The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Locality Description, Vegetation Evaluation and Insect Sampling
2.2. Data Analysis
3. Results
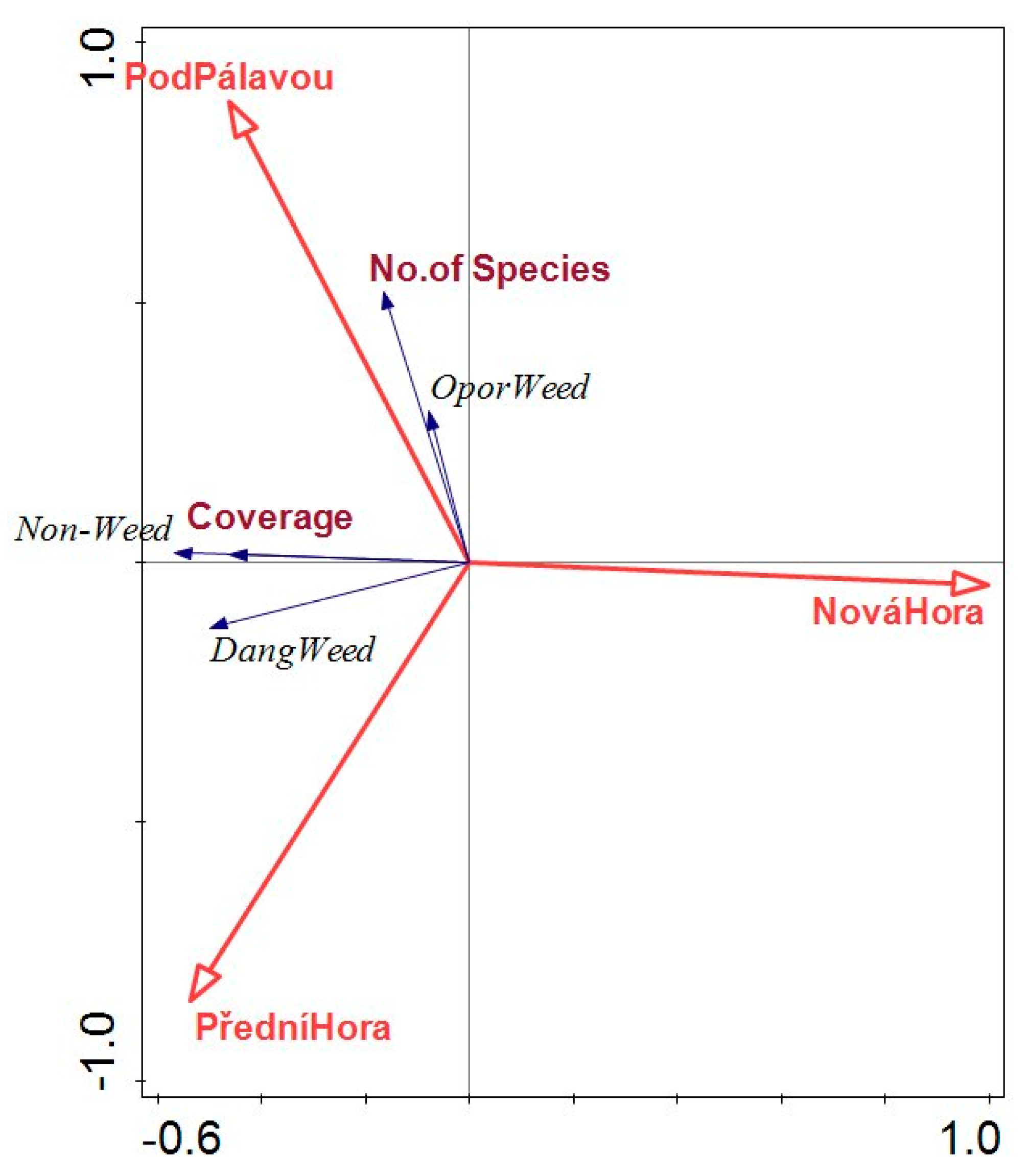
3.1. Vegetation Diversity and Number of Plant Species
3.2. Proportion of Grass, Leguminous and Other Dicotyledonous Species in Inter-Row Vegetation
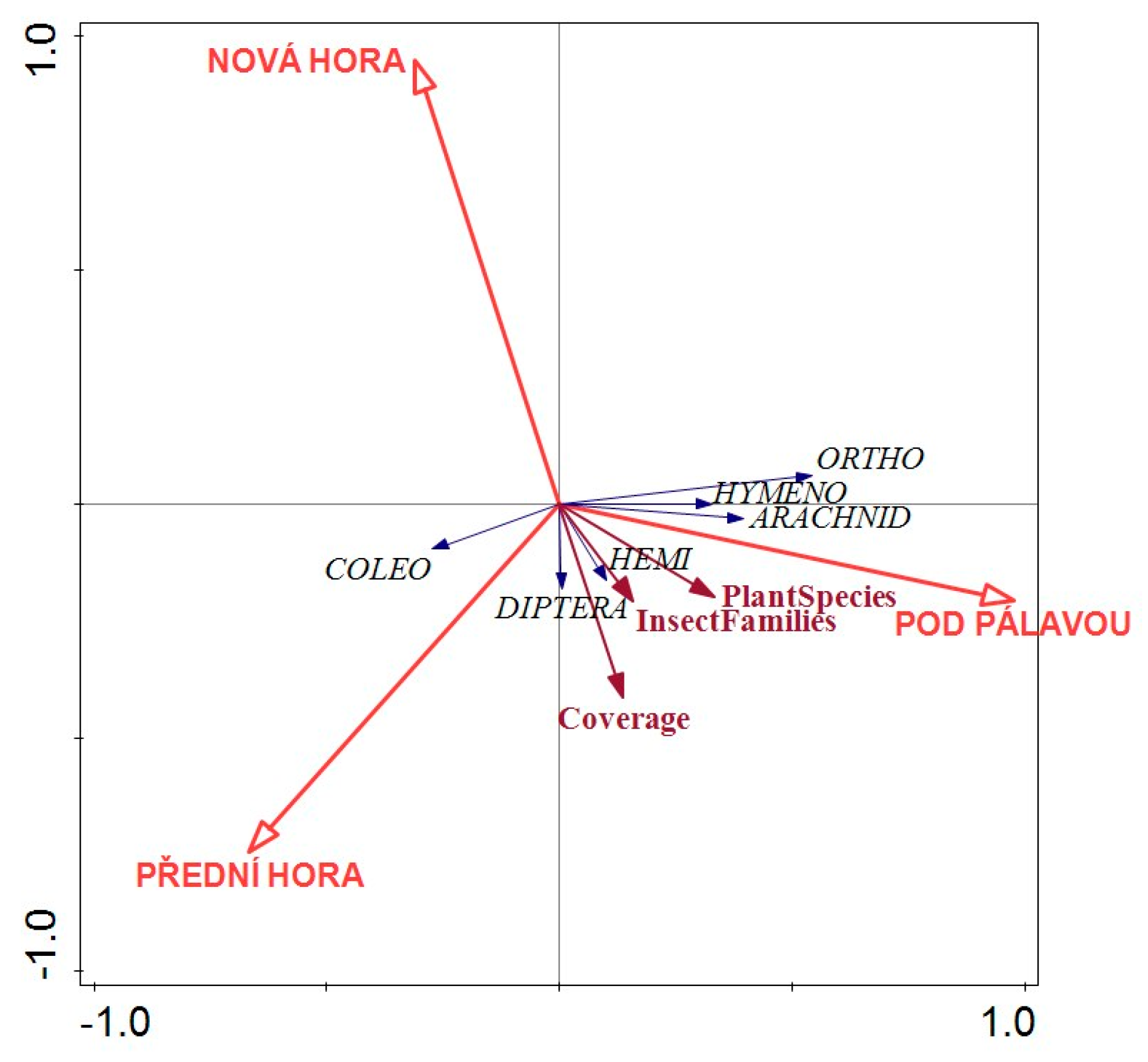
3.3. Insect Occurrence and Diversity
4. Discussion
4.1. Plant Vegetation Diversity and Number of Plant Species
4.2. Proportion of Grass, Leguminous and Other Dicotyledonous Species in Inter-Row Vegetation
4.3. Insect Occurrence and Diversity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altieri, M.A.; Nicholls, C.I. Biodiversity and Pest Management in Agroecosystems, 2nd ed.; Food Producss Press: New York, NY, USA, 2004; ISBN 15-602-2923-3. [Google Scholar]
- Báez, S.; Collins, S.L. Shrub Invasion Decreases Diversity and Alters Community Stability in Northern Chihuahuan Desert Plant Communities. PLoS ONE 2008, 3, e2332. [Google Scholar] [CrossRef] [PubMed]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J.M. Biodiversity and Ecosystem Functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Wilson, H.; Miles, A. Habitat Management in Vineyards: A Growers’ Manual for Enhancing Natural Enemies of Pests; Laboratory of Agroecology, University of California: Berkeley, CA, USA, 2010. [Google Scholar]
- Altieri, M.A.; Nicholls, C.I.; Ponti, L.; York, A. Designing biodiverse, pest-resilient vineyards through habitat management. Pract. Winery Vineyard 2005, 27, 16–30. [Google Scholar]
- Schellhorn, N.A.; Gagic, V.; Bommarco, R. Time will tell: Resource continuity bolsters ecosystem services. Trends Ecol. Evol. 2015, 30, 524–530. [Google Scholar] [CrossRef]
- Kelly, R.M.; Kitzes, J.; Wilson, H.; Merenlender, A. Habitat diversity promotes bat activity in a vineyard landscape. Agric. Ecosyst. Environ. 2016, 223, 175–181. [Google Scholar] [CrossRef]
