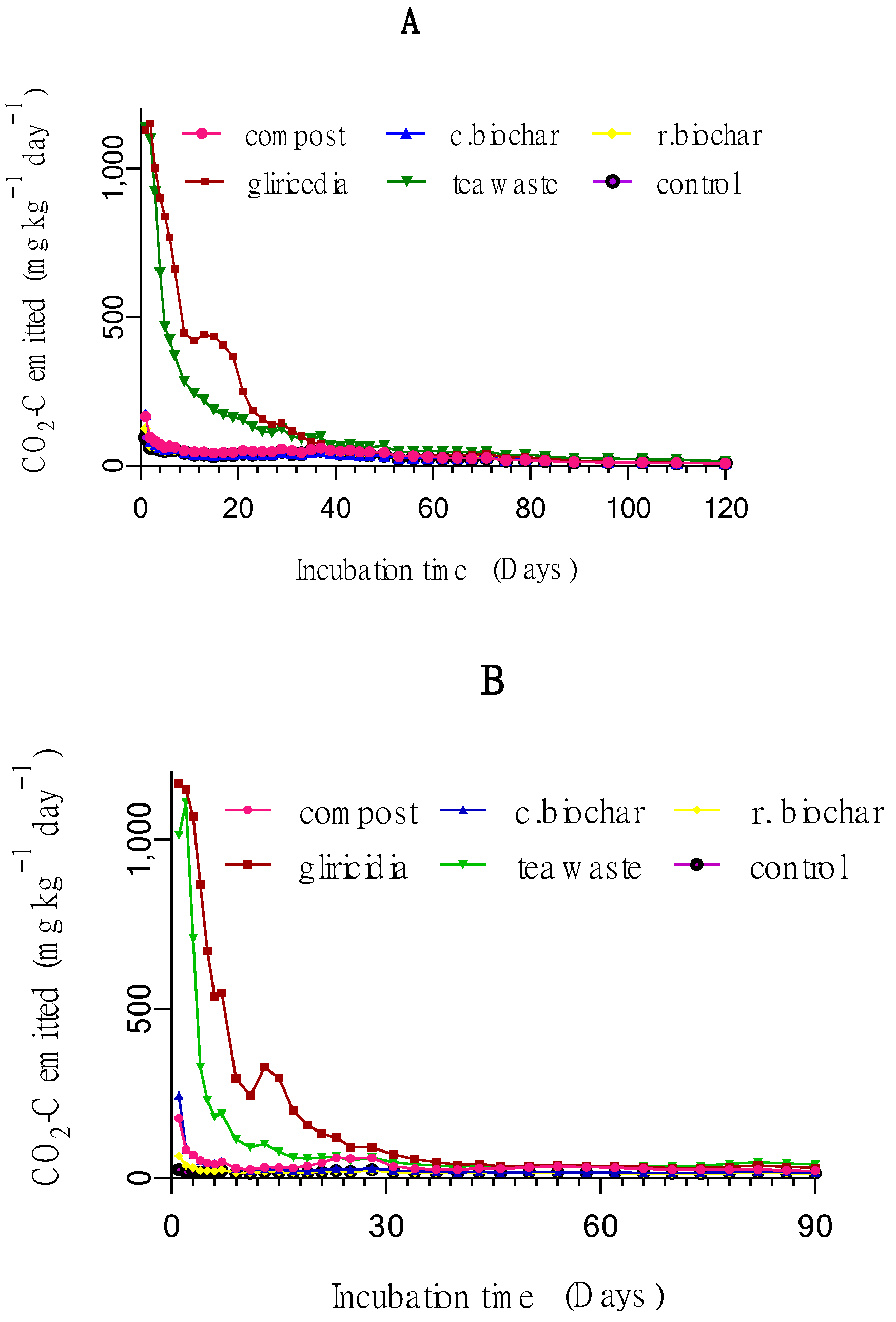
3.1.1. Daily CO2 Emission
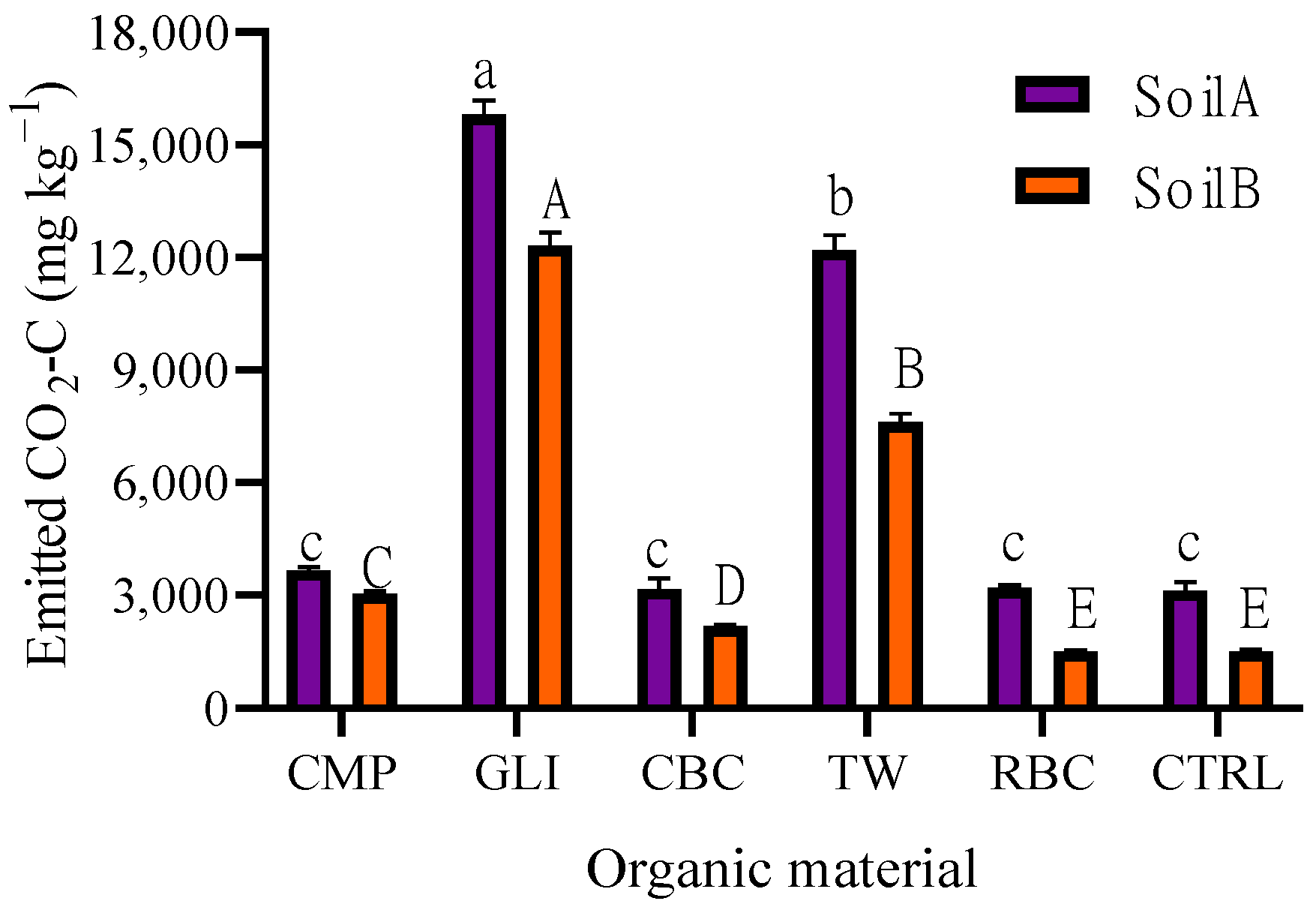
3.1.2. Cumulative CO2-C Emission
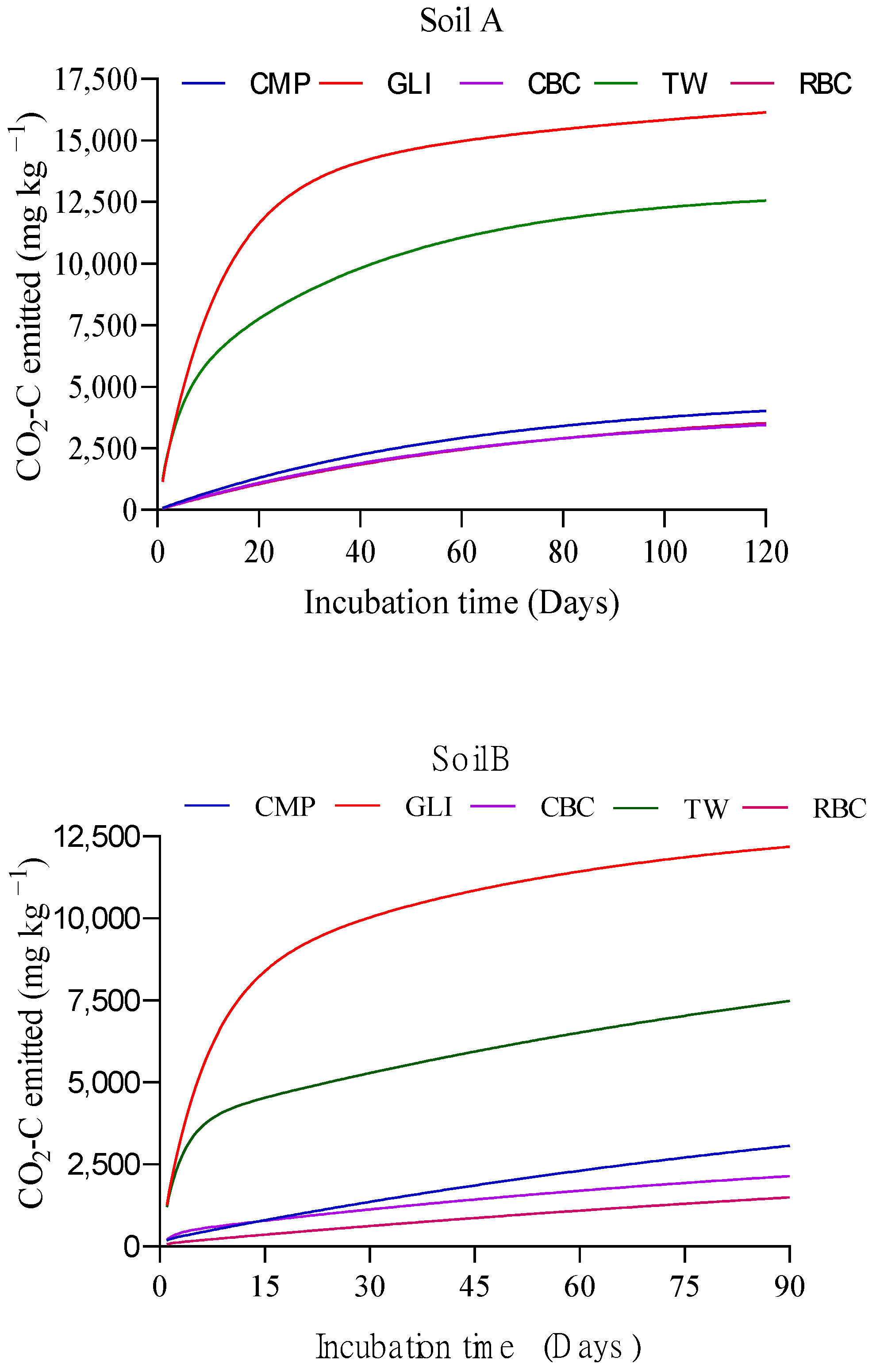
3.1.3. Kinetics of Carbon Mineralization
3.2. Microbial Biomass Changes
3.3. Soil Enzymes Activities
3.4. Correlation Analysis
4. Discussion
4.1. Carbon Mineralization Dynamics
4.1.1. Daily CO2 Emission
4.1.2. Cumulative CO2-C Emission
4.1.3. Kinetics of Carbon Mineralization
4.2. Microbial Biomass Changes
4.3. Soil Enzymes Activities
4.4. Correlation Analysis
4.5. Restoration Potential of Degraded Tea Soil
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sierra, C.A.; Trumbore, S.E.; Davidson, E.A.; Vicca, S.; Janssens, I. Sensitivity of Decomposition Rates of Soil Organic Matter with Respect to Simultaneous Changes in Temperature and Moisture. J. Adv. Model. Earth Syst. 2015, 7, 335–356. [Google Scholar] [CrossRef]
- International Tea Committee. Annual Bulletin of Statistics; ITC: London, UK, 2019. [Google Scholar]
- Abera, G.; Wolde-Meskel, E.; Bakken, L.R. Carbon and Nitrogen Mineralization Dynamics in Different Soils of the Tropics Amended with Legume Residues and Contrasting Soil Moisture Contents. Biol. Fertil. Soils 2012, 48, 51–66. [Google Scholar] [CrossRef]
- Saha, A.K.; Biswas, A.; Khan, A.Q.; Farazi, M.M.; Rahman, M.H. Improvement of Tea (Camellia Sinensis L.) Soil Properties by Growing Different Green Crops. Agriculturist 2014, 12, 34–38. [Google Scholar] [CrossRef]
- Anandacoomaraswamy, A.; Ekanayake, S.A.; Ananthacumaraswamy, S.; Chishom, A.; Jayasuriya, S. Effect of Land Degradation on Tea Productivity in Sri Lanka. In Soil Erosion Research for the 21st Century, Proceedings of the Int. Symp., Honolulu, HI, USA, 3–5 January 2001; Ascough, J.C., II, Flanagan, D.C., Eds.; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2001; pp. 75–78. [Google Scholar] [CrossRef]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Khalil, M.I.; Hossain, M.B.; Schmidhalter, U. Carbon and Nitrogen Mineralization in Different Upland Soils of the Subtropics Treated with Organic Materials. Soil Biol. Biochem. 2005, 37, 1507–1518. [Google Scholar] [CrossRef]
- Amoah-Antwi, C.; Kwiatkowska-Malina, J.; Thornton, S.F.; Fenton, O.; Malina, G.; Szara, E. Restoration of Soil Quality Using Biochar and Brown Coal Waste: A Review. Sci. Total Environ. 2020, 722, 1–21. [Google Scholar] [CrossRef]
- Moreno-Cornejo, J.; Zornoza, R.; Faz, A. Carbon and Nitrogen Mineralization during Decomposition of Crop Residues in a Calcareous Soil. Geoderma 2014, 230–231, 58–63. [Google Scholar] [CrossRef]
