Abstract
Guinea grass (Panicum maximum Jacq., renamed Megathyrsus maximus Jacq.) is a native forage plant in Africa of great economic value, but it was introduced in almost all tropical countries as a source of animal forage. Over the last decade, it was introduced in North arid regions of Africa (Morocco, Algeria, Tunisia, Libya, and Egypt) through authorized and unauthorized ways. It has two reproduction modes through sexual and apomictic ways. Besides its ability to provide high nutritive forage, guinea grass could affect the oases agroecosystems diversity due to its genetic aspects (apomixis and autotetraploidy) and eco-physiological traits (allelopathy effect and resistance to abiotic stress). That is why a review of genetic and eco-physiologic aspects of guinea grass is essential to investigate its potential introduction and management in new regions, particularly in arid and semiarid zones. In this paper, we review the most important traits of this plant that should be considered (polyploidy, apomixis, allelopathic effect, drought and salinity resistance, and invasion) for the potential success of guinea grass in integrated systems of forage/livestock.
1. Introduction
Guinea grass was previously known as Panicum maximum Jacq. In 2003, the subgeneric name, Megathyrsus, was added to generic rank and it was renamed Megathyrsus maximus (Jacq.) [1]. The grasses of Poaceae are ecologically dominant and are by far the most economically important family in the world [2,3,4]. The guinea grass is a perennial forage species belonging to this family. The guinea grass forms an agamic complex with Panicum infestum and Panicum trichocladum (tribe of Paniceae) and belongs to the subfamily Panicoideae of the Poaceae [5]. It is native to Africa (tropical origin), but this grass was distributed to almost all tropical countries as a source of animal forage due to its good forage quality, particularly as feed for beef [6]. Due to its high forage production, nutritional value, and increased adaptability to different ecological regions, guinea grass has been broadly introduced and exploited in most tropical and subtropical zones, such as Brazil, Japan, the USA, and Australia [5,7,8,9,10]. After its introduction to the subtropical rainfall zones (900 mm) in the last decade, it was cultivated in many subtropical arid and semiarid regions of North Africa and the Mideast.
Guinea grass is one of the most well-known potentials for the production of dry yield in subtropical and tropical regions, and it can reach annual production of 33 t/ha [11]. It presents high growth rates and biomass production, in part due to its C4 photosynthetic pathway (a mechanism to build up high concentrations of carbon dioxide in the chloroplasts of leaf cells) [12]. It is generally represented by autotetraploid biotypes (2n = 4x = 32) with facultative apomictic reproduction [13]. Among cultivars, there are vast variation in terms of yield potential, the forage quality, and the response to nutriments fertilization. Despite the guinea grass germplasm having a high level of diversity [14], only a few cultivars are used in North Africa. The cv. Mombaca from Brazil is the most commercialized and cultivated genotype in North African countries. This could be explained by the adaptive traits of this variety in the arid and semiarid zones (North African climates), characterized by drought, salinity, and winter hardiness. Hare et al. [15] reported that Mombaca and Tanzania cultivars survived in drought conditions. These cultivars were selected primarily for production traits such as leaf size, regrowing ability after harvest, and purity seed yield [16].
The Middle East and North African regions, where guinea grass is newly introduced, belong to Saharan, arid, and semiarid climates (map of arid and semiarid regions: https://arcg.is/0D8LjD, accessed on 10 April 2021). The agroecosystems of these regions are known by the oasis agriculture and the irrigated pastures. The North African oasis agriculture has always been threatened because of small exploitations, high management costs, the deficit of water, and high salinity levels. For that, the oasis agriculture in these regions has always been questionable. The most cultivated trees are the date palm (Phoenix dactylifera L.) and olive (Olea europaea L.), which offer important economic resources. For example, Tunisia is one of the world’s largest producers of olive oil [17] and date palm. Other fruit trees are cultivated in the oasis and arid zones, such as pomegranate, figs, peaches, apricots, etc. Regarding the forage species, there are many well-adapted species grown under the fruit trees, such as the perennial alfalfa (Medicago sativa L), the annual barley (Hordeum vulgare L.), the oats (Avena sativa L.), the sorghum (Sorghum bicolor L.), and the maize (Zea mays L.). In the last decade, the forage plant guinea grass was introduced to these agroecosystems and cultivated inside and outside of the oasis. In Tunisia, it was introduced by farmers in 2018 and distributed in different continental and coastal oases. In Libya, Egypt, Algeria, and Morocco, the introduction was prior to this date, with a license from the authorities. Up to now, the adaptation and the impact of this species on the biodiversity and sustainability of arid and semiarid agroecosystems as prospective studies are not well reported. However, guinea grass has a disadvantage in its ability to quickly spread and its capacity to become a weed plant in unexploited lands with disturbed soil. It is the main weed in sugarcane fields (similar to the oases ecosystems) because it grows well in shaded conditions [18]. It is also a colonizer of disturbed sites, including roadsides, and particularly untended areas. This robust grass may foster soil erosion in invaded areas [19,20]. Guinea grass is listed as invasive grass (Invasive Species Compendium; https://www.cabi.org/isc/datasheet/38666, accessed on 12 April 2021).
The knowledge of the nutritional requirements, polyploidy levels, and allelopathy effects of this forage species are extremely important for the best practice and the sustainability of both pasture and oasis agroecosystems in arid and semiarid regions. In this paper, we review the most important traits of this plant that should be considered (production, polyploidy, apomixis, allelopathy effect, drought resistance, and invasion).
