Abstract
Different types of soil respond variably to biochar amendment. Soil structure and fertility are properties which strongly affect the impacts of biochar on soil fertility and microbial activity. A pot experiment with lettuce was conducted to verify whether biochar amendment is more beneficial in sandy soil than in clay soil. The nutrient content (carbon and nitrogen), microbial biomass carbon, soil respiration, metabolic quotient, and plant biomass yield were determined. The treatments were prepared by mixing silty clay loam (Haplic Luvisol) with a quartz sand in ratios of 0%, 20%, 40%, 60%, 80%, and 100% of sand; the same six treatments were prepared and amended with biochar (12 treatments in total). Soil carbon and nitrogen, microbial biomass carbon, and soil respiration were indirectly dependent on the descending sand ratio, whereas the metabolic quotient increased with the ascending sand ratio. The biochar’s effects were positive for total carbon, microbial biomass carbon, metabolic quotient, and plant biomass in the sand-rich treatments. The maximum biochar-derived benefit in crop yield was found in the 100% sand + biochar treatment, which exhibited 24-fold (AGB) and 11-fold (root biomass) increases compared to the unamended treatment. The biochar application on coarse soil types with lower fertility was proven to be favorable.
1. Introduction
The soil structure is the key feature that controls microbially mediated processes of organic matter degradation in terrestrial ecosystems [1]. Soil texture is the important soil property that conditions the variability in the carbon (C) and nitrogen (N) turnover, and these differences have been assessed to be most significant between clay and sandy soils [2,3]. The content of microbial C compared to that of non-microbial C is higher (reaching up to 20% of oxidizable C) in clay soils than in coarse-textured soils [4]. Contrarily, activity of the microbial biomass can be twice as large in sandy or loam soils as in clay soils. Additionally, the amounts of mineralized N per microbial biomass unit are highest in the sandy soils [5]. Soil texture and organic matter content are the factors that control the soil functions, resilience, and fertility, even under the application of organic amendments, e.g., biochar [6]. There have been several studies that have shown that the dependence of the microbial properties (e.g., biomass) on the soil structure and fertility is strongly affected by the type of biochar amendment [7,8,9]. Slow-pyrolyzed, high-temperature biochars (>600 °C) significantly lower microbial biomass and enzyme activities in coarse-textured soil, whereas they do not in clay soils [7]. Crop productivity increased significantly (10% in average) in soils of both medium and coarse textured in comparison to unchanged clay soils in previous studies [8,9]. The most significant positive impacts on biomass and activity of soil microbiota were evidenced in sandy soils [10,11] rather than in clay soils [12]. Some authors have ascribed enhanced soil respiration to biochar-derived C rather than to available soil organic matter (SOM) [1]. Concurrently, biochar-stimulated mineralization of native soil organic carbon (SOC) is limited only to the low-C clay soils because stabilization of SOC by biochar-induced organomineral interactions are involved [2]. However, a strong beneficial effect was also reported for these [13], dependent on the biochar pyrolysis temperature. This is possibly because biochar properties, e.g., the temperature of pyrolysis, also play roles in putative adverse [14] or positive effects [15] in coarse-structured soils; nevertheless, many studies have shown the largest impact of biochar application occurring in acidic and sandy-textured soils, which suggests aggregating and moistening effects of biochars [9]. Several studies have compared the effects of biochars with specific characteristics on chemical (nutrient content and turnover) and biological (microbial biomass carbon (MBC), CO2 fluxes, diversity) properties of different types of soil (sand, loam, clay) under specific defined conditions [16,17]; however, some of them did not deliver clear results [18,19].
In this study, a short-term pot experiment with a test crop of lettuce (Lactuca sativa L. var. capitata L. cv. Brilliant) was designed and carried out to clarify the roles of soil texture and nutrient content in microbial and plant biomass production properties. In order to reduce the effects of various chemical, physical, and biological properties of particular soil types with different textures, as in other studies [20], we prepared a set of artificial soil types by mixing a silty clay loam, Haplic Luvisol, with a quartz sand in six specified decreasing ratios (6 treatments) ranging from 100% soil to 100% sand. We hypothesized that in this experimental setting, we would observe a positive effect of amendment of biochar from agricultural residues on soil in the respective selected properties, presuming the highest positive effects on soil microbial abundance and activity in the soil treatments with higher sand content.
2. Materials and Methods
2.1. Biochar Amendment, Soil and Pot Experiment Preparation
The biochar used in this study was prepared by pyrolysis of agricultural grain waste (cereal and sunflower husks) at moderate temperatures of approximately 600 °C. According to the manufacturer, the properties of the biochar were as follows: elements (in g·kg−1) –C 866, N 3.0, O 10.0, H 14.2; Ash550 °C 11.7%; salts 0.42%; pH (CaCl2) 8.5.
The growth substrates used for the reported pot experiment were prepared by mixing a quartz sand with topsoil (0–15 cm), a silty clay loam (according to USDA Textural Triangle), Haplic Luvisol (according to WRB soil classification [21]). This field soil was collected near the town of Troubsko, Czech Republic (49°10’28”N 16°29’32”E), in autumn 2018. The topsoil properties were determined before the start of the experiment as follows: soil macronutrients (in g·kg−1)—total carbon (TC) 7.0, total nitrogen (TN) 0.80, P 0.049, S 0.073, Ca 1.60, Mg 0.118, K 0.115; N forms (in mg·kg−1)—Nmineral 32.8, N-NO3 29.6, N-NH4 3.2; Si 220.0 g·kg−1; soil reaction = pH (CaCl2) 7.3. This heavy soil was chosen to ensure sufficiently different textures for all tested treatments depending on the sand:soil ratio.
In order to remove the coarse particles, the soil was sieved through a grid with a size of 2.0 mm. The sieved soil was mixed with fine quartz sand (0.1–1.0 mm; ≥95% SiO2) in the following ratios (w + w): (1) 100% sand; (2) 80% sand + 20% soil; (3) 60% sand + 40% soil; (4) 40% sand + 60% soil; (5) 20% sand + 80% soil; (6) 100% soil (Table 1). Each treatment was prepared in two versions: (A) without biochar; (B) with biochar in the amount of 32 g per 1 L of pot substrate, equaling 40 t·ha−1 (the dose was adopted from the method used by Sadowska et al. 2020 [22]). Here, 1 kg of each of the thoroughly mixed substrate treatments was used to fill experimental plastic pots (volume 1 L [23], top diameter 11 cm, bottom diameter 9 cm, height 13 cm); each treatment was carried out in 3 replicates (pots).