- Li, J.; Wu, X.; Gebremikael, M.T.; Wu, H.; Cai, D.; Wang, B.; Li, B.; Zhang, J.; Li, Y.; Xi, J. Response of Soil Organic Carbon Fractions, Microbial Community Composition and Carbon Mineralization to High-Input Fertilizer Practices under an Intensive Agricultural System. PLoS ONE 2018, 13, e0195144. [Google Scholar] [CrossRef]
- Ekanayake, P.B. Planting and Management of Shade Trees Green Manure Crops and Wind Belts. In Handbook on Tea; Zoysa, A.K., Ed.; Tea Research Institute of Sri Lanka: Talawakelle, Sri Lanka, 2008; pp. 86–93. [Google Scholar]
- Mohamed, M.T.; Zoysa, A.K. An Overview of Tea Industry in Sri Lanka. In Handbook on Tea; Zoysa, A.K., Ed.; Tea Research Institute of Sri Lanka: Talawakelle, Sri Lanka, 2008; pp. 4–9. [Google Scholar]
- Teutscherova, N.; Vazquez, E.; Santana, D.; Navas, M.; Masaguer, A.; Benito, M. Influence of Pruning Waste Compost Maturity and Biochar on Carbon Dynamics in Acid Soil: Incubation Study. Eur. J. Soil. Biol. 2017, 78, 66–74. [Google Scholar] [CrossRef]
- Pandit, N.R.; Mulder, J.; Hale, S.E.; Schmidt, H.P.; Cornelissen, G. Biochar from “Kon Tiki” Flame Curtain and Other Kilns: Effects of Nutrient Enrichment and Kiln Type on Crop Yield and Soil Chemistry. PLoS ONE 2017, 12, e0176378. [Google Scholar] [CrossRef]
- Schmidt, H.; Pandit, B.; Martinsen, V.; Cornelissen, G.; Conte, P.; Kammann, C. Fourfold Increase in Pumpkin Yield in Response to Low-Dosage Root Zone Application of Urine-Enhanced Biochar to a Fertile Tropical Soil. Agriculture 2015, 5, 723–741. [Google Scholar] [CrossRef]
- Tratsch, M.V.M.; Ceretta, C.A.; da Silva, L.S.; Ferreira, P.A.A.; Brunetto, G. Composition and Mineralization of Organic Compost Derived from Composting of Fruit and Vegetable Waste. Rev. Ceres 2019, 66, 307–315. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Juhos, K.; Biró, B.; Kocsis, T.; Pabar, S.A.; Varga, C.; Fekete, I. Effect of Detritus Manipulation on Different Organic Matter Decompositions in Temperate Deciduous Forest Soils. Forests 2020, 11, 675. [Google Scholar] [CrossRef]
- Laffely, A.; Erich, M.S.; Mallory, E.B. Evaluation of the CO2 Flush as a Soil Health Indicator. Appl. Soil Ecol. 2020, 154, 103594. [Google Scholar] [CrossRef]
- Dick, R.P. Soil Enzyme Activities as Indicators of Soil Quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; Volume 35, pp. 107–124. [Google Scholar] [CrossRef]
- Xue, D.; Yao, H.; Huang, C. Microbial Biomass, N Mineralization and Nitrification, Enzyme Activities, and Microbial Community Diversity in Tea Orchard Soils. Plant Soil 2006, 288, 319–331. [Google Scholar] [CrossRef]
- Pascual, J.A.; Garcıcia, C.; Hernandez, T.; Ayuso, M. Changes in the Microbial Activity of an Arid Soil Amended with Urban Organic Wastes. Biol. Fertil. Soils 1997, 24, 429–434. [Google Scholar] [CrossRef]
