Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions
Abstract
1. Introduction
2. Photosynthesis of Giant Reed in Optimal Growth Conditions
3. Photosynthesis of Giant Reed under Drought Conditions
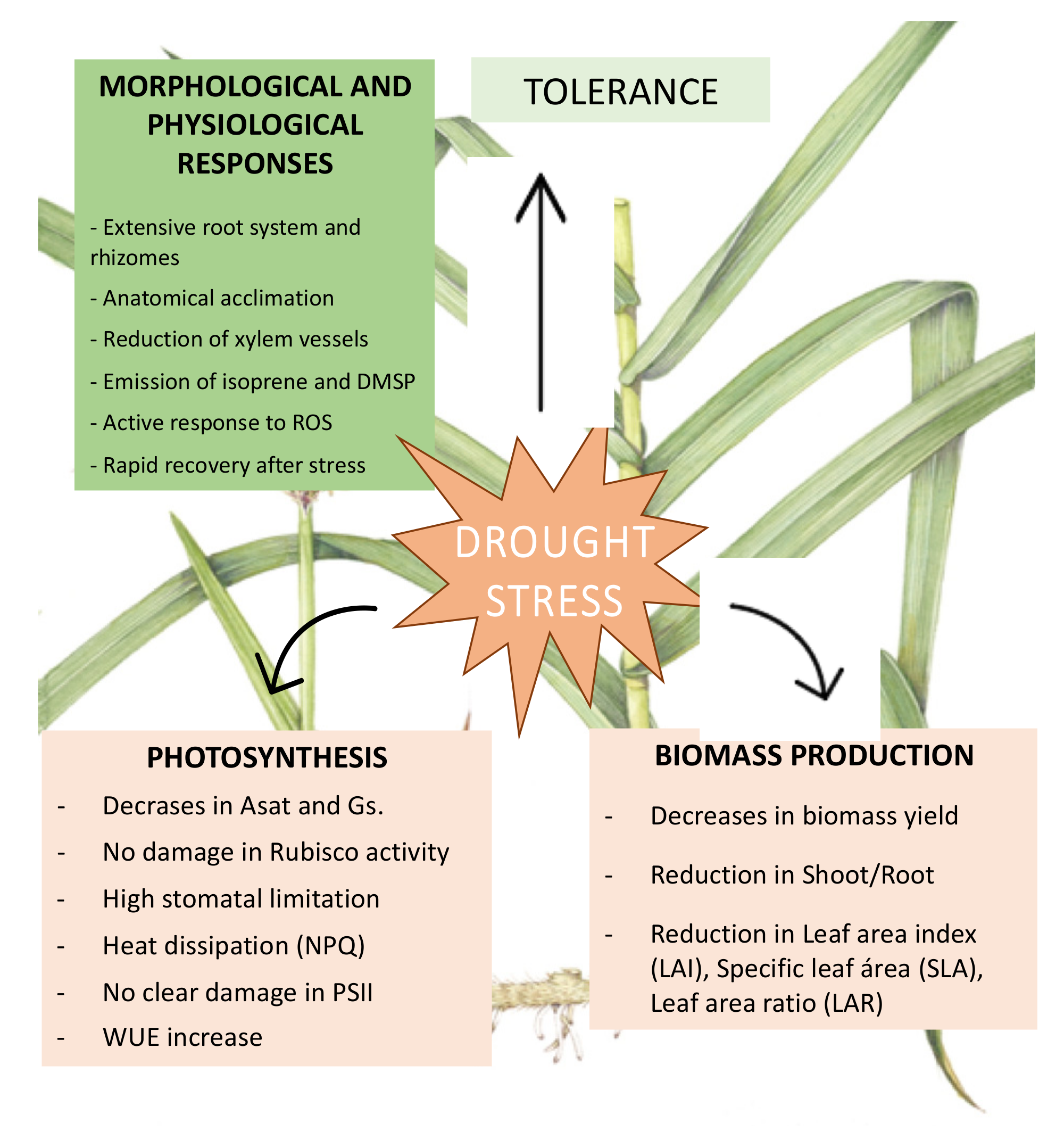
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perdue, R.E. Arundo donax—Source of musical reeds and industrial cellulose. Econ. Bot. 1958, 12, 368–404. [Google Scholar] [CrossRef]
- Mariani, C.; Cabrini, R.; Danin, A.; Piffanelli, P.; Fricano, A.; Gomarasca, S.; Dicandilo, M.; Grassi, F. Origin, diffusion and reproduction of the giant reed (Arundo donax L.): A promising weedy energy crop. Ann. Appl. Biol. 2010, 157, 191–202. [Google Scholar] [CrossRef]
- Amaducci, S.; Perego, A. Field evaluation of Arundo donax clones for bioenergy production. Ind. Crop. Prod. 2015, 75, 122–128. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass. Bioener. 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Mack, R.N. Evaluating the credits and debits of a proposed biofuel species: Giant reed (Arundo donax). Weed Sci. 2008, 56, 883–888. [Google Scholar] [CrossRef]
- Ren, L.; Eller, F.; Lambertini, C.; Guo, W.Y.; Brix, H.; Sorrel, B.K. Assessing nutrient responses and biomass quality for selection of appropriate paludiculture crops. Sci. Total Environ. 2019, 664, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Borin, M.; Barbera, A.C.; Milani, M.; Molari, G.; Zimbone, S.M.; Toscano, A. Biomass production and N balance of giant reed (Arundo donax L.) under high water and N input in Mediterranean environments. Eur. J. Agron. 2013, 51, 117–119. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Copani, V.; D’Agosta, G.M.; Sanzone, E.; Mantineo, M. First results on evaluation of Arundo donax L. clones collected in Southern Italy. Ind. Crop. Prod. 2006, 23, 212–222. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Scordia, D.; Sanzone, E.; Testa, G.; Copani, V. Response of giant reed (Arundo donax L.) to nitrogen fertilization and soil water availability in semi-arid Mediterranean environment. Eur. J. Agron. 2014, 60, 22–32. [Google Scholar] [CrossRef]
- Cosentino, S.L.; Patanè, C.; Sanzone, E.; Testa, G.; Scordia, D. Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 2016, 72, 56–69. [Google Scholar] [CrossRef]
- Mantineo, M.; D’Agosta, G.M.; Copani, V.; Patanè, C.; Cosentino, S.L. Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crop Res. 2009, 114, 204–213. [Google Scholar] [CrossRef]
- Nassi o Di Nasso, N.; Roncucci, N.; Bonari, E. Seasonal dynamics of aboveground and belowground biomass and nutrient accumulation and remobilization in giant reed (Arundo donax L.): A three-year study on marginal land. Bioenergy Res. 2013, 6, 725–736. [Google Scholar] [CrossRef]