Table 1.
Experimental treatments of soil and sand mixtures tested in the study.
The following controlled conditions were applied: test crop lettuce (Lactuca sativa L. var. capitata L.), cv. Brilliant; cultivation in growth chamber—full-spectrum stable white LED lighting, intensity 20,000 lx [24] ~200 µmol.m−2·s−1 [25]; photoperiod 12 h [26]; temperature 18/22 °C (night/day); relative humidity 70% [27]. A two-day sprouting of the lettuce seeds on wet filter paper preceded sowing to the depth of approximately 2 mm in each pot. After sowing, each pot was watered with 100 mL of distilled water. The 10-day-old seedlings were reduced to only one plant (the most robust) per pot. Pot placement in the growth chamber was randomized. Soil humidity was controlled and water content was maintained during the experiment. The pots were variably rotated once per week [28]. Six weeks after sowing, the plants were harvested [29]. A mixed soil sample was collected from each pot (5 subsamples were taken and amalgamated).
2.2. Plant Sampling and Biomass Determination
The lettuce shoots were cut at ground level, and the roots were gently cleaned of soil and washed with water [28]. The lettuce shoots and roots were dried at 60 °C to a constant weight, and dry AGB and root biomass were estimated gravimetrically by weighing on the analytical scales.
2.3. Soil Sampling and Preparation
The soil samples were taken after harvesting the lettuce (1 mixed sample per pot). Samples were homogenized by sieving them through a 2 mm mesh sieve under sterile conditions. The samples for MBC determination and respiration (basal and substrate-induced) measurement were stored at 4 °C for 14 days (based on the method [30]) before they were analyzed.
2.4. Soil Chemical, Biological, and Statistical Analyses
Soil properties were determined and the data obtained were statistically analyzed using the methods listed in Table 2, for which the specifications were identical to our previously published work [31]. The results of the Pearson’s correlation analysis were evaluated according to [32]: 0.5 < ρ < 0.7 meaning moderate correlation and 0.7 < ρ < 0.9 meaning strong correlation.

Table 2.
Methods used to determine the soil properties for statistical analysis with relevant references.
3. Results
3.1. General Assessment of the Effects of Biochar on Soil and Plant Characteristics
The results of the MANOVA analysis showed significant differences among all experimental treatments in most of the determined soil and plant properties at the level of p < 0.001 (with the exception of the dry root biomass, which differed at p = 0.001). The data can be found in the Appendix A, Table A1. A two-way ANOVA was carried out to evaluate the effects of either biochar (the first factor) or sand content (the second factor) on the determined soil properties; the results are mentioned below and displayed in the Appendix A, Table A2. A scheme with the results of the Pearson’s correlation analysis is shown in the Appendix A (Figure A1). The evaluation of the mutual dependence between the soil and plant properties and their values for each of the experimental treatments are shown in the Rohlf PCA biplot (Figure 1). The effects of the applied biochar in each experimental treatment were rated to identify potential beneficial or detrimental effects on soil properties.
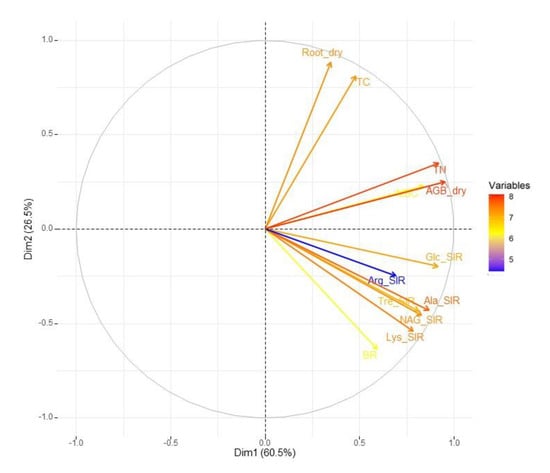
Figure 1.
Variable correlation PCA plot describing the relationships between all variables. Color scale (of arrows) from blue to red indicates the increasing ‘weight’ of variables.
3.2. Soil Nutrient Content, Plant Biomass
Soil TC and TN values in all treatments were indirectly related to the sand content and were increased by the biochar amendment (Figure 2A,C). The differences between BC and non-BC treatments (for each sand:soil ratio) were all significant. The greatest difference in TC was between the 100% sand and 100% sand + biochar treatments (Figure 2A). Except for treatments 100:0 and 80:20, 100:0 BC and 80:20 BC, 40:60 BC and 20:80 BC, there were no significant differences between treatments that differed by ±20% sand content (either unamended or plus BC). The TC value of the 100% soil + biochar showed no significant difference to the 60% sand + 40% soil, 40% sand + 60% soil, or 20% sand + 80% soil treatments (Figure 2A).
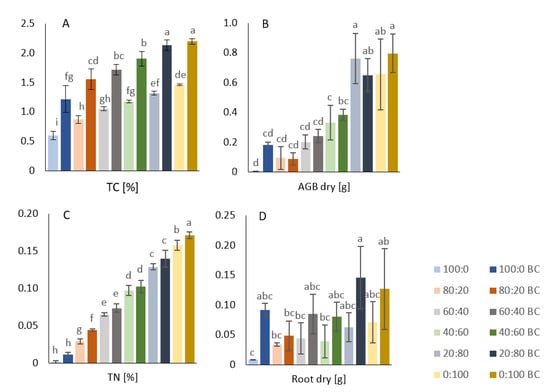
Figure 2.
(A) Total soil carbon. (B) Dry aboveground biomass. (C) Total soil nitrogen. (D) Dry root biomass. Average values shown with SD (error bars), treatments amended with biochar (dark colors) and unamended treatments (light colors) are sorted by decreasing sand:soil ratios (percentages ranging from 100:0 to 0:100). Different letters indicate statistically significant differences of MANOVA at p ≤ 0.05.