2. Morphology, Management, and Production
Guinea grass is a large tufted, fast-growing perennial species. It has a large phenotypic and agronomic variability, ranging in length from 0.5 to 3.5 m and in stem diameter from 5 to 10 mm. Based on the entire plant size, there are two main forms: a tall/medium tussock form, taller than 1.5 m at flowering, and a short tussock form [21]. It is characterized by short roots with creeping rhizome, erected culms, and nodes with hirsute. The leaves, with blade shape, are glabrous to pubescent (up to 35 mm in large). It has a panicle inflorescence (15 to 50 cm long). The spikelets are green to purple with a length of 3–4 mm. The seed production of guinea grass is ~1.7 to 3.1 million seeds/kg [18].
Guinea grass is a forage plant appropriate for pasture, cut-and-carry, silage, and hay [22]. The “cut and carry” was reported as the well-suited system, but it can be used as silage and hay as well. The long-term pasture guinea grass can be managed if it is grazed under 35 cm in height [22]. For good animal performance, the ideal rest period is to wait for the re-growth of 2.5 leaves/tiller [23]. Concerning the silage and hay managements, the best cutting should be between 60 and 90 cm in height, but for more acceptable quality it can be cut at up to 1.5 m [24]. The highest quality silage is achieved if the cutting is performed during pre-anthesis or anthesis [25]. Cutting at the three-leaf stage at 25 cm would offer maximum material yield and acceptable leaf/stem ratio [26].
The systems of forage production are often based on the forage mass [27]. Several studies reported higher rates of forage production of the guinea grass [27,28]. The maximum yielding guinea grasses, fertilized with 150 to 200 kg/ha of nitrogen, is around 18 to 21 t/ha per year [26,28,29]. The annual dry yield can reach 33 t/ha [30]. As with many other plants, the quantity and quality of forage are affected by genotypes and grazing management. For example, the insufficient crop systems management of these species induces an excessive stem growth that decreases the acceptability rate and cattle development [31]. Furthermore, the high light intensity activates the growth and production and increases the stem proportion [32]. Fernandes et al. [29] showed a high diversity of forage yield and nutritive value between 24 genotypes and reported that “Milênio” cultivar was the most productive of matter yield during two successive years. However, the most cultivated varieties (Mombaça and Tanzania) yielded 12.5 and 11 t/ha, respectively. The introduced variety in the North African region, Mombaça, is often cited as more productive than Tanzania (20.5 vs. 14.4 t/ha per year; [33]), and it is capable of producing high levels of forage quality [16]. Feeding and digestion studies of guinea grass reported the correlation between the neutral detergent fiber (NDF), the crude protein (CP), the blade size, and the leaf area [12]. The total digestible nutrients (TDNs) vary from 40% to about 60% of dry matter in guinea grass [34]. Table 1 summarizes the data collected from 15 papers referring to guinea grass and reported the ashes, CP, neutral detergent fiber, acid detergent fiber, and acid detergent lignin.

Table 1.
Range values of dry matter and chemical traits (% dry basis) of the guinea grass (leaves plus/or stems) collected from 15 papers. DM: dry matter, CP: crude protein, NDF: neutral detergent fiber, ADF: acid detergent fiber, ADL: acid detergent lignin.
3. Allelopathic Effect
An invasive species is an introduced organism that negatively affects its new environment through different ways, such as allelopathy. Allelopathy is a biological phenomenon by which a plant produces one or more molecules that affect the development of other organisms [50]. The Poaceae family has been described in many published investigations to exhibit evidence of allelopathic activity (simulative or inhibitive) [2]. The main allelopathic compounds are phenolic acids, hydroxamic acids, alkaloids, and quinines. The guinea grass is clump-forming plant that can produce smaller crowns near the mother plant parent that can be divided and removed to a new location. This characteristic accelerates its propagation in new zones. Despite the extensive research on the allelopathic compounds in the Poaceae species [2,4], little documentation has reported the allelopathy effect of guinea grass in literature. Chou [51] classified the P. maximum as an allelopathic species with other grasses from subtropical vegetation in Taiwan, such as Acroceras macrum, Cynodon dactylon, and Chloris gayana, that showed relatively pure stand in the fields. Reduced yield in the second crop was attributed primarily to an autointoxication mechanism. When considering the rapid spread of this forage and its unknown allelopathic effect, the oasis agroecosystems (high density and diversity of vegetation) could be influenced by its introduction. Silva et al. [52] reported that when it occurs with particular food crops, guinea grass is considered a destructive weed such as maize (Zea mays L.), citrus fruit, coffee, and sugarcane (Saccharum officinarum L.).
In the arid regions of the Middle East and North Africa, when it was newly introduced, the date palm (Phoenix dactylifera L.) was the most cultivated tree and is considered the key ecological and economic plant in the oasis agroecosystems [53]. The potential allelopathy of guinea grass on this target tree should be urgently studied and considered. Additionally, the alfalfa (Medicago sativa L.) was the first forage plant cultivated under the date palm trees in the oases and irrigated systems [54]. Alfalfa is one of the most important cultivated forage species worldwide, and it is well adapted to semiarid and arid regions [55].
To our best knowledge, no studies have been conducted on the interaction between Guinea grass and the main crops of the oasis agroecosystem, such as alfalfa (or date palm trees), to underline any potential allelopathy. Thus, it is crucial to study the activity type of guinea grass as a donor plant and its target species in combined crops. Three allelochemicals of guinea grass (p-Hydroxyphenyl acetic, trans-p-coumaric, and cis-p-coumaric acids) have been reported by Chou and Young [56].
Alternatively, the guinea grass could be a target plant of other forage Poaceae Cenchrus echinatus by modifying its germination, roots, and shoot length [57]. C. ciliaris has an extensive distribution in arid and semiarid lands [58]. In Tunisia, C. ciliaris ranges in broad bioclimatic gradient [59]. In addition, many guinea grass cultivars show medium levels of tolerance to shade [60], and the interaction between shade and nitrogen fertilization highly affects biomass yield and the nutritive value of Panicum cultivars. Thus, the implication for field conditions seems more complex.
