Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Phenotypic Characterization
2.3. DNA Extraction and Microsatellite Analysis
2.4. Population Structure
3. Results
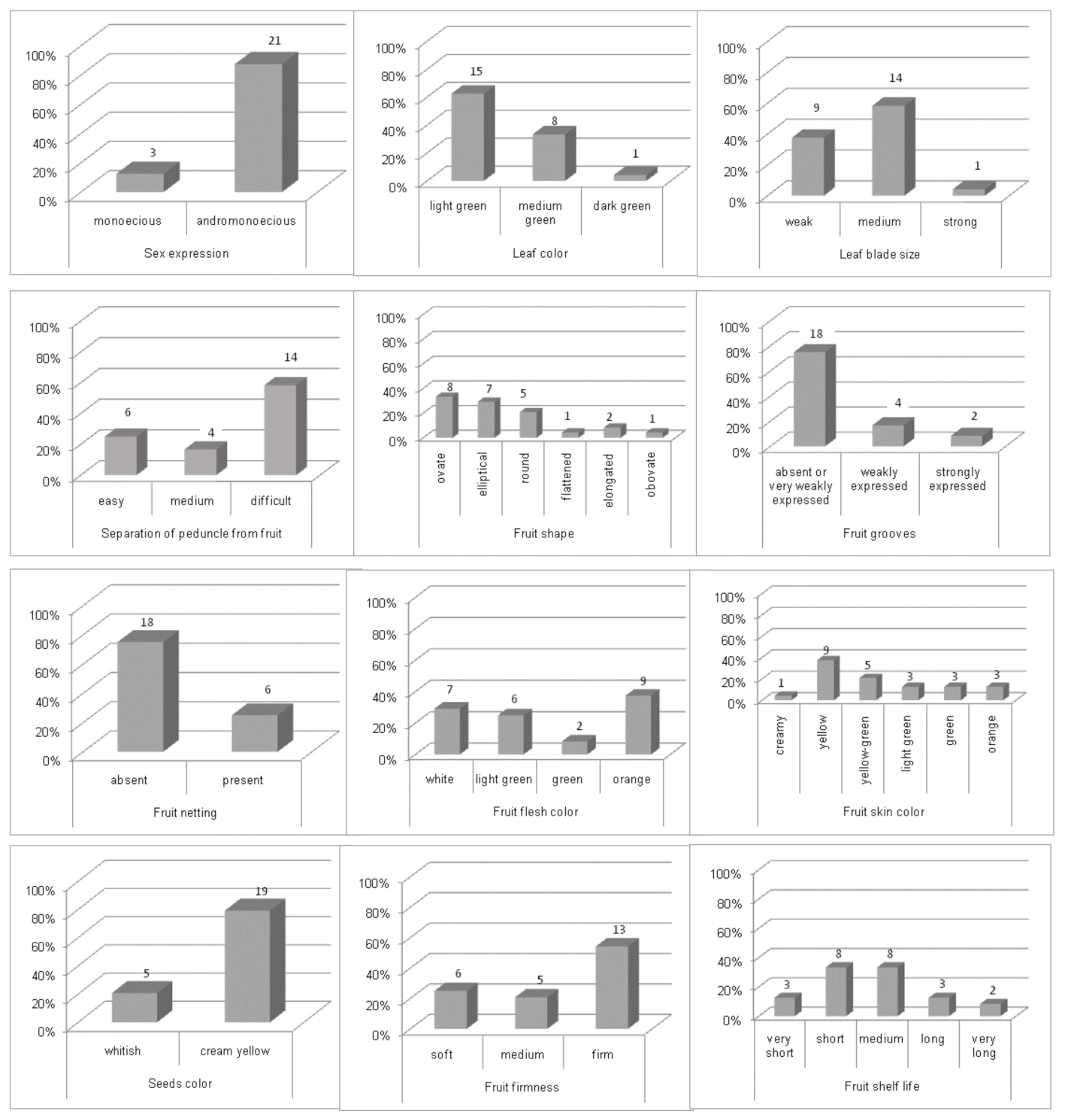
3.1. Phenotypic Characterization
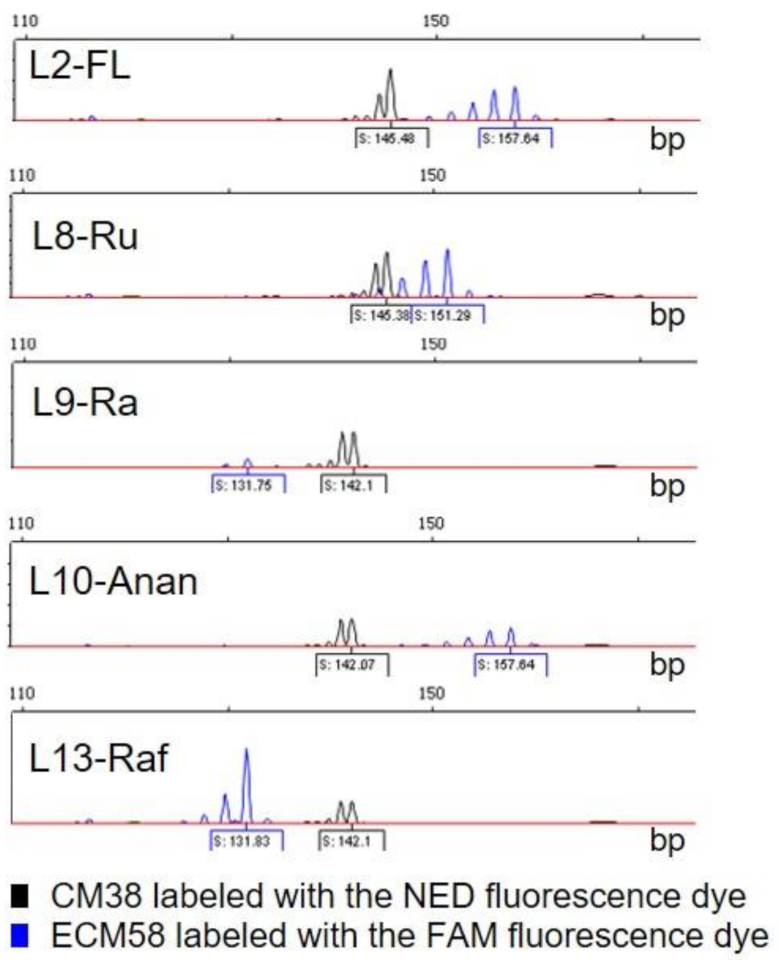
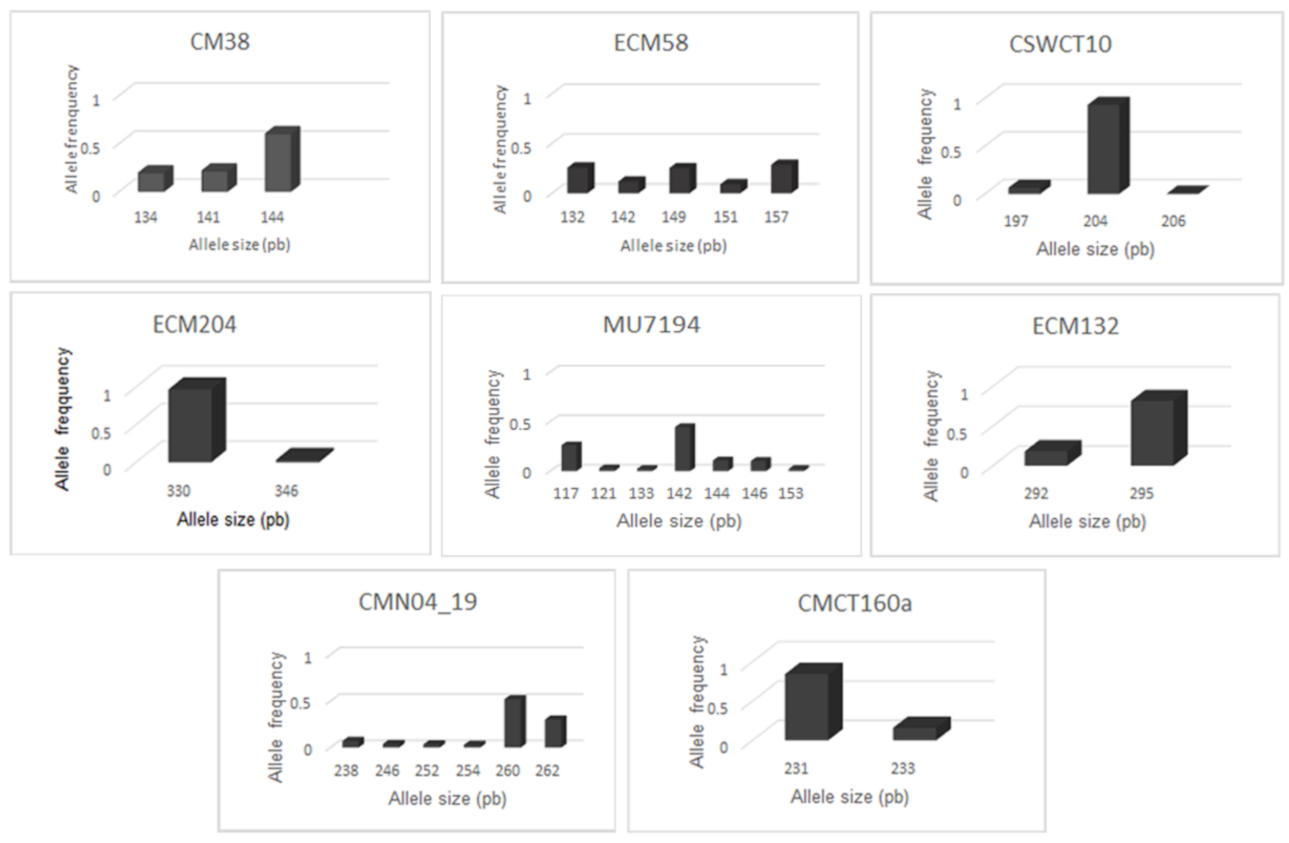
3.2. Molecular Characterization
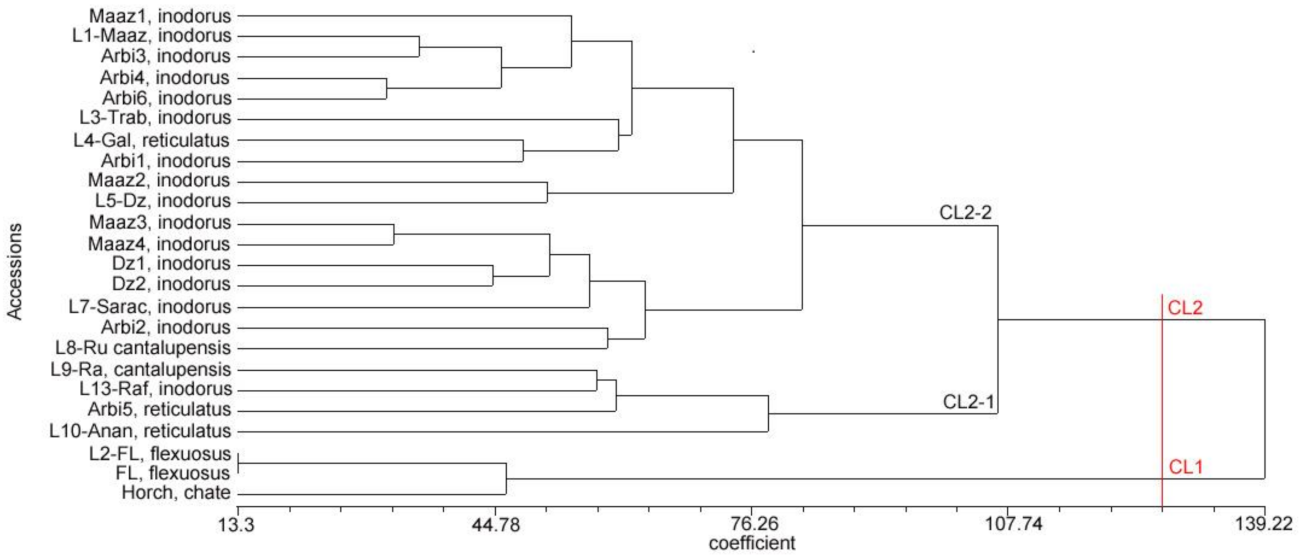
3.3. Combined Analysis of Phenotypic and Genotypic Data
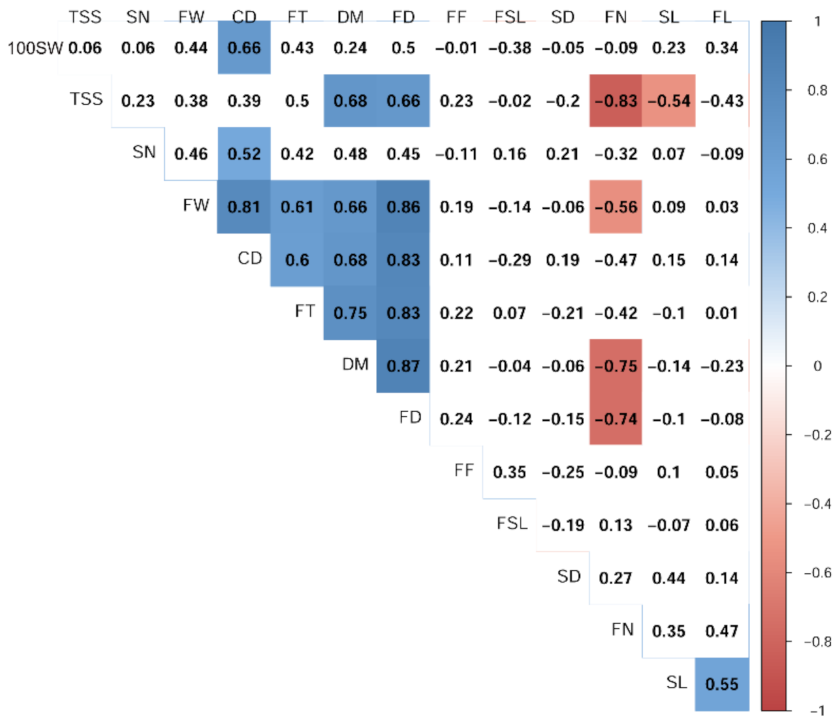
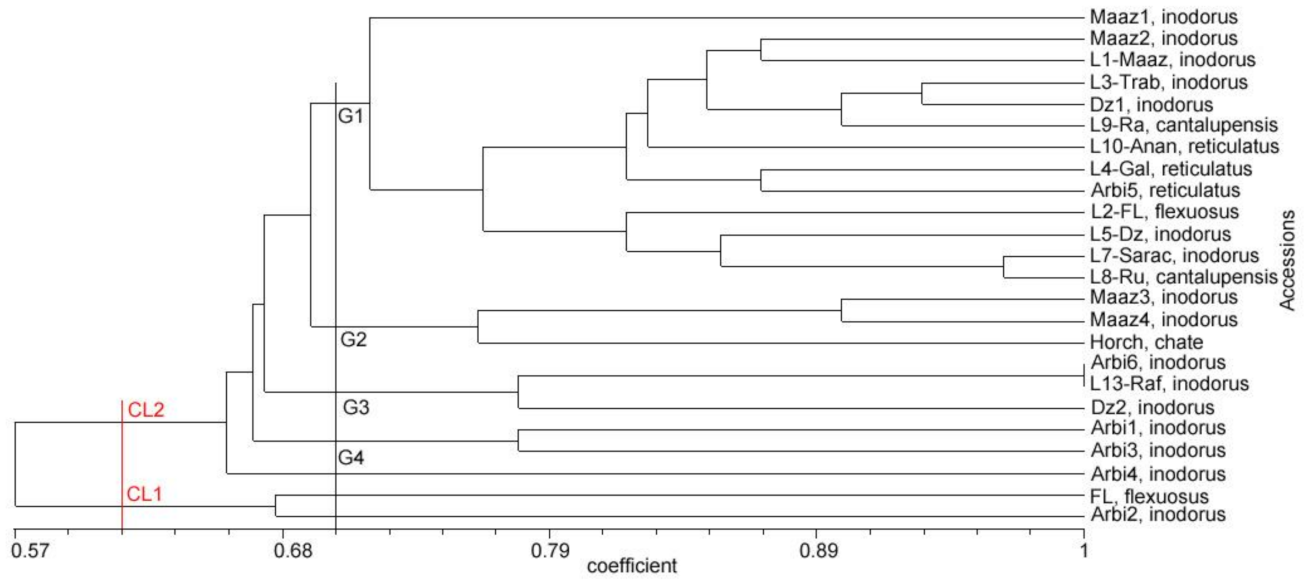
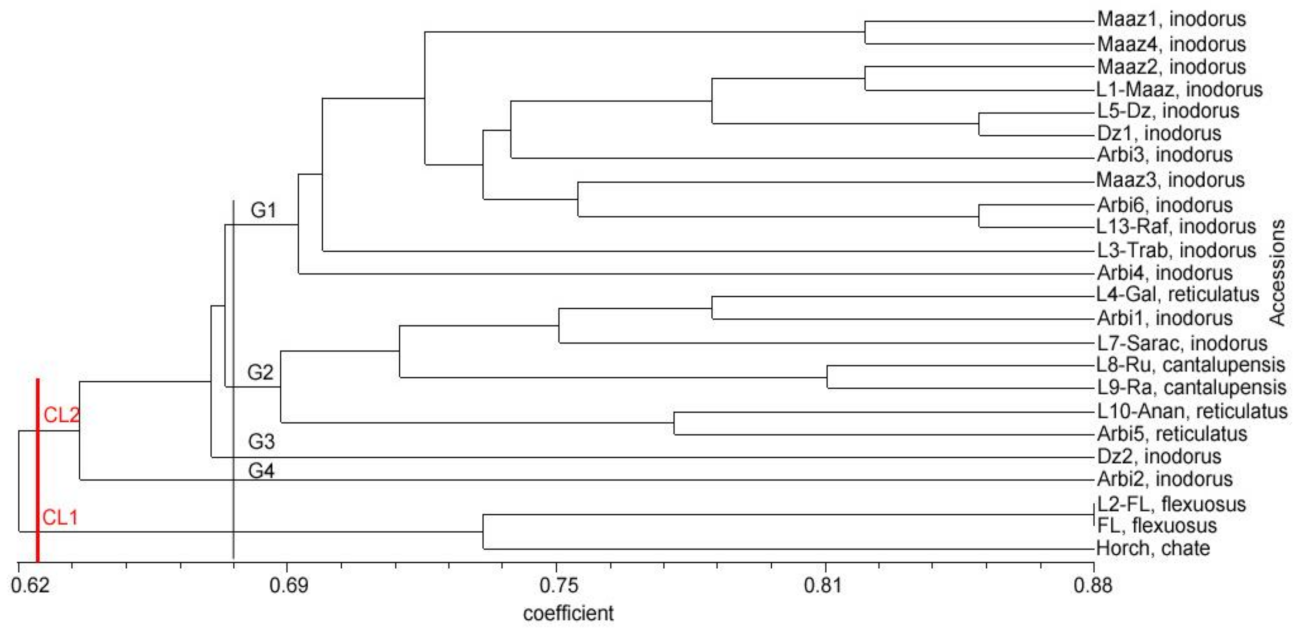
3.4. Relationships and Concordance between Phenotypic and Molecular Markers
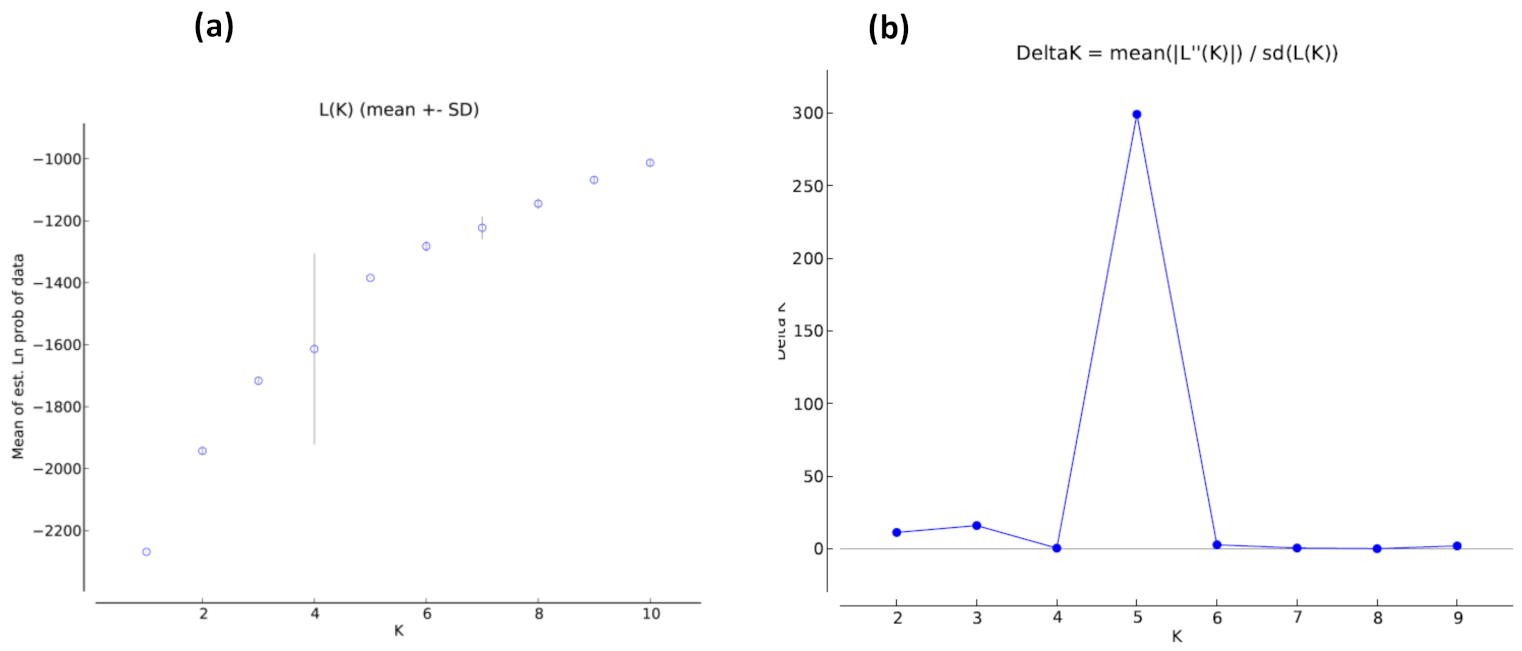
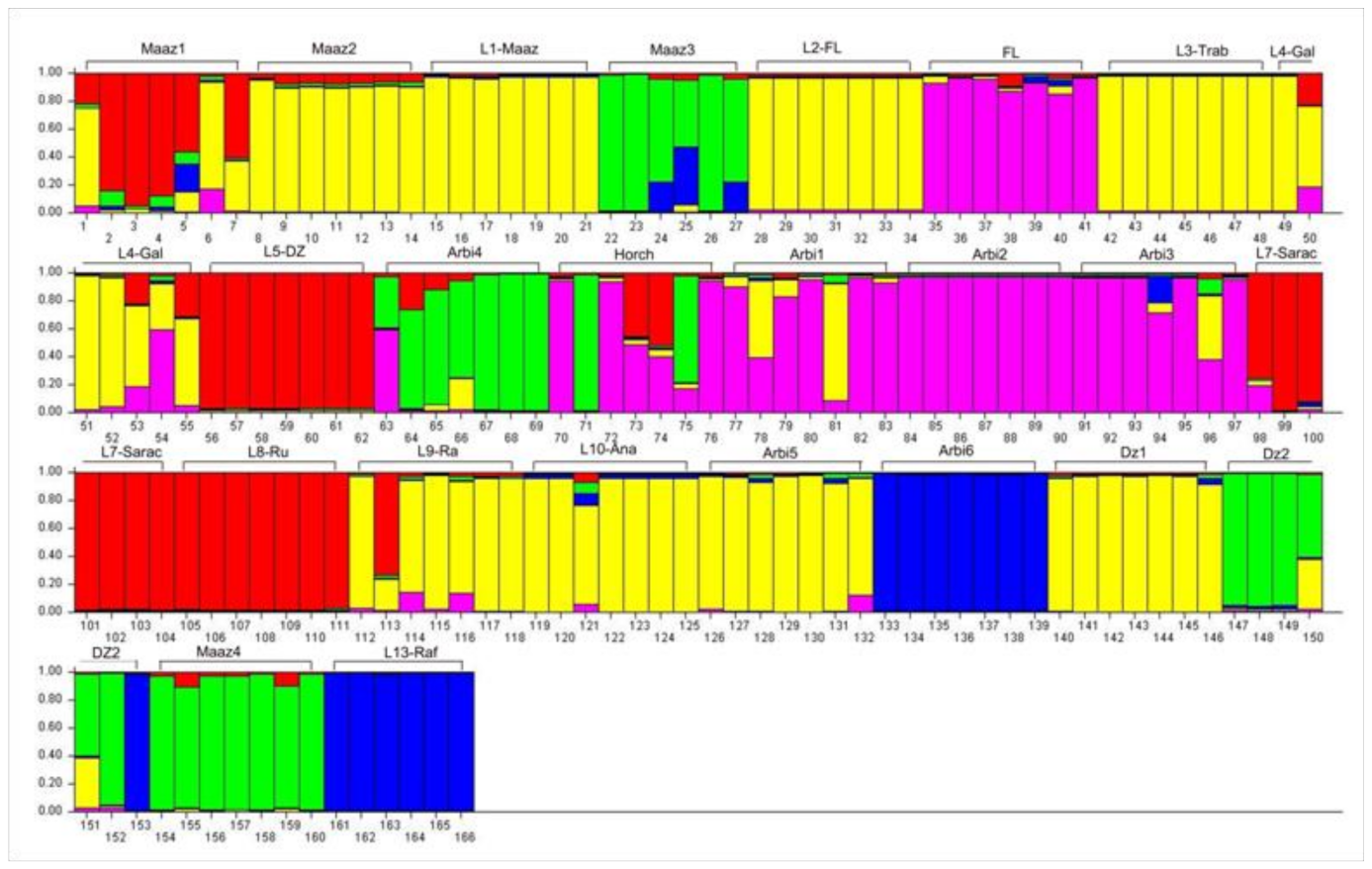