- Rusch, A.; Chaplin-Kramer, R.; Gardiner, M.M.; Hawro, V.; Holland, J.; Landis, D.; Thies, C.; Tscharntke, T.; Weisser, W.W.; Winqvist, C.; et al. Agricultural landscape simplification reduces natural pest control: A quantitative synthesis. Agric. Ecosyst. Environ. 2016, 221, 198–204. [Google Scholar] [CrossRef]
- Boller, E.F.; Häni, F.; Poehling, H.M. Ecological Infrastructures. Ideabook on Functional Biodiversity at the Farm Level; IOBCwprs Commission on Integrated Production Guidelines and Endorsement: Lindau, Switzerland, 2004. [Google Scholar]
- Winkler, K.; Wäckers, F.; Bukovinszkine-Kiss, G.; van Lenteren, J. Sugar resources are vital for Diadegma semiclausum fecundity under field conditions. Basic Appl. Ecol. 2006, 7, 133–140. [Google Scholar] [CrossRef]
- Kopta, T.; Pokluda, R.; Psota, V. Attractiveness of flowering plants for natural enemies. Hortic. Sci. 2012, 39, 89–96. [Google Scholar] [CrossRef]
- Thomson, L.J.; Hoffmann, A.A. Spatial scale of benefits from adjacent woody vegetation on natural enemies within vineyards. Biol. Control. 2013, 64, 57–65. [Google Scholar] [CrossRef]
- Gaigher, R.; Pryke, J.; Samways, M.J. High parasitoid diversity in remnant natural vegetation, but limited spillover into the agricultural matrix in South African vineyard agroecosystems. Biol. Conserv. 2015, 186, 69–74. [Google Scholar] [CrossRef]
- Smith, I.M.; Hoffmann, A.A.; Thomson, L.J. Ground cover and floral resources in shelterbelts increase the abundance of beneficial hymenopteran families. Agric. For. Ѐntomol. 2014, 17, 120–128. [Google Scholar] [CrossRef]
- Tscharntke, T.; Bommarco, R.; Clough, Y.; Crist, T.O.; Kleijn, D.; Rand, T.A.; Tylianakis, J.M.; van Nouhuys, S.; Vidal, S. Conservation biological control and enemy diversity on a landscape scale. Biol. Control. 2007, 43, 294–309. [Google Scholar] [CrossRef]