- Ramesh, T.; Bolan, N.S.; Kirkham, M.B.; Wijesekara, H.; Kanchikerimath, M.; Srinivasa Rao, C.; Sandeep, S.; Rinklebe, J.; Ok, Y.S.; Choudhury, B.U.; et al. Soil Organic Carbon Dynamics: Impact of Land Use Changes and Management Practices: A Review. Adv. Agron. 2019, 156, 1–107. [Google Scholar] [CrossRef]
- García, C.; Hernández, T.; Costa, F. Microbial Activity in Soils under Mediterranean Environmental Conditions. Soil Biol. Biochem. 1994, 26, 1185–1191. [Google Scholar] [CrossRef]
- Campos, J.A.; Peco, J.D.; García-Noguero, E. Antigerminative Comparison between Naturally Occurring Naphthoquinones and Commercial Pesticides. Soil Dehydrogenase Activity Used as Bioindicator to Test Soil Toxicity. Sci. Total Environ. 2019, 694, 1–7. [Google Scholar] [CrossRef] [PubMed]
- García-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-Term Effects of Municipal Solid Waste Compost Application on Soil Enzyme Activities and Microbial Biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Ghabbour, E.A.; Davies, G.; Misiewicz, T.; Alami, R.A.; Askounis, E.M.; Cuozzo, N.P.; Filice, A.J.; Haskell, J.M.; Moy, A.K.; Roach, A.C.; et al. National Comparison of the Total and Sequestered Organic Matter Contents of Conventional and Organic Farm Soils. Adv. Agron. 2017, 146, 1–35. [Google Scholar] [CrossRef]
- Fernandes, B.C.C.; Mendes, K.F.; Dias Júnior, A.F.; da Silva Caldeira, V.P.; da Silva Teófilo, T.M.; Silva, T.S.; Mendonça, V.; de Freitas Souza, M.; Silva, D.V. Impact of Pyrolysis Temperature on the Properties of Eucalyptus Wood-Derived Biochar. Materials 2020, 13, 5841. [Google Scholar] [CrossRef]
- Gaffar, S.; Dattamudi, S.; Baboukani, A.R.; Chanda, S.; Novak, J.M.; Watts, D.W.; Wang, C.; Jayachandran, K. Physiochemical Characterization of Biochars from Six Feedstocks and Their Effects on the Sorption of Atrazine in an Organic Soil. Agronomy 2021, 11, 716. [Google Scholar] [CrossRef]
- Nelson, D.; Sommers, L. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis; Sumner, D.L., Sparks, A., Page, E., Helmke, P., Loeppert, R., Soltanpour, P., Tabatabai, M., Johnston, M., Sumner, M., Eds.; Soil Science Society of America: Madison, WI, USA, 1996. [Google Scholar]
- Markus, D.K.; McKinnon, J.P.; Buccafuri, A.F. Automated Analysis of Nitrite, Nitrate, and Ammonium Nitrogen in Soils. Soil Sci. Soc. Am. J. 1985, 49, 1208–1215. [Google Scholar] [CrossRef]
- Masunga, R.H.; Uzokwe, V.N.; Mlay, P.D.; Odeh, I.; Singh, A.; Buchan, D.; De Neve, S. Nitrogen Mineralization Dynamics of Different Valuable Organic Amendments Commonly Used in Agriculture. Appl. Soil Ecol. 2016, 101, 185–193. [Google Scholar] [CrossRef]
- Mohan, D.; Abhishek, K.; Sarswat, A.; Patel, M.; Singh, P.; Pittman, C.U. Biochar Production and Applications in Soil Fertility and Carbon Sequestration a Sustainable Solution to Crop-Residue Burning in India. RSC Adv. 2018, 8, 508–520. [Google Scholar] [CrossRef]