3.5. Population Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitaker, T.W.; Davis, G.N. Cucurbits: Botany, Cultivation and Utilization; Interscience Publishers: New York, NY, USA, 1962; 249p. [Google Scholar]
- Pitrat, M. Melon Genetic Resources: Phenotypic Diversity and Horticultural Taxonomy. In Genetics and Genomics of Cucurbitaceae; Grumet, R., Katzir, N., Garcia-Mas, J., Eds.; Springer: Cham, Switzerland, 2016; Volume 3, pp. 25–60. [Google Scholar]
- McCreight, J.D.; Nerson, H.; Grumet, R. Melon (Cucumis melo L.). In Genetic Improvement of Vegetable Crops, 7th ed.; Kalloo, G., Berch, B.O., Eds.; Pergamon Press: Oxford, UK, 1993. [Google Scholar]
- Chikh-Rouhou, H.; Kacem, K.; Letaief, S.; Sta-Baba, R. Evaluation of Tunisian melon genetic resources to biotic stress under greenhouse conditions. In Proceedings of the XIth International Agriculture Symposium (AGROSYM 2020), Jahorina, East Sarajevo, 8–9 October 2020; Book of abstracts. p. 276. [Google Scholar]
- Chikh-Rouhou, H.; Garcés-Claver, A.; Sta-Baba, R.; González, V.; Daami-Remadi, M. Screening for resistance to race 1 of Fusarium oxysporum f.sp melonis in Tunisian melon cultivars using molecular markers. Comm. Agric. Appl. Biol. Sci. 2018, 83, 87–92. [Google Scholar]
- Chikh-Rouhou, H.; Ben Belgacem, A.M.; Sta-Baba, R.; Tarchoun, N.; Gómez-Guillamón, M.L. New source of resistance to Aphis gossypii in Tunisian melon accessions using phenotypic and molecular marker approaches. Phytoparasitica 2019, 47, 405–413. [Google Scholar] [CrossRef]
- M’nari, M.; Jebari, H.; Cherif, C. Study of strains of cucumber mosaic virus (CMV) on Tunisian melon and identification of resistant genotypes. Cah. Agric. 1993, 2, 60–62. [Google Scholar]
- Chikh-Rouhou, H.; Tlili, I.; Ilahy, R.; Chaar, H.; Sta-Baba, R. Evaluation de la diversité morphologique de quelques accessions locales de melon (Cucumis melo L.). Annales de l’INRAT 2020, 93, 171–183. [Google Scholar]
- Elbekkay, M.; Hamza, H.; Haddad, M.; Ferchichi, A.; Kik, C. Genetic erosion in melon (Cucumis melo): A case study from Tunisia. In Cucurbitaceae 2008, Proceedings of the IXth EUCARPIA Meeting on Genetics and Breeding of Cucurbitaceae, Avignon, France, 21–24 May 2008; Pitrat, M., Ed.; INRA: Avignon, France.