- Thomson, L.J.; McKenzie, J.; Sharley, D.J.; Nash, M.A.; Tsitsilas, A.; Hoffmann, A.A. Effect of woody vegetation at the landscape scale on the abundance of natural enemies in Australian vineyards. Biol. Control. 2010, 54, 248–254. [Google Scholar] [CrossRef]
- Nascimbene, J.; Zottini, M.; Ivan, D.; Casagrande, V.; Marini, L. Do vineyards in contrasting landscapes contribute to conserve plant species of dry calcareous grasslands? Sci. Total Environ. 2016, 545-546, 244–249. [Google Scholar] [CrossRef]
- Mania, E.; Isocrono, D.; Pedullà, M.; Guidoni, S. Plant Diversity in an Intensively Cultivated Vineyard Agroecosystem (Langhe, North-West Italy). S. Afr. J. Enol. Vitic. 2015, 36, 378–388. [Google Scholar] [CrossRef]
- Hall, R.M.; Penke, N.; Kriechbaum, M.; Kratschmer, S.; Jung, V.; Chollet, S.; Guernion, M.; Nicolai, A.; Burel, F.; Fertil, A.; et al. Vegetation management intensity and landscape diversity alter plant species richness, functional traits and community composition across European vineyards. Agric. Syst. 2020, 177, 102706. [Google Scholar] [CrossRef]
- Ragasová, L.; Kopta, T.; Winkler, J.; Pokluda, R. Assessing Diversity Levels in Selected WineRegions of South Moravia (Czech Republic). Pol. J. Environ. Stud. 2020, 29, 1315–1321. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie: Grundzüge der Vegetationskunde [Phytosociology: Fundamentals of Vegetation Science], 3rd ed.; Springer: Vienna, Austria, 1964. (In German) [Google Scholar]
- Urban, J.; Šarapatka, B. Ekologické Zemědělství [Ecological Agriculture]; MŽP: Praha, Czech Republic, 2003; (In Czech). ISBN 80-7212-274-6.
- AMET—Sdružení Litschmann & Suchý. Available online: http://www.amet.cz/ (accessed on 5 January 2021).