- Stanford, G.; Smith, S.J. Nitrogen Mineralization Potentials of Soils. Soil Sci. Soc. Am. J. 1972, 36, 465–472. [Google Scholar] [CrossRef]
- Molina, J.A.E.; Hadas, A.; Clapp, C.E. Computer Simulation of Nitrogen Turnover in Soil and Priming Effect. Soil Biol. Biochem. 1990, 22, 349–353. [Google Scholar] [CrossRef]
- Sparling, G.P.; Feltham, C.W.; Reynolds, J.; West, A.W.; Singleton, P. Estimation of Soil Microbial c by a Fumigation-Extraction Method: Use on Soils of High Organic Matter Content, and a Reassessment of the Kec-Factor. Soil Biol. Biochem. 1990, 22, 301–307. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform Fumigation, and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Jenkinson, D.S. Determination of Microbial Biomass Carbon and Nitrogen in Soil. In Advances in Nitrogen Cycling in Agricultural Systems; Wilson, J.R., Ed.; CAB International: Wallingford, UK, 1988; pp. 368–386. [Google Scholar]
- Casida, L.; Klein, D.; Santoro, T. Soil Dehydrogenase Activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Ladd, J.N. Origin and Range of Enzymes in Soil. In Soil Enzymes; Burns, R., Ed.; Academic Press: London, UK, 1978; pp. 51–96. [Google Scholar]
- Abdelhafez, A.A.; Abbas, M.H.H.; Attia, T.M.S.; El Bably, W.; Mahrous, S.E. Mineralization of Organic Carbon and Nitrogen in Semi-Arid Soils under Organic and Inorganic Fertilization. Environ. Technol. Innov. 2018, 9, 243–253. [Google Scholar] [CrossRef]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic Coating on Biochar Explains Its Nutrient Retention and Stimulation of Soil Fertility. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.V.; Miralles De Imperial, R.; Calvo, R.; Garcia, M.C.; Leon-Cfreces, C.; Delgado, M.M. Carbon Mineralisation Kinetics of Poultry Manure in Two Soils. Soil Res. 2012, 50, 222–228. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Case, S.D.C.; Alberti, G.; McNamara, N.P.; Zavalloni, C.; Vervisch, B.; delle Vedove, G.; De Neve, S. C Mineralization and Microbial Activity in Four Biochar Field Experiments Several Years after Incorporation. Soil Biol. Biochem. 2014, 78, 195–203. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar Accelerates Organic Matter Degradation and Enhances N Mineralisation during Composting of Poultry Manure without a Relevant Impact on Gas Emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Soil Restoration Using Composted Plant Residues: Effects on Soil Properties. Soil Tillage Res. 2009, 102, 109–117. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rahman, M.M.; Biswas, J.C.; Miah, M.M.U.; Akhter, S.; Maniruzzaman, M.; Choudhury, A.K.; Ahmed, F.; Shiragi, M.H.K.; Kalra, N. Carbon Mineralization and Carbon Dioxide Emission from Organic Matter Added Soil under Different Temperature Regimes. Int. J. Recycl. Org. Waste Agric. 2017, 6, 311–319. [Google Scholar] [CrossRef]