These results were influenced by the different soil particulate conditions of the treatments. Decreases in sand content (from 100% to 0%) led to the relatively high increases in TC values of the biochar-amended treatments in comparison to the treatments without biochar (the Appendix A, Figure A2A). This trend is apparent in the sharper slope of the regression curve of the dependence of TC on soil content (%) in the BC treatments as compared to the slightly slope in biochar-untreated treatments. However, the two-way ANOVA detected an equally significant (p < 0.001) effect of either the biochar or sand:soil ratio on the values and differences in TC. The TC content in the soil correlated highly positively with soil TN (ρ = 0.71) and also with the dry root biomass (ρ = 0.71), which suggested that a surplus of biochar-derived C in the soil enhanced the plant (root) growth. TC agonism with the dry root biomass is also shown on the PCA plot (Figure 1).
The TN content showed no significant differences (at a significance level of p ≤ 0.05) between most of the treatments, with the same sand:soil ratio and either with or without biochar amendment (with the exception of the 80% sand + 20% soil and the 100% soil). The treatments that differed by ±20% sand content exhibited significant differences when either unamended or biochar-amended (Figure 2C).
The decreasing sand content (from 100% to 0%, by −20%) led to a relatively smaller increase, coupled with a slighter regression curve slope (the Appendix A, Figure A2B) in the TN of biochar-amended treatments as compared to the sharper regression curve slope with the rise of the TN values in the treatments without biochar. TN strongly correlated with dry (ρ = 0.90) AGB; the agonism of these properties is also shown via the PCA biplot (Figure 1). As such, we anticipated the growth stimulated by increased N assimilation in plants under higher available soil TN. The two-way ANOVA again revealed equally significant (p < 0.001) effects of either biochar or sand:soil ratio on the values and differences in TN.
The dry root biomass showed no significant differences at a certain sand:soil ratio between the two treatments (with or without biochar addition)—see Figure 2D. However, the 20:80 BC treatment differed significantly from the 80:20 BC in dry root biomass. This is evidence of only a weak effect of the various sand:soil ratios (without biochar) on the root biomass, whereas the biochar may enhance these contrasts. The two-way ANOVA results were supportive and revealed a significant (p < 0.001) effect of biochar on the values and differences in the dry root biomass, but it was less significantly (p < 0.05) affected by the sand:soil factor.
A high positive correlation of soil TC content and dry root biomass (ρ = 0.71) and moderately strong correlation between soil TC content and dry ABG (ρ = 0.61) supported the presumed relation between plant biomass and the biochar amendment in the soil. Further, the calculation showed the increase in proportion between plant biomass values of the biochar-treated and plant biomass values of untreated treatments (Figure 3A,B). We suggest that the biochar’s beneficial fertilizing effect on the plant biomass reached the maximum positive effect in the 100% sand sample.
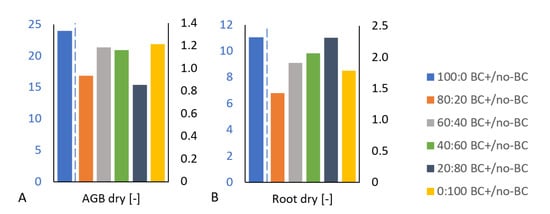
Figure 3.
(A) Dry aboveground biomass and (B) dry root biomass trends, i.e., the ratio (of average values) between the plant biomass of the biochar-amended (BC+) and the plant biomass of the unamended (no-BC) treatments, sorted according to decreasing sand:soil ratios (aligned from 100:0 to 0:100). Note: The blue (left) axis belongs to the blue bar (100:0 treatment), while the black (right) axis belongs to the other bars (treatments).
3.3. Microbial Biomass and Respiration Activity
We observed significant differences in the MBC values related to the sand:soil ratio in both the biochar-amended and unamended treatments, with the exception of the comparison between the 80% sand + 20% soil and the 60% sand + 40% soil treatments (both BC-treated and untreated). No significant difference in MBC was found between the 20:80 BC and 0:100 BC (Figure 4A). The MBC values of 100% sand, 80% sand + 20% soil, and 60% sand + 40% soil did not significantly differ in pairs of unamended and amended treatments with the same sand:soil ratio (Figure 4A). Significant differences in MBC related to the biochar addition were detected in the untreated 20% sand + 80% soil and 100% soil treatments as compared to the respective 20:80 BC and 0:100 BC treatments (Figure 4A). The two-way ANOVA revealed an equally significant (p < 0.001) effect of either biochar or sand:soil ratio, as well as a significant (p < 0.001) synergic effect of both factors. The MBC showed the highest positive correlation with the AGB dry (ρ = 0.82), and the increase in MBC values was coupled with increasing crop yields. The concordant trends for MBC and dry AGB in the variable correlation PCA plot (Figure 1) corroborated the correlation analysis results.
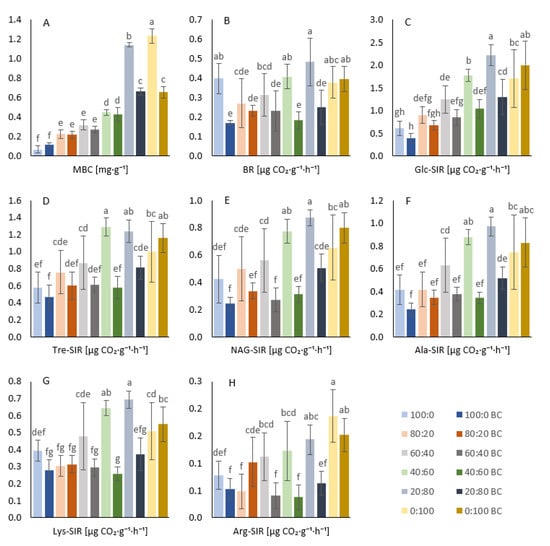
Figure 4.
Microbial biomass carbon values (A). Soil basal values (B). Respiration induced by D-glucose (C), D-trehalose (D), N-acetyl-β-D-glucosamine (E), L-alanine (F), L-lysine (G), and L-arginine (H). Average values shown with standard error of mean (error bars), treatments amended with biochar (dark colors) and unamended (light colors) are sorted by decreasing sand:soil ratios (from 100:0 to 0:100). Different letters indicate statistically significant differences of MANOVA at p ≤ 0.05.