- Doxon, E.D.; Davis, C.A.; Fuhlendorf, S.D. Comparison of two methods for sampling invertebrates: Vacuum and sweep-net sampling. J. Field Ornithol. 2011, 82, 60–67. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination (Version 5.0); Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Miglécz, T.; Valkó, O.; Török, P.; Deák, B.; Kelemen, A.; Donkó, Á.; Drexler, D.; Tóthmérész, B. Establishment of three cover crop mixtures in vineyards. Sci. Hortic. 2015, 197, 117–123. [Google Scholar] [CrossRef]
- Tardy, F.; Moreau, D.; Dorel, M.; Damour, G. Trait-based characterisation of cover plants’ light competition strategies for weed control in banana cropping systems in the French West Indies. Eur. J. Agron. 2015, 71, 10–18. [Google Scholar] [CrossRef]
- Cunha-Blum, J.; Oki, Y.; Solar, R.; Fernandes, G.W. More is not always better: Responses of the endemic plant Vellozia nanuzae to additional nutrients. Acta Bot. Bras. 2020, 34, 487–496. [Google Scholar] [CrossRef]
- David, T.I.; Storkey, J.; Stevens, C.J. Understanding how changing soil nitrogen affects plant–pollinator interactions. Arthropod-Plant Interact. 2019, 13, 671–684. [Google Scholar] [CrossRef]
- Dehariya, P.; Mishra, D.; Dhakad, R.; Kumar, A. Studies on Different Levels of Nitrogen Application on Growth and Yield of Amaranthus (Amaranthus tricolor L.). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1423–1427. [Google Scholar] [CrossRef]
- Toungos, M.; Babayola, M.; Shehu, H.; Kwaga, Y.; Bamai, N. Effects of Nitrogen Fertilizer on the Growth of Vegetable Amaranths (Amaranthus cruensis L.) in Mubi, Adamawa State Nigeria. Asian J. Adv. Agric. Res. 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. Legumes Soil Health Sustain. Manag. 2018, 487–510. [Google Scholar] [CrossRef]
- Kishi, R.N.I.; Júnior, R.F.G.; Val-Moraes, S.P.; Kishi, L.T. Soil Microbiome and Their Effects on Nutrient Management for Plants. Probiotics Agroecosyst. 2017, 117–143. [Google Scholar] [CrossRef]
- Miransari, M. Soil microbes and the availability of soil nutrients. Acta Physiol. Plant. 2013, 35, 3075–3084. [Google Scholar] [CrossRef]