- Rahman, F.; Rahman, M.; Rahman, G.K.M.M.; Hossain, A.T.M.S.; Miah, G. Effect of Organic and Inorganic Fertilizers and Rice Straw on Carbon Sequestration and Soil Fertility under a Rice—Rice Cropping Pattern. Carbon Manag. 2016, 7, 41–53. [Google Scholar] [CrossRef]
- Ali, I.; Nabi, G. Soil Carbon and Nitrogen Mineralization Dynamics Following Incorporation and Surface Application of Rice and Wheat Residues. Soil Environ. 2016, 35, 207–215. [Google Scholar]
- Mishra, A.; Kumar, N.; Kumar, R.; Kumar, R.; Tomar, D. Mineralization of Carbon, Nitrogen, Phosphorus and Sulphur from Different Organic Wastes in Silty Clay Loam Soils. J. Appl. Nat. Sci. 2016, 8, 16–22. [Google Scholar] [CrossRef]
- Wu, H.; Hu, J.; Shaaban, M.; Xu, P.; Zhao, J.; Hu, R. The Effect of Dolomite Amendment on Soil Organic Carbon Mineralization Is Determined by the Dolomite Size. Ecol. Process. 2021, 10. [Google Scholar] [CrossRef]
- Mohanty, S.; Nayak, A.K.; Kumar, A.; Tripathi, R.; Shahid, M.; Bhattacharyya, P.; Raja, R.; Panda, B.B. Carbon and Nitrogen Mineralization Kinetics in Soil of Rice-Rice System under Long Term Application of Chemical Fertilizers and Farmyard Manure. Eur. J. Soil Biol. 2013, 58, 113–121. [Google Scholar] [CrossRef]
- Zacháry, D.; Filep, T.; Jakab, G.; Varga, G.; Ringer, M.; Szalai, Z. Kinetic Parameters of Soil Organic Matter Decomposition in Soils under Forest in Hungary. Geoderma Reg. 2018, 14, e00187. [Google Scholar] [CrossRef]
- Puttaso, P.; Namanusart, W.; Thumanu, K.; Kamolmanit, B.; Brauman, A.; Lawongsa, P. Assessing the Effect of Rubber (Hevea Brasiliensis (Willd. Ex A. Juss.) Muell. Arg.) Leaf Chemical Composition on Some Soil Properties of Differently Aged Rubber Tree Plantations. Agronomy 2020, 10, 1871. [Google Scholar] [CrossRef]
- Marzi, M.; Shahbazi, K.; Kharazi, N.; Rezaei, M. The Influence of Organic Amendment Source on Carbon and Nitrogen Mineralization in Different Soils. J. Soil Sci. Plant Nutr. 2020, 20, 177–191. [Google Scholar] [CrossRef]
- Cai, A.; Feng, W.; Zhang, W.; Xu, M. Climate, Soil Texture, and Soil Types Affect the Contributions of Fine-Fraction-Stabilized Carbon to Total Soil Organic Carbon in Different Land Uses across China. J. Environ. Manag. 2016, 172, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Zaharah, A.R.; Bah, A.R. Patterns of Decomposition and Nutrient Release by Fresh Gliricidia (Gliricidia Sepium) Leaves in an Ultisol. Nutr. Cycl. Agroecosyst. 1999, 55, 269–277. [Google Scholar] [CrossRef]