4. Apomixis and Polyploidy Levels
The P. maximum belongs to agamic complexes, which are assemblages of multiple polyploid and agamospermous forms that are typically the products of hybrid crosses. The apomixis is the asexual reproduction via seed. In fact, the apomictic species can produce seeds via unique fertilization of the polar nuclei (pseudogamy) or without fertilization (autonomously) [61]. The apomixis in guinea grass is due to its characteristics of considerable meiotic irregularity and chromosome non-disjunction in microsporogenesis [62], and it influences the formation and maintenance of new cytotypes in nature. Additionally, the polyploid levels in guinea grass are originated by fertilization with the unreduced gametes. The obtained hybrids from different polyploidy levels have a lower rate of sexuality [13]. The guinea grass is generally represented by tetraploid biotypes with 2n = 4x = 32 chromosomes that reproduce by facultative apomixis [13]. The obligate sexuality was found only in diploid types [8,14,62]. The chromosome numbers in Guinea grass that have been reported are 2n = 16 and 32 [8], 2n = 18 [63], 2n = 32 and 44 [64], and 2n = 32 and 48 [62] (Figure 1).
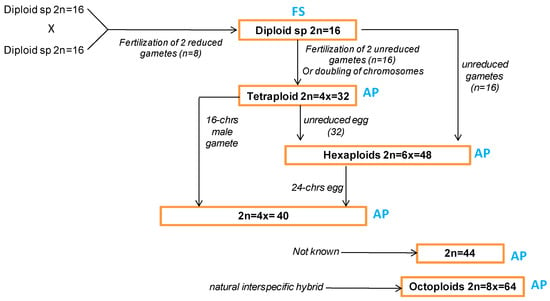
Figure 1.
Diverse gene pools according to chromosomes number reported in guinea grass species and their probable origins. The reproduction paths are indicated as FS: fully sexual and AP: apomictic. This flow chart is generated based on 5 references [10,11,47,48,56].
The 48-chromosome plants and the 32-chromosome plants have differences in flowering dates [8]. Combes and Pernes [65] studied guinea grass accessions from Africa (Kenya and Tanzania) and found two groups: (i) tetraploid (4x = 32) and apomictic, or (ii) diploid (2n = 16) and fully sexual. The crosses between plants with different chromosomal numbers or with distinct meiotic behavior frequently produce infertile progeny [14]. The sexual mode of reproduction has been reported for this species at the diploid (2n = 16) level [65], while the tetraploid plants are mainly apomictic [62]. As a result, the natural populations of guinea grass are characterized by diploid-sexual and tetraploid-apomictic gene pools. However, the genetic exchanges among the two groups are possible by (i) tetraploidization, by crosses between 2x × 4x crosses, (ii) temporary phase of sexuality and hybridization at the tetraploid level, and (iii) haploidization and sexual return to the diploid level [5]. Considering these genetic aspects (high levels of apomictic reproduction in tetraploid pools and limits in sexual reproduction), the recent introduction of this forage plant into the agroecosystems of arid and semiarid regions could tend to the homogenization of the crop and threaten the forage resource sustainability.
The autotetraploid guinea grass is similar to the cultivated forage alfalfa (Medicago sativa) [14,62,66], as both individual plants have more than four sets of chromosomes from the same parental species. However, the natural interspecific crossing between guinea grass and other species such as P. infestum and P. trichocladum has been described as another origin of the genetic diversity and the existence of the agamic complex formed by guinea grass [14]. This interspecific crossing seems to be rarely observed since it is not well described in the literature.
5. Guinea Grass and Drought
The populations in North African countries live under water stress, and the groundwater (depth > 500 m) is the only source of water supply for most of the local demand (agricultural, industry, tourism, and domestic) [67]. Considering the effect of water stress on guinea grass, several studies have been conducted on the subject, a great majority of which reported the potential resistance of this plant [68,69,70,71,72]. They focused on leaf elongation, biomass production, growth, forage quality, and antioxidant responses and other traits under the deficit of water. The net photosynthesis of guinea grass could decrease to zero due to water stress application during the vegetative and reproductive stages [68]. The water stress decreased biomass production affects the stoichiometric homeostasis in Panicum maximum up to 16% [73]. Furthermore, the water stress could delay the elongation of stem and flowering [70]. In response to water stress, guinea grass clipped at larger heights showed a greater reduction in leaf and biomass compared to plants clipped at lower heights [74]. Stressed plants of three guinea grass populations showed a significant reduction in leaf water potentials and relative water contents compared to normally watered plants [75]. Water deficit also reduces seasonal production of guinea grass (cv. Mombaça) [76,77], though little is recognized about the critical values required for its development and the effect of water availability for yearly production [78]. Guinea grass mainly grows in tropical and subtropical areas with more than 900 mm of rainfall on a wide range of soils or in irrigated regions. It is also affected by the seasonality, as are other forage plants. When it is irrigated, the guinea grass attains high production in the spring and summer seasons [79]. To sustain agriculture in arid and semiarid zones, some studies indicated that the antioxidant system of drought resistance could be stimulated in guinea grass by inoculating with PGPB (plant-growth promoting bacteria) [20] or macro-polymeric inoculants of Bacillus strains [80].