- Nascimbene, J.; Marini, L.; Ivan, D.; Zottini, M. Management Intensity and Topography Determined Plant Diversity in Vineyards. PLoS ONE 2013, 8, e76167. [Google Scholar] [CrossRef]
- Vencill, W.K.; Nichols, R.L.; Webster, T.; Soteres, J.K.; Mallory-Smith, C.; Burgos, N.R.; Johnson, W.; McClelland, M.R. Herbicide Resistance: Toward an Understanding of Resistance Development and the Impact of Herbicide-Resistant Crops. Weed Sci. 2012, 60, 2–30. [Google Scholar] [CrossRef]
- Castillo, P.; Rapoport, H.F.; Palomares-Rius, J.E.; Díaz, R.M.J. Suitability of weed species prevailing in Spanish vineyards as hosts for root-knot nematodes. Eur. J. Plant Pathol. 2007, 120, 43–51. [Google Scholar] [CrossRef]
- Novara, A.; Gristina, L.; Saladino, S.; Santoro, A.; Cerdà, A. Soil erosion assessment on tillage and alternative soil managements in a Sicilian vineyard. Soil Tillage Res. 2011, 117, 140–147. [Google Scholar] [CrossRef]
- Goldammer, T. Grape Grower’s Handbook, 3rd ed.; APEX Publishers: Centreville, VA, USA, 2018; ISBN 978-0967521268. [Google Scholar]
- Sulas, L.; Mercenaro, L.; Campesi, G.; Nieddu, G. Different Cover Crops Affect Nitrogen Fluxes in Mediterranean Vineyard. Agron. J. 2017, 109, 2579–2585. [Google Scholar] [CrossRef]
- Czech Government Order on Conditions for Implementation of Agri-Environmental-Climate Measures and on Amendment to Government Order No. 79/2007 Coll., On Conditions for Implementation of Agri-Environmental Measures, as Amended. (Nařízení Vlády o Podmínkách Provádění Agroenvironmentálně-Klimatických Opatření a o Změně Nařízení Vlády č. 79/2007 Sb., o Podmínkách Provádění Agroenvironmentálních Opatření, ve Znění Pozdějších Předpisů). Available online: https://www.zakonyprolidi.cz/cs/2007-79 (accessed on 6 January 2021).
- Finch, C.U.; Sharp, W.C. Covercrops in California Orchards and Vineyards; US Department of Agriculture: Washington, DC, USA, 1976; p. 25.