- Turrión, M.B.; Lafuente, F.; Mulas, R.; López, O.; Ruipérez, C.; Pando, V. Effects on Soil Organic Matter Mineralization and Microbiological Properties of applying Compost to Burned and Unburned Soils. J. Environ. Manag. 2012, 95, 1–6. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Z. Recalcitrant Carbon Controls the Magnitude of Soil Organic Matter Mineralization in Temperate Forests of Northern China. For. Ecosyst. 2018, 5. [Google Scholar] [CrossRef]
- Kocsis, T.; Kotroczó, Z.; Kardos, L.; Biró, B. Optimization of Increasing Biochar Doses with Soil–Plant–Microbial Functioning and Nutrient Uptake of Maize. Environ. Technol. Innov. 2020, 20, 101191. [Google Scholar] [CrossRef]
- Blagodatsky, S.A.; Heinemeyer, O.; Richter, J. Estimating the Active and Total Soil Microbial Biomass by Kinetic Respiration Analysis. Biol. Fertil. Soils 2000, 32, 73–81. [Google Scholar] [CrossRef]
- Li, J.; Zhao, B.-Q.; Li, X.-Y.; Jiang, R.-B.; Bing, S.H. Effects of Long-Term Combined Application of Organic and Mineral Fertilizers on Microbial Biomass, Soil Enzyme Activities and Soil Fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar] [CrossRef]
- Sparling, G.P. Ratio of Microbial Biomass Carbon to Soil Organic Carbon as a Sensitive Indicator of Changes in Soil Organic Matter. Aust. J. Soil Res. 1992, 30, 195–207. [Google Scholar] [CrossRef]
- Garcia-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Soil Biology & Biochemistry Suitability of Enzyme Activities for the Monitoring of Soil Quality Improvement in Organic Agricultural Systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Wolińska, A.; Zofia, S. Dehydrogenase Activity in the Soil Environment; Intech: London, UK, 2012; Volume I, p. 38. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota—A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Kujur, M.; Kumar Patel, A. Kinetics of Soil Enzyme Activities under Different Ecosystems: An Index of Soil Quality. Chil. J. Agric. Res. 2014, 74, 96–104. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme Activities in Soil. In Nucleic Acids and Proteins in Soil; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 257–311. [Google Scholar]
- Oladele, S.O. Effect of Biochar Amendment on Soil Enzymatic Activities, Carboxylate Secretions and Upland Rice Performance in a Sandy Clay Loam Alfisol of Southwest Nigeria. Sci. Afr. 2019, 4, e00107. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Pascual, J.; Moreno, J.L.; Ros, M. Microbial Activity in Soils of SE Spain Exposed to Degradation and Desertification Processes. Strategies for Their Rehabilitation. In Research and Perspectives of Soil Enzymology in Spain; Garcia-Izquierdo, C., Hernandez, M.T., Eds.; CEBAS CSIC: Espinardo, Murcia, Spain, 2000; pp. 93–143. [Google Scholar]

| Soil Property | High C Soil (SA) | Low C Soil (SB) |
|---|---|---|
| Soil texture | Silty clay | Clay loam |