When water stress increased, there was a variation in the diverse morphological and physiological traits of guinea grass (e.g., leaf size and stomatal conductance) [52]. As a physiological mechanism of resistance, the guinea grass increased the leaf stomata resistance in order to decrease the transpiration rate [60]. At the plant morphology level, some reports consider that the guinea grass is well adapted to drought stress and presents high adaptation to the most varied edaphoclimatic conditions due to the created clumps and the strong root system [81]. Additionally, under water stress associated with warming, guinea grass increases some biochemical compounds such as photosynthetic pigments, the enzymatic compounds SOD (superoxide dismutase) and APX (ascorbate peroxidase) [72], glutathione, and the osmo-regulator proline [20]. The increase in SOD and APX under abiotic stresses such as drought is a mechanism to prevent the damage from the increased reactive oxygen species (ROS) levels produced due to the stress. As well, the accumulation of PS II activity of guinea grass under water stress preserves membrane stability [72]. In addition, the non-enzymatic, antioxidant-like proline is accumulated by the plants to maintain cellular water homeostasis and to prevent the damage from ROS interaction with plant key molecules, such as DNA, proteins, and lipids. Therefore, shorter period of drought will not affect the survival of guinea grass under a future scenario of climate change [72]. However, the effect of water deficit depends on the growth stage of the plant.
6. Guinea Grass and Salinity
As are most of the arid regions in the world, the cultivated lands in North Africa and the Mideast are affected by progressive and consecutive development of soil salinity and irrigation water. A detailed understanding of the effects of salinity on guinea grass is needed. In fact, the salinity of soil imposes ionic (ion toxicity and imbalance) and osmotic stress (water deficiency) by lowering the soil water potential [82]. Few studies have been designed to elucidate the impacts of salt stress on nutritive, physiological, and morphological traits of this forage plant. Panicum species are known to be tolerant to salt stress. At the germination stage, it seems that a low or moderate salinity (EC 12 and 16) showed a stimulating effect on guinea grass [83]. As an adaptive strategy, plants improve water use efficiency (WUE) and reduce transpiration rate (stomatal limitation) under salt stress, which reflects the elevated water maintenance ability of Panicum [82]. However, the photosynthetic performance is reduced due to the stomata and other biochemical limitations [84]. This forage plant can support salinity when it is planted in a mixture of soil and sand (soil 70%, sand 30%) [85]. A high hemicellulose level was observed in the salinity level of 3.0 dS m−1 [86]. As an alternative to the salt water, the magnetized seawater showed encouraging results in the irrigation on guinea grass [87]. Guinea grass is clustered with the tolerant plants that could be used in saline soils for appropriate management [88]. In saline soil with an electrical conductivity of 11 dS m−1 and among five forage species (Panicum maximum, Setaria sphacelata, Euchlaena mexicana, Brachiaria brizantha and Cynodon plectostachyus), the guinea grass showed the highest level of tolerance. Yet, in saline soil, the application of 20 t/ha manure increased the production, the CP, and the digestible nutrient of guinea grass. Based on this previous data, the guinea grass could be an alternative solution, particularly in high salt cultivated lands.
7. Conclusions
The nutritive values, the perenniality, and the adaptive potentials of guinea grass are the criteria providing an advantage, when compared to many other forage crops. This forage species seems to be tolerant to the major abiotic stresses (water deficit and salinity). However, the introduction of guinea grass in semiarid and arid regions (particularly in the irrigated agroecosystems) and its management recommendations seem to work well only under specific circumstances. Considering the existing vegetation, the allelopathic interactions of this plant with the native crops and its apomixis effects on the agro-diversity in the North African agroecosystems need to be well studied and considered. No study documented its palatability compared to the forages offered in these regions (alfalfa, barley, oats, and sorghum, etc.). Future investigations should aim to concentrate on these issues, under arid and semiarid conditions, to maximize the potential success of guinea grass in the integrated systems of forage/livestock.
Author Contributions
M.A.B. conceived the article and drafted the manuscript with substantial input from W.E. Both authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank all the farmers contacted in Tunisia, for permission to visit their properties and for assistance with the prospection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Simon, B.K.; Jacobs, S.W.L. Megathyrsus, a new generic name for Panicum subgenus Megathyrsus. Austrobaileya 2003, 6, 571–574. [Google Scholar]
- Sánchez-Moreiras, A.M.; Weiss, O.A.; Reigosa-Roger, M.J. Allelopathic evidence in the Poaceae. Bot. Rev. 2003, 69, 300–319. [Google Scholar] [CrossRef]
- Heywood, V.H.; Moore, D.M.; Richardson, I.B.K.; Stearn, W.T. Flowering Plants of the World; Oxford University Press: Oxford, UK, 1978; Volume 336. [Google Scholar]
- Favaretto, A.; Scheffer-Basso, S.M.; Perez, N.B. Allelopathy in Poaceae species present in Brazil. A review. Agron. Sustain. Dev. 2018, 38, 1–12. [Google Scholar] [CrossRef]
- Savidan, Y.; Pernès, J. Diploid-tetraploid-dihaploid cycles and the evolution of Panicum maximum Jacq. Evolution 1982, 36, 596–600. [Google Scholar] [PubMed]
- Aganga, A.A.; Tshwenyane, S. Potentials of guinea grass (Panicum maximum) as forage crop in livestock production. Pak. J. Nutr. 2004, 3, 1–4. [Google Scholar]
- de Sousa, A.C.B.; Jank, L.; de Campos, T.; Sforça, D.A.; Zucchi, M.I.; de Souza, A.P. Molecular diversity and genetic structure of guineagrass (Panicum maximum Jacq.), a tropical pasture grass. Trop. Plant Biol. 2011, 4, 185–202. [Google Scholar] [CrossRef]
- Nakajima, K.; Komatsu, T.; Mochizuki, N.; Suzuki, S. Isolation of diploid and tetraploid sexual plants in guineagrass (Panicum maximum Jacq.). Jpn. J. Breed. 1979, 29, 228–238. [Google Scholar] [CrossRef]
- Duke, J.A. Panicum maximum Jacq. (Poaceae: Guineagrass, Hamilgrass). 1983. Available online: http//www.hort.purdue.edu/newcrop/duke_energy/Panicum_maximum.html (accessed on 10 April 2021).