- Ruiz-Colmenero, M.; Bienes, R.; Marques, M. Soil and water conservation dilemmas associated with the use of green cover in steep vineyards. Soil Tillage Res. 2011, 117, 211–223. [Google Scholar] [CrossRef]
- Bolduc, E.; Buddle, C.M.; Bostanian, N.J.; Vincent, C. Ground-Dwelling Spider Fauna (Araneae) of Two Vineyards in Southern Quebec. Environ. Ѐntomol. 2005, 34, 635–645. [Google Scholar] [CrossRef]
- Bruggisser, O.T.; Schmidt-Entling, M.H.; Bacher, S. Effects of vineyard management on biodiversity at three trophic levels. Biol. Conserv. 2010, 143, 1521–1528. [Google Scholar] [CrossRef]
- Caprio, E.; Nervo, B.; Isaia, M.; Allegro, G.; Rolando, A. Organic versus conventional systems in viticulture: Comparative effects on spiders and carabids in vineyards and adjacent forests. Agric. Syst. 2015, 136, 61–69. [Google Scholar] [CrossRef]
- Fiera, C.; Ulrich, W.; Popescu, D.; Bunea, C.-I.; Manu, M.; Nae, I.; Stan, M.; Markó, B.; Urák, I.; Giurginca, A.; et al. Effects of vineyard inter-row management on the diversity and abundance of plants and surface-dwelling invertebrates in Central Romania. J. Insect Conserv. 2020, 24, 175–185. [Google Scholar] [CrossRef]
- Birkhofer, K.; Rusch, A.; Andersson, G.K.; Bommarco, R.; Dänhardt, J.; Ekbom, B.; Jönsson, A.; Lindborg, R.; Olsson, O.; Rader, R.; et al. A framework to identify indicator species for ecosystem services in agricultural landscapes. Ecol. Indic. 2018, 91, 278–286. [Google Scholar] [CrossRef]
- Maelfait, J.P.; Hendrickx, F. Spiders as bio-indicators of anthropogenic stress in natural and semi-natural habitats in Flanders (Belgium): Some recent developments. In Proceedings of the 17th European Colloquium of Arachnology, Edinburgh 1997; Selden, P.A., Ed.; British Arachnological Society: Bucks, UK, 1998. [Google Scholar]
- Ossamy, S.; Elbanna, S.M.; Orabi, G.M.; Semida, F.M. Assessing the potential role of spider as bioindicators in Ashtoum el Gamil Natural Protected Area, Port Said, Egypt. Indian J. Arachnol. 2016, 5, 100–112. [Google Scholar]
- Wäckers, F. Assessing the suitability of flowering herbs as parasitoid food sources: Flower attractiveness and nectar accessibility. Biol. Control. 2004, 29, 307–314. [Google Scholar] [CrossRef]