| Bulk density (g cm−3) | 1.58 | 1.66 |
| pH (H2O) | 3.96 ± 0.08 | 3.56 ± 0.07 |
| pH (KCl) | 3.46 ± 0.03 | 3.32 ± 0.04 |
| pH (CaCl2) | 3.62 ± 0.12 | 3.34 ± 0.03 |
| Electrical conductivity (µS cm−1) | 102.4 ± 5.6 | 174.1 ± 8.4 |
| Total carbon (%) | 3.44 | 1.87 |
| Organic C (%) | 3.04 | 1.43 |
| Total Nitrogen (%) | 0.24 | 0.12 |
| Total sulphur (%) | 0.07 | 0.06 |
| KCl extractable NH4–N (mg kg−1) | 68 ± 8 | 64 ± 3 |
| KCl extractable NO3–N (mg kg−1) | 79 ± 4 | 45 ± 2 |
| NH4OAc extractable K (mg kg−1) | 41 ± 4 | 64 ± 4 |
| Available P (Bray) (mg kg−1) | 194 ± 6 | 144 ± 5 |
| NH4OAc extractable Mg (mg kg−1) | 98 ± 3 | 73 ± 2 |
| NH4OAc extractable Ca (mg kg−1) | 312 ± 14 | 172 ± 11 |
| Organic Material | Total (g kg−1) | % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | N | S | P | K # | Mg # | Ca # | C/N | C/P | C/S | Moisture | |
| Compost | 234.3 | 11.7 | 1.7 | 3.4 | 0.34 | 0.09 | 0.20 | 20 | 32 | 65 | 12.90 |
| Gliricidia | 478.0 | 41.2 | 3.0 | 2.3 | 0.23 | 0.22 | 1.03 | 12 | 208 | 159 | 77.20 |
| Charged BC | 642.7 | 6.6 | 1.2 | 4.9 | 0.49 | 0.25 | 0.41 | 97 | 131 | 536 | 39.80 |
| Tea waste | 466.3 | 11.4 | 2.0 | 2.3 | 0.23 | 0.14 | 0.22 | 41 | 203 | 233 | 9.50 |
| Raw BC | 667.4 | 5.8 | 1.5 | 5.3 | 0.53 | 0.24 | 0.38 | 115 | 126 | 445 | 4.10 |
| Soil | Organic Amendment | First-Order Model Cmin = C0 (1 − e−kt) | Parallel First-Order Model Cmin = Cf (1 − e−kft) + Cs (1 − e−kst) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C0 | k | R2 | SEE | Cf | Cs | kf | ks | R2 | SE | ||
| mg kg−1 | day−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | mg kg−1 | |||||
| 3.44% carbon soil (Silty clay) | Compost | 4691.07 | 0.0162 | 0.9950 | 83.73 | 2217.56 | 2473.50 | 0.0162 | 0.0162 | 0.9950 | 85.85 |
| Gliricidia | 15,349.54 | 0.0707 | 0.9944 | 317.31 | 13,228.33 | 4590.96 | 0.0870 | 0.0084 | 0.9991 | 230.39 | |
| CBC | 4070.22 | 0.0156 | 0.9954 | 67.94 | 1924.98 | 2145.24 | 0.0156 | 0.0156 | 0.9953 | 69.66 | |
| Tea waste | 11,514.35 | 0.0595 | 0.9479 | 693.61 | 4432.87 | 8571.35 | 0.2660 | 0.0247 | 0.9996 | 62.23 | |
| Raw biochar | 4371.99 | 0.0137 | 0.9976 | 50.47 | 2078.31 | 2293.68 | 0.0137 | 0.0137 | 0.9988 | 51.75 | |
| 1.87% carbon soil (Clay loam) | Compost | 4504.63 | 0.0122 | 0.9923 | 80.99 | 175.76 | 5983.69 | 1.5697 | 0.0073 | 0.9991 | 39.97 |
| Gliricidia | 11,367.72 | 0.0919 | 0.9750 | 489.31 | 7687.30 | 5459.07 | 0.1624 | 0.0193 | 0.9991 | 118.46 | |
| CBC | 2199.87 | 0.0265 | 0.9505 | 128.59 | 395.37 | 3322.02 | 0.6022 | 0.0083 | 0.9972 | 17.59 | |
| Tea waste | 6358.44 | 0.1004 | 0.8214 | 676.85 | 3703.11 | 7196.00 | 0.3705 | 0.0083 | 0.9974 | 83.76 | |
| Raw biochar | 2570.42 | 0.0094 | 0.9954 | 30.75 | 75.94 | 3902.74 | 0.9621 | 0.0050 | 0.9997 | 07.76 | |