- Escribano, S.; Lazaro, A.; Cuevas, H.E.; Lopez-Sesé, A.I.; Staub, J.E. Spanish melons (Cucumis melo L.) of the Madrid provenance: A unique germplasm reservoir. Genet. Resour. Crop Evol. 2012, 59, 359–373. [Google Scholar] [CrossRef]
- Fergany, M.; Kaur, B.; Monforte, A.J.; Pitrat, M.; Lecoq, C.H.; Dhillon, N.P.S.; Dhaliwal, S.S. Variation in melon (Cucumis melo) landraces adapted to the humid tropics of southern India. Genet. Resour Crop. Evol. 2011, 55, 225–243. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Monforte, A.J.; Arus, P. Phylogenetic relationships among Cucumis species based on the ribosomal internal transcribed spacer sequence and microsatellite markers. Plant. Syst. Evol. 2004, 248, 191–203. [Google Scholar] [CrossRef]
- Dhillon, N.P.S.; Singh, J.; Fergany, M.; Monforte, A.J.; Sureja, A.K. Phenotypic and molecular diversity among landraces of snapmelon (Cucumis melo var. momordica) adapted to the hot and humid tropics of eastern India. Plant Genet. Resour. 2009, 7, 291–300. [Google Scholar] [CrossRef]
- Singh, D.; Leskovar, D.I.; Sharma, S.P.; Sarao, N.K.; Vashisht, V.K. Genetic diversity and inter-relationship among Indian and exotic melons based on fruit morphology, quality components and microsatellite markers. Physiol. Mol. Biol. Plants. 2020, 26, 985–1002. [Google Scholar] [CrossRef]
- Chikh-Rouhou, H.; Tlili, I.; Ilahy, R.; R’him, T.; Sta-Baba, R. Fruit quality assessment and characterization of melon genotypes. Int. J. Veg. Sci. 2021, 27, 3–19. [Google Scholar] [CrossRef]
- Keneni, G.; Jarso, M.; Wolabu, T.; Dino, G. Extent and pattern of genetic diversity for morpho-agronomic traits in Ethiopian highl and pulse landraces II. Faba bean (Vicia faba L.). Genet. Resour. Crop Evol. 2005, 52, 551–561. [Google Scholar] [CrossRef]
- Neuhausen, S.L. Evaluation of restriction fragment length polymorphism in Cucumis melo. Theor. Appl. Genet. 1992, 83, 379–384. [Google Scholar] [CrossRef]
- Lopez-Sesé, A.I.; Staub, J.; Katzir, N.; Gómez-Guillamón, M.L. Estimation of between and within accession variation in selected Spanish melon germplasm using RAPD and SSR markers to assess strategies for large collection evaluation. Euphytica 2003, 127, 41–51. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Seros, M.O.; Gomez-Paniagua, H.; De Vicente, M.C. Comparing AFLP, RAPD and RFLP markers for measuring genetic diversity in melon. Theor. Appl. Genet. 2000, 101, 860–864. [Google Scholar] [CrossRef]
- Merheb, J.; Pawełkowicz, M.; Branca, F.; Bolibok-Bragoszewska, H.; Skarzynska, A.; Plader, A.; Chalak, L. Characterization of Lebanese germplasm of snake melon (Cucumis melo subsp. melo var. flexuosus) using morphological traits and SSR Markers. Agronomy 2020, 10, 1293. [Google Scholar]
- Lakshmana Reddy, D.C.; Sudurashini, K.V.; Anand, C.R.; Aswath, C.; Avinash, K.N.; Nandini, H.; Sreenivasa Rao, E. Genetic diversity and population structure of Indian melon (Cucumis melo L.) landraces with special reference to disease and insect resistance loci. Plant Breed. 2016, 13, 384–390. [Google Scholar] [CrossRef]
- Trimech, R.; Zaouali, Y.; Boulila, A.; Chabchoub, L.; Ghezal, I.; Boussaid, M. Genetic variation in Tunisian melon (Cucumis melo L.) germplasm as assessed by morphological traits. Plant Genet. Resour. 2013, 60, 1621–1628. [Google Scholar] [CrossRef]
- Henane, I.; Slimane, R.; Jebari, H. SSR-based genetic diversity analysis of Tunisian varieties of melon (Cucumis melo L.) and Fakous (Cucumis melo var. flexuosus). Int. J. Adv. Res. 2015, 3, 727–734. [Google Scholar]
- Trimech, R.; Afif, M.; Boussaid, M. Genetic diversity of Tunisian melon (Cucumis melo. L) landraces and their relationships with introduced varieties as assessed by simple-sequence repeat (SSR) markers. Afr. J. Biotec. 2015, 14, 86–95. [Google Scholar]
- SAS. Statistical Analysis System, version V.9.1. SAS/ STAT Users Guide. SAS Publishing: North Carolina, NY, USA, 1990. [Google Scholar]
- Ihaka, R.; Gentleman, R. R: A Language for data analysis and graphics. J. Comput. Graph Stat. 1996, 5, 299–314. [Google Scholar]
- Sokal, R.R.; Michener, C.D. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Rodríguez-Maza, M.J.; Garcés-Claver, A.; Park, S. A versatile PCR marker for pungency in Capsicum spp. Mol. Breed. 2012, 30, 889–898. [Google Scholar] [CrossRef]
- Fukino, N.; Sakata, Y.; Kunihsa, M.; Matsumoto, S. Characterisation of novel simple sequence repeat (SSR) markers for melon (Cucumis melo L.) and their use for genotype identification. J. Hortic. Sci. Biotec. 2007, 82, 330–334. [Google Scholar] [CrossRef]