The microbial biomass determined the microbial activity in the soil, which was measured as the soil respiration. An assumptive basal respiration (BR) relation with the content of sand in the substrate was, however, not observed (Figure 4B). The only significant increase in the BR value due to the sand content was detected in the 20% sand + 80% soil treatment (compared to other unamended treatments) and in the 100% soil + biochar treatment (compared to the other amended treatments). We found it contradictory that the respiration activity of soil microorganisms did not increase with both the increase in the soil content and the microbial biomass (Figure 4A). Nevertheless, the BR values adjusted to the amount of MBC (i.e., metabolic quotient) showed a clearer picture, which (except for outlying values of the 40% sand + 60% soil treatments, both BC-treated and untreated) evidenced a descending respiration rate with decreasing sand content in the pot substrate (Figure 5A).
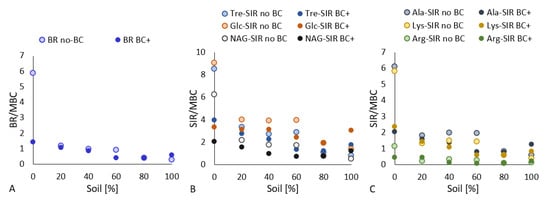
Figure 5.
The dependence of the metabolic (basal (A) and substrate-induced (B,C) quotients (respiration and MBC ratio) on the sand:soil ratio (expressed as a percentage of soil) and biochar amendment.
Not all biochar-amended treatments showed decreased BR as compared to the unamended treatments. BR was significantly lowered due to biochar application only in the 100% sand, 40% sand + 60% soil, and 20% sand + 80% soil samples as compared to the untreated treatments. The metabolic quotient qCO2 in the biochar-treated treatments directly responded to the decreasing sand content (Figure 5A). Only the BR value of the 100% soil + biochar treatment did not follow this trend.
As documented by the agonisms among all types of respiration in the variable correlation PCA plot (Figure 1) and in the Pearson’s correlation plot (Figure A1, the respiration induced by various sugar substrates (D-trehalose (Tre-SIR), D-glucose (Glc-SIR), N-acetyl-β-D-glucosamine (NAG-SIR)) and amino acid substrates (L-alanine (Ala-SIR), L-lysine (Lys-SIR), L-arginine (Arg-SIR)) showed very similar results. Soil respiration (SIR) values did not significantly decrease with biochar amendment in the 100% sand and 80% sand + 20% soil treatments, whereas NAG-, Ala-, Lys-, and Arg-SIR values were significantly lowered by biochar application to the soil, with all having a lower sand:soil ratio (60:40, 40.60, 20:80, except for the 100% soil (Figure 4E–H). Glc-SIR and Tre-SIR were significantly lower in the 40:60 BC and 20:80 BC samples as compared to the unamended 40:60 and 20:80 samples (see Figure 4C,D). Of interest was the fact that all SIRs showed comparable, insignificantly variable values for both the 100% soil and 100% soil + biochar samples. The sand:soil ratio from 100:0 to 60:40 among the respective treatment (both BC-treated and untreated) samples demonstrated mostly insignificant differences in SIR values (except for Glc-SIR, Tre-SIR, Ala-SIR 100:0 vs. 60:40, and Lys-SIR, Arg-SIR 80:20 vs. 60:40). Contrarily, the treatments showed significantly lower SIRs as compared to the respective 40:60, 20:80, and 0:100 treatments (with and without biochar) (Figure 4C–H). While the Glc-SIR and Lys-SIR in the 100% soil were significantly lower as compared to the 20% sand + 80% soil treatment, all SIRs in the 100% soil + biochar were significantly increased in comparison to the 20% sand + 80% soil + biochar and the other higher sand:soil ratios.
However, substrate-induced qCO2 was also lowered due to the biochar amendment. Both the amended and unamended treatments followed an opposite trend to soil-substrate-induced respiration: the direct decrease in qCO2 related to the descending amount of sand in the substrate (Figure 5B,C). Nevertheless, the two-way ANOVA showed an equally significant (p < 0.001) effect of either the biochar or sand:soil ratio, as well as a significant (p < 0.001) synergic effect on both factors of the values and differences in all determined types of soil respiration.
4. Discussion
It is known that the maximum capacity of soil to store C (both organic and total) is determined by soil type (and the proportion of clay) [1]. The field soil used in this study was silty clay loam (Haplic Luvisol). This soil is (according to FAO’s Harmonized World Soil Database) moderately rich in C (on average), whereas the quartz sand contained neither organic matter nor TC. The mixing of soil and sand in variable ratios simulated the TC distribution and retention by soil types differing in their particulate fractions. Close positive relationships between the fine formulation of a soil and the amounts of TC and TN in the topsoil were observed [36]. Herath et al. reported that the soil formulation (coarse or fine fractions of soil) affected the input efficiency of biochar C into the SOM [37]. Free articulate SOM (in contrast to a heavy fraction of the soil) was considered to make labile biochar-derived SOC available, which is inconsistent with stable C in the form of biochar [37].
Several studies [38,39,40,41] have referred to the variable effects of biochar amendment to the soil on the plant biomass yield in relation to soil texture. These studies mostly reported a positive effect of biochar on the plant biomass yield, being more apparent than the effect of soil texture. However, our results imply that dry AGB was more affected by the changing sand:soil ratio, whereas the dry root biomass responded more to the biochar application (Figure 2B,D). Nevertheless, the contribution of biochar addition to the plant biomass was the most significant with the highest content of sand in the pot substrate (Figure 3A,B). Bruun et al. (2014) also reported that biochar treatment resulted in the greatest crop yield in coarse sandy soil due to the improved root growth [42].
There were previous studies [42,43] that reported both increased water retention in sandy soil and increased drainage combined with decreased plant-available water in biochar-treated clay–loam soil. However, some authors referred to the contrarian role of soil type in the beneficial effect of biochar under water limitation [44]. With respect to these observations, we cannot exclude that diverse effects of biochar in either sand-dominant soil or clay-dominant soil on dry biomass were due to short-term fluctuations in the soil moisture in this pot experiment.