- Impagliazzo, A.; Mori, M.; Fiorentino, N.; Di Mola, I.; Ottaiano, L.; De Gianni, D.; Nocerino, S.; Fagnano, M. Crop growth analysis and yield of a lignocellulosic biomass crop (Arundo donax L.) in three marginal areas of Campania region. Ital. J. Agron. 2017, 12, 7. [Google Scholar] [CrossRef]
- Dahl, J.; Obernberger, I. Evaluation of the combustion characteristics of four perennial energy crops (Arundo donax, Cynara cardunculus, Miscanthus x Giganteus and Panicum virgatum). In Proceedings of the 2nd World Conference on Biomass for Energy, Industry and Climate Protection, Rome, Italy, 10–14 May 2004. [Google Scholar]
- Pilu, R.; Bucci, A.; Badone, F.C.; Landoni, M. Giant reed (Arundo donax L.): A weed plant or a promising energy crop? Afr. J. Biotechnol. 2012, 11, 9163–9174. [Google Scholar] [CrossRef]
- Maishanu, S.M.; Hussani, H.B.N. Studies on factors affecting biogas generation from Pistia stratiote. In Proceedings of the 32nd Annual Conference of Nigeria Society for Microbiology, Online Conference, 26–30 April 2021; University of Ilorin: Ilorin, Nigeria, 1991. [Google Scholar]
- Jones, M.B.; Finnan, J.; Hodkinson, T.R. Morphological and physiological traits for higher biomass production in perennial rhizomatous grasses grown on marginal land. GCB Bioenergy 2015, 7, 375–385. [Google Scholar] [CrossRef]
- Sage, R.F. The evolution of C4 photosynthesis. New Phytol. 2004, 161, 341–370. [Google Scholar] [CrossRef] [PubMed]
- Rossa, B.; Rüffers, A.V.; Naidoo, G.; von Willert, D.J. Arundo donax L. (Poaceae)—A C3 species with unusually high photosynthetic capacity. Plant Biol. 1998, 111, 216–221. [Google Scholar] [CrossRef]
- Sánchez, E.; Gil, S.; Azcón-Bieto, J.; Nogués, S. The response of Arundo donax L. (C3) and Panicum virgatum (C4) to different stresses. Biomass. Bioenergy 2016, 85, 335–345. [Google Scholar] [CrossRef]
- Haworth, M.; Cosentino, S.L.; Marino, G.; Brunetti, C.; Scordia, D.; Testa, G.; Riggi, E.; Avola, G.; Loreto, F.; Centritto, M. Physiological responses of Arundo donax ecotypes to drought: A common garden study. GCB Bioenergy 2017, 9, 132–143. [Google Scholar] [CrossRef]
- Haworth, M.; Catola, S.; Marino, G.; Brunetti, C.; Michelozzi, M.; Riggi, E.; Avola, G.; Cosentino, S.L.; Loreto, F.; Centritto, M. Moderate drought stress induces increased foliar dimethylsulphoniopropionate (DMSP) concentration and isoprene emission in two contrasting ecotypes of Arundo donax. Front. Plant Sci. 2017, 8, 1016. [Google Scholar] [CrossRef]
- Mann, J.J.; Barney, J.N.; Kyser, G.B.; Di Tomaso, J.M. Miscanthus x giganteus and Arundo donax shoot and rhizome tolerance of extreme moisture stress. GCB Bioenergy 2013, 5, 693–700. [Google Scholar] [CrossRef]
- Webster, R.J.; Driever, S.M.; Kromdijk, J.; McGrath, J.; Leakey, A.D.B.; Siebke, K.; Demetriades-Shah, T.; Bonnage, S.; Peloe, T.; Lawson, T.; et al. High C3 photosynthetic capacity and high intrinsic water use efficiency underlies the high productivity of the bioenergy grass Arundo Donax. Sci. Rep. 2016, 6, 20694. [Google Scholar] [CrossRef] [PubMed]