- Fernandez-Silva, I.; Eduardo, I.; Blanca, J.; Esteras, C.; Pico, B.; Nuez, F.; Arus, P.; Garcia-Mas, J.; Monforte, A.J. Bin mapping of genomic and EST-derived SSRs in melon (Cucumis melo L.). Theor. Appl. Genet. 2008, 118, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Diaz, A.; Fergany, M.; Formisano, G. A consensus linkage map for molecular markers and Quantitative Trait Loci associated with economically important traits in melon (Cucumis melo L.). BMC Plant Biol. 2011, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Mallor, C.; Arnedo-Andrés, M.S.; Garcés-Claver, A. Assessing the genetic diversity of Spanish Allium cepa landraces for onion breeding using microsatellite markers. Sci. Hortic. 2014, 170, 24–31. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skalnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Yildiz, M.; Akgul, N.; Sensoy, S. Morphological and Molecular Characterization of Turkish Landraces of Cucumis melo L. Not. Bot. Horti. Agrobo. 2014, 42, 51–58. [Google Scholar] [CrossRef]
- Mantel, N.A. The detection of disease clustering and generalized regression approach. Cancer Res. 1967, 27, 209–220. [Google Scholar]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics. 2000, 155, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Evanno, G.G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software structure: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Omari, S.; Kamenir, Y.; Benichou, J.I.C.; Pariente, S.; Sela, H.; Perl-Treves, R. Landraces of snake melon, an ancient Middle Eastern crop, reveal extensive morphological and DNA diversity for potential genetic improvement. BMC Genet. 2018, 19, 34. [Google Scholar]
- Chikh-Rouhou, H.; Gómez-Guillamón, M.L.; González, V.; Sta-Baba, R.; Garcés-Claver, A. Cucumis melo L. in Tunisia: Unexploited sources of resistance to Fusarium wilt. Horticulturae 2021. submitted. [Google Scholar]
- Malik, A.A.; Vashisht, V.K.; Singh, K.; Sharma, A.; Singh, D.K.; Singh, H.; Monforte, A.J.; McCreight, J.D.; Dhillon, N.P.S. Diversity among melon (Cucumis melo L.) landraces from the Indo-Gangetic plains of India and their genetic relationship with USA melon cultivars. Genet. Resour. Crop Evol. 2014, 61, 1189–1208. [Google Scholar] [CrossRef]
- Soltani, F.; Akashi, Y.; Kashi, A.; Zamani, Z.; Mostofi, Y.; Kato, K. Characterization of Iranian melon landraces of Cucumis melo L. groups flexuosus and dudaim by analysis of morphological characters and random amplified polymorphic DNA. Breed. Sci. 2010, 60, 34–45. [Google Scholar] [CrossRef][Green Version]
- Aragão, F.A.S.; Torres Filho, J.; Nunes, G.H.S.; Queiróz, M.A.; Bordallo, P.N.; Buso, G.S.C.; Ferreira, M.A.; Costa, Z.P.; Bezerra Neto, F. Genetic divergence among accessions of melon from traditional agriculture of the Brazilian Northeast. Genet. Mol. Res. 2013, 12, 6356–6371. [Google Scholar] [CrossRef]
- Scarano, D.; Rubio, F.; Ruiz, J.J.; Rao, R.; Corrado, G. Morphological and genetic diversity among and within common bean (Phaseolus vulgaris L.) landraces from the Campania region (Southern Italy). Sci. Hortic. 2014, 180, 72–78. [Google Scholar] [CrossRef]
- Tanksley, S.D.; McCouch, S.R. Seed banks and molecular maps: Unlocking genetic potential from the wild. Science 1997, 277, 1063–1066. [Google Scholar] [CrossRef]
- Jianbin, H.; Panqiao, W.; Qiong, L.; Yan, S. Microsatellite analysis of genetic relationships between wild and cultivated melons in Northwest and Central China. Mol. Biol. Rep. 2014, 41, 7723–7728. [Google Scholar]
- Stone, S.; Boyhan, G. Inter- and intra cultivar variation of heirloom and open-pollinated watermelon cultivars. HortScience 2019, 54, 212–220. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Rosenberg, N.A. On the size distribution of private microsatellite alleles. Theor. Popul. Biol. 2011, 80, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kalinowski, S.T. Counting alleles with rarefaction: Private alleles and hierarchical sampling designs. Conserv. Genet. 2004, 5, 539–543. [Google Scholar] [CrossRef]
- Yildiz, M.; Ekbicb, E.; Kelesc, D.; Sensoy, S.; Abak, K. Use of ISSR, SRAP, and RAPD markers to assess genetic diversity in Turkish melons. Sci. Hortic. 2011, 130, 349–353. [Google Scholar] [CrossRef]
- Sensoy, S.; Buyukalaca, S.; Abak, K. Evaluation of genetic diversity in Turkish melons (Cucumis melo L.) based on phenotypic characters and RAPD markers. Genet.Resour.Crop Evol. 2007, 54, 1351–1356. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea (Vignaun guiculata L.Walp.) germplasm collections using phenotypic traits and SNP markers. BMC Genet. 2020, 21, 110–126. [Google Scholar] [CrossRef] [PubMed]
- Schaal, B.A.; Hayworth, D.A.; Olsen, K.M.; Rauscher, J.T.; Smith, W.A. Phylogeographic studies in plants: Problems and prospects. Molec. Ecol. 1998, 7, 465–474. [Google Scholar] [CrossRef]
- Guidoti, D.T.; Gonela, A.; Vidigal, M.C.; Conrado, T.V.; Romani, I. Interrelationship between morphological, agronomic and molecular characteristics in the analysis of common bean genetic diversity. Acta Sci. Agron. 2018, 40, 1–9. [Google Scholar] [CrossRef]