MBC is the amount of C content in the living biomass of soil biota (mostly bacteria and fungi). It represents an SOC fraction, which is susceptible to changes in various soil properties, including N limitations, residue and nutrient management, plant diversity, soil moisture, temperature, and soil texture [45].
Some studies have shown that the same types of soil, each with different fractions, were affected in turnover of C through the microbial biomass [3,4,5]. These authors [7,19] referred to greater biochar-derived increases of total microbial biomass in sandy soils compared to clay soils. Although we did not observe a significant contribution of biochar to MBC in sand-rich soil treatments, the MBC values of higher-clay treatments were less improved by biochar. In fact, the biochar induced a negative priming effect on microbial abundance in low sand content substrates (Figure 4A, Section 3.2). We assumed that the sand:soil ratio was the trigger between the positive (sand) and negative (clay) effects of biochar on MBC in the pot substrates. Liu et al. (2016) referred to a similar positive biochar-derived effect on soil microbial traits in coarse soils, while negative effects were observed in fine-textured soils [21]. The study, carried out in field luvisoil (a clay-rich soil type) inferred that MBC was lowered by amendment with biochar, which corroborates our previous findings [46].
BR is an indicator of the catabolic activities of soil organisms (mostly microbes). This may be estimated as CO2 produced non-specifically within the entire soil microbiome [47] or as a selectively induced microbial response to a defined energy source [48]. It is known that CO2 production is soil-texture-dependent; nevertheless, the referenced respiration responses in different types of soil may differ. Longer-term (90 days) enhancement of C decomposition in soils with more clay was reported by Gregorich et al. (1991) [49], whereas Franzluebbers et al. (1996) observed the opposite over a much shorter period (21 days) [49]. The soil microbial biomass was more active in coarse-textured soils than in fine-textured soils. We experienced a decrease in the metabolic quotient in our mid-length experiment (compared to the above-mentioned two studies) when sand content in the pot substrate was lowered stepwise (Figure 5). Hassing et al. (1994) and Franzluebbers et al. (1996) reported very similar results to ours; they noted doubled activity of the biomass in average sandy or loam soil than in an average clay soil [5,49]. The above-described principles of microbial respiration can be affected by soil texture and were found to be similar in the biochar-treated soils. Liu et al. (2016) referred to significant positive effects of biochar application on soil CO2 fluxes in coarse-textured soils [20]. We contrasted these results with observed insignificant changes in the substrates with ≥80% sand, which was still beneficial due to prevention of the negative biochar priming effect on respiration. Contrarily, significant negative effects were observed in biochar-treated soils with fine textures (high clay content) [20] and in our substrate treatments characterized by 40–80% of soil (silty clay loam) (Figure 4C–H, Section 3.2). The above-mentioned results were mutually evaluated and a weak indirect relationship was found between respiration values and the sand:soil ratio. Zhou et al. (2017) referred to a similar reduction in qCO2 with biochar amendment and also to the reduction in clay soils (as compared to sandy soils) [19]. Our observations are listed in Section 3.2 (Figure 5A–C).
Neither the soil respiration nor metabolic quotient was higher in the biochar-amended soil; however, qCO2 was directly related to the sand content and indirectly related to the microbial biomass in both biochar-treated and untreated treatments. The specific respiration rate (qCO2) was determined by soil microbial biomass as well [9]. Glc-SIR and Ala-SIR rather strongly correlated with TN (ρ = 0.72 and 0.57, respectively) and the dry AGB (ρ = 0.72 and 0.61, respectively), and thus we presumed that a high clay content promoted nutrient stabilization. Singh et al. (2014) also observed C stabilization and protection from mineralization in soils with higher clay content [13]; therefore, we deduced that the observed stabilization may benefit plants’ nutrient uptake and higher absolute respiration values.
This experiment primarily aimed to only investigate and evaluate theoretical application of biochar on different simulated artificial soil types. The biochar application was expected to alleviate adverse effects of sand- and soil-based substrates (with variable sand:soil ratios) on the nutrient content, microbial abundance, and fertility. The main outcome of this study was evaluation of the potential of biochar application for sandy soil fertilization and also the possible reclamation and protection of lands affected by wind erosion. We deduce this from the positive effects of biochar application on soil microbial activity (and the weakest negative priming effect on microbial abundance) with the treatments containing higher sand content. We also observed the best improvement of plant biomass yield in the biochar-treated pot substrate with high sand content.
5. Conclusions
Based on the performed pot experiment, the following points were concluded.
Soil TC and TN were positively affected by the soil content in the pot substrate (prepared by mixing silty clay loam (Haplic Luvisol) with a quartz sand) and by biochar addition. The amendment of biochar to the substrate led to a proportionally larger increase in the C levels (and a lower increase in N levels) with an ascending soil:sand ratio in comparison to the unamended treatments. This observation was supportive evidence for the known biochar function of enhanced C sequestration. Morover, the increacing N content could be indicative of the enhancing effect of biochar on the rate of nitrification in soil.
The MBC was positively affected by the soil content in the pot substrate, whereas the biochar amendment decreased MBC in the 20% sand + 80% soil and the 100% soil treatments. The other treatments showed no change. The BR and SIR showed that neither the absolute values of evolved CO2 nor the metabolic quotient (qCO2) were lower in the biochar-treated soil. This can be explained as a partially negative effect of biochar on the abundance of soil microflora, whereas the microbial activity was presumably unchanged.
Calculation of the proportional increase in the plant biomass values, i.e., comparison between biochar-amended and untreated treatments, showed that biochar had a beneficial effect on the plant biomass. It reached the maximum value at 100% sand, with 24-fold higher values for AGB and 11-fold for root biomass. However, this positive affect of biochar should be attributed to positive effects on soil physicochemical properties, biological processes, nutrient transformation, and the enhanced access of nutrients derived by the biochar material, which is more fertile in comparison to the quartz sand.
Although the biochar application on the coarse soil type showed the most beneficial effects in the 100% sand treatment (compared to the other treatments), this approach cannot be recommended as cost-effective, yet it leaves the option for further testing of possible beneficial effects in some specified types of biochar-based technology (e.g., water availability improvement, reclamation of specifically endangered or damaged soils) in agriculture.