- Smith, R.L. Seed dormancy in Panicum maximum Jacq. Trop. Agric. 1979, 56, 233–239. [Google Scholar]
- Jank, L. Melhoramento e seleção de variedades de Panicum maximum. In Proc 12 th Simposio Sobre Manejo da Pastagem; FEALQ: Piracicaba, Brazil, 1995; pp. 21–58. [Google Scholar]
- Batistoti, C.; Lempp, B.; Jank, L.; Morais, M.D.G.; Cubas, A.C.; Gomes, R.A.; Ferreira, M.V.B. Correlations among anatomical, morphological, chemical and agronomic characteristics of leaf blades in Panicum maximum genotypes. Anim. Feed Sci. Technol. 2012, 171, 173–180. [Google Scholar] [CrossRef]
- Savidan, Y. Chromosomal and embryological analyses in sexual x apomictic hybrids of Panicum maximum jacq. Theor. Appl. Genet. 1980, 58, 153–156. [Google Scholar] [CrossRef]
- Jank, L.; Calixto, S.; Costa, J.C.G.; Savidan, Y.H.; Curvo, J.B.E. Catalog of the characterization and evaluation of the Panicum maximum germplasm: Morphological description and agronomical performance. Campo Grande Embrapa Gado de Corte 1997, 68, 53. [Google Scholar]
- Hare, M.D.; Phengphet, S.; Songsiri, T.; Sutin, N. Botanical and agronomic growth of two Panicum maximum cultivars, Mombasa and Tanzania, at varying sowing rates. Trop. Grasslands-Forrajes Trop. 2014, 2, 246–253. [Google Scholar] [CrossRef]
- Jank, L.; Costa, J.C.G.; Savidan, Y.H.; do Valle, C.B. New Panicum maximum Cultivars for Diverse Ecosystems in Brazil. In Proceedings of the 17th International Grassland Congress, Palmerston, New Zealand, 8–21 February 1993. [Google Scholar]
- Deddabi, O.S.; Montemurro, C.; Ben Maachia, S.; Ben Amar, F.; Fanelli, V.; Gadaleta, S.; El Riachy, M.; Chehade, A.; Siblini, M.; Boucheffa, S.; et al. A Hot Spot of Olive Biodiversity in the Tunisian Oasis of Degache. Diversity 2020, 12, 358. [Google Scholar] [CrossRef]
- Ecoport. Available online: http://www.ecoport.org (accessed on 12 April 2021).
- Shashikanth, V.S.; Shekara, B.G.; Somashekhar, K.S.; Krishnappa, M.R. performance of guinea grass varieties in southern dry zone of Karnataka. Forage Res. 2013, 39, 147–149. [Google Scholar]
- Dahipahle, A.V.; Bhagat, S.B.; Shinde, D.B.; Mahadkar, U.V.; Gangawane, S.B. Performance of Guinea Grass Varieties in North Konkan Zone of Maharashtra. In Proceedings of the 23rd International Grassland Congress (Sustainable use of Grassland Resources for Forage Production, Biodiversity and Environmental Protection), New Delhi, India, 20–24 November 2015. [Google Scholar]
- Cook, B.G.; Pengelly, B.C.; Brown, S.D.; Donnelly, J.L.; Eagles, D.A.; Franco, M.A.; Hanson, J.; Mullen, B.F.; Partridge, I.J.; Peters, M. Tropical Forages: An Interactive Selection Tool; CSIRO/DPI&F/CIAT/ILRI: Brisbane, Australia, 2005. [Google Scholar]
- FAOG Index. A Searchable Catalogue of Grass and Forage Legumes; FAO: Rome, Italy, 2010. [Google Scholar]
- Adeyemi, I.G.; Soetan, K.O. Preliminary Investigation of Some Serum Biochemical Parameters of Confined Nigerian Cattle Breeds in Ibadan, South-West Nigeria Fed with Some Conventional and Non-Conventional Feedstuffs. Food Sci. Qual. Manag. 2018, 77, 59–65. [Google Scholar]
- Phimmasan, H. Evaluation of Tropical Forages as Feeds for Growing Rabbits. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005. [Google Scholar]
- Sarwatt, S.V.; Mussa, M.A.; Kategile, J.A. The nutritive value of ensiled forages cut at three stages of growth. Anim. Feed Sci. Technol. 1989, 22, 237–245. [Google Scholar] [CrossRef]
- Da Silveira, M.C.T.; Júnior, D.N.; Da Cunha, B.A.L.; Difante, G.S.; Pena, K.S.; Da Silva, S.C.; Sbrissia, A.F. Effect of cutting interval and cutting height on morphogenesis and forage accumulation of guinea grass (Panicum maximum). Trop. Grasslands 2010, 44, 103–108. [Google Scholar]
- Da Silva, S.C.; Bueno, A.A.O.; Carnevalli, R.A.; Silva, G.P.; Chiavegato, M.B. Nutritive value and morphological characteristics of Mombaça grass managed with different rotational grazing strategies. J. Agric. Sci. 2019, 157, 592–598. [Google Scholar] [CrossRef]
- Muir, J.P.; Jank, L. Guineagrass. Warm-Season (C4) Grasses 2004; The American Society of Agronomy: Madison, WI, USA, 2004; Volume 45, pp. 589–621. [Google Scholar]
- Fernandes, F.D.; Ramos, A.K.B.; Jank, L.; Carvalho, M.A.; Martha, G.B., Jr.; Braga, G.J. Forage yield and nutritive value of Panicum maximum genotypes in the Brazilian savannah. Sci. Agric. 2014, 71, 23–29. [Google Scholar] [CrossRef]