- Romero-Munar, A.; Baraza, E.; Cifre, J.; Achir, C.; Gulías, J. Leaf plasticity and stomatal regulation determines the ability of Arundo donax plantlets to cope with water stress. Photosynthetica 2018, 56, 698–706. [Google Scholar] [CrossRef]
- Erickson, J.E.; Soikaew, A.; Sollenberger, L.E.; Bennet, J.M. Water use and water-use efficiency of three perennial bioenergy grass crops in Florida. Agriculture 2012, 2, 325–338. [Google Scholar] [CrossRef]
- Nackley, L.L.; Vogt, K.A.; Kim, S.H. Arundo donax water use and photosynthetic responses to drought and elevated CO2. Agr. Water Manag. 2014, 136, 13–22. [Google Scholar] [CrossRef]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and water stress effects on biomass production in different Arundo donax L. clones. Bioenergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef]
- Sánchez, E.; Lino, G.; Arias, C.; Serrat, X.; Nogués, S. Photosynthesis, resource acquisition and growth responses of two biomass crops subjected to water stress. J. Plant Sci. 2018, 6, 68–86. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Della Rocca, G.; Centritto, M.; Parenti, A.; Monti, A. Giant reed genotypes from temperate and arid environments show different response mechanisms to drought. Physiol. Plant. 2018, 163, 490–501. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Riggi, E.; Avola, G.; Brunetti, C.; Scordia, D.; Testa, G.; Gomes, M.T.G.; Loreto, F.; Cosentino, S.L.; et al. The effect of summer drought on the yield of Arundo donax is reduced by the retention of photosynthetic capacity and leaf growth later in the growing season. Ann. Bot. 2019, 124, 567–579. [Google Scholar] [CrossRef]
- Papazoglou, E.G.; Karantounias, G.A.; Vemmos, S.N.; Bouranis, D.L. Photosynthesis and growth responses of giant reed (Arundo donax L.) to heavy metals Cd and Ni. Environ. Int. 2005, 31, 243–249. [Google Scholar] [CrossRef]
- Triana, F.; Nassi o Di Nasso, N.; Ragaglini, G.; Roncucci, N.; Bonari, E. Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthus x giganteus Greed et Deu.) in a Mediterranean environment. GCB Bioenergy 2015, 7, 811–819. [Google Scholar] [CrossRef]
- Mohr, H.; Schopfer, P. Plant Physiology; Springer: Berlin, Germany, 1995; p. 629. [Google Scholar]
- Dohleman, F.G.; Heaton, E.A.; Leakey, A.D.B.; Long, S.P. Does greater leaf-level photosynthesis explain the larger solar energy conversion efficiency of Miscanthus relative to switchgrass? Plant. Cell Environ. 2009, 32, 1525–1537. [Google Scholar] [CrossRef]
- Wullschleger, S.D. Biochemical limitations to carbon assimilation in C3 plants—A retrospective analysis of the A/Ci curves from 109 species. J. Exp. Bot. 1993, 44, 907–920. [Google Scholar] [CrossRef]