- Buide, M.L. Pollination Ecology of Silene acutifolia (Caryophyllaceae): Floral Traits Variation and Pollinator Attraction. Ann. Bot. 2005, 97, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kephart, S.; Reynolds, R.J.; Rutter, M.T.; Fenster, C.B.; Dudash, M.R. Pollination and seed predation by moths on Silene and allied Caryophyllaceae: Evaluating a model system to study the evolution of mutualisms. New Phytol. 2006, 169, 667–680. [Google Scholar] [CrossRef] [PubMed]
- De Castro, C.V.; Hoffmeister, T.S. Friend or foe? A parasitic wasp shifts the cost/benefit ratio in a nursery pollination system impacting plant fitness. Ecol. Evol. 2020, 10, 4220–4232. [Google Scholar] [CrossRef] [PubMed]
- Patt, J.M.; Hamilton, G.C.; Lashomb, J.H. Foraging success of parasitoid wasps on flowers: Interplay of insect morphology, floral architecture and searching behavior. Ѐntomol. Exp. Appl. 1997, 83, 21–30. [Google Scholar] [CrossRef]
- Kevan, P.G. Parasitoid Wasps as Flower Visitors in the Canadian High Arctic. J. Pest Sci. 1973, 46, 3–7. [Google Scholar] [CrossRef]
- Müller, H. Die Befruchtung der Blumen Durch Insekten und Die Gegenseitigen Anpassungen Beider; Engelmann: Leipzig, Germany, 1873; Volume VIII, p. 478. [Google Scholar]
- Györfi, J. Beiträge zur Biologie und Ökologie der Schlupfwespen (Ichneumonidae). Z. Angew. Entomol. 2009, 51, 142–147. [Google Scholar] [CrossRef]
- Gonçalves, F.; Carlos, C.; Aranha, J.; Torres, L. Does habitat heterogeneity affect the diversity of epigaeic arthropods in vineyards? Agric. For. Ѐntomol. 2018, 20, 366–379. [Google Scholar] [CrossRef]
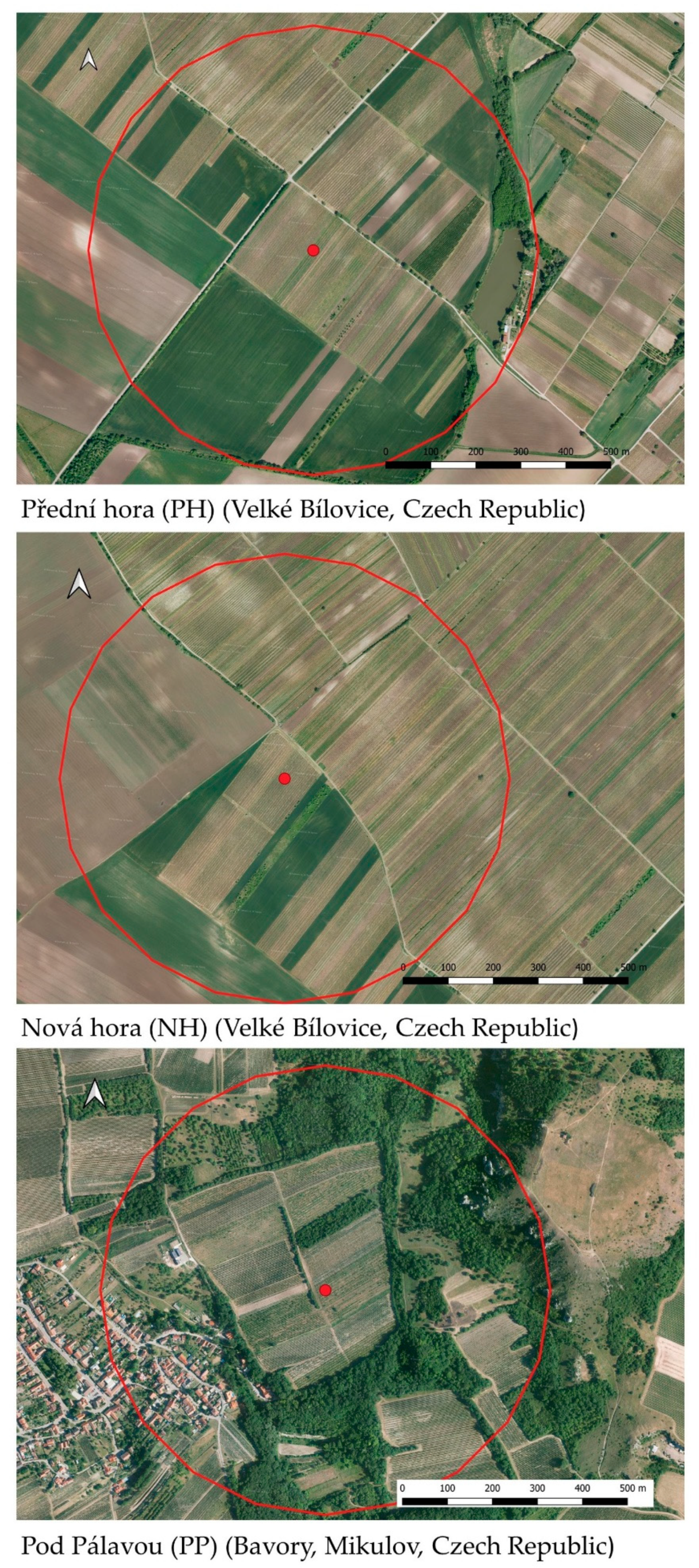


| Vineyard Site | May | June | July | August | |||||
|---|---|---|---|---|---|---|---|---|---|
| t (°C) a | Rainfall (mm) b | t (°C) | Rainfall (mm) | t (°C) | Rainfall (mm) | t (°C) | Rainfall (mm) | ||
| Pod Pálavou | 2018 | 18.90 | 42.78 | 22.1 | 39.06 | 22.3 | 36.58 | 24.4 | 13.33 |
| 2019 | 13.1 | 104.47 | 23.6 | 26.97 | 21.8 | 97.03 | 22.1 | 63.86 | |
| Přední hora, Nová hora | 2018 | 18.2 | 87.78 | 20.6 | 43.23 | 21.7 | 58.08 | 24 | 24.09 |
| 2019 | 12.4 | 155.43 | 22.9 | 95.7 | 21.1 | 101.31 | 21.7 | 113.85 | |
| Category | Description | Example (General) |
|---|---|---|