- Freitas, K.R.; Rosa, B.; Ruggiero, J.A.; do Nascimento, J.L.; Heinemam, A.B.; Macedo, R.F.; Naves, M.A.T.; de Oliveira, I.P. Avaliação da composição químico-bromatológica do capim Mombaça (Panicum maximum Jacq.) submetido a diferentes doses de nitrogênio. Biosci. J. 2007, 23, 1–10. [Google Scholar]
- Benvenutti, M.A.; Gordon, I.J.; Poppi, D.P.; Crowther, R.; Spinks, W.; Moreno, F.C. The horizontal barrier effect of stems on the foraging behaviour of cattle grazing five tropical grasses. Livest. Sci. 2009, 126, 229–238. [Google Scholar] [CrossRef]
- Deinum, B.; Sulastri, R.D.; Zeinab, M.H.J.; Maassen, A. Effects of light intensity on growth, anatomy and forage quality of two tropical grasses (Brachiaria brizantha and Panicum maximum var. trichoglume). NJAS Wageningen J. Life Sci. 1996, 44, 111–124. [Google Scholar] [CrossRef]
- Cecato, U.; Machado, A.O.; Martins, E.N.; Pereira, L.A.F.; Barbosa, M.A.A.D.F.; dos Santos, G.T. Evaluation of production and any physiological characteristics of genotypes of Panicum maximum Jacq. under two cutting heights. Rev. Bras. Zootec. 2000, 29, 660–668. [Google Scholar] [CrossRef]
- Johnson, W.L.; Ordoveza, A.L.; Hardison, W.A.; Castillo, L.S. The nutritive value of Panicum maximum (Guinea grass) II. Digestibility by cattle and water buffaloes, related to season and herbage growth stage. J. Agric. Sci. 1967, 69, 161–170. [Google Scholar] [CrossRef]
- Rokomatu, I.; Aregheore, E.M. Effects of supplementation on voluntary dry matter intake, growth and nutrient digestibility of the Fiji Fantastic sheep on a basal diet of Guinea grass (Panicum maximum). Livest. Sci. 2006, 100, 132–141. [Google Scholar] [CrossRef]
- Khan, N.A.; Farooq, M.W.; Ali, M.; Suleman, M.; Ahmad, N.; Sulaiman, S.M.; Cone, J.W.; Hendriks, W.H. Effect of species and harvest maturity on the fatty acids profile of tropical forages. JAPS 2015, 25, 739–746. [Google Scholar]
- Hare, M.D.; Tatsapong, P.; Phengphet, S. Herbage yield and quality of Brachiaria cultivars, Paspalum atratum and Panicum maximum in north-east Thailand. TG Trop. Grasslands 2009, 43, 65. [Google Scholar]
- Hare, M.D.; Phengphet, S.; Songsiri, T.; Sutin, N. Effect of nitrogen on yield and quality of Panicum maximum cvv. Mombasa and Tanzania in Northeast Thailand. Trop. Grasslands-Forrajes Trop. 2015, 3, 27–33. [Google Scholar] [CrossRef]
- Paciullo, D.S.C.; Gomide, C.A.D.M.; de Castro, C.R.T.; Maurício, R.M.; Fernandes, P.B.; Morenz, M.J.F. Morphogenesis, biomass and nutritive value of Panicum maximum under different shade levels and fertilizer nitrogen rates. Grass Forage Sci. 2017, 72, 590–600. [Google Scholar] [CrossRef]
- Ajayi, F.T.; Babayemi, O.J.; Taiwo, A.A. Effects of supplementation of Panicum maximum with four herbaceous forage legumes on performance, nutrient digestibility and nitrogen balance in West African dwarf goats. Anim. Sci. J. 2008, 79, 673–679. [Google Scholar] [CrossRef]
- Galindo, F.S.; Buzetti, S.; Teixeira Filho, M.C.M.; Dupas, E. Rates and sources of nitrogen fertilizer application on yield and quality of Panicum maximum cv. Mombasa. Idesia 2019, 37, 67–73. [Google Scholar] [CrossRef]
- Jimoh, S.O.; Amisu, A.A.; Dele, P.A.; Ojo, V.O.A.; Adeyemi, T.A.; Olanite, J.A. Effects of Animal Manures and Cutting Height on the Chemical Composition of Two Panicum maximum Varieties (Local and Ntchisi) Harvested at Different Stages of Growth. Pertanika J. Trop. Agric. Sci. 2019, 42, 359–3769. [Google Scholar]
- Brown, W.F.; Adjei, M.B. Urea ammoniation effects on the feeding value of guineagrass (Panicum maximum) hay. J. Anim. Sci. 1995, 73, 3085–3093. [Google Scholar] [CrossRef]
- Pieterse, P.A.; Rethman, N.F.G.; Van Bosch, J. Production, water use efficiency and quality of four cultivars of Panicum maximum at different levels of nitrogen fertilisation. Trop. Grasslands 1997, 31, 17–123. [Google Scholar]
- Adebisi, I.A.; Ajibike, A.B.; Muraina, T.O.; Alalade, J.A.; Oladepo, O. Performance and Nutrient Digestibility of West African Dwarf goats fed Panicum maximum supplemented with Gmelina arborea leaves mixture. Niger. J. Anim. Sci. 2016, 18, 518–524. [Google Scholar]
- Abdi, H.; Tessema, Z.; Mengistu, U.; Sisay, F. Effect of nitrogen fertilizer application on nutritive value of Cenchrus ciliaris and Panicum maximum grown under irrigation at Gode, Somali Region. J. Nutr. Food Sci. 2015, S11, 1–6. [Google Scholar] [CrossRef]
- Braz, T.G.D.S.; Martuscello, J.A.; Jank, L.; da Fonseca, D.M.; Resende, M.D.V.; Evaristo, A.B. Genotypic value in hybrid progenies of Panicum maximum Jacq. Ciência Rural 2017, 47. [Google Scholar] [CrossRef][Green Version]