- Osborne, B.A.; Garrett, M.K. Quantum yields for CO2 uptake in some diploid and tetraploid plant species. Plant. Cell Environ. 1983, 6, 135–144. [Google Scholar] [CrossRef]
- Long, S.P.; Postl, W.F.; Bolhàr-Nordenkampf, H.R. Quantum yields for uptake of carbon-dioxide in C-3 vascular plants of contrasting habitats and taxonomic groupings. Planta 1993, 189, 226–234. [Google Scholar] [CrossRef]
- Hidalgo, M.; Fernandez, J. Biomass production of ten populations of giant reed (Arundo donax L.) under the environmental conditions of Madrid (Spain). In Biomass for Energy and Industry, Proceeding of the First World Conference, Sevilla, Spain, 5–9 June 2000; Kyritsis, S., Beenackers, A.A.C.M., Helm, P., Grassi, A., Chiaramonti, D., Eds.; James & James (Science Publishers) Ltd.: London, UK, 2000; pp. 1881–1884. [Google Scholar]
- Nazli, R.I.; Tansi, V.; Öztürk, H.H.; Kusvuran, A. Miscanthus, switchgrass, giant reed and bulbous canary grass as potential bioenergy crops in a semi-arid Mediterranean environment. Ind. Crop. Prod. 2018, 125, 9–23. [Google Scholar] [CrossRef]
- Ahmad, R.; Liow, P.S.; Spencer, D.F.; Jasieniuk, M. Molecular evidence for a single genetic clone of invasive Arundo donax in the United States. Aquat. Bot. 2008, 88, 113–120. [Google Scholar] [CrossRef]
- Hardion, L.; Verlaque, R.; Saltonstall, K.; Leriche, A.; Vila, B. Origin of the invasive Arundo donax (Poaceae): A trans-Asian expedition in herbaria. Ann. Bot. 2014, 114, 455–462. [Google Scholar] [CrossRef]
- Pilu, R.; Cassani, E.; Landoni, M.; Badone, F.C.; Passera, A.; Cantaluppi, E.; Corno, L.; Adani, F. Genetic characterization of an Italian Giant Reed (Arundo donax L.) clones collection: Exploiting clonal selection. Euphytica 2014, 162. [Google Scholar] [CrossRef]
- Touchell, D.H.; Ranney, T.G.; Panthee, D.R. Genetic diversity, cytogenetics and biomass yields among taxa of giant reed (Arundo species). J. Am. Soc. Hort. Sci. 2016, 141, 256–263. [Google Scholar] [CrossRef]
- Fabbrini, F.; Ludovisi, R.; Alasia, O.; Flexas, J.; Douthe, C.; Ribas-Carbó, M.; Robson, P.; Taylor, G.; Scarascia-Mugnozza, G.; Keurentjes, J.J.B.; et al. Characterization of phenology, physiology, morphology and biomass traits across a broad Euro-Mediterranean ecotypic panel of the lignocellulosic feedstock Arundo donax. GCB Bioenergy 2019, 11, 152–170. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Haworth, M.; Marino, G.; Cosentino, S.L.; Brunetti, C.; De Carlo, A.; Avola, G.; Riggi, E.; Loreto, F.; Centritto, M. Increased free abscisic acid during drought enhances stomatal sensitivity and modifies stomatal behaviour in fast growing giant reed (Arundo donax L.). Environ. Exp. Bot. 2018, 147, 116–124. [Google Scholar] [CrossRef]
- Kørup, K.; Laerke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Munnich, C.; Didion, T.; Jensen, E.S.; Martensson, L.M.; Jørgensen, U. Biomass production and water use efficiency in perennial grasses during and after drought stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef]