| Dangerous weeds | large plants in low amounts, very high ability to reproduce (noxious, invasive) | Amaranthus retroflexus L., Atriplex spp., Avena fatua L. Chenopodium sp., Cirsium arvense (L.) Scop., Datura stramonium L., Echinochloa crus-galli (L.) P. B., Elytrigia repens (L.) Nevski, Hyoscyamus niger L., Rumex obtusifolius L., R. crispus L. |
| Opportunistic weeds | most common weeds, mid-growth, integrated in vegetation cover, not problematic, easily controlled by preventive measures, only problem when over-reproduced | Capsella bursa-pastoris (L.) Med., Centaurea cyanus L., Mercurialis annua L., Papaver rhoeas L., Polygonum aviculare L., Setaria sp., Stellaria media (L.) Vill., Thlapsi arvense L., Viola arvensis Murray |
| Non-harmful weeds | large group of small plant species, with ground growth, no problems even when over- reproduced, controlled by common agrotechnical practices | Anagallis arvensis L., Daucus carota L., Geranium pusillum Burm.fil., Medicago lupulina L., Trifolium pratense L., Valerianella locusta (L.) Laterr., Veronica spp. |
| Proportion of Non-Crop Vegetation a (%) | Average b Coverage Rate (%) | No. of Plant Species c | Total Number of Plant Species d | No. of Invertebrates’ Families e | ||||
|---|---|---|---|---|---|---|---|---|
| S.E. | S.E. | S.E. | ||||||
| Přední Hora | 7 | 71.21 | 4.13 | 9.08 | 0.77 | 17 | 10.25 | 0.76 |
| Nová Hora | 2 | 52.08 | 5.41 | 7.79 | 0.59 | 21 | 8.96 | 0.90 |
| Pod Pálavou | 42 | 68.96 | 1.90 | 11.04 | 0.61 | 30 | 11.08 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragasová, L.; Kopta, T.; Winkler, J.; Šefrová, H.; Pokluda, R. The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region. Agronomy 2021, 11, 1073. https://doi.org/10.3390/agronomy11061073
Ragasová L, Kopta T, Winkler J, Šefrová H, Pokluda R. The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region. Agronomy. 2021; 11(6):1073. https://doi.org/10.3390/agronomy11061073
Chicago/Turabian StyleRagasová, Lucia, Tomáš Kopta, Jan Winkler, Hana Šefrová, and Robert Pokluda. 2021. "The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region" Agronomy 11, no. 6: 1073. https://doi.org/10.3390/agronomy11061073
APA StyleRagasová, L., Kopta, T., Winkler, J., Šefrová, H., & Pokluda, R. (2021). The Effect of the Proportion of Adjacent Non-Crop Vegetation on Plant and Invertebrate Diversity in the Vineyards of the South Moravian Region. Agronomy, 11(6), 1073. https://doi.org/10.3390/agronomy11061073