- Da Silva, E.B.; Carneiro, M.S.D.S.; Furtado, R.N.; Lopes, M.N.; Braga, M.D.M. Chemical composition of Panicum maximum ‘BRS Zuri’subjected to levels of salinity and irrigation depths. Revista Ciência Agronômica 2020, 51. [Google Scholar] [CrossRef]
- Fajemilehin, S.O.K.; Babayemi, O.J.; Fagbuaro, S.S. Effect of anhydrous magnesium sulphate fertilizer and cutting frequency on yield and chemical composition of Panicum maximum. African J. Biotechnol. 2008, 7, 907–911. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Chou, C.-H. Allelopathic researches in the subtropical vegetation in Taiwan. Comp. Physiol. Ecol. 1980, 5, 222–234. [Google Scholar]
- Silva, A.C., Jr.; Goncalves, C.G.; Scarano, M.C.; Pereira, M.R.R.; Martins, D. Effect of glyphosate on guineagrass submitted to different soil water potential. Planta Daninha 2018, 36. [Google Scholar] [CrossRef]
- Hamza, H.; Jemni, M.; Benabderrahim, M.A.; Mrabet, A.; Touil, S.; Othmani, A.; Salah, M. Ben Date Palm Status and Perspective in Tunisia. In Date Palm Genetic Resources and Utilization; Springer Science & Bussines Media: Dordrecht, The Netherlands, 2015; pp. 193–221. [Google Scholar]
- Benabderrahim, M.A.; Elfalleh, W.; Belayadi, H.; Haddad, M. Effect of date palm waste compost on forage alfalfa growth, yield, seed yield and minerals uptake. Int. J. Recycl. Org. Waste Agric. 2018, 7. [Google Scholar] [CrossRef]
- Benabderrahim, M.A.; Guiza, M.; Haddad, M. Genetic diversity of salt tolerance in tetraploid alfalfa (Medicago sativa L.). Acta Physiol. Plant. 2020, 42, 1–11. [Google Scholar] [CrossRef]
- Young, C.C.; Chou, T.C. Autointoxication in residues of Asparagus officinalis L. Plant Soil 1985, 85, 385–393. [Google Scholar] [CrossRef]
- Nascimento, E.A.; Terrones, M.G.H.; Morais, S.A.L.; Chang, R.; Andrade, G.A.; Santos, D.Q.; Pereira, B.H.A. Allelopathic activity of Cenchrus echinatus L. extracts on weeds and crops. Allelopath. J. 2009, 24, 363–372. [Google Scholar]
- Jackson, J. Is there a relationship between herbaceous species richness and buffel grass (Cenchrus ciliaris)? Austral Ecol. 2005, 30, 505–517. [Google Scholar] [CrossRef]
- Ziegler, A.D.; Warren, S.D.; Perry, J.L.; Giambelluca, T.W. Reassessment of revegetation strategies for Kaho’olawe Island, Hawai’i. Rangel. Ecol. Manag. Range Manag. Arch. 2000, 53, 106–113. [Google Scholar] [CrossRef][Green Version]
- Santiago-Hernández, F.; López-Ortiz, S.; Ávila-Reséndiz, C.; Jarillo-Rodríguez, J.; Pérez-Hernández, P.; de Dios Guerrero-Rodríguez, J. Physiological and production responses of four grasses from the genera Urochloa and Megathyrsus to shade from Meliaazedarach L. Agrofor. Syst. 2016, 90, 339–349. [Google Scholar] [CrossRef]
- Hojsgaard, D.; Hörandl, E. The rise of apomixis in natural plant populations. Front. Plant Sci. 2019, 10, 358. [Google Scholar] [CrossRef]
- Warmke, H.E. Apomixis in Panicum maximum. Am. J. Bot. 1954, 41, 5–11. [Google Scholar] [CrossRef]
- de Wet, J.M.J. Chromosome Numbers of a few South African Grasses. Cytologia 1954, 19, 97–103. [Google Scholar] [CrossRef]
- Moffett, A.A.; Hurcombe, R. Chromosome numbers of South African grasses. Heredity 1949, 3, 369–373. [Google Scholar] [CrossRef]
- Combes, D.; Pernes, J. Variations in chromosome numbers in Panicum maximum Jacq. in relation to method of reproduction. Compte Rendu Hebdomadaire Seances Academie Sciences 1970, 270, 782–785. [Google Scholar]
- Lara, L.A.D.C.; Santos, M.F.; Jank, L.; Chiari, L.; Vilela, M.D.M.; Amadeu, R.R.; dos Santos, J.P.R.; Pereira, G.D.S.; Zeng, Z.-B.; Garcia, A.A.F. Genomic Selection with Allele Dosage in Panicum maximum Jacq. G3 Genes Genomes Genet. 2019, 9, 2463–2475. [Google Scholar] [CrossRef]
- Hamed, Y.; Hadji, R.; Redhaounia, B.; Zighmi, K.; Bâali, F.; El Gayar, A. Climate impact on surface and groundwater in North Africa: A global synthesis of findings and recommendations. Euro-Mediterranean J. Environ. Integr. 2018, 3, 1–15. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Páez, A. Effect of water deficit applied at different growth stages of Panicum maximum Jacq. Revista Facultad Agronomía Universidad Zulia 1996, 13, 79–93. [Google Scholar]
- Ng, T.T.; Wilson, J.R.; Ludlow, M.M. Influence of water stress on water relations and growth of a tropical (C4) grass, Panicum maximum var. trichoglume. Funct. Plant Biol. 1975, 2, 581–595. [Google Scholar] [CrossRef]