| Soil | Organic Material | MBC | MBN | SMQ |
|---|---|---|---|---|
| µg C g−1 ± SEM | µg N g−1 ± SEM | % ± SEM | ||
| Soil A | Compost | 662.3 ± 22.0 a | 70.03 ± 4.45 b | 2.18 ± 0.07 a |
| Gliricidia | 692.3 ± 14.4 a | 90.68 ± 3.41 a | 2.28 ± 0.05 a | |
| Charged biochar | 687.7 ± 18.6 a | 85.53 ± 9.75 ab | 2.26 ± 0.06 a | |
| Tea waste | 672.7 ± 32.1 a | 82.34 ± 7.64 ab | 2.21 ± 0.10 a | |
| Raw biochar | 579.2 ± 13.0 b | 50.80 ± 2.31 c | 1.90 ± 0.04 b | |
| Control | 375.0 ± 29.1 c | 45.95 ± 4.47 c | 1.24 ± 0.10 c | |
| Soil B | Compost | 390.5 ± 10.2 ab | 58.89 ± 7.45 ab | 2.73 ± 0.07 ab |
| Gliricidia | 409.0 ± 16.4 a | 74.98 ± 8.36 a | 2.86 ± 0.12 a | |
| Charged biochar | 409.0 ± 29.3 a | 68.51 ± 7.37 ab | 2.86 ± 0.21 a | |
| Tea waste | 386.1 ± 13.6 ab | 69.29 ± 7.58 a | 2.70 ± 0.10 ab | |
| Raw biochar | 340.7 ± 14.5 b | 48.12 ± 3.55 bc | 2.38 ± 0.10 b | |
| Control | 211.8 ± 11.8 c | 45.90 ± 4.22 b | 1.48 ± 0.10 c |
| Parameter | DH | MBC | MBN | CAT | Ccum | OC% |
|---|---|---|---|---|---|---|
| DH | 1.000 | 0.524 ** | 0.192 | 0.542 ** | −0.156 | 0.349 * |
| MBC | 1.000 | 0.397 * | 0.702 ** | 0.452 ** | 0.826 ** | |
| MBN | 1.000 | 0.245 | 0.529 ** | 0.137 | ||
| CAT | 1.000 | 0.080 | 0.626 ** | |||
| Ccum | 1.000 | 0.328 | ||||
| OC% | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liyanage, L.R.M.C.; Sulaiman, M.F.; Ismail, R.; Gunaratne, G.P.; Dharmakeerthi, R.S.; Rupasinghe, M.G.N.; Mayakaduwa, A.P.; Hanafi, M.M. Carbon Mineralization Dynamics of Organic Materials and Their Usage in the Restoration of Degraded Tropical Tea-Growing Soil. Agronomy 2021, 11, 1191. https://doi.org/10.3390/agronomy11061191
Liyanage LRMC, Sulaiman MF, Ismail R, Gunaratne GP, Dharmakeerthi RS, Rupasinghe MGN, Mayakaduwa AP, Hanafi MM. Carbon Mineralization Dynamics of Organic Materials and Their Usage in the Restoration of Degraded Tropical Tea-Growing Soil. Agronomy. 2021; 11(6):1191. https://doi.org/10.3390/agronomy11061191
Chicago/Turabian StyleLiyanage, Liyana Rallage Mahesh Chaminda, Muhammad Firdaus Sulaiman, Roslan Ismail, Gamini Perera Gunaratne, Randombage Saman Dharmakeerthi, Minninga Geethika Neranjani Rupasinghe, Amoda Priyangi Mayakaduwa, and Mohamed M. Hanafi. 2021. "Carbon Mineralization Dynamics of Organic Materials and Their Usage in the Restoration of Degraded Tropical Tea-Growing Soil" Agronomy 11, no. 6: 1191. https://doi.org/10.3390/agronomy11061191
APA StyleLiyanage, L. R. M. C., Sulaiman, M. F., Ismail, R., Gunaratne, G. P., Dharmakeerthi, R. S., Rupasinghe, M. G. N., Mayakaduwa, A. P., & Hanafi, M. M. (2021). Carbon Mineralization Dynamics of Organic Materials and Their Usage in the Restoration of Degraded Tropical Tea-Growing Soil. Agronomy, 11(6), 1191. https://doi.org/10.3390/agronomy11061191