Author Contributions
Conceptualization, M.B., T.H. and J.H.; methodology, M.B., T.H. and J.H.; software, T.H., T.B.; validation, M.B., T.H. and E.K.; formal analysis, O.L.; investigation, J.H.; resources, O.L., J.E.; data curation, T.B., E.K., M.R.; writing—original draft preparation, M.B., J.H.; writing—review and editing, J.H., J.E., L.S.; visualization, T.H., A.K.; supervision, J.E., M.R.; project administration, M.B.; funding acquisition, M.B., A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Technology Agency of the Czech Republic, the project TH03030319: ‘‘Promoting the functional diversity of soil organisms by applying classical and modified stable organic matter while preserving the soil’s production properties”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Detailed results after multivariate analysis of variance MANOVA (ANOVA separately).
Table A1.
Detailed results after multivariate analysis of variance MANOVA (ANOVA separately).
| Response Variable | F Test | Significance (p) |
|---|---|---|
| MBC | F (11,96) = 650.10 | <0.001 *** |
| BR | F (11,132) = 17.35 | <0.001 *** |
| Glc-SIR | F (11,132) = 42.68 | <0.001 *** |
| Tre-SIR | F (11,132) = 24.47 | <0.001 *** |
| NAG-SIR | F (11,132) = 27.25 | <0.001 *** |
| Ala-SIR | F (11,132) = 29.89 | <0.001 *** |
| Lys-SIR | F (11,132) = 29.99 | <0.001 *** |
| Arg-SIR | F (11,132) = 23.79 | <0.001 *** |
| TC | F (11,60) = 135.10 | <0.001 *** |
| TN | F (11,60) = 614.18 | <0.001 *** |
| AGB_dry | F (11,24) = 20.25 | <0.001 *** |
| Root_dry | F (11,24) = 4.30 | 0.001 ** |
Statistical difference at the level: ** p < 0.01, *** p < 0.001.
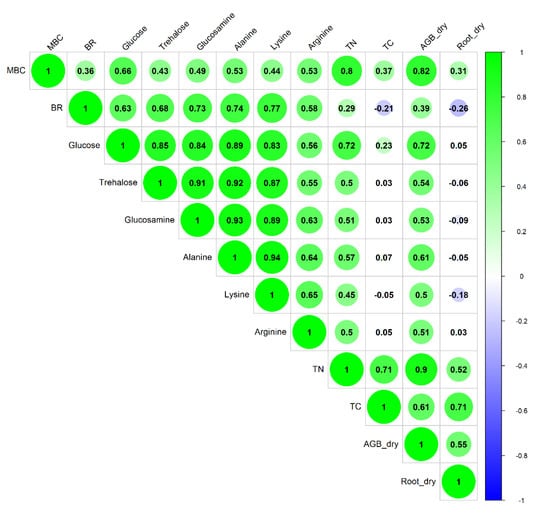
Figure A1.
Correlation Matrix of Soil Properties (numbers indicate the Pearson’s correlation coefficient).
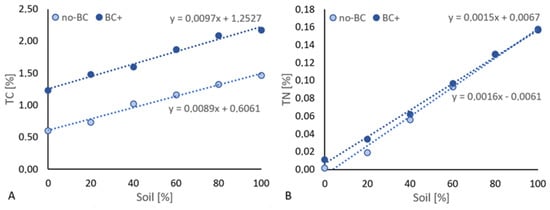
Figure A2.
The relationship of total carbon (A) and total nitrogen (B) and the sand:soil ratio (expressed as a percentage of soil). Data series are described by regression curves and equations.

Table A2.
Results of two-way analysis of variance ANOVA.
Table A2.
Results of two-way analysis of variance ANOVA.
| Response: TC | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 6.938 | 1.388 | 115.872 | <0.001 *** |
| Biochar | 1 | 9.036 | 9.036 | 754.544 | <0.001 *** |
| Sand:soil to Biochar | 5 | 0.069 | 0.014 | 1.144 | 0.347 |
| Residuals | 60 | 0.719 | 0.012 | ||
| Response: TN | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 0.217 | 0.043 | 664.444 | <0.001 *** |
| Biochar | 1 | 0.002 | 0.002 | 31.577 | <0.001 *** |
| Sand:soil to Biochar | 5 | 1.74 × 10−4 | 3.50 × 10−5 | 0.535 | 0.749 |
| Residuals | 61 | 0.004 | 0.000 | ||
| Response: MBC | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 11.994 | 2.399 | 1244.090 | <0.001 *** |
| Biochar | 1 | 0.577 | 0.577 | 299.330 | <0.001 *** |
| Sand:soil to Biochar | 5 | 2.153 | 0.431 | 223.280 | <0.001 *** |
| Residuals | 96 | 0.185 | 0.002 | ||
| Response: BR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 0.355 | 0.071 | 9.883 | <0.001 *** |
| Biochar | 1 | 0.625 | 0.625 | 87.139 | <0.001 *** |
| Sand:soil to Biochar | 5 | 0.372 | 0.074 | 10.365 | <0.001 *** |
| Residuals | 132 | 0.947 | 0.007 | ||
| Response: Glc-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 34.403 | 6.881 | 69.538 | <0.001 *** |
| Biochar | 1 | 4.849 | 4.849 | 49.008 | <0.001 *** |
| Sand:soil to Biochar | 5 | 5.578 | 1.116 | 11.274 | <0.001 *** |
| Residuals | 132 | 13.061 | 0.099 | ||
| Response: Tre-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 5.639 | 1.128 | 28.476 | <0.001 *** |
| Biochar | 1 | 2.265 | 2.265 | 57.194 | <0.001 *** |
| Sand:soil to Biochar | 5 | 2.632 | 0.526 | 13.292 | <0.001 *** |
| Residuals | 132 | 5.228 | 0.040 | ||
| Response: NAG-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 3.085 | 0.617 | 29.608 | <0.001 *** |
| Biochar | 1 | 1.733 | 1.733 | 83.151 | <0.001 *** |
| Sand:soil to Biochar | 5 | 1.315 | 0.263 | 12.623 | <0.001 *** |
| Residuals | 132 | 2.751 | 0.021 | ||
| Response: Ala-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 4.181 | 0.836 | 33.827 | <0.001 *** |
| Biochar | 1 | 1.961 | 1.961 | 79.328 | <0.001 *** |
| Sand:soil to Biochar | 5 | 1.575 | 0.315 | 12.740 | <0.001 *** |
| Residuals | 132 | 3.263 | 0.025 | ||
| Response: Lys-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 1.071 | 0.214 | 22.042 | <0.001 *** |
| Biochar | 1 | 0.908 | 0.908 | 93.458 | <0.001 *** |
| Sand:soil to Biochar | 5 | 0.839 | 0.168 | 17.263 | <0.001 *** |
| Residuals | 132 | 1.283 | 0.010 | ||
| Response: Arg-SIR | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 0.176 | 0.035 | 27.024 | <0.001 *** |
| Biochar | 1 | 0.059 | 0.059 | 45.128 | <0.001 *** |
| Sand:soil to Biochar | 5 | 0.069 | 0.014 | 10.669 | <0.001 *** |
| Residuals | 132 | 0.172 | 0.001 | ||
| Response: AGB dry | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 2.487 | 0.498 | 24.809 | < 0.001 *** |
| Biochar | 1 | 0.021 | 0.021 | 1.820 | 0.190 |
| Sand:soil to Biochar | 5 | 0.079 | 0.016 | 1.368 | 0.271 |
| Residuals | 24 | 0.279 | 0.012 | ||
| Response: Root dry | Df | Sum Sq | Mean Sq | F value | p (>F) |
| Sand:soil | 5 | 0.020 | 0.004 | 3.736 | 0.012 * |
| Biochar | 1 | 0.026 | 0.026 | 23.593 | <0.001 *** |
| Sand:soil to Biochar | 5 | 0.005 | 0.001 | 0.998 | 0.440 |
| Residuals | 24 | 0.026 | 0.001 |
Statistical difference at the level: * p < 0.05, *** p < 0.001.