- Wilson, J.R.; Ng, T.T. Influence of water stress on parameters associated with herbage quality of Panicum maximum var. trichoglume. Aust. J. Agric. Res. 1975, 26, 127–136. [Google Scholar] [CrossRef]
- Ludlow, M.M.; Ng, T.T. Leaf elongation rate in Panicum maximum var. trichoglume following removal of water stress. Funct. Plant Biol. 1977, 4, 263–272. [Google Scholar] [CrossRef]
- Borjas-Ventura, R.; Alves, L.R.; de Oliveira, R.; Martínez, C.A.; Gratão, P.L. Impacts of warming and water deficit on antioxidant responses in Panicum maximum Jacq. Physiol. Plant. 2019, 165, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Viciedo, D.O.; de Mello Prado, R.; Martínez, C.A.; Habermann, E.; de Cássia Piccolo, M. Short-term warming and water stress affect Panicum maximum Jacq. stoichiometric homeostasis and biomass production. Sci. Total Environ. 2019, 681, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Páez, A.; González, O.M.E.; Yrausquín, X.; Salazar, A.; Casanova, A. Water stress and clipping management effects on guineagrass: I. Growth and biomass allocation. Agron. J. 1995, 87, 698–706. [Google Scholar] [CrossRef]
- Klar, A.E.; Usberti, J.A., Jr.; Henderson, D.W. Differential Responses of Guinea Grass Populations to Drought Stress 1. Crop Sci. 1978, 18, 853–857. [Google Scholar] [CrossRef]
- de Souza, É.M.; Isepon, O.J.; Alves, J.B.; Bastos, J.F.P.; Lima, R.C. Effects of irrigation and nitrogen fertilization on dry matter yield of Panicum maximum cultivars. Rev. Bras. Zootec. 2005, 34, 1146–1155. [Google Scholar]
- Carnevalli, R.A.; Da Silva, S.C.; Bueno, A.A.D.O.; Uebele, M.C.; Bueno, F.O.; Hodgson, J.; Silva, G.N.; Morais, J.P.G. Herbage production and grazing losses in Panicum maximum cv. Mombaça under four grazing managements. Trop. Grasslands 2006, 40, 165. [Google Scholar]
- de Araujo, L.C.; Santos, P.M.; Rodriguez, D.; Pezzopane, J.R.M. Key factors that influence for seasonal production of Guinea grass. Sci. Agric. 2018, 75, 191–196. [Google Scholar] [CrossRef]
- de Jesus, F.L.F.; Sanches, A.C.; de Souza, D.P.; Mendonça, F.C.; Gomes, E.P.; Santos, R.C.; Santos, J.E.O.; da Silva, J.L.B. Seasonality of biomass production of irrigated Mombaça ‘Guinea grass’. Acta Agric. Scand. Sect. B Soil Plant Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Mendoza-Labrador, J.; Romero-Perdomo, F.; Hernández, J.-P.; Uribe, D.; Buitrago, R.B. Enhancement of drought tolerance on guinea grass by dry alginate macrobeads as inoculant of Bacillus strains. bioRxiv 2019, 761056. [Google Scholar] [CrossRef]
- Kissmann, K.G.; Groth, D. Plantas Infestantes e Nocivas–Tomo 1; BASF Brasileira SA: São Paulo, Brazil, 1997. [Google Scholar]
- Hussain, T.; Koyro, H.-W.; Zhang, W.; Liu, X.; Gul, B.; Liu, X. Low Salinity Improves Photosynthetic Performance in Panicum antidotale Under Drought Stress. Front. Plant Sci. 2020, 11, 481. [Google Scholar] [CrossRef]
- Malaviya, D.R.; Roy, A.K.; Anand, A.; Choubey, R.N.; Baig, M.J.; Dwivedi, K.; Kushwaha, N.; Kaushal, P. Salinity tolerance of Panicum maximum genotypes for germination and seedling growth. Range Manag. Agrofor. 2019, 40, 227–235. [Google Scholar]
- Ashraf, M. Relationships between leaf gas exchange characteristics and growth of differently adapted populations of Blue panicgrass (Panicum antidotale Retz.) under salinity or waterlogging. Plant Sci. 2003, 165, 69–75. [Google Scholar] [CrossRef]
- Sawen, D.; Lekitoo, M.N.; Kayadoe, M.; Yoku, O.; Djunaedi, M. Respon Produksi Rumput Gajah (Pennisetum purpureum), Benggala (Panicum maximum) dan Setaria (Setaria spacelata) terhadap Perbedaan Salinitas. Jurnal Riset Agribisnis Dan Peternakan 2020, 5, 20–29. [Google Scholar] [CrossRef]
- Da Silva, E.B. Composição Mineral e Química em Panicum Maximum cv. brs Zuri Submetida a Diferentes Níveis de Salinidade e Lâminas de Irrigação. Ph.D. Thesis, Universidade Federal do Ceará, Fortaleza, Brasil, 2017. [Google Scholar]
- Alfaidi, M.A.; Al-Toukhy, A.A.; Al-Zahrani, H.S.; Howladar, M.M. Effect of irrigation by magnetized sea water on guinea grass (Panicum maximum) leaf content of chlorophyll a, b, carotenoids, Pigments, protein & proline. Adv. Environ. Biol. 2017, 11, 73–84. [Google Scholar]
- Kusmiyati, F. Turnitin-Nfluence of Rice Straw Mulch on Saline Soil: Forage Production, Feed Quality and Feed Intake by Sheep. J. ISAAS 2018, 22, 42–51. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).