- Haworth, M.; Centritto, M.; Giovannelli, A.; Marino, G.; Proietti, N.; Capitani, D.; De Carlo, A.; Loreto, F. Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 2017, 9, 119–131. [Google Scholar] [CrossRef]
- Pipatsitee, P.; Eiumnoh, A.; Praseartkul, P.; Taota, K.; Kongpugdee, S.; Sakulleerungroj, K.; Cha-um, S. Application of infrared thermography to assess cassava physiology under water deficit condition. Plant Prod. Sci. 2018, 21, 398–406. [Google Scholar] [CrossRef]
- Velikova, V.; Brunetti, C.; Tattini, M.; Doneva, D.; Ahrar, M.; Tsonev, T.; Stefanova, M.; Ganeva, T.; Gori, A.; Ferrini, F.; et al. Physiological significance of isoprenoids and phenylpropanoids in drought response of Arundinoideae species with contrasting habitats and metabolism. Plant Cell Environ. 2016, 39, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Cao, Y.; Tang, S.; Lu, Y.; Luo, S.; Ma, Y. Effects of drought stress and rewatering on physiological characteristics of Arundo donax var. Versicolor. Sci. Soil Water Conserv. 2020, 18, 59–66. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Villani, M.; Volterrani, M.; Guglielminetti, L.; Scartazza, A. Inter- and intraspecific variability in physiological traits and post-anoxia recovery of photosynthetic efficiency in grasses under oxygen deprivation. Physiol. Plant. 2017, 161, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Pompeiano, A.; Landi, M.; Meloni, G.; Vita, F.; Guglielminetti, L.; Guidi, L. Allocation pattern, ion partitioning, and chlorophyll a fluorescence in Arundo donax L. in responses to salinity stress. Plant Biosyst. 2017, 151, 613–622. [Google Scholar] [CrossRef]
- Pompeiano, A.; Remorini, D.; Vita, F.; Guglielminetti, L.; Miele, S.; Morini, S. Growth and physiological response of Arundo donax L. to controlled drought stress and recovery. Plant Biosyst. 2017, 151, 906–914. [Google Scholar] [CrossRef]
- Pompeiano, A.; Huarancca Reyes, T.; Moles, T.M.; Guglielminetti, L.; Scartazza, A. Photosynthetic and growth responses or Arundo donax L. plantlets under different oxygen deficiency stresses and reoxygenation. Front. Plant Sci. 2019, 10, 408. [Google Scholar] [CrossRef] [PubMed]
| T | Asat | Gs | E | WUE | Experimental Conditions | Reference |
|---|---|---|---|---|---|---|
| WW | 23.6 | 0.56–0.68 | A two-year field trial of giant reed under different nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. | [11] | ||
| WW | ~33~38 | 0.548–0.770 | Two well-established ecotypes (more than 15 years) from Sicily (Italy) in an experimental field. | [22] | ||
| WW | 25–30 | 0.3–0.6 | Rhizomes collected from Morocco, Sicily, and Florence and planted in plots in an experimental field. | [22] | ||
| WW | ~38 | 0.750–0.800 | Two well-established ecotypes (Central Italy and Morocco) with irrigation | [23] | ||