| Code | Local Name | Locality Origin | Horticultural Group | Type | Description |
|---|---|---|---|---|---|
| Maaz1 | Maazoun | Chott Mariem-Sousse | inodorus | Landrace | Sweet fruit without aroma; yellow skin |
| Maaz2 | Maazoun | Menzel Chaker-Sfax | inodorus | Landrace | Sweet fruit without aroma, yellow-green skin |
| Maaz3 | Maazoun | Wardanine-Monastir | inodorus | Landrace | Sweet fruit without aroma; yellow-green skin |
| Maaz4 | Maazoun | Sidi Bouzid | inodorus | Landrace | Sweet fruit without aroma; yellow-green skin |
| Arbi1 | Arbi | Jammel-Monastir | inodorus | Landrace | Sweet fruit without aroma, yellow skin |
| Arbi2 | Arbi | Jendouba | inodorus | Landrace | Fruit without aroma; creamy-white skin |
| Arbi3 | Arbi | Jammel-Monastir | inodorus | Landrace | Sweet fruit without aroma; orange skin |
| Arbi4 | Arbi | Jammel-Monastir | inodorus | Landrace | Sweet fruit without aroma; yellow skin |
| Arbi5 | Arbi | Kairouan | reticulatus | Landrace | Fruit with aroma, netted skin |
| Arbi6 | Arbi | El Jem-Mahdia | inodorus | Landrace | Sweet fruit without aroma, yellow skin |
| Dz1 | Dziri | Manzel Kamel-Monastir | inodorus | Landrace | Sweet fruit without aroma; light-green skin |
| Dz2 | Dziri-arbi | Sidi Banour-Mahdia | inodorus | Landrace | Sweet fruit without aroma of light-green color |
| FL | Fakous Salem | Wardanine- Monastir | flexuosus | Landrace | Elongated fruit, nonsweet, eaten raw before ripeness |
| Horch | Harchay | Wardanine- Monastir | chate | Landrace | Fruit small-sized, nonsweet, eaten raw before ripeness |
| L1-Maaz | Maazoun | - | inodorus | Breeding line | Sweet fruit without aroma, skin of yellow-green color |
| L2-FL | Fakous | - | flexuosus | Breeding line | Elongated fruit, nonsweet, eaten raw before ripeness |
| L3-Trab | Trabelsi | - | inodorus | Breeding line | Sweet fruit without aroma of orange color |
| L4-Gal | Galaoui | - | reticulatus | Breeding line | Sweet fruit with aroma, netted/corked skin |
| L5-Dz | Dziri | - | inodorus | Breeding line | Sweet fruit without aroma; light-green skin |
| L7-Sarac | Sarachika | - | inodorus | Breeding line | Sweet Fruit without aroma; yellow skin |
| L8-Ru | RD | - | cantalupensis | Breeding line | Sweet fruit with aroma and grooves |
| L9-Ra | Rupa | - | cantalupensis | Breeding line | Sweet fruit with aroma and grooves |
| L10-Anan | Ananas | - | reticulatus | Breeding line | Sweet fruit with aroma, netted skin |
| L13-Raf | V4 | - | inodorus | Breeding line | Sweet fruit without aroma; orange skin |
| DM (days) | SD (mm) | FN | SL (cm) | FW (g) | FL (cm) | FD (cm) | CD (cm) | FT (cm) | TSS (°Brix) | SN | 100-SW (g) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| inodorus group | ||||||||||||
| Maaz1 | 139.3 ± 4.6 | 9.4 ± 1.1 | 3.2 ± 0.2 | 178.3 ± 17.5 | 881.0 ± 190.7 | 13.0 ± 2.1 | 11.1 ± 1.4 | 4.9 ± 0.7 | 2.7 ± 0.5 | 9.8 ± 0.6 | 410.7 ± 85.9 | 5.5 ± 2.5 |
| Maaz2 | 150.0 ± 6.92 | 7.3 ± 0.5 | 2.4 ± 0.5 | 168.7 ± 28.2 | 1063.4 ± 101.8 | 14.8 ± 1.1 | 13.5 ± 0.8 | 5.4 ± 1.5 | 4.5 ± 1.1 | 10.2 ± 1.1 | 767.7 ± 193.4 | 6.5 ± 0.3 |
| Maaz3 | 139.3 ± 4.61 | 7.0 ± 1.1 | 3.2 ± 0.5 | 112.3 ± 6.8 | 735.8 ± 56.6 | 11.2 ± 0.8 | 10.6 ± 1.1 | 4.5 ± 0.5 | 3.2 ± 0.7 | 10.9 ± 0.5 | 393.3 ± 84.1 | 6.0 ± 0.5 |
| Maaz4 | 133.0 ± 1.73 | 7.5 ± 0.9 | 3.1 ± 0.4 | 158.0 ± 15.8 | 845.3 ± 75.1 | 12.0 ± 1.2 | 9.8 ± 0.2 | 4.2 ± 0.8 | 2.6 ± 0.2 | 9.5 ± 0.6 | 404.7 ± 53.86 | 5.1 ± 0.6 |
| Arbi1 | 137.3 ± 8.08 | 9.0 ± 2.5 | 2.0 ± 1.0 | 180.3 ± 6.5 | 915.5 ± 220.9 | 14.1 ± 2.2 | 10.2 ± 1.9 | 5.7 ± 0.9 | 2.4 ± 0.6 | 9.8 ± 0.1 | 693.3 ± 110.1 | 7.5 ± 0.3 |
| Arbi2 | 136.7 ± 4.61 | 12.6 ± 0.5 | 2.1 ± 0.8 | 197.7 ± 17.7 | 544.7 ± 62.4 | 11.9 ± 1.6 | 9.4 ± 0.5 | 5.3 ± 0.8 | 2.2 ± 0.4 | 8.4 ± 0.2 | 428.3 ± 143.5 | 7.3 ± 0.5 |
| Arbi3 | 135.3 ± 12.7 | 11.3 ± 1.2 | 2.7 ± 0.3 | 172.3 ± 14.7 | 831.8 ± 40.5 | 16.8 ± 6.2 | 10.7 ± 0.5 | 5.6 ± 0.4 | 2.5 ± 0.2 | 11.2 ± 1.1 | 571.7 ± 110.1 | 6.9 ± 0.8 |
| Arbi4 | 146.0 ± 10.5 | 8.9 ± 1.5 | 2.7 ± 1.2 | 146.0 ± 20.1 | 1035.1 ± 111.9 | 15.9 ± 2.5 | 11.3 ± 1.7 | 5.6 ± 0.2 | 2.8 ± 0.3 | 11.1 ± 1.0 | 529.3 ± 52.5 | 6.4 ± 1.2 |
| Arbi6 | 130.0 ± 3.46 | 9.5 ± 1.1 | 3.0 ± 0.3 | 136.0 ± 22.5 | 955.5 ± 102.3 | 17.1 ± 2.6 | 11.4 ± 0.9 | 5.8 ± 0.5 | 2.6 ± 1.0 | 11.5 ± 1.5 | 500.0 ± 97.7 | 7.2 ± 0.2 |
| Dz1 | 154.0 ± 3.26 | 10.3 ± 0.8 | 2.4 ± 0.5 | 171.0 ± 28.8 | 823.2 ± 67.5 | 18.5 ± 4.5 | 11.1 ± 2.1 | 5.1 ± 0.5 | 3.1 ± 0.7 | 11.3 ± 1.4 | 415.0 ± 99.1 | 5.1 ± 0.9 |
| Dz2 | 150.0 ± 6.92 | 8.5 ± 1.2 | 2.3 ± 0.3 | 177.3 ± 11.6 | 702.0 ± 70.1 | 11.5 ± 0.3 | 9.8 ± 1.6 | 5.5 ± 0.4 | 2.3 ± 0.7 | 9.0 ± 1.1 | 414.7 ± 53.5 | 6.6 ± 0.8 |
| L1-Maaz | 150.3 ± 14.8 | 9.9 ± 0.8 | 2.1 ± 0.7 | 179.7 ± 6.5 | 971.7 ± 204.5 | 12.3 ± 1.6 | 12.0 ± 1.4 | 5.1 ± 1.4 | 3.7 ± 0.6 | 11.0 ± 1.9 | 596.7 ± 66.3 | 6.6 ± 0.4 |