References
- Van Veen, J.A.; Kuikman, P.J. Soil Structural Aspects of Decomposition of Organic Matter by Micro-Organisms. Biogeochemistry 1990, 11, 213–233. [Google Scholar] [CrossRef]
- Ladd, J.N.; Amato, M.; Parsons, J.W. Studies on nitrogen immobilization and mineralization in calcarerous, soils-III. Concentration and distribution of nitrogen derived from the soil biomass. In Proceedings of the IAEA/FAO/SSF Symposium, Braunschweig, Germany, 6–10 September 1976; pp. 301–310. [Google Scholar]
- Van Veen, J.A.; Ladd, J.N.; Amato, M. Turnover of carbon and nitrogen through the microbial biomass in a sandy loam and a clay soil incubated with [14C(U)] glucose and [15N](NH4) 2So4 under different moisture regimes. Soil Biol. Biochem. 1985, 17, 747–756. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Voroney, R.P.; Kachanoski, R.G. Turnover of carbon through the microbial biomass in soils with different texture. Soil Biol. Biochem. 1991, 23, 799–805. [Google Scholar] [CrossRef]
- Hassink, J. Effect of soil texture on the size of the microbial biomass and on the amount of C and N mineralized per unit of microbial biomass in dutch grassland soils. Soil Biol. Biochem. 1994, 26, 1573–1581. [Google Scholar] [CrossRef]
- Ajayi, A. Biochar-Induced Changes in Soil Resilience: Effects of Soil Texture and Biochar Dosage. Pedosphere 2017, 27, 236–247. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, A.F.; Ji, C.Y.; Joseph, S.; Bian, R.J.; Li, L.Q.; Pan, G.X.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions-a meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Cheng, H.; Hill, P.W.; Bastami, M.S.; Jones, D.L. Biochar stimulates the decomposition of simple organic matter and suppresses the decomposition of complex organic matter in a sandy loam soil. GCB Bioenergy 2017, 9, 1110–1121. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L. Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci. Rep. 2014, 4, 3687. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Durenkamp, M.; Nobili, M.; Lin, Q.; Devonshire, B.J.; Brookes, P.C. Microbial biomass growth, following incorporation of biochars produced at 350 °C or 700 °C, in a silty-clay loam soil of high and low pH. Soil Biol. Biochem. 2013, 57, 513–523. [Google Scholar] [CrossRef]
- Dempster, D.N.; Gleeson, D.B.; Solaiman, Z.M.; Jones, D.L.; Murphy, D.V. Decreased soil microbial biomass and nitrogen mineralisation with Eucalyptus biochar addition to a coarse textured soil. Plant Soil 2012, 354, 311–324. [Google Scholar] [CrossRef]
- Kolton, M.; Meller Harel, Y.; Pasternak, Z.; Graber, E.R.; Elad, Y.; Cytryn, E. Impact of biochar application to soil on the root-associated bacterial community structure of fully developed greenhouse pepper plants. Appl. Environ. Microbiol. 2011, 77, 4924–4930. [Google Scholar] [CrossRef]
- Khadem, A.; Raiesi, F. Responses of microbial performance and community to corn biochar in calcareous sandy and clayey soils. Appl. Soil Ecol. 2017, 114, 16–27. [Google Scholar] [CrossRef]
- Singh, G.; Mavi, M.S. Impact of addition of different rates of rice-residue biochar on C and N dynamics in texturally diverse soils. Arch. Agron. Soil Sci. 2018, 64, 1419–1431. [Google Scholar] [CrossRef]
- Li, X.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Zhou, H.M.; Zhang, D.X.; Wang, P.; Liu, X.Y.; Cheng, K.; Li, L.Q.; Zheng, J.W.; Zhang, X.H.; Zheng, J.F.; Crowley, D.; et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A Meta-analysis. Agric. Ecosyst. Environ. 2017, 239, 80–89. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. Gcb Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- WRB Soil Classification, ISBN 978-92-5-108369-7 (print), E-ISBN 978-92-5-108370-3 (PDF). Available online: http://www.fao.org/3/i3794en/I3794en.pdf (accessed on 22 April 2021).