| WS | 18–25 | 0.2–0.3 | Two well-established ecotypes (Central Italy and Morocco) rain-fed for 6 weeks. | [23] | ||
| WW | 11.91 | − | 3.36 | 3.54 | Field capacity (20–35% moisture v/v; 0.0 MPa). Established cohort (16 weeks old; 8 weeks of treatment). Containers in greenhouse in California (USA). | [24] |
| WS | 0.52 | 0.13 | 4.03 | Mild drought (9% moisture). Established cohort (16 weeks old; 8 weeks of treatment). Containers in greenhouse in California (USA). | [24] | |
| WS | 0.12 | 0.16 | 0.75 | Severe drought (5% moisture). Established cohort (16 weeks old; 8 weeks of treatment). Containers in greenhouse in California (USA). | [24] | |
| WW | 30.2 | Natural stand located in Portugal. | [25] | |||
| WW | 28.3 | 0.295 | 3.3 | Plants collected in San Martí Sarroca (Spain). | [21] | |
| WW | ~20~28 | 0.170 | Pot experiment with plantlets of a commercial clone. | [26] | ||
| WS | 15.45 | 0.067 | Progressive drought for 66 days (until 20%FC) in plantlets. | [26] | ||
| WW | ~30 | ~1.1 | Wild population from Florida (USA). | [27] | ||
| WW | 27.13 | 0.644 | 6.31 | 4.2 | Pot experiment. Rhizomes from California (USA). | [28] |
| WW | ~23~27 | 0.250–0.450 | Different clones in pots in greenhouse conditions. | [29] | ||
| WW | ~27 | ~0.5 | ~9 | ~3 | Plants in pots in greenhouse conditions. | [30] |
| WS | ~18 | ~0.15 | ~3.7 | ~5.5 | Mild stress in pots in greenhouse conditions. | [30] |
| WS | ~9 | ~0.8 | ~1.8 | ~6.3 | Several stresses in pots in greenhouse conditions. | [30] |
| WW | 20–21 | 0.38–0.39 | 5.6 | Two ecotypes (Central Italy and Morocco) planted in rhizotrons. | [31] | |
| WS | 14.1–14.4 | 0.153–0.157 | 3.2–2.9 | Two ecotypes (Central Italy and Morocco) planted in rhizotrons. | [31] | |
| WW | 25–30 | 0.5-0.7 | Rhizomes collected from Morocco, Sicily, and Florence and planted in plots in an experimental field. | [32] | ||
| WW | 15.3–34.0 | Pot experiment in experimental fields; A. donax L. from Greece. | [33] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, E.; Rivera-Vargas, P.; Serrat, X.; Nogués, S. Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions. Agronomy 2021, 11, 1089. https://doi.org/10.3390/agronomy11061089
Sánchez E, Rivera-Vargas P, Serrat X, Nogués S. Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions. Agronomy. 2021; 11(6):1089. https://doi.org/10.3390/agronomy11061089
Chicago/Turabian StyleSánchez, Elena, Pablo Rivera-Vargas, Xavier Serrat, and Salvador Nogués. 2021. "Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions" Agronomy 11, no. 6: 1089. https://doi.org/10.3390/agronomy11061089
APA StyleSánchez, E., Rivera-Vargas, P., Serrat, X., & Nogués, S. (2021). Arundo donax L.: How High Photosynthetic Capacity Is Maintained under Water Scarcity Conditions. Agronomy, 11(6), 1089. https://doi.org/10.3390/agronomy11061089