| L3-trab | 134.7 ± 7.02 | 9.3 ± 1.1 | 2.4 ± 0.7 | 213.3 ± 18.4 | 801.4 ± 200.9 | 20.0 ± 9.2 | 9.0 ± 1.6 | 4.4 ± 0.8 | 2.5 ± 0.5 | 9.7 ± 0.3 | 622.0 ± 80.0 | 6.7 ± 1.5 |
| L5-Dz | 147.3 ± 11.5 | 10.4 ± 0.7 | 2.7 ± 0.6 | 158.3 ± 17.5 | 1050.9 ± 38.9 | 16.9 ± 3.2 | 10.6 ± 2.1 | 5.8 ± 1.2 | 2.6 ± 1.0 | 9.6 ± 0.9 | 955.3 ± 171.7 | 4.1 ± 0.9 |
| L7-Sarac | 149.0 ± 12.1 | 7.7 ± 1.2 | 2.7 ± 0.1 | 127.3 ± 8.5 | 790.6 ± 124.8 | 12.1 ± 2.8 | 11.1 ± 2.0 | 4.5 ± 0.5 | 3.1 ± 0.3 | 9.8 ± 0.5 | 466.0 ± 66.9 | 7.5 ± 1.0 |
| L13-Raf | 159.0 ± 3.4 | 8.7 ± 0.6 | 2.3 ± 0.3 | 136.0 ± 12.3 | 1373.0 ± 110.7 | 13.9 ± 1.8 | 13.90.8 ± | 6.7 ± 0.6 | 3.8 ± 0.3 | 11.5 ± 1.3 | 485.3 ± 77.1 | 9.5 ± 1.2 |
| cantalupensis group | ||||||||||||
| L8-Ru | 132.0 ± 3.5 | 6.8 ± 1.0 | 1.9 ± 1.0 | 92.7 ± 8.7 | 436.1 ± 67.4 | 9.8 ± 0.6 | 9.7 ± 0.7 | 4.2 ± 0.2 | 3.3 ± 0.4 | 9.7 ± 0.7 | 384.7 ± 86.4 | 6.0 ± 0.5 |
| L9-Ra | 139.3 ± 9.2 | 10.5 ± 1.3 | 2.2 ± 0.9 | 175.7 ± 25.01 | 1101.1 ± 48.5 | 13.1 ± 0.9 | 11.2 ± 2.0 | 5.7 ± 0.6 | 2.9 ± 0.3 | 11.8 ± 1.1 | 382.3 ± 67.1 | 6.9 ± 0.8 |
| reticulatus group | ||||||||||||
| Arbi5 | 130.0 ± 3.4 | 6.5 ± 1.0 | 2.4 ± 0.9 | 195.7 ± 15.2 | 1489.6 ± 238.0 | 29.4 ± 9.2 | 12.9 ± 3.0 | 6.5 ± 1.8 | 2.9 ± 0.6 | 8.8 ± 0.4 | 421.3 ± 82.6 | 10.7± 1.5 |
| L4-Gal | 142.0 ± 8.0 | 12.4 ± 2.2 | 2.7 ± 0.8 | 133.7 ± 14.16 | 1025.4 ± 123.1 | 14.1 ± 0.7 | 11.41.3 ± | 5.9 ± 0.6 | 2.7 ± 0.4 | 10.1 ± 0.4 | 674.0 ± 68.8 | 6.7 ± 0.4 |
| L10-Anan | 144.7 ± 9.2 | 9.4 ± 1.5 | 1.8 ± 0.5 | 196.7 ± 21.3 | 1765.2 ± 259.7 | 16.7 ± 3.9 | 12.4 ± 3.5 | 5.5 ± 1.5 | 2.9 ± 0.8 | 9.5 ± 0.2 | 410.7 ± 70.6 | 5.9 ± 1.2 |
| flexuosus group | ||||||||||||
| L2-FL | 103.3 ± 4.1 | 11.0 ± 0.6 | 35.0 ± 5.0 | 190.0 ± 25.8 | 204.9 ± 32.7 | 29.3 ± 3.1 | 4.2 ± 1.1 | 4.0 ± 1.0 | 1.9 ± 0.5 | 4.9 ± 0.6 | 301.0 ± 35.5 | 5.6 ± 0.6 |
| FL | 105.3 ± 5.5 | 11.0 ± 0.5 | 36.7 ± 6.0 | 226.7 ± 25.2 | 203.3 ± 15.3 | 38.3 ± 3.5 | 4.4 ± 0.5 | 3.7 ± 1.1 | 2.0 ± 0.5 | 4.7 ± 0.5 | 213.0 ± 24.1 | 5.3 ± 0.5 |
| chate group | ||||||||||||
| Horch | 103.3 ± 3.0 | 7.3 ± 0.6 | 8.0 ± 1.0 | 112.3 ± 6.8 | 84.0 ± 16.4 | 4.3 ± 0.5 | 3.0 ± 0.3 | 1.9 ± 0.5 | 0.5 ± 0.1 | 7.4 ± 0.4 | 197.3 ± 18.3 | 2.7 ± 0.3 |
| CV | 5.5 | 11.9 | 37.6 | 18.6 | 41.9 | 46.5 | 17.7 | 22.5 | 23.1 | 10.6 | 36.3 | 25.4 |
| F value | 11.5 ** | 7.4 ** | 92.4 ** | 3.6 ** | 3.9 ** | 9.61 ** | 6.8 ** | 2.4 * | 4.4 ** | 9.3 ** | 2.46 * | 2.66 * |
| SSR Locus | Ch | Primer Sequences | N | Size Range (bp) | G | Ho | He | I | Fis | PIC |
|---|---|---|---|---|---|---|---|---|---|---|
| CM38 | 10 | F:TAGCATCTGATCGGAAAACC R:CAACTTCATCCGCCAAGAAT | 3 | 134–144 | 6 | 0.17 | 0.19 | 0.96 | 0.06 | 0.57 |
| ECM58 | 1 | F:TTGAAGCTTCTTCACCTTCTCTTT R:CACCCCACAAGGGTTCAATA | 5 | 132–157 | 11 | 0.19 | 0.23 | 1.50 | 0.18 | 0.76 |
| CSWCT10 | 3 | F:AGATCGGAATTGAAAAAG R:AAAGGGGCTTCCTCTCTA | 3 | 197–206 | 4 | 0.05 | 0.03 | 0.26 | −0.68 | 0.13 |
| ECM204 | 7 | F:CTCTCTTCATTTCCCCTCGTT R:TGGCCTGGAAAGTAAGGGTAT | 2 | 330–346 | 3 | 0.01 | 0.02 | 0.14 | 0.21 | 0.06 |
| MU7194 | 4 | F:TACTCCTCGCTGATCTTCCC R:AAAGGAAGAAGCGCACAAAA | 7 | 117–153 | 12 | 0.13 | 0.22 | 1.48 | 0.42 | 0.71 |
| ECM132 | 6 | F:CCATTCGTCAAGCAAAGCTAC R:CAAAGGGTCCCTCAATTTCTC | 2 | 292–295 | 3 | 0.10 | 0.09 | 0.48 | −0.06 | 0.30 |
| CMN04_19 | 9 | F:TTCTTCCCACCAAACCTACG R:AAATGGCAGAGAGCGAGAAA | 6 | 238–262 | 11 | 0.08 | 0.14 | 1.31 | 0.48 | 0.66 |
| CMCT160a | 11 | F:GTCTCTCTCCCTTATCTTCCA R:ACGGTGTTTGGTGTGAGAAG | 2 | 231–233 | 3 | 0.04 | 0.04 | 0.43 | −0.27 | 0.26 |
| Total | 30 | ------ | 53 | ----- | ----- | ------ | ----- | ------ | ||
| Mean | 3.75 | ------ | ------ | 0.10 | 0.12 | 0.82 | 0.04 | 0.43 |
| SV | df | SS | MS | Est.var | PV (%) | p-Value |
|---|---|---|---|---|---|---|
| Among populations | 23 | 794.763 | 34.555 | 4.763 | 75 | 0.01 |
| Within populations | 142 | 229.333 | 1.615 | 1.615 | 25 | 0.01 |
| Total | 165 | 1024.096 | 6.378 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chikh-Rouhou, H.; Mezghani, N.; Mnasri, S.; Mezghani, N.; Garcés-Claver, A. Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers. Agronomy 2021, 11, 1121. https://doi.org/10.3390/agronomy11061121
Chikh-Rouhou H, Mezghani N, Mnasri S, Mezghani N, Garcés-Claver A. Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers. Agronomy. 2021; 11(6):1121. https://doi.org/10.3390/agronomy11061121
Chicago/Turabian StyleChikh-Rouhou, Hela, Najla Mezghani, Sameh Mnasri, Neila Mezghani, and Ana Garcés-Claver. 2021. "Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers" Agronomy 11, no. 6: 1121. https://doi.org/10.3390/agronomy11061121
APA StyleChikh-Rouhou, H., Mezghani, N., Mnasri, S., Mezghani, N., & Garcés-Claver, A. (2021). Assessing the Genetic Diversity and Population Structure of a Tunisian Melon (Cucumis melo L.) Collection Using Phenotypic Traits and SSR Molecular Markers. Agronomy, 11(6), 1121. https://doi.org/10.3390/agronomy11061121