- Sadowska, U.; Domagała-Świątkiewicz, I.; Żabiński, A. Biochar and Its Effects on Plant–Soil Macronutrient Cycling during a Three-Year Field Trial on Sandy Soil with Peppermint (Mentha piperita L.). Part I: Yield and Macro Element Content in Soil and Plant Biomass. Agronomy 2020, 10, 1950. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W.; Biber, M. Evaluation of the potential of bio-fertilizers as a source of nutrients and heavy metals by means of the exhaustion lettuce test. J. Elem. 2018, 23. [Google Scholar] [CrossRef]
- Bankole, A.; Umebese, C.E.; Feyisola, R.T.; Bamise, O. Influence of salicylic acid on the growth of lettuce (Lactuca sativa var longifolia) during reduced leaf water potential. J. Appl. Sci. Environ. Manag. 2018, 22, 543. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Cervera-Mata, A.; Pastoriza, S.; Rufián-Henares, J.Á.; Párraga, J.; Martín-García, J.M.; Delgado, G. Impact of spent coffee grounds as organic amendment on soil fertility and lettuce growth in two Mediterranean agricultural soils. Arch. Agron. Soil Sci. 2018, 64, 790–804. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Xylia, P.; Anastasiou, M.; Pantelides, I.; Tzortzakis, N. Effects of Ascophyllum nodosum seaweed extracts on lettuce growth, physiology and fresh-cut salad storage under potassium deficiency. J. Sci. Food Agric. 2018, 98, 5861–5872. [Google Scholar] [CrossRef]
- Iocoli, G.A.; Zabaloy, M.C.; Pasdevicelli, G.; Gómez, M.A. Use of biogas digestates obtained by anaerobic digestion and co-digestion as fertilizers: Characterization, soil biological activity and growth dynamic of Lactuca sativa L. Sci. Total Environ. 2019, 647, 11–19. [Google Scholar] [CrossRef]
- Trinchera, A.; Baratella, V.; Rinaldi, S.; Renzaglia, M.; Marcucci, A.; Rea, E. Greenhouse Lettuce: Assessing Nutrient Use Efficiency Of Digested Livestock Manure As Organic N-Fertilizer. In Proceedings of the II International Symposium on Organic Greenhouse Horticulture; ISHS: Leuven, Belgium, 2018. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Holatko, J.; Hammerschmiedt, T.; Datta, R.; Baltazar, T.; Kintl, A.; Latal, O.; Pecina, V.; Sarec, P.; Novak, P.; Balakova, L.; et al. Humic Acid Mitigates the Negative Effects of High Rates of Biochar Application on Microbial Activity. Sustainability 2020, 12, 9524. [Google Scholar] [CrossRef]
- Hinkle, D.E.; Wiersma, W.; Jurs, S.G. Applied Statistics for the Behavioral Sciences, 5th ed.; Houghton Mifflin: Boston, MA, USA, 2003. [Google Scholar]
- ISO_10694. Soil Quality-Determination of Organic and Total Carbon after Dry Combustion (Elemental Analysis); International Organization for Standardization: Geneve, Switzerland, 1995. [Google Scholar]
- ISO_13878. Soil Quality-Determination of Total Nitrogen Content by Dry Combustion (Elemental Analysis); International Organization for Standardization: Geneve, Switzerland, 1998. [Google Scholar]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A Rapid Microtiter Plate Method To Measure Carbon Dioxide Evolved from Carbon Substrate Amendments so as To Determine the Physiological Profiles of Soil Microbial Communities by Using Whole Soil. Appl. Environ. Microbiol 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Hassink, J. The capacity of soils to preserve organic C and N by their association with clay and silt particles. Plant Soil 1997, 191, 77–87. [Google Scholar] [CrossRef]
- Herath, S.; Camps Arbestain, M.; Hedley, M.; Hale, R.; Kaal, J. Fate of biochar in chemically- and physically-defined soil organic carbon pools. Org. Geochem. 2014, 73. [Google Scholar] [CrossRef]
- Castellini, M.; Giglio, L.; Niedda, M.; Palumbo, A.D.; Ventrella, D. Impact of biochar addition on the physical and hydraulic properties of a clay soil. Soil Tillage Res. 2015, 154, 1–13. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S.; Torn, M.S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8, 8. [Google Scholar] [CrossRef]
- Huang, R.; Tian, D.; Liu, J.; Lu, S.; He, X.H.; Gao, M. Responses of soil carbon pool and soil aggregates associated organic carbon to straw and straw-derived biochar addition in a dryland cropping mesocosm system. Agric. Ecosyst. Environ. 2018, 265, 576–586. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Bruun, E.W.; Petersen, C.T.; Hansen, E.; Holm, J.K.; Hauggaard-Nielsen, H. Biochar amendment to coarse sandy subsoil improves root growth and increases water retention. Soil Use Manag. 2014, 30, 109–118. [Google Scholar] [CrossRef]
- Jahan, S.; Iqbal, S.; Rasul, F.; Jabeen, K. Efficacy of biochar as soil amendments for soybean (Glycine max L.) morphology, physiology, and yield regulation under drought. Arab. J. Geosci 2020, 13, 356. [Google Scholar] [CrossRef]
- Aller, D.; Rathke, S.; Laird, D.; Cruse, R.; Hatfield, J. Impacts of fresh and aged biochars on plant available water and water use efficiency. Geoderma 2017, 307, 114–121. [Google Scholar] [CrossRef]
- Jiang-shan, Z.; Jian-fen, G.; Guang-shui, C.; Wei, Q. Soil microbial biomass and its controls. J. For. Res. 2005, 16, 327–330. [Google Scholar] [CrossRef]
- Brtnicky, M.; Dokulilova, T.; Holatko, J.; Pecina, V.; Kintl, A.; Latal, O.; Vyhnanek, T.; Prichystalova, J.; Datta, R. Long-Term Effects of Biochar-Based Organic Amendments on Soil Microbial Parameters. Agronomy 2019, 9, 747. [Google Scholar] [CrossRef]
- Anderson, J.P.E.; Page, A.L.; Miller, R.H.; Keeney, D.R. Soil Respiration. In Methods of Soil Analysis, Part 2, 2nd ed.; Page, A.L., Ed.; ASA and SSSA: Madison, WI, USA, 1982; Volume 2, pp. 831–871. [Google Scholar]
- Anderson, J.P.E.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Franzluebbers, A.J.; Haney, R.L.; Hons, F.M.; Zuberer, D.A. Active fractions of organic matter in soils with different texture. Soil Biol. Biochem. 1996, 28, 1367–1372. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).